Abstract
Finland is the second largest oat producer in Europe. Despite the existing knowledge of phenolics in oat, there is little information on the phenolic composition of oats from Finland. The aim of the study was to investigate the concentrations of free and bound phenolic acids, as well as avenanthramides in eight Finnish cultivars of husked oat (Avena sativa L.). Seven phenolic acids and one phenolic aldehyde were identified, including, in decreasing order of abundance: p-coumaric, ferulic, cinnamic, syringic, vanillic, 2,4-dihydroxybenzoic, and o-coumaric acids and syringaldehyde. Phenolic acids were mostly found as bound compounds. Significant varietal differences (p < 0.05) were observed in the cumulative content of phenolic acids, with the lowest level found in cv. ‘Viviana’ (1202 ± 52.9 mg kg–1) and the highest in cv. ‘Akseli’ (1687 ± 80.2 mg kg–1). Avenanthramides (AVNs) 2a, 2p, and 2f were the most abundant. Total AVNs levels ranged from 26.7 ± 1.44 to 185 ± 12.5 mg kg–1 in cv. ‘Avetron’ and ‘Viviana’, respectively.
Keywords: Avena sativa L., avenanthramides, dietary fiber, Finnish oats, phenolic compounds
Introduction
Oat is a grain crop belonging to the family of Poaceae (or Gramineae).1 Two main species of oat are produced in the world: Avena sativa L. and Avena nuda L.; the former, known as common oat, is the most cultivated.2Avena sativa L. is extensively grown in the cool and moist regions of Northern Europe and North America.3 Finland and Poland are the biggest producers of oat within the European Union.4 Oat-derived foods, e.g. ready to eat breakfast products, granola bars, oat-based cookies, and beverages, are commercially available across the world, although oat is mainly consumed as whole grain cereal due to the health benefits it provides as such.5 The increasing interest of consumers toward whole grain oat is mainly driven by its advantageous macronutrient composition: lipids with a high degree of unsaturation, e.g. oleic and linoleic acids account for approximately 40 and 36% of total fatty acids, respectively;1 proteins with a favorable composition of essential amino acids;6 dietary fiber with a high content of β-glucan (2–8.5% w/w of oat seed), a soluble type of fiber that is able to lower plasma cholesterol by increasing the fecal excretion of bile acids.7 An accruing body of evidence suggests that the protective effects of whole grain cereals against the development of noncommunicable diseases (NCD), including cardiovascular diseases and diabetes, are partly due to the presence of bioactive compounds, e.g. phenolic compounds, which are bound to and work synergistically with the dietary fibers.8,9 In an 8-week placebo controlled study of 80 healthy overweight/obese volunteers, Vitaglione et al. (2015)10 showed that subjects consuming whole grain wheat had greater excretion of ferulic acid and dihydroferulic acid in urine and lower plasma concentrations of markers of inflammation, e.g. PAI-1 (plasminogen activator inhibitor 1).
Phenolic compounds are secondary products of the plant metabolism and are structurally characterized by the presence of at least one aromatic ring bearing one or more hydroxyl groups.11 They vary greatly in the molecular size, from simple monomers, to complex polymers, and can be divided into several subclasses, e.g. phenolic acids, flavonoids, stilbenes, coumarins, and tannins.12 The level of phenolic compounds in whole grain cereals is influenced by the grain type, variety, and part of the grain analyzed.13 Researchers have demonstrated that phenolic acids are among the most abundant phytochemicals in oat.11,14 Phenolic compounds, particularly phenolic acids, are found in cereals either free or bound to cell wall components.15 The free phenolics are mainly located in the vacuoles of the pericarp and, being soluble, can be extracted with organic solvents.16 In cereals, including oat, only a small fraction of phenolic compounds exists in the free form.17 Bound phenolic compounds form ether linkages with lignin and ester linkages with structural macromolecules such as polysaccharides and proteins.11 Bound phenolics can be extracted following processes of alkaline and/or acid hydrolysis.18 In addition to simple phenolic compounds, oat provides avenanthramides (AVNs), which are a group of hydroxycinnamoylanthranilate alkaloids unique to oat among the cultivated cereals.19 It is estimated that more than 25 AVNs are found in oat, although the most common forms are esters of 5-hydroxyanthranilic acid with caffeic (2c aka C), p-coumaric (2p aka A), and ferulic (2f aka B) acids.20 Avenanthramides were found to have anti-inflammatory effects and ameliorate skin disorders.21 Several factors, e.g. genotype, abiotic, and biotic stressors, can influence the concentrations of AVNs in oat to a large extent. As a result, it is important to identify varieties of oat with high levels of AVNs, so as to provide consumers with raw materials of enhanced nutritional value.
Finland is a major world producer of oat, with yields exceeding one million tonnes in 2016.22 Nevertheless, data on the composition of Finnish oat cultivars are very limited. Rainakari et al. (2016)23 measured the dietary fiber content of oat products from Finland. The investigation did not include phenolic compounds and took no notice of varietal differences, as based on commercial products. The European project “Avena Genetic Resources for Quality in Human Consumption”24 investigated a wide range of European cultivars of Avena, yet cultivars from Finland were not included. Murariu et al. (2013)25 performed an extensive investigation of 117 cultivars of Avena from several European countries, including the cultivar (cv) ‘Ivory’ from Sweden and Estonia. The study concluded that the Nordic agricultural conditions are ideal to produce oat; however, cultivars from Finland were not characterized. As Finland is a large producer and exporter of oat, compositional information on Finnish oat cultivars will provide data that are important both scientifically and commercially. The goal of the present research was to investigate the differences in the macro- and non-nutrient (phytochemical) composition of husked oat (Avena sativa L.) from eight Finnish cultivars: ‘Akseli’, ‘Avetron’, ‘Peppi’, ‘Ivory’, ‘Marika’, ‘Riina’, ‘Rocky’, and ‘Viviana’. The study aimed to measure the fat and dietary fiber compositions of the selected cultivars, as well as to characterize the phenolic profile of the samples and to differentiate between free and bound phenolic acids, based on the extraction procedures. In addition, the study evaluated how the selected genotypes influenced the concentrations of AVNs.
Materials and Methods
Chemicals
General laboratory reagents were purchased from VWR International Oy (Helsinki, Finland). Methyl tert-butyl ether (MTBE), ethyl acetate, acetic acid (glacial), and sodium sulfate (anhydrous, granular, ≥ 99%) were of analytical grade. Methanol and acetonitrile were of LC-MS grade. Hydrochloric acid (reagent grade, 37%) and sodium hydroxide (pellets pure) were purchased from Sigma-Aldrich, Inc. (Gillingham, England). Water was purified in loco with a Milli-Q water purification system (Millipore Co.). Analytical standards of 2,4-dihydroxy benzoic acid, vanillic acid, syringic acid, syringaldehyde, cinnamic acid, ferulic acid, o-coumaric acid, and p-coumaric acid were purchased from Sigma-Aldrich (Gillingham, England). Analytical standards of avenanthramides 2c, 2p, and 2f were purchased from ReseaChem GmbH (Burgdorf, Switzerland).
Plant Materials and Sample Preparation
A set of eight cultivars of husked oat (Avena sativa L) was provided by The Natural Resources Institute Finland (Luke). The selected cultivars are named: ‘Akseli’, ‘Avetron’, ‘Peppi’, ‘Ivory’, ‘Marika’, ‘Riina’, ‘Rocky’, and ‘Viviana’. The samples were collected from the farmers’ network in the Sastamala area (61°20′25″ N, 022°54′35″ E), Southwestern Finland. A representative silo sample of 1 kg was taken from the harvest and delivered to the laboratory. Oats were grown and harvested in the year 2016. Husked oats (oat kernels) were milled with a Retsch ZM-100 ultracentrifugal mill with a 0.5 mm screen insert (Düsseldorf, Germany), placed in sealed plastic bags, and stored at room temperature (20.3 ± 2 °C) under vacuum until analysis.
Macronutrient Analysis
Routine proximate analytical procedures were employed to determine the macronutrient composition of the oat samples. Three independent samples of each cultivar were analyzed. The dry matter (DM) was determined following the official AOAC method (925.10).26 Dietary fiber was measured following the official AOAC method (991.43).27 Total fat was determined by adaptation of the Folch procedure.28 Samples (0.5 g) were suspended in 5 mL of extraction mixture solvent (MTBE/methanol, 3/1, (v/v)), and agitated on an orbital shaker at RT for 20 min. The supernatants were separated by centrifugation (5 min; 700g; 18 °C). The remaining pellets were suspended in 2 mL of extraction mixture solvent (MTBE/methanol/water, 4/1.2/1, (v/v)) and stirred on vortex for 1 min. The supernatants were separated by centrifugation (5 min; 700g; 18 °C) and combined with the other supernatants. After adding 1.25 mL of water to the pooled supernatants and stirring for 1 min on vortex at RT, the samples were centrifuged (5 min; 700g; 18 °C) to induce phase separation. The organic phases containing lipids were collected and weighed after evaporation of the solvent under a stream of nitrogen.
Extraction of Phenolic Acids
Phenolic acids were extracted as described by Multari et al. (2016)29 and Neacsu et al. (2015).30 Four independent samples of each cultivar were analyzed. The term total phenolic acids indicates the sum of the cumulative free and bound phenolic acids. As syringaldehyde was the only phenolic aldehyde detected, it was considered as a phenolic acid in the data analysis for simplicity reasons.
Extraction of Free Phenolic Acids
Samples of milled husked oats (approximately 0.1 g) were suspended in HCl (3 mL; 0.2 M) and extracted into EtOAc (6 mL), and the layers were separated by centrifugation (5 min; 1800g; 18 °C). The extraction was repeated twice, and the EtOAc extracts were combined and left to stand over sodium sulfate (anhydrous) and then filtered. The solvent was removed under reduced pressure at a temperature not exceeding 40 °C. Then, the extracts were dissolved in methanol (1 mL) for UPLC-MS analysis. The remaining aqueous fraction, obtained after EtOAc extraction, was neutralized with NaOH (approximately 0.2 mL; 4 M) and freeze-dried.
Extraction of Bound Phenolic Acids
The freeze-dried pellets were suspended in NaOH (3 mL; 1 M) and stirred at room temperature for 4 h under nitrogen. The pH was reduced to 2 with HCl (approximately 0.4 mL; 10 M), and the samples were extracted into EtOAc (6 mL). This was repeated twice. The EtOAc extracts were combined, and the solvent was removed under reduced pressure at a temperature not exceeding 40 °C. The extracts were dissolved in methanol (1 mL) for UPLC-MS analysis. The pH of the remaining aqueous fractions was brought to 7 with NaOH (approximately 1.9 mL; 4 M), and the aqueous fractions were freeze-dried. Then, the dried aqueous fractions were suspended in HCl (3 mL; 2 M) and incubated at 95 °C for 30 min with intermittent mixing. The samples were cooled and extracted with EtOAc (6 mL). This was repeated twice. The EtOAc extracts were combined, and the solvent was removed under reduced pressure at a temperature not exceeding 40 °C. Extracts were dissolved in methanol (1 mL) for UPLC-MS analysis.
Extraction of Avenanthramides
Avenanthramides were extracted by adapting the method from Bryngelsson et al. (2002).31 Briefly, milled oat samples (5.0 g) were extracted twice with 80% methanol (35 mL) for 30 min at RT using a magnetic stirrer. Then, samples were centrifuged (10 min; 600g; 18 °C), and the supernatants were dried under reduced pressure at a temperature not exceeding 40 °C. Extracts were dissolved in methanol (2 mL), filtered through PTFE membrane filters (Pall Corporation, Port Washington, NY, USA), and analyzed by HPLC. Three independent samples of each cultivar were analyzed. Avenanthramides that contain the avenalumoyl structure instead of the hydroxycinnamoyl structure, i.e. type II avenanthramides, are referred to as avenalumins (ALs) in the text. Exemplatonary avenanthramide structures are found as Supporting Information (SI 2).
UPLC-MS Analysis of Phenolic Acids
The liquid chromatography separation of the phenolic acids was obtained adapting the method from Multari et al. (2016)29 and Neacsu et al. (2015).30 It was performed on an UPLC-PDA-ESI-MS system consisting of a Waters Acquity UPLC in combination with a Waters 2996 PDA detector and a Waters Quattro Premier mass spectrometer (Waters Corp., Milford, MA). The column used was a Kinetex C18 column (100 × 4.6 mm; 2.6 μm i.d.; 100 Å) from Phenomenex (Torrance, USA). The mobile-phase solvents were water containing 0.1% acetic acid (A) and acetonitrile containing 0.1% acetic acid (B). The gradient used to separate the different phenolic compounds was as follows: 10% B (0–1.5 min), 55% B (1.5–16.5 min), 80% B (16.5–30.0 min), 10% B (30.0–32.0 min). The flow rate was 840 μL min–1, the injection volume was 10 μL, and the PDA was set at 210–600 nm. After splitting, the LC eluent (400 μL min–1) was directed into the mass spectrometer equipped with an electrospray interface. The mass spectrometer was run in both negative and positive ion modes with the following source settings: capillarity voltage, 3.0 kV (ES+) and 5.0 kV (ES–); cone voltage, 15.0 V (ES+) and 22.0 V (ES–); extractor voltage 3.0 V (ES+) and 4.0 V (ES–); source temperature, 120 °C; desolvation temperature, 300 °C; desolvation gas flow, 700 L/h; cone gas flow, 100 L/h. Ions were scanned across the range of m/z 120–450. All the phenolic compounds were identified using the UV spectra and the parent ions (m/z obtained from both positive ion scan [M + H]+ and negative ion scan [M – H]−). The quantification of the phenolic compounds by PDA was performed using external standards. The list of the individual phenolic compounds identified by UPLC-PDA-MS is included as Supporting Information (SI 1).
HPLC-DAD Analysis of Avenanthramides
Avenanthramides were identified and quantified as described by Mattila et al. (2005).32 The system employed was an Agilent 1100 high-performance liquid chromatograph equipped with a diode array detector (HPLC-DAD) (Agilent, Santa Clara, CA, USA). The HPLC pumps, autosampler, column oven, and diode array system were operated by the ChemStation computer program. The analytical column was Phenomenex Kinetex C18 (100 × 3.0 mm; 5 μm i.d.; 100 Å); the column oven was set at 35 °C. The mobile phase consisted of 0.05 M phosphate buffer (A) at pH 2.4 and methanol (B) with the following gradient: 5–60% B in 50 min; 60–90% B in 6 min. The flow was set at 0.6 mL min–1. Avenanthramides were quantitated at the wavelength of 350 nm. In addition, to AVNs 2c, 2p, and 2f, two unknown AVNs (AVNa and AVNb) and five avenalumins, i.e. type II AVNs, were tentatively identified according to their UV spectra (SI 2) and quantitated using AVN 2p calibration curve, due to the lack of commercial standards.
Statistical Analysis
Data reported are mean of minimum triplicate observations, and values were expressed as mean ± SD. The statistical analysis was carried out using SPSS 23.0 for Windows (IBM, Armonk, NY, USA). The Shapiro–Wilk test was applied to verify the normal distribution of the variables. When the statistical distribution was not normal, a logarithmic transformation of the variables was performed. The Levene’s test was applied to detect possible nonhomogeneity of the variances. The data were analyzed using One-Way-Analysis of Variance (ANOVA) to compare the groups, and the Tukey’s test was performed to allow for multiple comparisons. Differences among groups were considered significant at p < 0.05. Not detected (n/d) values were not included in the statistical analysis.
Results and Discussion
Macronutrient Composition
The macronutrient composition of the oat samples is shown in Table 1. The dry matter (DM) accumulation exhibited significant differences among the oat cultivars (p ≤ 0.001), ranging from 882 ± 1.37 g kg–1 in cv. ‘Peppi’ to 915 ± 14.8 g kg–1 in cv. ‘Viviana’. The average DM content in these oat samples was 89.98%, which is in line with previous investigations.14 The fat content varied significantly (p ≤ 0.001) among the oats, with the highest value observed for cv. ‘Avetron’ (58.8 ± 1.75 g kg–1 DM). All the oats had a fat content above 40 g kg–1 DM, apart from cv. ‘Marika’ that presented a fat content of 38.7 ± 0.22 g kg–1 DM. These results agree with earlier studies, in which an average fat content of about 50 g kg–1 DM was reported.33 Noteworthy is the investigation carried out by Hu et al. (2014),34 in which oat from Sweden, a country sharing with Finland common botanical and agronomic characteristics, had a fat content of 51.3 g kg–1 DM. Oat has been traditionally considered as a good source of vegetable fats, which are mainly located in the endosperm.5 Apart from maize, oat provides more fats than other cereals,1 being an excellent source of energy and unsaturated fatty acids. The quality and quantity of oil in oat can be influenced by several factors, e.g. extraction method, cultivar, and storage conditions. However, studies performed on oat oil reported that husked oats contain more than 70% of unsaturated fatty acids,35 and that palmitic, oleic, and linoleic acids are the main fatty acids regardless of the genetic and environmental factors.1,36
Table 1. Macronutrient Composition of the Oat Samplesa.
| cultivar | moisture | dry matter | fat | dietary fiber |
|---|---|---|---|---|
| Akseli | 95.7 ± 1.75 c,b | 904 ± 1.75 a,b | 46.1 ± 1.35 e | 350 ± 6.50 a |
| Avetron | 109 ± 1.74 a,b | 891 ± 1.74 c,b | 58.8 ± 1.75 a | 284 ± 7.77 e,f |
| Ivory | 111 ± 0.67 a,b | 889 ± 0.67 c,b | 43.7 ± 0.57 f,e | 289 ± 2.10 d,e,f |
| Marika | 89.8 ± 3.33 c,b | 910 ± 3.33 a,b | 38.7 ± 0.22 g | 319 ± 2.32 b,c |
| Peppi | 118 ± 1.37 a | 882 ± 1.37 c | 50.1 ± 0.69 d | 326 ± 2.22 b |
| Riina | 100 ± 1.35 b | 900 ± 1.35 b | 52.1 ± 0.13 d,c | 314 ± 5.03 b,c |
| Rocky | 92.9 ± 2.40 c,b | 907 ± 2.40 a,b | 54.0 ± 0.38 b,c | 284 ± 3.74 f |
| Viviana | 84.5 ± 14.8 c | 915 ± 14.8 a | 56.2 ± 0.77 b | 312 ± 1.36 c |
Data (g kg–1 DM) is presented as mean ± SD and represents mean of minimum three independent measurements. Values with unlike letters (a-g) within the same column differ significantly (p < 0.05)
Dietary fiber (DF) has long been considered critical for the physiological effects resulting from the consumption of whole grain oat.37 The content of total dietary fiber from the oat samples is reported in Table 1. Among the samples, cv. ‘Akseli’ had the highest content of dietary fiber 350 ± 6.50 g kg–1 DM, which statistically differed from all the other oats (p < 0.001). The average dietary fiber level of the oats analyzed in this study was 31%, which is higher than most of the oat samples analyzed in analogous investigations.1,38 This difference can be ascribed to the presence, in the selected oats, of the husk, the outer covering of the seed that is rich in dietary fiber.39 The husks can account for up to 30% of the total kernel weight and are rich in hemicellulose, lignin, and cellulose.40 Conversely, naked oats (free from the external husk) are richer in lipids, proteins, and starch.41
Identification and Quantification of Phenolic Acids
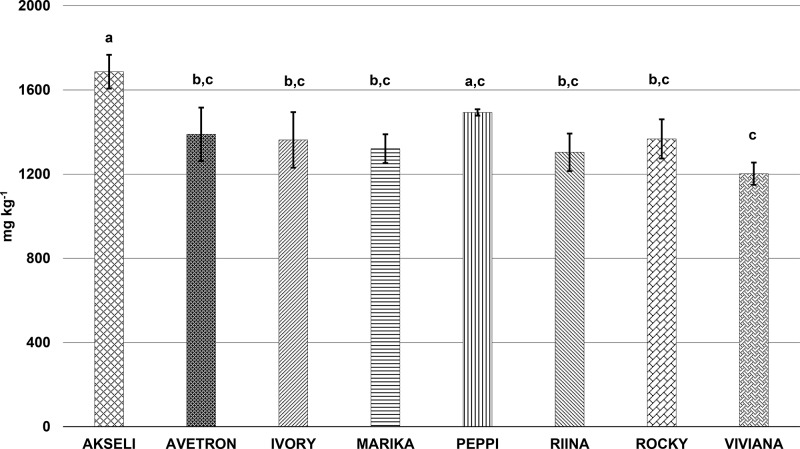
The total content of phenolic acids from the selected Finnish oats statistically differed among the samples (p < 0.001) (Figure 1) and ranged from 1202 ± 52.9 to 1687 ± 80.2 mg kg–1. The concentrations of phenolic acids in the cultivars studied were, in the following order: ‘Akseli’ > ‘Peppi’ > ‘Rocky’ > ‘Avetron’ > ‘Ivory’ > ‘Marika’ > ‘Riina’ > ‘Viviana’. Bound phenolic acids markedly outweighed the free in the cumulative content. Bound phenolic acids accounted for more than 99% of the total phenolic acids in all the cultivars (Table 2). Nevertheless, statistically significant differences were identified (p < 0.01), with cv. ‘Peppi’ showing the highest share of bound phenolic acids (99.5 ± 0.03%). It is widely recognized that phenolic acids mostly occur as bound compounds in cereals, including oats.11 They are generally linked by ester and ether bonds to cell wall polysaccharides.13 This finds further corroboration in the present investigation. The cultivars ‘Akseli’ and ‘Peppi’ showed the highest content of both dietary fiber (350 ± 6.5 and 326 ± 2.22 kg–1 DM, respectively) and cumulative bound phenolic acids (1684 ± 80.2 and 1493 ± 15.1 mg kg–1, respectively). Rolled oats are oat groats produced industrially from dehusked kernels, by cutting, steaming, and flaking.23,42 Since the present investigation showed that most of the phenolic acids were bound to fiber, rolling oat into flakes can substantially decrease the phenolic content of oat. Conversely, low processed oat can serve as a natural and cost-effective source of functional ingredients. The corroborated association between dietary fiber and bioactive phenolic compounds could enhance the interest of the scientific community and consumers toward the selected oat cultivars. Across the samples, ferulic acid and p-coumaric acid contributed to the majority of bound acids. Ferulic acid also provided the greatest share of free phenolic acids. Nevertheless, regardless the contribution of ferulic acid, the absolute values of free phenolic acids were low, averaging 2.90 ± 0.69 mg kg–1, i.e. 0.21 ± 0.02% of the cumulative phenolic acid content. Previous investigations carried out on oat confirmed that ferulic acid and p-coumaric acid prevailed in the bound form.43
Figure 1.
Total phenolic acid content of the oat cultivars. Results are expressed as sum of the cumulative free and bound phenolic acids and represent mean of four independent measurements. Values with unlike letters (a-c) differ significantly (p < 0.05).
Table 2. Content of Individual Phenolic Acids of the Oat Cultivarsa.
| 2,4-dihydroxy benzoic acid |
vanillic acid |
syringic acid |
syringaldehyde |
cinnamic acid |
ferulic acid |
o-coumaric acid |
p-coumaric acid |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| free | bound | free | bound | free | bound | free | bound | free | bound | free | bound | free | bound | free | bound | cumulative free phenolics | cumulative bound phenolics | |
| Akseli | n/d | n/d | n/d | 7.1 ± 0.96 a | 2.11 ± 0.11 a | 6.3 ± 0.1 c | n/d | n/d | n/d | 12.1 ± 1.59 a | 0.6 ± 0.4 b | 829 ± 73.8 a | n/d | 3.78 ± 0.65 a,b | n/d | 826 ± 53.0 a | 2.71 ± 0.35 b,c,d | 1684 ± 80.2 a |
| Avetron | n/d | 4.67 ± 0.56 b | n/d | 4.86 ± 0.62 a | 2.04 ± 0.12 a | 8.0 ± 0.62 a,b | n/d | n/d | n/d | 9.2 ± 1.2 a,b,c | n/d | 586 ± 68.0 b,c,d | n/d | 2.84 ± 0.24 a,b | n/d | 771 ± 69.4 a,b | 2.04 ± 0.12 d | 1387 ± 127 b,c |
| Ivory | n/d | 5.71 ± 0.64 a,b | n/d | 6.01 ± 0.71 a | n/d | 7.61 ± 0.71 b | n/d | n/d | n/d | 7.65 ± 0.82 b,c | 2.55 ± 0.26 a | 618 ± 33.1 b,c,d | n/d | 2.30 ± 0.59 b,c | n/d | 713 ± 65.6 a,b,c | 2.55 ± 0.26 c,d | 1360 ± 132 b,c |
| Marika | n/d | n/d | n/d | 4.59 ± 0.58 a | n/d | 6.17 ± 0.54 c | 1.13 ± 0.08 a | n/d | n/d | 7.17 ± 0.56 c | 0.8 ± 0.2 b | 709 ± 38.2 a,c | n/d | 2.39 ± 0.92 b,c | n/d | 590 ± 35.4 c | 1.93 ± 0.16 d | 1319 ± 68.4 b,c |
| Peppi | n/d | 6.65 ± 0.35 a | n/d | 7.05 ± 0.37 a | n/d | 3.89 ± 0.16 d | n/d | n/d | n/d | n/d | 5.0 ± 0.2 a | 731 ± 8.41 a,c | n/d | 2.54 ± 0.46 b,c | n/d | 737 ± 13.9 a,b,c | 5.0 ± 0.2 a,b | 1488 ± 15.1 a,b |
| Riina | n/d | n/d | n/d | n/d | 1.92 ± 0.13 a | 8.9 ± 0.5 a | 1.69 ± 0.15 a | n/d | n/d | 9.57 ± 2.21 a,b,c | n/d | 626 ± 59.2 c,d | n/d | 1.68 ± 0.92 c,b | n/d | 654 ± 66.1 b | 3.61 ± 0.19 b,c,d | 1300 ± 89.1 b,c |
| Rocky | n/d | n/d | 0.3 ± 0.1 | 5.77 ± 0.23 a | 2.18 ± 0.13 a | n/d | 1.11 ± 0.25 a | n/d | n/d | 11.7 ± 2.5 a,b | 0.7 ± 0.2 b | 747 ± 68.0 a,c | n/d | 1.11 ± 0.05 c | n/d | 598 ± 55.6 b,c | 4.29 ± 0.28 b,c | 1363 ± 93.1 b,c |
| Viviana | n/d | n/d | n/d | 4.76 ± 0.7 a | n/d | 6.45 ± 0.53 c,b | n/d | n/d | n/d | 9.2 ± 1.11 a,b,c | 1.03 ± 0.3 b | 532 ± 27.3 d | n/d | 1.69 ± 0.58 c,b | n/d | 646 ± 43.7 b,c | 1.03 ± 0.3 e | 1201 ± 52.9 c |
Data (mg kg–1) is presented as mean ± SD and represents mean of four independent measurements. n/d = not detected (i.e., below the detection level). Values with unlike letters (a-d) within the same column differ significantly (p < 0.05).
Table 2 shows the content of individual phenolic acids identified in the oat samples (exemplary UPLC chromatograms are included as Supporting Information, SI 1). Eight different compounds were found. The selected oats differed in their qualitative profiles, as only four phenolic acids were ubiquitously identified across the samples: ferulic acid, o-coumaric acid, p-coumaric acid, and syringic acid. Ferulic and p-coumaric acids were the major compounds. Both of them were mainly identified in the bound form. Ferulic acid and p-coumaric acid accounted each for about 45% of the total phenolic acid content (Figure 2). Syringic acid was also identified in all the samples yet with marked differences between free and bound forms: cv. ‘Rocky’ contained syringic acid only as free (2.18 ± 0.13 mg kg–1), while cvs ‘Marika’ and ‘Viviana’ had only the bound form (6.17 ± 0.54 and 6.45 ± 0.53 mg kg–1, respectively). Syringic acid represented about 0.54 ± 0.2% of the total phenolic content. Vanillic acid (on average 0.42 ± 0.14% of the total phenolic content) was identified in all the oat samples in the bound form, apart from cv. ‘Rocky’ where it was identified as both free and bound compound (0.3 ± 0.1 and 5.77 ± 0.23 mg kg–1, respectively) and cv. ‘Riina’ where it was absent. Cinnamic acid (0.72 ± 0.2% of the total phenolic acid content) was found across the samples in the bound form, but it was not detected in cv. ‘Peppi’. Syringaldehyde was identified as free compounds in cvs ‘Riina’, ‘Rocky’, and ‘Marika’, while 2,4-dihydroxybenzoic acid was identified in cvs ‘Peppi’, ‘Ivory’, and ‘Avetron’ as bound compound.
Figure 2.
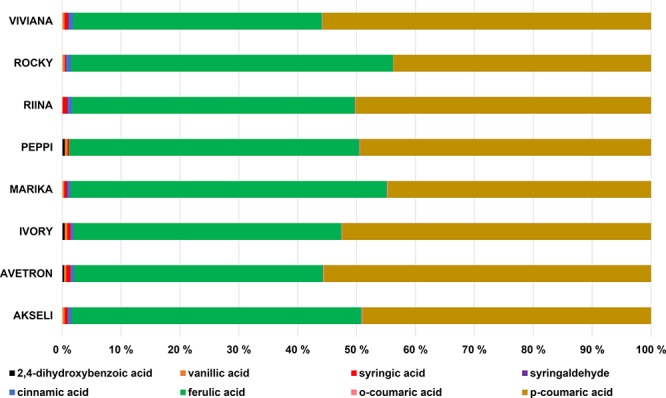
Phenolic acids in oat cultivars reported as individual percentages of the total phenolic acid content.
The selected Finnish oats were markedly different in the quantitative profiles, as the concentrations of the individual phenolic acids varied significantly across the cultivars, not including vanillic acid. For example, bound cinnamic acid in cv. ‘Rocky’ (11.7 ± 2.5 mg kg–1) was about 35% higher than in cv. ‘Ivory’ (7.65 ± 0.82 mg kg–1) (p < 0.05); bound ferulic acid in cv. ‘Askeli’ (829 ± 73.8 mg kg–1) was about 35% higher than in cv. ‘Viviana’ (532 ± 27.3 mg kg–1) (p < 0.001); bound p-coumaric acid in cv. ‘Askeli’ (826 ± 53.0 mg kg–1) was about 29% higher than in cv. ‘Marika’ (590 ± 35.4 mg kg–1) (p < 0.001). The attentive observation of the data suggests that oats were rich in hydroxycinnamates, e.g. ferulic acid, o-coumaric acid, and p-coumaric acid. This is in agreement with previous investigations that attested the ubiquity of hydroxycinnamates in whole grain cereals.44 In addition, ferulic acid and p-coumaric acid were described as the most abundant phenolic compounds in oat by analogous studies.45 Noteworthy is the investigation carried out by Cai et al. (2012),46 in which the concentrations of ferulic and p-coumaric acids increased several folds after fungal fermentation of oat. During fermentation, fungi produce different types of enzymes that can soften the kernel structure and release the phenolic compounds bound to the cell wall.11 Cai et al. (2012)46 showed that ferulic and p-coumaric acids are mainly present as bound phenolics in oats, as corroborated by the present study. In addition, enzymes might degrade polymeric phenolic compounds during the fermentation process.47 To some extent, an analogous outcome might be achieved by performing processes of hydrolysis at high temperatures to enhance the extractability of bound phenolic compounds, as carried out herein. Flavonoids are important antioxidants in foods but traditionally have been detected in low quantities in cereals.11 In the current study, several flavonoids were investigated, e.g. quercetin, kaempferol, isorhamnetin, and rhamnetin; however, they were not detected in any of the samples (data not shown).
Identification and Quantification of Avenanthramides (AVNs)
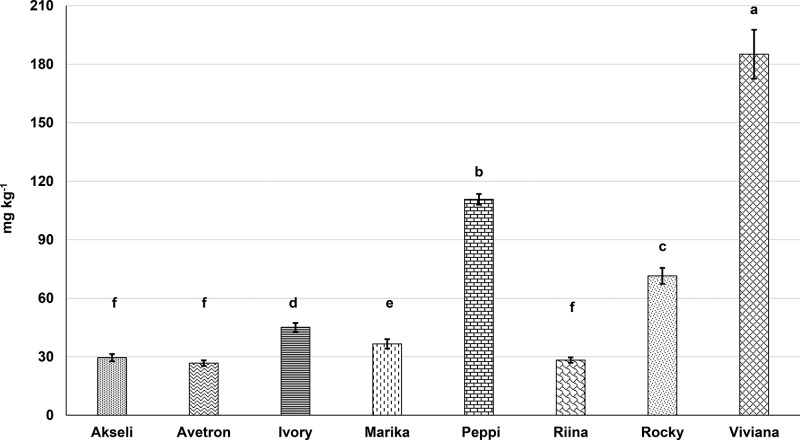
In addition to simple phenolic compounds, ten AVNs were detected in the selected oats (Table 3). An explanatory chromatogram of oat AVNs and the corresponding UV spectra is provided as Supporting Information (SI 2). Total levels of AVN ranged from 26.7 ± 1.44 to 185 ± 12.5 mg kg–1 (Figure 3). Cv. ‘Viviana’ showed the highest total AVN content, with levels of AVN 2c, 2p, and 2f at 39.2 ± 5.14, 29.6 ± 5.13, and 21.9 ± 0.35 mg kg–1, respectively. On the contrary, cv. ’Avetron’ presented the lowest total AVN content, with the concentrations of AVNs 2c, 2p, and 2f being 3.50 ± 0.45, 6.0 ± 0.29, and 4.82 ± 0.31 9 mg kg–1, respectively. The total content of AVNs differed markedly across the samples (p < 0.05), with up to 7-fold difference among the selected cultivars, implying that the variety has a great effect on the concentration of AVNs in oat. This observation is consistent with the investigation of Chen et al. (2018),2 which detected very different levels of AVNs among nine varieties of oat from China. Several studies showed that AVNs 2c, 2p, and 2f are the main kind of AVNs found in oat.48 This in agreement with the present investigation. Nevertheless, the literature provides inconsistent data on the concentration of AVNs in oats. Xie et al. (2017)49 reported a total AVN content (as sum of 2c, 2p, and 2f) for Canadian oats averaging 36 mg kg–1. Chen et al. (2018)2 showed the variety ‘Longyan’ from China to provide about 146 mg kg–1 of total AVNs (sum of 2c, 2p, and 2f). It is clear that location, climate, variety, processing methods, and their interactions, are all factors playing a significant role in the process of AVN biosynthesis. Investigations on model systems suggest that AVNs might exert beneficial effects, e.g. anti-inflammatory activity, on human health.50,51 Results from the present investigation indicate that to gain additional health benefits from the consumption of oats, the selection of genotype is an essential factor in the production of oats with high AVN content.
Table 3. Content of Individual Avenanthramides of the Oat Cultivarsa.
| 2c | 2p | 2f | AVNa | AVNb | AL 1 | AL 2 | AL 3 | AL 4 | AL 5 | cumulat 2c+2p+2f | cumulat ALs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akseli | 5.22 ± 0.75 e | 6.90 ± 0.26 e,f | 4.69 ± 0.06 f | n/d | n/d | n/d | 3.84 ± 0.58 c | 1.55 ± 0.15 c | 3.31 ± 0.06 e,f | 3.99 ± 0.15 e,f | 16.8 ± 0.98 f | 12.7 ± 0.89 d |
| Avetron | 3.50 ± 0.48 f | 6.00 ± 0.29 f | 4.82 ± 0.31 f | n/d | 1.20 ± 0.10 d | n/d | 2.33 ± 0.23 c | 1.83 ± 0.21 c | 2.92 ± 0.46 f | 4.09 ± 0.35 e,d,f | 14.3 ± 1.01 g | 11.2 ± 0.5 d |
| Ivory | 7.19 ± 0.64 d | 8.40 ± 0.32 d,e | 12.3 ± 0.42 d | 1.62 ± 0.12 c | 2.34 ± 0.06 c | n/d | 2.27 ± 0.15 c | 1.69 ± 1.00 c | 4.33 ± 0.21 d,e | 4.98 ± 0.17 d | 27.8 ± 1.36 d | 13.3 ± 0.7 d |
| Marika | 5.97 ± 0.84 d,e | 7.14 ± 0.31 e,f | 8.89 ± 0.36 e | n/d | 2.07 ± 0.12 c | 1.12 ± 0.15 d | 1.89 ± 0.25 c | 1.61 ± 0.15 c | 3.18 ± 0.06 e,f | 4.69 ± 0.15 d,e | 22.0 ± 1.53 e | 12.5 ± 0.8 d |
| Peppi | 19.0 ± 0.46 b | 20.9 ± 1.14 b | 18.5 ± 0.93 b | 3.00 ± 0.22 b | 2.98 ± 0.10 b | 4.58 ± 0.15 b | 11.4 ± 0.32 b | 4.14 ± 0.15 b | 12.5 ± 0.64 b | 13.6 ± 0.53 b | 58.5 ± 1.57 b | 46.3 ± 0.9 b |
| Riina | 3.57 ± 0.23 f | 6.80 ± 0.40 f | 4.47 ± 0.23 f | n/d | 1.11 ± 0.10 d | 1.21 ± 0.15 d | 2.39 ± 0.12 c | 1.82 ± 0.23 c | 3.38 ± 0.45 e,f | 3.52 ± 0.56 f | 14.8 ± 0.76 f,g | 12.3 ± 0.6 d |
| Rocky | 10.2 ± 0.70 c | 10.9 ± 0.51 c | 16.9 ± 0.64 c | 2.41 ± 0.31 b | 3.12 ± 0.10 b | 3.14 ± 0.38 c | 4.07 ± 0.40 c | 4.18 ± 0.46 b | 8.65 ± 0.75 c | 7.84 ± 0.56 c | 38.0 ± 1.71 c | 27.9 ± 2.0 c |
| Viviana | 39.2 ± 5.14 a | 29.6 ± 5.13 a | 21.9 ± 0.35 a | 5.68 ± 0.23 a | 4.84 ± 0.35 a | 15.3 ± 2.29 a | 26.8 ± 3.57 a | 5.74 ± 0.50 a | 15.6 ± 2.00 a | 20.5 ± 0.29 a | 90.7 ± 5.71 a | 83.9 ± 6.5 a |
Data (mg kg–1) is presented as mean ± SD and represents mean of three independent measurements. n/d = not detected (i.e., below the detection level). Values with unlike letters (a-g) within the same column differ significantly (p < 0.05). AVNs 2c, 2f, and 2p are esters of 5-hydroxyanthranilic acid with caffeic (2c aka C), p-coumaric (2p aka A), and ferulic (2f aka B) acids. AVNa and AVNb are unknown avenanthramides.
Figure 3.
Total avenanthramides content of the oat cultivars. Results are expressed as mean of three independent measurements. Values with unlike letters (a-f) differ significantly (p < 0.05).
In conclusion, the selected Finnish oats showed favorable macro- and non-nutrient (phytochemical) profiles, due to their content in fats, which are likely to be unsaturated,1 dietary fiber, and phytochemicals, i.e. phenolic acids and avenanthramides (AVNs). Accruing evidence suggests that phenolic acids are mainly present as insoluble esters instead of aglycones (free forms), with hydroxycinnamates being esterified to hemicellulose arabinoxylans in the cereal cell wall.44 In this study, seven phenolic acids, one phenolic aldehyde, and ten avenanthramides were identified and quantified from eight Finnish oat cultivars. Some of the phytochemicals were found at high concentrations, e.g. ferulic acid, p-coumaric acid, AVNs 2c and 2p. These compounds are known for their beneficial health effects, e.g. anti-inflammatory activity, hypoglycemic effects, cardiovascular protection.51,52 Almost all the phenolic acids were identified in the bound form. The selected cultivars differed greatly in the phytochemical composition. The cv. ‘Akseli’ showed the highest content of phenolic acids, whereas cv. ‘Viviana’ had the highest content of avenanthramides. From a commercial standpoint, these two cultivars provide new opportunities for local food producers and manufacturers, as oat breeders could selectively grow them, due to their high levels of bioactive compounds. The cultivars ‘Akseli’ and ‘Viviana’ might be employed in the formulation of functional products, which could ameliorate the diet of targeted consumers. From a nutritional standpoint, their consumption could have a beneficial impact on colon health.53 Human intervention studies showed that fiber from whole grain cereals can deliver phenolic compounds into the lower gut and that the release of phenolic acids by gut microbiota can increase the abundance of Firmicutes, Bacteroidetes, and Lactobacilli, by a two-way interaction mechanism.10 It is acknowledged that phenolic metabolites lower the colonic pH value and modulate the composition of the gut microbiota.54 While the release of bound phenolic compounds by the action of gut microbiota is being studied, actual knowledge on the bioavailability of phenolics from whole grain oat is limited and inconsistent. The metabolic fate of phenolic compounds depends largely on their level of esterification to cell wall polysaccharides. In general, the selected Finnish oats were particularly rich in dietary fiber and fiber-bound phenolic acids. Further research is required to elucidate the bioaccessibility and bioavailability of phenolic compounds from whole grain oats and to advance the knowledge on their processing as raw materials of added-value for food products. As the levels of total and individual phenolic acids and AVNs varied considerably across the samples, the present study provides information to shape the future oat breeding programs within Finland and the Northern regions of Europe.
Acknowledgments
Mrs. Riitta Henriksson in Luke Jokioinen is thanked for the excellent technical assistance.
Glossary
Abbreviations
- AOAC
American Organization of Analytical Chemists
- AVNs
avenanthramides
- cvs.
cultivars
- cv.
cultivar
- DF
dietary fiber
- DM
dry matter
- MTBE
methyl tert-butyl ether
- PAI-1
plasminogen activator inhibitor
- RT
retention time
- SD
standard deviation
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jafc.7b05726.
Author Contributions
S.M. designed and performed all the experiments reported in this study, apart from the fat analysis, carried out the UPLC-PDA-MS analysis of the phenolic compounds, analyzed data, performed the statistical analysis, and wrote the manuscript; J.-P.S. supervised the analytical work and revised the manuscript; P.O.-C. performed the fat analysis; J.-M.P. provided the oat samples, carried out the analysis of AVNs, and revised the manuscript; V.H. was responsible for the collection of the oat samples; B.Y. revised the manuscript. All the authors approved the final version of the manuscript for publication.
The authors acknowledge the financial support from Tekes–the Finnish Funding Agency for Innovation in the project Sustainable utilization of Andean and Finnish crops/Perucrop” (project decision number 1084/31/2016). The project is cofunded by Finnish companies, the University of Turku, and The Natural Resource Institute Finland.
The authors declare no competing financial interest.
Supplementary Material
References
- Halima N. B.; Saad R. B.; Khemakhem B.; Fendri M.; Abdelkafi S. Oat (Avena Sativa L.): Oil and Nutriment Compounds Valorization for Potential Use in Industrial Applications. J. Oleo Sci. 2015, 64 (9), 915–932. 10.5650/jos.ess15074. [DOI] [PubMed] [Google Scholar]
- Chen C.; Wang L.; Wang R.; Luo X.; Li Y.; Li J.; Li Y.; Chen Z. Phenolic Contents, Cellular Antioxidant Activity and Antiproliferative Capacity of Different Varieties of Oats. Food Chem. 2018, 239, 260–267. 10.1016/j.foodchem.2017.06.104. [DOI] [PubMed] [Google Scholar]
- Valkama E.; Salo T.; Esala M.; Turtola E. Grain Quality and N Uptake of Spring Cereals as Affected by Nitrogen Fertilization in Northern Conditions: A Meta-Analysis. Agric. Food Sci. 2013, 22 (2), 208–222. [Google Scholar]
- Daou C.; Zhang H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11 (4), 355–365. 10.1111/j.1541-4337.2012.00189.x. [DOI] [Google Scholar]
- Rasane P.; Jha A.; Sabikhi L.; Kumar A.; Unnikrishnan V. S. Nutritional Advantages of Oats and Opportunities for Its Processing as Value Added Foods - a Review. J. Food Sci. Technol. 2015, 52 (2), 662–675. 10.1007/s13197-013-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunilkumar B. A.; Leonova S.; Oste R.; Olsson O. Identification and Characterization of High Protein Oat Lines from a Mutagenized Oat Population. J. Cereal Sci. 2017, 75, 100–107. 10.1016/j.jcs.2017.03.003. [DOI] [Google Scholar]
- Capuano E. The Behavior of Dietary Fiber in the Gastrointestinal Tract Determines Its Physiological Effect. Crit. Rev. Food Sci. Nutr. 2017, 57 (16), 3543–3564. 10.1080/10408398.2016.1180501. [DOI] [PubMed] [Google Scholar]
- Padayachee A.; Day L.; Howell K.; Gidley M. J. Complexity and Health Functionality of Plant Cell Wall Fibers from Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57 (1), 59–81. 10.1080/10408398.2013.850652. [DOI] [PubMed] [Google Scholar]
- Abuajah C. I.; Ogbonna A. C.; Osuji C. M. Functional Components and Medicinal Properties of Food: A Review. J. Food Sci. Technol. 2015, 52 (5), 2522–2529. 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaglione P.; Mennella I.; Ferracane R.; Rivellese A. A.; Giacco R.; Ercolini D.; Gibbons S. M.; La Storia A.; Gilbert J. A.; Jonnalagadda S.; et al. Whole-Grain Wheat Consumption Reduces Inflammation in a Randomized Controlled Trial on Overweight and Obese Subjects with Unhealthy Dietary and Lifestyle Behaviors: Role of Polyphenols Bound to Cereal Dietary Fiber. Am. J. Clin. Nutr. 2015, 101 (2), 251–261. 10.3945/ajcn.114.088120. [DOI] [PubMed] [Google Scholar]
- Bei Q.; Liu Y.; Wang L.; Chen G.; Wu Z. Improving Free, Conjugated, and Bound Phenolic Fractions in Fermented Oats (Avena Sativa L.) with Monascus Anka and Their Antioxidant Activity. J. Funct. Foods 2017, 32, 185–194. 10.1016/j.jff.2017.02.028. [DOI] [Google Scholar]
- Maqsood S.; Benjakul S.; Abushelaibi A.; Alam A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13 (6), 1125–1140. 10.1111/1541-4337.12106. [DOI] [Google Scholar]
- Gangopadhyay N.; Hossain M. B.; Rai D. K.; Brunton N. P. A Review of Extraction and Analysis of Bioactives in Oat and Barley and Scope for Use of Novel Food Processing Technologies. Molecules 2015, 20 (6), 10884–10909. 10.3390/molecules200610884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Qiu S.; Gan J.; Li Z.; Nirasawa S.; Yin L. New Insights into the Antioxidant Activity and Components in Crude Oat Oil and Soybean Oil. J. Food Sci. Technol. 2016, 53 (1), 808–815. 10.1007/s13197-015-1991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Sharma S. Bioactive Components and Functional Properties of Biologically Activated Cereal Grains: A Bibliographic Review. Crit. Rev. Food Sci. Nutr. 2017, 57 (14), 3051–3071. 10.1080/10408398.2015.1085828. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Sang S. Phytochemicals in Whole Grain Wheat and Their Health-Promoting Effects. Mol. Nutr. Food Res. 2017, 61 (7), 1600852. 10.1002/mnfr.201600852. [DOI] [PubMed] [Google Scholar]
- Antonini E.; Lombardi F.; Alfieri M.; Diamantini G.; Redaelli R.; Ninfali P. Nutritional Characterization of Naked and Dehulled Oat Cultivar Samples at Harvest and after Storage. J. Cereal Sci. 2016, 72, 46–53. 10.1016/j.jcs.2016.09.016. [DOI] [Google Scholar]
- Lee J.; Chan B. L. S.; Mitchell A. E. Identification/Quantification of Free and Bound Phenolic Acids in Peel and Pulp of Apples (Malus Domestica) Using High Resolution Mass Spectrometry (HRMS). Food Chem. 2017, 215, 301–310. 10.1016/j.foodchem.2016.07.166. [DOI] [PubMed] [Google Scholar]
- Antonini E.; Diamantini G.; Ninfali P. The Effect of Mechanical Processing on Avenanthramide and Phenol Levels in Two Organically Grown Italian Oat Cultivars. J. Food Sci. Technol. 2017, 54 (8), 2279–2287. 10.1007/s13197-017-2665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Li M.; Ling A.; jin; Hu X.; Ma Z.; Liu L.; Li Y. Effects of Genotype and Environment on Avenanthramides and Antioxidant Activity of Oats Grown in Northwestern China. J. Cereal Sci. 2017, 73, 130–137. 10.1016/j.jcs.2016.12.005. [DOI] [Google Scholar]
- Redaelli R.; Dimberg L.; Germeier C. U.; Berardo N.; Locatelli S.; Guerrini L. Variability of Tocopherols, Tocotrienols and Avenanthramides Contents in European Oat Germplasm. Euphytica 2016, 207 (2), 273–292. 10.1007/s10681-015-1535-8. [DOI] [Google Scholar]
- FAOSTAT http://www.fao.org/faostat/en/#data/QC (accessed Feb 26, 2018).
- Rainakari A.-I.; Rita H.; Putkonen T.; Pastell H. New Dietary Fibre Content Results for Cereals in the Nordic Countries Using AOAC 2011.25 Method. J. Food Compos. Anal. 2016, 51, 1–8. 10.1016/j.jfca.2016.06.001. [DOI] [Google Scholar]
- Avena Genetic Resources for Quality In Human Consumption (AVEQ). http://aveq.julius-kuehn.de/aveq/ (accessed Feb 26, 2018).
- Murariu D.; Placinta D. D.; Germeier C. U.; Annamaa K.; Antonomova N.; Bulinska-Radomska Z.; Kordulasinska I.; Koenig J.; Terzi V. Quality Characteristics of European Avena Genetic Resources Collections. Romanian Agric. Res. 2013, 30, 45–50. [Google Scholar]
- Bradley R. L.Moisture and Total Solids Analysis. In Food Analysis; Springer: Boston, MA, 2010; pp 85–104. [Google Scholar]
- McCleary B. V.; Rossiter P. Measurement of Novel Dietary Fibers. J. AOAC Int. 2004, 87 (3), 707–717. [PubMed] [Google Scholar]
- Folch J.; Lees M.; Sloane Stanley G. H. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226 (1), 497–509. [PubMed] [Google Scholar]
- Multari S.; Neacsu M.; Scobbie L.; Cantlay L.; Duncan G.; Vaughan N.; Stewart D.; Russell W. R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64 (41), 7800–7811. 10.1021/acs.jafc.6b00926. [DOI] [PubMed] [Google Scholar]
- Neacsu M.; Vaughan N.; Raikos V.; Multari S.; Duncan G. J.; Duthie G. G.; Russell W. R. Phytochemical Profile of Commercially Available Food Plant Powders: Their Potential Role in Healthier Food Reformulations. Food Chem. 2015, 179, 159–169. 10.1016/j.foodchem.2015.01.128. [DOI] [PubMed] [Google Scholar]
- Bryngelsson S.; Mannerstedt-Fogelfors B.; Kamal-Eldin A.; Andersson R.; Dimberg L. H. Lipids and Antioxidants in Groats and Hulls of Swedish Oats (Avena Sativa L). J. Sci. Food Agric. 2002, 82 (6), 606–614. 10.1002/jsfa.1084. [DOI] [Google Scholar]
- Mattila P.; Pihlava J.-M.; Hellström J. Contents of Phenolic Acids, Alkyl- and Alkenylresorcinols, and Avenanthramides in Commercial Grain Products. J. Agric. Food Chem. 2005, 53 (21), 8290–8295. 10.1021/jf051437z. [DOI] [PubMed] [Google Scholar]
- Li H.; Qiu J.; Liu C.; Ren C.; Li Z. Milling Characteristics and Distribution of Phytic Acid, Minerals, and Some Nutrients in Oat (Avena Sativa L.). J. Cereal Sci. 2014, 60 (3), 549–554. 10.1016/j.jcs.2014.08.004. [DOI] [Google Scholar]
- Hu X.-Z.; Zheng J.-M.; Li X.; Xu C.; Zhao Q. Chemical Composition and Sensory Characteristics of Oat Flakes: A Comparative Study of Naked Oat Flakes from China and Hulled Oat Flakes from Western Countries. J. Cereal Sci. 2014, 60 (2), 297–301. 10.1016/j.jcs.2014.05.015. [DOI] [Google Scholar]
- van den Broeck H. C.; Londono D. M.; Timmer R.; Smulders M. J. M.; Gilissen L. J. W. J.; van der Meer I. M. Profiling of Nutritional and Health-Related Compounds in Oat Varieties. Foods 2016, 5 (1), 2. 10.3390/foods5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C.; Simsek S.; Thavarajah D.; Thavarajah P.; Ohm J.-B. Detailed Composition Analyses of Diverse Oat Genotype Kernels Grown in Different Environments in North Dakota. Cereal Chem. 2013, 90 (6), 572–578. 10.1094/CCHEM-09-12-0111-R. [DOI] [Google Scholar]
- Ho H. V. T.; Sievenpiper J. L.; Zurbau A.; Blanco Mejia S.; Jovanovski E.; Au-Yeung F.; Jenkins A. L.; Vuksan V. The Effect of Oat β-Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and ApoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116 (8), 1369–1382. 10.1017/S000711451600341X. [DOI] [PubMed] [Google Scholar]
- Hübner F.; O’Neil T.; Cashman K. D.; Arendt E. K. The Influence of Germination Conditions on Beta-Glucan, Dietary Fibre and Phytate during the Germination of Oats and Barley. Eur. Food Res. Technol. 2010, 231 (1), 27–35. 10.1007/s00217-010-1247-1. [DOI] [Google Scholar]
- Biel W.; Jacyno E.; Kawecka M. Chemical Composition of Hulled, Dehulled and Naked Oat Grains. S. Afr. J. Anim. Sci. 2014, 44 (2), 189–197. 10.4314/sajas.v44i2.12. [DOI] [Google Scholar]
- Shewry P. R.; Piironen V.; Lampi A.-M.; Nystrom L.; Li L.; Rakszegi M.; Fras A.; Boros D.; Gebruers K.; Courtin C. M.; et al. Phytochemical and Fiber Components in Oat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56 (21), 9777–9784. 10.1021/jf801880d. [DOI] [PubMed] [Google Scholar]
- Givens D. I.; Davies T. W.; Laverick R. M. Effect of Variety, Nitrogen Fertiliser and Various Agronomic Factors on the Nutritive Value of Husked and Naked Oats Grain. Anim. Feed Sci. Technol. 2004, 113 (1), 169–181. 10.1016/j.anifeedsci.2003.11.009. [DOI] [Google Scholar]
- Decker E. A.; Rose D. J.; Stewart D. Processing of Oats and the Impact of Processing Operations on Nutrition and Health Benefits. Br. J. Nutr. 2014, 112, S58–S64. 10.1017/S000711451400227X. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Liu C.; Luo S.; Chen J.; Gong E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS One 2016, 11 (8), e0161086. 10.1371/journal.pone.0161086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.; Garcia A.; Combet E.. Colonic Metabolism of Bioactive Molecules; Potential Impact of Dietary Fiber. In Dietary Fiber and Health; Cho S., Almeida N., Eds.; CRC Press: Boca Raton, FL, 2012; pp 209–234. [Google Scholar]
- Van Hung P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56 (1), 25–35. 10.1080/10408398.2012.708909. [DOI] [PubMed] [Google Scholar]
- Cai S.; Wang O.; Wu W.; Zhu S.; Zhou F.; Ji B.; Gao F.; Zhang D.; Liu J.; Cheng Q. Comparative Study of the Effects of Solid-State Fermentation with Three Filamentous Fungi on the Total Phenolics Content (TPC), Flavonoids, and Antioxidant Activities of Subfractions from Oats (Avena Sativa L.). J. Agric. Food Chem. 2012, 60 (1), 507–513. 10.1021/jf204163a. [DOI] [PubMed] [Google Scholar]
- Abd Razak D. L.; Abd Rashid N. Y.; Jamaluddin A.; Sharifudin S. A.; Long K. Enhancement of Phenolic Acid Content and Antioxidant Activity of Rice Bran Fermented with Rhizopus Oligosporus and Monascus Purpureus. Biocatal. Agric. Biotechnol. 2015, 4 (1), 33–38. 10.1016/j.bcab.2014.11.003. [DOI] [Google Scholar]
- Boz H. Phenolic Amides (Avenanthramides) in Oats - A Review. Czech J. Food Sci. 2015, 33 (5), 399–404. 10.17221/696/2014-CJFS. [DOI] [Google Scholar]
- Xie Z.; Mui T.; Sintara M.; Ou B.; Johnson J.; Chu Y.; O’shea M.; Kasturi P.; Chen Y. Rapid Quantitation of Avenanthramides in Oat-Containing Products by High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry (HPLC-TQMS). Food Chem. 2017, 224, 280–288. 10.1016/j.foodchem.2016.12.079. [DOI] [PubMed] [Google Scholar]
- Sang S.; Chu Y. Whole Grain Oats, More than Just a Fiber: Role of Unique Phytochemicals. Mol. Nutr. Food Res. 2017, 61 (7), 1600715. 10.1002/mnfr.201600715. [DOI] [PubMed] [Google Scholar]
- Burton-Freeman B. Postprandial Metabolic Events and Fruit-Derived Phenolics: A Review of the Science. Br. J. Nutr. 2010, 104, S1–S14. 10.1017/S0007114510003909. [DOI] [PubMed] [Google Scholar]
- Ozdal T.; Sela D. A.; Xiao J.; Boyacioglu D.; Chen F.; Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8 (2), 78. 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G.; Clifford M. N. Colonic Metabolites of Berry Polyphenols: The Missing Link to Biological Activity?. Br. J. Nutr. 2010, 104 (S3), S48–S66. 10.1017/S0007114510003946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.