Abstract
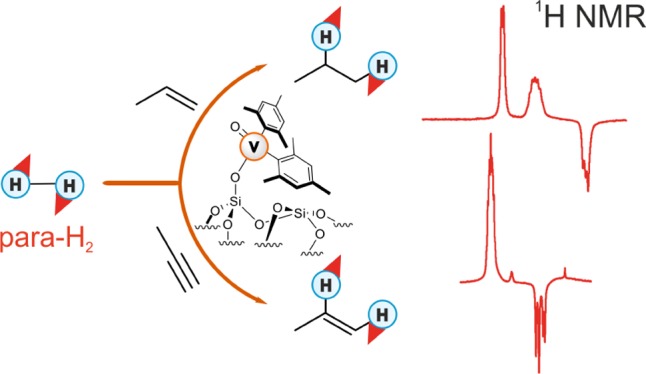
Parahydrogen can be used in catalytic hydrogenations to achieve substantial enhancement of NMR signals of the reaction products and in some cases of the reaction reagents as well. The corresponding nuclear spin hyperpolarization technique, known as parahydrogen-induced polarization (PHIP), has been applied to boost the sensitivity of NMR spectroscopy and magnetic resonance imaging by several orders of magnitude. The catalyst properties are of paramount importance for PHIP because the addition of parahydrogen to a substrate must be pairwise. This requirement significantly narrows down the range of the applicable catalysts. Herein, we study an efficient silica-supported vanadium oxo organometallic complex (VCAT) in hydrogenation and dehydrogenation reactions in terms of efficient PHIP production. This is the first example of group 5 catalyst used to produce PHIP. Hydrogenations of propene and propyne with parahydrogen over VCAT demonstrated production of hyperpolarized propane and propene, respectively. The achieved NMR signal enhancements were 200–300-fold in the case of propane and 1300-fold in the case of propene. Propane dehydrogenation in the presence of parahydrogen produced no hyperpolarized propane, but instead the hyperpolarized side-product 1-butene was detected. Test experiments of other group 5 (Ta) and group 4 (Zr) catalysts showed a much lower efficiency in PHIP as compared to that of VCAT. The results prove the general conclusion that vanadium-based catalysts and other group 4 and group 5 catalysts can be used to produce PHIP. The hydrogenation/dehydrogenation processes, however, are accompanied by side reactions leading, for example, to C4, C2, and C1 side products. Some of the side products like 1-butene and 2-butene were shown to appear hyperpolarized, demonstrating that the reaction mechanism includes pairwise parahydrogen addition in these cases as well.
Introduction
Parahydrogen-induced polarization (PHIP) is a nuclear spin hyperpolarization technique based on the use of parahydrogen-enriched H2 (para-H2) in catalytic reactions.1−3 Upon hydrogenations with para-H2, strong NMR signals of specific shape amplified by several orders of magnitude can be observed, revealing the high nuclear spin hyperpolarization in the reaction products. The hyperpolarized products obtained this way can be used for substantial sensitivity boosting in NMR spectroscopy and magnetic resonance imaging, which often suffer from an insufficient sensitivity. PHIP does not require remarkably sophisticated equipment necessary in other spin hyperpolarization methods like dynamic nuclear polarization (DNP).4 The efficiency of PHIP, however, crucially depends on the catalyst-mediated para-H2 interaction with substrates. The para-H2 addition process must be pairwise, meaning that the two hydrogen atoms must follow each other throughout the catalytic cycle and end up together in the same product molecule. The requirement of the pairwise mechanism, therefore, provides a unique type of labeling, which is sensitive to pairwise catalytic processes. It also sets, however, some limitations on the catalytic system if one wishes to get strong spin hyperpolarization by using PHIP. So far, the most efficient catalysts providing high contribution of the pairwise mechanism are dissolved precious metal complexes, which are unfortunately difficult to rapidly separate from the hyperpolarized substances. As solid heterogeneous catalysts are easy to separate from reaction mixtures, heterogeneous hydrogenation using parahydrogen is considered as an efficient approach for producing pure hyperpolarized gases and liquids. For the last decade, many heterogeneous catalytic systems were found to produce PHIP in hydrogenations with parahydrogen.5−9 In most cases, the catalysts were based on the group 8 metal nanoparticles10−12 or complexes supported on oxides.7−9,13 Metal nanoparticle-based catalysts provided high stability but moderate enhancements, whereas immobilized complexes are typically unstable under reaction conditions.8,9
Vanadium-based catalysts are used in a number of important industrial processes.14−17 Recent developments led to a vanadium oxo organometallic complex supported on silica as a new efficient catalyst for non-oxidative dehydrogenation of propane (Scheme 1).18 This catalyst, designated in the text as VCAT, demonstrates high activity, selectivity, and stability, and it is well-characterized under propane dehydrogenation reaction conditions. On the other hand, VCAT can be potentially used in hydrogenation reactions, because hydrogenation reaction is the reverse of dehydrogenation, and thus, it can be catalyzed by the same catalyst. In principle, sequential dehydrogenation–hydrogenation can also lead to the spin hyperpolarization if para-H2 addition proceeds in a pairwise manner, as was reported previously.8,11,19,20 This route of production of hyperpolarized substances is referred to in the literature as “pairwise replacement”.19,20 Thus, propane dehydrogenation over VCAT may produce hyperpolarized propane. Thus, both hydrogenation and dehydrogenation using VCAT in the presence of para-H2 are of great interest to explore. At the same time, observation of hyperpolarization effects can provide important mechanistic information about these chemical reactions catalyzed by VCAT.
Scheme 1. VCAT Structure.

Herein, we show that surface-grafted vanadium complex VCAT can provide hyperpolarized compounds in hydrogenations with parahydrogen. In particular, it is shown that in spite of a relatively low activity, this catalyst demonstrates high stability over long reaction runs, providing continuous production of hyperpolarized propane in propene hydrogenation with parahydrogen. It is demonstrated that the production of hyperpolarized propane takes place over a wide range of temperatures (250–500 °C). On the other hand, propane dehydrogenation in the presence of para-H2 led to the observation of hyperpolarization effects for the side-product 1-butene, whereas propane itself remained not polarized, implying that the H2 pairwise replacement process is likely inefficient. It is shown that in comparison to several other surface-grafted complexes of group 5 and 4 metals (Ta- and Zr-based complexes), VCAT catalyst demonstrates higher activity and efficiency in the production of hyperpolarized substances. It was demonstrated that the catalytic activity of VCAT catalyst in hydrogenations is the highest among the examined catalysts. In contrast to group 5 catalysts (VCAT and Ta-based one), Group 4 catalysts (Zr-based) were more active at low temperatures (below 0 °C), but their activity was still low.
Experimental Section
Catalysts
The VCAT catalyst was prepared by the synthetic procedure described elsewhere.18 Prior to grafting reactions, the molecular precursor [V(=O)(Mes)3] was synthesized in two steps.21,22 A mixture of the molecular complex (300 mg, 0.70 mmol) and the support SiO2-(700) (2 g) in pentane (10 mL) was stirred at 25 °C for 2 h. After filtration, the solid, VCAT, was washed five times with pentane. The resulting red powder was dried under vacuum (10–5 Torr). FTIR, UV–vis, and solid state NMR (1H and 13C) spectra (Figures S6–S8) as well as the metal loading of 1.4 wt % determined by elemental analysis were in agreement with literature data.18
In addition, several other group 4 and 5 catalysts presented in Table 1 were tested. These catalysts were synthesized according to the methods described in ref (23). All catalyst treatments were performed under an Ar atmosphere.
Table 1. Labels and Structures of Other Studied Group 4 and 5 Complexes.
PHIP Experiments
The parahydrogen-enriched H2 was produced using Bruker parahydrogen generator BPHG-90 (the actual parahydrogen content in H2 was ca. 80–90%). This gas is referred to as para-H2 in the text. NMR experiments were performed on a Bruker AV-300 spectrometer operating at 300 MHz 1H resonance frequency.
In most cases, investigated hydrogenation and dehydrogenation reactions in the presence of para-H2 were carried out in a quartz reactor operating in the temperature range from 25 to 650 °C outside the NMR magnet (Figure 1, right branch). The gas mixture after the reactor was flowing through a capillary into a 10 mm NMR tube inside the NMR magnet to acquire 1H NMR spectra. This experimental procedure involving adiabatic transfer of the products of reaction with para-H2 from low to high magnetic fields leads to the so-called ALTADENA24 effect. In a common run, 3–5 mg of catalyst packed in the reactor were used. The gas flow rate through the reactor was 2.1 or 4.1 scc/s.
Figure 1.
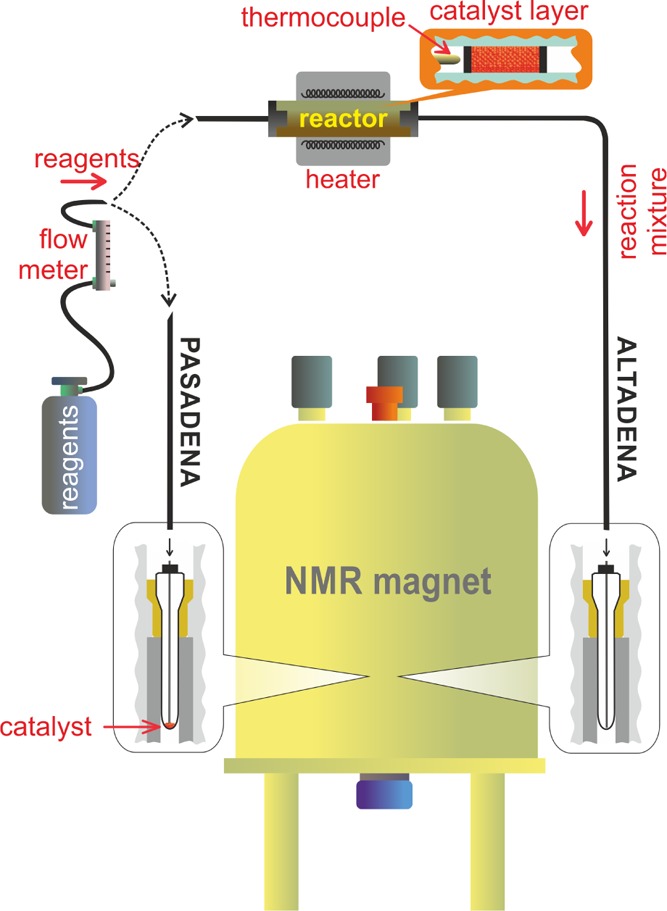
Experimental setup and procedure. The reagent gas flow controlled by a flowmeter was supplied either to the packed bed reactor outside and then to the empty sample tube inside the NMR magnet (right branch, ALTADENA experiment), or directly into the sample tube with the catalyst inside the NMR magnet (left branch, PASADENA experiment).
In selected experiments, when heating to high temperatures was not required, the reactions with para-H2 were performed inside the NMR magnet in a 10 mm tube containing 3–5 mg of catalyst (Figure 1, left branch). The NMR spectra were acquired during the reaction. The gaseous reagents were passed through the catalyst layer at the bottom of the tube using a 1/16″ Teflon capillary with the flow rate of 2.1 scc/s in the temperature range from −20 to +130 °C. As products were formed in the high magnetic field, the so-called PASADENA1 hyperpolarization effect was observed in this case.
In propene hydrogenation with VCAT, several gas mixtures of differing propene/para-H2 ratios were used in the experiments: 1/3, 1/4, and 1/6. In the experiments with other substrates (propane, propyne, 1,3-butadiene, and 1-butyne) and other catalysts (Table 1), the substrate/para-H2 ratio was 1/4.
Results
To the best of our knowledge, neither group 4 nor group 5 heterogeneous catalysts are presented in the literature in the context of PHIP studies. Herein, main results are obtained for the vanadium VCAT catalyst (Scheme 1). For comparison, other group 5 and additionally group 4 catalysts were tested in hydrogenations with para-H2, see Table 1 and Supporting Information. Thus, the following discussion of the results is divided into two parts describing VCAT results first and comparing it to other catalysts, respectively.
VCAT Catalyst
Propene Hydrogenation
1H NMR spectra acquired for propene hydrogenation catalyzed by the VCAT catalyst at 350 °C are shown in Figure 2. The use of para-H2 as a reagent led to the enhanced NMR signals of the product propane molecules when the gas mixture was flowing through the catalyst at 4.1 scc/s, Figure 2a. The signals appeared in the form of two multiplets of opposite signs; negative sign (emission) for methyl group and positive sign (absorption) for methylene group. For comparison, the spectrum representing thermally polarized signals acquired after an abrupt termination of gas flow followed by a 5 s relaxation delay is shown in Figure 2b. The signal enhancements can be seen clearly from the comparison to the thermal 1H NMR spectrum. The observation of the enhanced signals provides an additional insight into the mechanism of hydrogenation over the VCAT catalyst because hyperpolarized signals indicate the addition of para-H2 molecules to propene in a pairwise manner. The contribution of the pairwise mechanism to the overall propene hydrogenation process was estimated from the observed enhancements as 1–2%. We should note, however, that this estimate constitutes a lower bound because nuclear spin relaxation processes decreasing originally produced hyperpolarization were not taken into account.
Figure 2.

1H NMR spectra of the gaseous reaction mixture from propene hydrogenation catalyzed by VCAT. Spectrum (a) was detected while the propane was produced in the reactor at 350 °C and the reagent gas flow rate of 4.1 scc/s and the mixture was flowing through the sample tube, whereas spectrum (b) was detected 5 s after the gas flow was abruptly stopped. The initial propene/para-H2 ratio in the reagent gas mixture was equal to 1/4.
It should be noted that some less-pronounced PHIP effects in the NMR spectra were also evident for reagent propene molecules. Indeed, the ratios of the amplitudes of H1b and H1c signals to the amplitude of H1a signal in Figure 2a reveal a significant difference compared to the corresponding ratios in the spectrum of thermally polarized propene in Figure 2b. H1b and H1c signals have much lower intensity in comparison to H1a signal in the former case. This observation indicates that in addition to propene hydrogenation the replacement of two vicinal hydrogens in the reagent propene molecules by hydrogens of a para-H2 molecule takes place. It also gives a new insight to the mechanism of propene hydrogenation over VCAT and side processes accompanying it. Such pairwise replacement in propene molecules was reported earlier with Rh, Pt, and Ir metal nanoparticles,8,19 and possible mechanisms leading to this effect are discussed in the literature.19
The hyperpolarization levels were slightly dependent on the composition of the reagent gas mixture, with higher content of parahydrogen providing on average slightly higher hyperpolarization levels, Figure 3. This was likely influenced by the conversion between para-H2 and ortho-H2 accompanying the hydrogenation over VCAT. The higher concentration of para-H2 resulted in higher probability that parahydrogen is added to propene before it is converted to orthohydrogen. The higher flow rates on average also resulted in higher detected hyperpolarization as the influence of the nuclear relaxation during the gas transport is reduced, Figure 3b,c.
Figure 3.

1H NMR signal enhancements measured as a function of the reactor temperature for hyperpolarized propane produced in propene hydrogenation with para-H2 over VCAT. Average enhancement values over CH2 and CH3 groups are presented. The bar charts shown in (a–c) correspond to the reagent mixtures with 1/3, 1/4, and 1/6 propene/para-H2 ratios, respectively. The reagent gas flow rates in the reactor are indicated in the legends.
We note that catalytic activity of VCAT in propene hydrogenation was evident clearly at 250 °C and higher temperatures. In the activity tests, the temperature was increased stepwise from 50 to 500 °C under continuous flow of reagent gas mixtures having propene/para-H2 ratio of 1/4 and 1/6 (Figure 4). Experiments were also done with the mixture of 1/3 propene/para-H2 ratio, but conversion of propene was hard to quantify precisely in this case (below 1%). The initial activity increased slightly in time over the period of ca. 2 h at the elevated temperature of 500 °C and reached some constant value, which was in accord with previous observations in the study of catalytic performance over this catalyst in propane dehydrogenation.18 For a stability check, the temperature was decreased from 500 to 300 °C and then gradually increased to 500 °C again. The catalytic activity was preserved at the same level, which confirmed the thermal stability of the catalyst after a 2 h activation treatment. In the course of the activation, the color of the catalyst changed from orange to black.
Figure 4.

Propene conversion as a function of temperature in propene hydrogenation over VCAT. The results are shown for the reagent gas mixtures of (a) 1/4 and (b) 1/6 propene/p-H2 ratios and two different gas flow rates (2.1 and 4.1 scc/s).
Importantly, PHIP signal amplitudes changed accordingly with the changes of the temperature described above: the higher the yield, the stronger the signal from the hyperpolarized propane. This means that the hyperpolarization degree was not significantly dependent on the temperature, and the signal intensity was determined majorly by the number of produced molecules. The observation serves as an extra evidence of the active center stability under reaction conditions. In the vast majority of cases, immobilized complexes are unstable at such high temperatures,8,9 which is clear from irreversible changes of hyperpolarization degree of the products. Our experience in the field tells that group 8 metal complexes (Rh, Ir, and Pd) tend to degrade rapidly under propene hydrogenation conditions above 150 °C with the formation of metal nanoparticles, whereas VCAT demonstrates high stability even at 500 °C. These results suggest that vanadium species have been transformed to tripodal V(III) (Scheme S1 in Supporting Information) upon the heat treatment, because such isolated species are expected to be stable similarly to unsaturated tripodal tantanlum surface species.25 Furthermore, they can easily conduct oxidative addition of H2 molecules (Scheme S2). The catalytic cycle is completed after an insertion of propene into the resulting H–V–H center, followed by the reductive elimination of propane. The propane molecule should be hyperpolarized in this case as the hydrogenation is pairwise (see Scheme S2 in Supporting Information). Recently, a similar vanadium complex (VIII(Mes)3·THF) has been grafted onto silica and investigated in alkene and alkyne hydrogenations.26 This bipodal vanadium complex was catalytically active in 1-octene hydrogenation at 70 °C and diphenyl acetylene at 100 °C. The mechanism of these reactions was proposed to be either oxidative addition/reductive elimination or heterolytic activation. However, no direct evidence was given. For VCAT at relatively low temperatures (<250 °C), no significant hyperpolarization was observed, while the catalytic activity was low. This result may indicate that at the low temperatures VCAT can undergo a transfer of hydride to the support,25 giving a bipodal vanadium(V) oxo species (Scheme S1) which can complete the catalytic cycle only by nonpairwise heterolytic activation (Scheme S3). We note, however, that the formation of the tripodal V(III) at high temperatures is a tentative conclusion based on the literature data and our experience with group 5 catalysts. More data about the nature of the active species can be obtained by using a combination of ex situ, in situ, and operando characterization methods, which will be addressed in our future study of VCAT. Such experiments are beyond the scope of this paper, which describes mostly the observation of hyperpolarization effects with this catalyst.
Finally, we can conclude that the 1H NMR signal enhancements observed for propane in the propene hydrogenation with parahydrogen are on the order of 100–300-fold, depending on the experimental conditions (Figure 3). These numbers are lower than the theoretically expected ones (ca. 104-fold at 7 T), but they do demonstrate that orders of magnitude signal enhancements are possible with VCAT. As mentioned above, the enhancements can be converted into the fractions of pairwise addition in the overall hydrogenation process, which constitute the lowest values possible, providing the fraction of pairwise addition on the order of 1–2%. The nuclear relaxation processes in this case may lead to a severe underestimation of the pairwise addition fractions, especially considering that the vanadium center is paramagnetic in certain oxidation states. The paramagnetic relaxation can significantly accelerate the nuclear spin relaxation, leading to quenching of hyperpolarization. In the case of VCAT, however, this process seems to be not extremely fast as to prevent an observation of substantial nuclear hyperpolarization in propene hydrogenation.
Propane Dehydrogenation
Propane dehydrogenation over VCAT was carried out using the propane/para-H2 mixture with the ratio of the reagents equal to 1/4. In principle, dehydrogenation in the presence of para-H2 molecules may result in hyperpolarized products if pairwise hydrogen replacement or dehydrogenation followed by rehydrogenation take place.11,19,20 Formally, these processes can lead to hyperpolarized propane or propene as represented in Scheme 2.
Scheme 2. Formal Mechanism of Pairwise Replacement Leading to the Formation of Hyperpolarized Propane.

In our experiments, signals of hyperpolarized products at 4.7 and 5.7 ppm in 1H NMR spectra were initially detected upon heating to 400 °C when the reaction mixture was passed through a VCAT layer at the flow rate of 2.1 scc/s (Figure 5). These signals were also observed at 450, 500, and 550 °C at the same flow rate. As it was determined from a thorough analysis of the NMR spectra, these signals correspond to hyperpolarized 1-butene, namely to the CH (5.7 ppm) and CH2 (4.7 ppm) groups of its vinyl fragment. This was an interesting and unexpected result indicating that complex side processes take place during the interaction of propane molecules with VCAT in the presence of para-H2. The NMR spectra show also the presence of ethylene along with 1-butene in the temperature range 400–550 °C. The formation of the C2 and C4 reaction products from C3 propane molecules on VCAT under these conditions implies likely that the reaction mechanism includes a C–C coupling step resulting in C6 chain which in turn is split into C2 and C4 parts. Alternatively, the C4 and C2 hydrocarbons could be formed as a result of metathesis of two propene molecules. At the same time, no signals of 1-butene were detected in the 1H NMR spectra of reaction mixture if the reaction was carried out at 600 and 650 °C. Moreover, at these high temperatures, propene was the main reaction product, while ethylene and methane were detected as side products (Figure 5e,f). At these temperatures, the thermodynamics is favorable for the formation of propene, while formation of C1 methane and C2 ethylene is the result of propane molecule splitting. The change in the catalytic behavior of VCAT with the temperature was also apparent from the propane conversion, which for the mixture flowing at 2.1 scc/s increased as follows: 1–2% at 400 °C, 9% at 600 °C, and 14% at 650 °C. It should be noted that after cooling down the reactor from 650 to 450 °C the formation of hyperpolarized 1-butene as well as ethylene was again observed, implying that the catalyst was stable under applied high temperature conditions.
Figure 5.
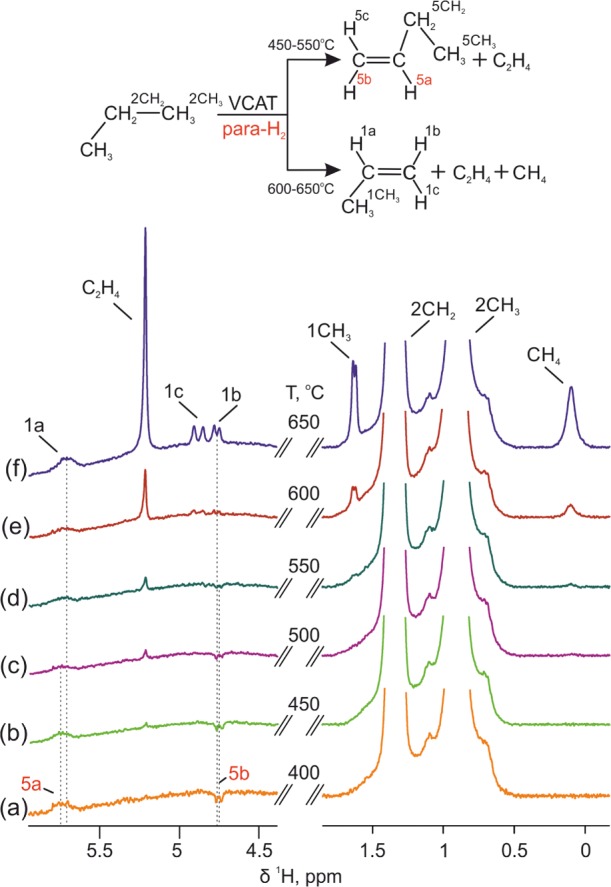
1H NMR spectra of reaction mixture formed in propane dehydrogenation on VCAT when the propane/para-H2 (1/4) gas mixture was passed through the reactor at different rector temperatures of (a) 400, (b) 450, (c) 500, (d) 550, (e) 600, and (f) 650 °C.
It should be noted that the observed hyperpolarization of 1-butene protons at the double carbon–carbon bond, H5a and H5b, is rather unexpected because we would instead expect to see hyperpolarization of propane or propene as represented in Scheme 2. The mechanism of hyperpolarized 1-butene formation is unclear, but undoubtedly, it includes pairwise addition of two hydrogen atoms originating from one para-H2, which is evidenced by the observation of the PHIP effect itself. It is possible that the dehydrogenation goes deeper, and a triple carbon–carbon bound is formed, which is then hydrogenated with para-H2. Indeed, in the tests with 1-butyne hydrogenation catalyzed by VCAT at 500 °C it was shown that hyperpolarized 1-butene can be formed from 1-butyne and para-H2 (see Figure S1 in Supporting Information). However, in this case the enhanced hyperpolarization signals are observed for all hydrogens at the 1-butene double bond, whereas in propane dehydrogenation only H5a and H5b hydrogens got hyperpolarized (Figure 5a). This test indicates that there are likely some differences in the mechanisms of formation of hyperpolarized 1-butene in the propene dehydrogenation and in the 1-butyne hydrogenation.
The NMR signal enhancement for H5a hydrogen of 1-butene was about 25 in the propane dehydrogenation, which was determined by comparing the amplitudes of the hyperpolarized and thermal signals (see Figure S2 in Supporting Information). This value corresponds to less than 1% fraction of the pairwise addition, meaning that the pairwise replacement of hydrogens is not very efficient in the complex process of 1-butene formation from propane, which must also include processes such as oligomerization or metathesis. In an earlier study, some of us have demonstrated the possibility to observe PHIP for C4 oligomerization products during acetylene hydrogenation with para-H2 over palladium nanoparticles,11 wherein the signal enhancements generated for H2a of 1-butene molecule were an order of magnitude higher than the value observed for the same product produced over VCAT in propane dehydrogenation in this work. Additionally, in the case of VCAT, PHIP was observed only for hydrogens H5a and H5b but not for H5c (i.e., for the pair of protons cis to each other with respect to the double bond), whereas there was no such selectivity in the case of Pd nanoparticles. Apparently, the pathways of C4 product formation and hyperpolarization significantly differ for these catalytic systems.
In contrast, propane signals did not reveal any noticeable hyperpolarization under propane dehydrogenation conditions, implying that the pairwise hydrogen replacement process19 drawn in Scheme 2 for propane is not efficient. However, one should consider that the polarized signals of propane could be possibly masked by the strong signals of thermally polarized propane. The inefficiency of pairwise hydrogen replacement in propane is in accord with previously reported tests performed using Pt and Ir nanoparticles as catalysts.19
Propyne Hydrogenation
A significant hyperpolarization of propene was observed in the hydrogenation of propyne with para-H2 over VCAT, Figure 6.
Figure 6.

1H NMR spectra of the reaction mixture produced in propyne hydrogenation with para-H2 over VCAT at 500 °C. Spectrum (a) was detected in the continuous flow mode using a single scan, whereas (b) is detected using 256 scan accumulations after the flow was abruptly stopped. The reagent mixture of 1/4 propyne/para-H2 ratio was used, which was supplied to the reactor at the flow rate of 2.1 scc/s.
The measured NMR signal enhancement for proton H1a of produced propene is ca. 1300, which is an order of magnitude higher compared to that observed for methyl and methylene groups of propane in propene hydrogenation. In contrast to propene hydrogenation, however, VCAT was much less active in propyne hydrogenation, providing typical reaction yields of less than 1% under similar experimental conditions. Moreover, the higher temperatures around 500 °C were required to observe detectable signals of the hyperpolarized propene.
In addition to propene, hyperpolarized 2-butene was visible in the 1H NMR spectra acquired from the reaction mixture in continuous reaction mode (Figure 6a). Moreover, thermally polarized ethylene was also detected (Figure 6b). Such observations imply that like in propane dehydrogenation the side reactions leading to the C4 and C2 hydrocarbons took place, meaning that propyne hydrogenation over the VCAT catalyst is accompanied either by oligomerization and hydrogenolysis processes or metathesis because the initial feed contained only the C3 hydrocarbon. Because the high temperatures were used, to verify that the hyperpolarized butene and propene were produced over VCAT, but not due to pyrolysis, we performed test experiments with no catalyst loaded in the reactor but otherwise under the same reaction conditions (same temperatures and flow rates). No hyperpolarized/thermally polarized butene or propene products were detected at the same level of accuracy, implying that the catalytic action of VCAT was the source of the hyperpolarized substances.
Other Group 4 and 5 Catalysts
Some selected catalysts based on immobilized complexes of other group 4 and group 5 elements were tested in hydrogenation reactions with para-H2. The structures of the catalysts are shown in Table 1.
Tantalum belongs to the same group as vanadium. Immobilized TaCAT complex was studied in the hydrogenation of propene in the temperature range of 25–400 °C. The complex was found to become active at 200 °C in propene hydrogenation with parahydrogen. Moreover, the formation of hyperpolarized propane was detected at this temperature by acquiring 1H NMR spectra of the flowing reaction mixture in the ALTADENA-type experiments (see Figure S3, Supporting Information). The signals of the hyperpolarized propane were also detected when the reaction was carried out at 250–400 °C. The measured signal enhancement factors and propene conversion data are presented in Figure 7.
Figure 7.

Enhancement factors and propene conversions obtained in propene hydrogenation with para-H2 over TaCAT in the temperature range of 200–400 °C. The gas reagent flow rate was 2.1 scc/s. The enhancement factors are calculated for protons of CH3 group of propane.
It can be seen that the signal enhancement factors varied in the range of 70–90, which means that the enhancement was practically constant in the examined temperature range. The conversion, however, changed significantly with the highest value at 200–250 °C (4%) and the lowest value at 350–400 °C (1%). Thus, TaCAT produced lower levels of hyperpolarization (Scheme S4 in Supporting Information) but comparable reaction yields as compared to the data for VCAT catalyst (Figures 3 and 4). The decrease in the activity at the high temperatures is likely due to partial transformation of bipodal monohydride Ta–H species to tripodal Ta species,25 which are inactive in hydrogenations (Scheme S5 in Supporting Information).
ZrCAT immobilized complex was used as an example of group 4-based catalyst in propene hydrogenation with para-H2. This complex became active at 150 °C reaction temperature, and hyperpolarized propane was detected in 1H NMR spectra of the reaction mixture under continuous flow conditions (Figure S4 in Supporting Information). It was found that temperature increase to 300–500 °C leads to decrease in the hyperpolarization of propane and deactivation of the catalyst. The signal enhancement factor measured for the hyperpolarized propane was 20 at 150 °C and 6 at 300 °C. At the same time, no propane signals were detected at 400 °C or higher. This observation likely indicates a restructuring of the surface species (Scheme S6 in Supporting Information). The hyperpolarization is likely produced by bipodal zirconium bis-hydride species (Scheme S7 in Supporting Information). The number of such species declines with the temperature because of a gradual transformation to tripodal zirconium monohydride. The mechanism of the hydrogenation reaction with the latter species is such that the hydrogen insertion cannot be pairwise, (Scheme S8 in Supporting Information) and consequently, no hyperpolarization of propane is expected. At the higher temperature (400 °C), the zirconium completely transforms to tetrapodal species without any possibility to insert propene.
It should be noted that in addition to propane, hyperpolarized 1-butene was formed at 300–350 °C over ZrCAT. However, the temperature increase led to vanishing of 1-butene at 400 °C as well from the reaction mixture. The enhancement factor for 1-butene 1H NMR signal was likely very high, because corresponding thermal signals were not observed after 128 accumulations when the reaction mixture flow was stopped. We note also that thermally polarized 1,3-butadiene was detected in 1H NMR spectrum of the reaction mixture when the flow was stopped (Figure S4b* in Supporting Information). The observation of 1,3-butadiene suggests that the hyperpolarized 1-butene produced in continuous flow conditions could be formed by hydrogenation of 1,3-butadiene, which is initially generated by the reaction of propene with ZrCAT catalyst. This assumption is supported by the production of hyperpolarized 1-butene with a 1H NMR signal shape similar to that detected in propene hydrogenation when 1,3-butadiene hydrogenation with para-H2 was tested at 300 °C with the same catalyst (Figure S5 in Supporting Information).
Discussion
The observation of PHIP effects demonstrated in this work for the hydrogenations of propene, propyne, and 1-butyne with para-H2 over VCAT constitutes a solid evidence for the presence of pairwise addition mechanistic pathways in these reactions. This is an important feature of VCAT, making vanadium-based catalysts worth considering for an efficient production of hyperpolarized substances. It is also interesting in terms of understanding of the mechanistic aspects of hydrogenation catalyzed by the complex. Basically, two ways of para-H2 activation can lead to the pairwise addition and eventually to the formation of the spin hyperpolarization. In the first case, the hydrogenation proceeds through the oxidative addition of para-H2.27 This way is well-exemplified by the formation of dihydride compounds from transition metal complexes and para-H2 molecules in homogeneous hydrogenations. In the second case, the reaction proceeds through a heterolytic activation of molecular hydrogen, meaning that the charge separation occurs in para-H2 activation.6,28 This way is characteristic, for instance, for oxide-based catalytic systems like activated ZnO,29 in which one of the hydrogen atoms from the para-H2 molecule goes to the metal ion whereas another goes to the oxygen. The presence of PHIP on propane produced from propene and para-H2 may be the result of any of these two mechanistic pathways as soon as the vanadium center can interact to form a dihydride complex, and also, this center contains oxygen as a ligand which may facilitate the heterolytic activation.
It is worth to note that to produce hyperpolarized propane efficiently in propene hydrogenation with para-H2, the time scale of transfer of the two hydrogens to the substrate should be relatively fast compared to the lifetime of the hyperpolarization. In other words, the transfer time should be preferably much shorter than the time during which the hyperpolarization is destroyed. Depending on conditions, the NMR signal enhancements observed for CH2 and CH3 groups of propane produced over VCAT varied in the range of 100–350-fold. The reaction temperature had only a weak effect on the enhancement factors (Figure 3). This observation indicates that the reaction step responsible for the transfer of para-H2 to the substrate is not the rate-limiting one or it is rate-limiting but always much faster than the nuclear spin relaxation. We note also that the amplitude of ortho-H2 signal visible in the NMR spectra was relatively low and temperature-independent in the studied hydrogenations over VCAT, indicating the absence of a fast ortho–para conversion process in the studied temperature range and implying that the addition of H2 to VCAT is likely an irreversible step.
In addition, mechanistic aspects of propene hydrogenation over VCAT are reflected by the observed weak hyperpolarization of H1a, H1b, and H1c hydrogens of propene when it was hydrogenated to propane with para-H2 (Figure 2). This observation most likely indicates the presence of side reactions leading to pairwise replacement of pairs of hydrogens at the double bond in propene with the ones originating from para-H2. Such processes were observed in several studies with supported Rh, Pt, and Pd metal catalysts and in situ reduced immobilized complexes.8,11,19,20 On the other hand, we cannot entirely exclude the polarization transfer from para-H2 to the catalyst-bound propene on the catalytic center, which may also occur as the reaction was performed at the low magnetic field, that is, under conditions which are known to lead to the signal amplification by reversible exchange effect (SABRE).30
On the basis of the enhancement factors, the contribution of pairwise addition of para-H2 to propene in the overall hydrogenation process catalyzed by VCAT is around 1–2%. This value is the lower limit estimate of the contribution, because relaxation of the hyperpolarization is hard to account for correctly in the evaluation, and the actual value of the pairwise contribution can be significantly higher. This estimated value is comparable to those observed in propene hydrogenation over immobilized Vaska’s complex and the immobilized Wilkinson’s complex reduced in situ.7−9 VCAT, however, has the highest stability compared to the Rh and Ir immobilized complexes. Supported Pt and Rh metal catalysts also provide comparable values of the pairwise addition in propene hydrogenation with para-H2.5
As was discussed above, the NMR signal enhancements are practically unchanged in the temperature range of 250–500 °C, meaning that the temperature does not significantly influence the contribution of pairwise addition to the overall propene hydrogenation over VCAT. On the other hand, the temperature had an effect on the propene conversion. In particular, it can be seen that largest propene conversions of 7 and 9% at 2.1 scc/s flow rate were observed at 250 °C for 1/4 and 1/6 propene/para-H2 mixtures, respectively. At the same time, the propene conversion rapidly drops with increasing temperature, and at 500 °C, the conversion did not exceed 3% for both mixtures. This observation most likely spotlights the shift of thermodynamic equilibrium to the formation of propene and H2 at such a high temperature because of the exothermic nature of the hydrogenation reaction, when hydrogenation is no longer favorable.
We should note that other immobilized complexes of group 4 and 5 elements (Ta and Zr) studied in this work have shown only weak activity in propene hydrogenation. Some promising conversion of propene to hyperpolarized propane was obtained with TaCAT, but the NMR signal enhancement of the product was several times lower as compared to what was observed with VCAT. The hyperpolarization of propane produced with ZrCAT was in turn several times lower than for TaCAT. Therefore, among all studied catalysts VCAT was the best catalyst to produce hyperpolarized propane, which also demonstrated a noticeable stability over long reaction runs.
Interestingly, switching the substrate hydrogenated with para-H2 from propene to propyne had a significant effect on both the observed NMR signal enhancement of the reaction product, propene, and the catalytic activity of VCAT. A dramatically increased signal enhancement observed for the product molecules of 1300–1500-fold was recorded, which is almost an order of magnitude larger value as compared to the signal enhancement for propane in propene hydrogenation with para-H2. These values correspond to at least 10–12% of pairwise addition. Thus, the contribution of pairwise addition to the overall hydrogenation of propyne over VCAT is significant, especially if we take into account the effects of relaxation processes leading to underestimation of the fraction of pairwise addition. The significant difference in the NMR signal enhancements observed in the hydrogenations of propene and propyne indicates a significant difference in hydrogenation mechanisms for these substrates. A detailed elucidation of these differences is currently not possible as it requires sophisticated in situ VCAT studies not accessible at the current state-of-the-art, and this is beyond the scope of this article. We note, however, that such a significant enhancement considerably distinguishes this complex from previously studied heterogeneous catalysts. Indeed, hydrogenation on supported metal catalysts or oxides usually provides NMR signal enhancements between 100–200, while the use of immobilized Ir catalysts makes it possible to obtain enhancements of about 500–700-fold under similar experimental conditions.5,6 Catalytic activity of VCAT in propyne hydrogenation was much lower as compared to that in propene hydrogenation with the VCAT catalyst. Propyne conversion was less than 1% in the temperature range from 250–500 °C. Indeed, in propyne hydrogenation, reliably detectable NMR signals of thermally polarized propene after an abrupt stop of the reaction mixture flow were acquired upon heating up to 500 °C after extensive scan accumulations, whereas in propene hydrogenation under the similar reaction conditions propane was easily detected with a single scan.
The propane dehydrogenation over VCAT in the presence of para-H2 did not lead to a production of hyperpolarized propane. This initial expectation was based on the idea that pairwise replacement may be an efficient process taking place on the catalyst as shown in Scheme 2. Instead, we detected hyperpolarized 1-butene produced due to side processes taking place during the activation of propane molecules in the presence of para-H2. In addition to 1-butene, ethylene and methane molecules were detected in the NMR spectra of reaction mixture as side products (Figure 5). The formation of the C4 hydrocarbon product indicated the presence of propane oligomerization or metathesis processes taking place over VCAT, whereas the presence of C2 and C1 hydrocarbons indicates that carbon chain scission is also taking place. The main reaction product at temperatures above 500 °C was nonpolarized propene.
Conclusions
In conclusion, we have examined a new silica-supported vanadium oxo organometallic complex VCAT in hydrogenation and dehydrogenation reactions in the presence of para-H2. It was found that the catalyst provides the formation of hyperpolarized propane in propene hydrogenation by means of PHIP, demonstrating significant NMR signal enhancements of more than two orders of magnitude compared to thermal polarization. This is the first observation of PHIP effects over vanadium-based catalysts. On the other hand, no hyperpolarized propane was detected in propane dehydrogenation in the presence of para-H2, indicating that pairwise replacement of two hydrogens from propane with the ones originating from para-H2 is not an efficient process over VCAT. Instead, the side-product 1-butene was found hyperpolarized in this process, illuminating complexity of the dehydrogenation process.
Substantial hyperpolarization of more than three orders of magnitude was produced by VCAT as a catalyst in propyne hydrogenation with para-H2. Such a high enhancement factor deserves considering it as a record value observed among heterogeneous catalysts like immobilized complexes and supported metal catalysts of various metals known to produce PHIP. VCAT demonstrates also exceptionally high stability over long use in reaction conditions. It also has a relatively high activity in comparison to other group 4 and 5 immobilized complexes (Ta, Zr), which, however, is not supreme, with the best result obtained of around 9% conversion in the continuous flow mode in propene hydrogenation. We believe that further investigations of vanadium-based immobilized complexes are of significant importance for new breakthrough developments in the field of production of hyperpolarized substances in hydrogenations with para-H2.
Acknowledgments
I.V.S. thanks RFBR (16-33-60198) and I.V.K. and V.V.Z. thank RFBR (17-54-33037) for financial support of the research. The authors thank FASO Russia (0333-2017-0002) for basic funding. V.V.Z. thanks University of Oulu (Kvantum Institute) for financial support.
Glossary
Abbreviations
- ALTADENA
adiabatic longitudinal transport after dissociation engenders nuclear alignment
- DNP
dynamic nuclear polarization
- PASADENA
parahydrogen and synthesis allow dramatically enhanced nuclear alignment
- para-H2
parahydrogen
- PHIP
parahydrogen-induced polarization
- rf
radio frequency
- SABRE
signal amplification by reversible exchange
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpcc.7b12069.
1-butyne hydrogenation and propane dehydrogenation over VCAT; propene and 1,3-butadiene hydrogenation over ZrCAT; and propene hydrogenation over TaCAT (PDF)
Russian Foundation for Basic research, RFBR (17-54-33037, 16-33-60198). FASO Russia (0333-2017-0002). University of Oulu (Kvantum Institute).
The authors declare no competing financial interest.
Supplementary Material
References
- Bowers C. R.; Weitekamp D. P. Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. 10.1021/ja00252a049. [DOI] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 1986, 57, 2645–2648. 10.1103/physrevlett.57.2645. [DOI] [PubMed] [Google Scholar]
- Natterer J.; Bargon J. Parahydrogen Induced Polarization. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 31, 293–315. 10.1016/s0079-6565(97)00007-1. [DOI] [Google Scholar]
- Leggett J.; Hunter R.; Granwehr J.; Panek R.; Perez-Linde A. J.; Horsewill A. J.; McMaster J.; Smith G.; Köckenberger W. A Dedicated Spectrometer for Dissolution DNP NMR Spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 5883–5892. 10.1039/c002566f. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Zhivonitko V. V.; Skovpin I. V.; Barskiy D. A.; Koptyug I. V.. Hyperpolarization Methods in NMR Spectroscopy; Kuhn L. T., Ed.; Topics in Current Chemistry; Springer: Berlin, Heidelberg, 2013; Vol. 338, pp 123–180. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Barskiy D. A.; Salnikov O. G.; Khudorozhkov A. K.; Bukhtiyarov V. I.; Prosvirin I. P.; Koptyug I. V. Parahydrogen-Induced Polarization (PHIP) in Heterogeneous Hydrogenation over Bulk Metals and Metal Oxides. Chem. Commun. 2014, 50, 875–878. 10.1039/c3cc44939d. [DOI] [PubMed] [Google Scholar]
- Skovpin I. V.; Zhivonitko V. V.; Prosvirin I. P.; Khabibulin D. F.; Koptyug I. V. Gas-Phase Hydrogenation with Parahydrogen Over Immobilized Vaska’s Complex. Z. Phys. Chem. 2016, 231, 575–592. 10.1515/zpch-2016-0824. [DOI] [Google Scholar]
- Skovpin I. V.; Zhivonitko V. V.; Koptyug I. V. Parahydrogen-Induced Polarization in Heterogeneous Hydrogenations over Silica-Immobilized Rh Complexes. Appl. Magn. Reson. 2011, 41, 393–410. 10.1007/s00723-011-0255-z. [DOI] [Google Scholar]
- Skovpin I. V.; Zhivonitko V. V.; Kaptein R.; Koptyug I. V. Generating Parahydrogen-Induced Polarization Using Immobilized Iridium Complexes in the Gas-Phase Hydrogenation of Carbon–Carbon Double and Triple Bonds. Appl. Magn. Reson. 2013, 44, 289–300. 10.1007/s00723-012-0419-5. [DOI] [Google Scholar]
- Zhivonitko V. V.; Kovtunov K. V.; Beck I. E.; Ayupov A. B.; Bukhtiyarov V. I.; Koptyug I. V. Role of Different Active Sites in Heterogeneous Alkene Hydrogenation on Platinum Catalysts Revealed by Means of Parahydrogen-Induced Polarization. J. Phys. Chem. C 2011, 115, 13386–13391. 10.1021/jp203398j. [DOI] [Google Scholar]
- Zhivonitko V. V.; Skovpin I. V.; Crespo-Quesada M.; Kiwi-Minsker L.; Koptyug I. V. Acetylene Oligomerization over Pd Nanoparticles with Controlled Shape: A Parahydrogen-Induced Polarization Study. J. Phys. Chem. C 2016, 120, 4945–4953. 10.1021/acs.jpcc.5b12391. [DOI] [Google Scholar]
- Kovtunov K. V.; Beck I. E.; Zhivonitko V. V.; Barskiy D. A.; Bukhtiyarov V. I.; Koptyug I. V. Heterogeneous Addition of H2 to Double and Triple Bonds over Supported Pd Catalysts: a Parahydrogen-Induced Polarization Technique Study. Phys. Chem. Chem. Phys. 2012, 14, 11008–11014. 10.1039/c2cp40690j. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Zhivonitko V. V.; Corma A.; Koptyug I. V. Parahydrogen-Induced Polarization in Heterogeneous Hydrogenations Catalyzed by an Immobilized Au(III) Complex. J. Phys. Chem. Lett. 2010, 1, 1705–1708. 10.1021/jz100391j. [DOI] [Google Scholar]
- Bauer G.; Güther V.; Hess H.; Otto A.; Roidl O.; Roller H.; Sattelberger S.. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: 2000, pp 49–69. [Google Scholar]
- Chalupka K.; Thomas C.; Millot Y.; Averseng F.; Dzwigaj S. Mononuclear pseudo-Tetrahedral V Species of VSiBEA Zeolite as the Active Sites of the Selective Oxidative Dehydrogenation of Propane. J. Catal. 2013, 305, 46–55. 10.1016/j.jcat.2013.04.020. [DOI] [Google Scholar]
- Cheng L.; Ferguson G. A.; Zygmunt S. A.; Curtiss L. A. Structure–Activity Relationships for Propane Oxidative Dehydrogenation by Anatase-Supported Vanadium Oxide Monomers and Dimers. J. Catal. 2013, 302, 31–36. 10.1016/j.jcat.2013.02.012. [DOI] [Google Scholar]
- Takahara I.; Saito M.; Inaba M.; Murata K. Dehydrogenation of Propane over a Silica-Supported Vanadium Oxide Catalyst. Catal. Lett. 2005, 102, 201–205. 10.1007/s10562-005-5856-4. [DOI] [Google Scholar]
- Szeto K. C.; Loges B.; Merle N.; Popoff N.; Quadrelli A.; Jia H.; Berrier E.; De Mallmann A.; Delevoye L.; Gauvin R. M.; et al. Vanadium Oxo Organometallic Species Supported on Silica for the Selective Non-oxidative Dehydrogenation of Propane. Organometallics 2013, 32, 6452–6460. 10.1021/om400795s. [DOI] [Google Scholar]
- Zhou R.; Zhao E. W.; Cheng W.; Neal L. M.; Zheng H.; Quiñones R. E.; Hagelin-Weaver H. E.; Bowers C. R. Parahydrogen-Induced Polarization by Pairwise Replacement Catalysis on Pt and Ir Nanoparticles. J. Am. Chem. Soc. 2015, 137, 1938–1946. 10.1021/ja511476n. [DOI] [PubMed] [Google Scholar]
- Burueva D. B.; Salnikov O. G.; Kovtunov K. V.; Romanov A. S.; Kovtunova L. M.; Khudorozhkov A. K.; Bukhtiyarov A. V.; Prosvirin I. P.; Bukhtiyarov V. I.; Koptyug I. V. Hydrogenation of Unsaturated Six-Membered Cyclic Hydrocarbons Studied by the Parahydrogen-Induced Polarization Technique. J. Phys. Chem. C 2016, 120, 13541–13548. 10.1021/acs.jpcc.6b03267. [DOI] [Google Scholar]
- Gambarotta S.; Floriani C.; Chiesi-Villa A.; Guastini C. A Tri-σ-aryl Vanadium(III) Derivative–Structural Determination of Trimesitylvanadium(III)-Tetrahydrofuran. J. Chem. Soc., Chem. Commun. 1984, 886–887. 10.1039/c39840000886. [DOI] [Google Scholar]
- Vivanco M.; Ruiz J.; Floriani C.; Chiesivilla A.; Rizzoli C. Chemistry of the Vanadium Carbon sigma Bond .2. Oxovanadium(IV) and Oxovanadium(V) Containing Metal-to-Carbon sigma Bonds. Organometallics 1993, 12, 1802–1810. 10.1021/om00029a042. [DOI] [Google Scholar]
- Norsic S.; Larabi C.; Delgado M.; Garron A.; de Mallmann A.; Santini C.; Szeto K. C.; Basset J.-M.; Taoufik M. Low Temperature Hydrogenolysis of Waxes to Diesel Range Gasoline and Light Alkanes: Comparison of Catalytic Properties of Group 4, 5 and 6 Metal Hydrides Supported on Silica–Alumina. Catal. Sci. Technol. 2012, 2, 215–219. 10.1039/c1cy00256b. [DOI] [Google Scholar]
- Pravica M. G.; Weitekamp D. P. Net NMR Alignment by Adiabatic Transport of Para-Hydrogen Addition-Products to High Magnetic-Field. Chem. Phys. Lett. 1988, 145, 255–258. 10.1016/0009-2614(88)80002-2. [DOI] [Google Scholar]
- Saggio G.; de Mallmann A.; Maunders B.; Taoufik M.; Thivolle-Cazat J.; Basset J.-M. Synthesis, Characterization, and Reactivity of the Highly Unsaturated Silica-Supported Trisiloxy Tantalum: (SiO)3Ta(III). Organometallics 2002, 21, 5167–5171. 10.1021/om020376g. [DOI] [Google Scholar]
- Sohn H.; Camacho-Bunquin J.; Langeslay R. R.; Ignacio-de Leon P. A.; Niklas J.; Poluektov O. G.; Liu C.; Connell J. G.; Yang D.; Kropf J.; et al. Isolated, Well-Defined Organovanadium(III) on Silica: Single-Site Catalyst for Hydrogenation of Alkenes and Alkynes. Chem. Commun. 2017, 53, 7325–7328. 10.1039/c7cc01876b. [DOI] [PubMed] [Google Scholar]
- Eisenberg R.; Eisenschmid T. C.; Chinn M. S.; Kirss R. U. Parahydrogen-Induced Polarization and Polarization Transfer in Hydrogenation and Oxidative Addition-Reactions. Adv. Chem. Ser. 1992, 230, 47–74. 10.1021/ba-1992-0230.ch004. [DOI] [Google Scholar]
- Zhivonitko V. V.; Sorochkina K.; Chernichenko K.; Kótai B.; Földes T.; Pápai I.; Telkki V.-V.; Repo T.; Koptyug I. Nuclear Spin Hyperpolarization with ansa-Aminoboranes: A Metal-Free Perspective for Parahydrogen-Induced Polarization. Phys. Chem. Chem. Phys. 2016, 18, 27784–27795. 10.1039/c6cp05211h. [DOI] [PubMed] [Google Scholar]
- Anderson A. B.; Nichols J. A. Hydrogen on zinc oxide. Theory of its Heterolytic Adsorption. J. Am. Chem. Soc. 1986, 108, 4742–4746. 10.1021/ja00276a010. [DOI] [Google Scholar]
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; Khazal I. G.; Lopez-Serrano J.; Williamson D. C. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



