Abstract
Purpose of review
Type 2 diabetes is associated with a characteristic dyslipidemia that may exacerbate cardiovascular risk. The causes of, and the effects of new antihyperglycemia medications on, this dyslipidemia, are under investigation. In an unexpected reciprocal manner, lowering LDL-cholesterol with statins slightly increases the risk of diabetes. Here we review the latest findings.
Recent findings
The inverse relationship between LDL-cholesterol and diabetes has now been confirmed by multiple lines of evidence. This includes clinical trials, genetic instruments using aggregate single nucleotide polymorphisms, as well as at least eight individual genes – HMGCR, NPC1L1, HNF4A, GCKR, APOE, PCKS9, TM6SF2, and PNPLA3 – support this inverse association. Genetic and pharmacologic evidence suggest that HDLcholesterol may also be inversely associated with diabetes risk. Regarding the effects of diabetes on lipoproteins, new evidence suggests that insulin resistance but not diabetes per se may explain impaired secretion and clearance of VLDL-triglycerides. Weight loss, bariatric surgery, and incretin-based therapies all lower triglycerides, whereas SGLT2 inhibitors may slightly increase HDL-cholesterol and LDL-cholesterol.
Summary
Diabetes and lipoproteins are highly interregulated. Further research is expected to uncover new mechanisms governing the metabolism of glucose, fat, and cholesterol. This topic has important implications for treating type 2 diabetes and cardiovascular disease.
Keywords: diabetes, HDL, LDL, statins, triglycerides
INTRODUCTION
Type 2 diabetes has long been known to be associated with a characteristic dyslipidemia, including high triglycerides, small/dense LDL particles, and low levels of HDL-cholesterol [1]. However, the mechanisms underlying the effects of diabetes on dyslipidemia are incompletely understood. Moreover, recent data have revealed a surprising converse association, whereby some lipoproteins appear to impact diabetes diagnosis or severity (Fig. 1). The goals of this article are to review the data surrounding the effects of lipoproteins on diabetes risk, and to summarize new updates within the last 2 years on the effects of diabetes on lipoproteins.
FIGURE 1.
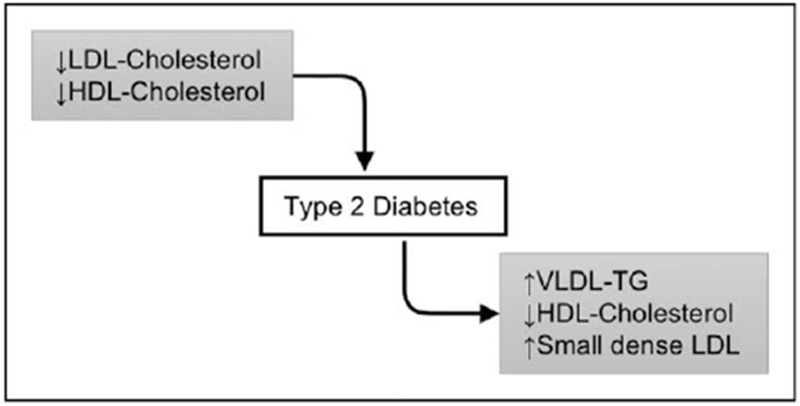
Diabetes and lipoprotein metabolism are inter-regulated. Low levels of LDL-cholesterol and HDL-cholesterol increase type 2 diabetes risk and worsen glycemia. Type 2 diabetes is accompanied by abnormalities of lipoprotein metabolism.
LOW LDL-CHOLESTEROL IS LINKED TO INCREASED DIABETES RISK
Several years ago, it was revealed that statins, widely used for LDL lowering, are associated with a slightly higher risk of diabetes [2,3,4▪]. This risk is statin dose-dependent [5]. In patients with established diabetes, statin usage is associated with increased hemoglobin A1c (HbA1c), though the effect is small [6,7]. These findings provoked the question of whether inhibition of HMGCR – the statin mechanism of action – was to blame for impaired glycemia. Indeed, genetic variants in the HMGCR gene that lower LDL-cholesterol tend to be associated with slightly increased diabetes risk [8,9,10▪▪]. However, it remained unclear whether this was specific to HMGCR, or was triggered by other methods of LDL-cholesterol modulation. Support for the latter came from Fall et al. [11], who used a genetic instrument containing 140 single nucleotide polymorphisms (SNPs) – in genes other than HMGCR – to show that higher LDL-cholesterol is associated with a lower diabetes risk, although an earlier study using 31 SNPs found no effect on diabetes risk [12]. A summary of the effects of lipoproteins on diabetes is shown in Table 1.
Table 1.
Effects of lipoproteins on type 2 diabetes
| References | Drug/genes | Effect on lipoprotein | Effect on T2D/glycemia |
|---|---|---|---|
| [2,3,4▪,5–7] | Statins | LDL-C ↓ | T2D ↑, HbA1c↑ |
| [18–24,25▪▪] | PCSK9 inhibitors | LDL-C ↓ | ↔ or HbA1c↑ |
| [11] | 140 SNPs | LDL-C ↑ | T2D ↓ |
| [13] | 130 SNPs | LDL-C ↑ | T2D ↓ |
| [12] | 31 SNPs | LDL-C ↑ | ↔ |
| [14] | 113 SNPs | LDL-C ↓ | T2D ↑ |
| [8,9,10▪▪] | HMGCR | LDL-C ↓ | T2D ↑ |
| [10▪▪] | NPC1L1 | LDL-C ↓ | T2D ↑ |
| [14] | HNF4A | LDL-C ↓ | T2D ↑ |
| [14] | GCKR | LDL-C ↓ | T2D ↑ |
| [14–16] | APOE | LDL-C ↓ | T2D ↑ |
| [9,10▪▪,14,17] | PCSK9 | LDL-C ↓ | T2D ↑ |
| [14,26] | TM6SF2 | LDL-C ↓ | ↔ or T2D ↑ |
| [14,26] | PNPLA3 | LDL-C ↓ | ↔ or T2D ↑ |
| [27] | APOB | LDL-C ↓ | ↔ |
| [35,36▪▪] | CETP inhibitors | HDL-C ↑, LDL-C ↓ | T2D ↓, Glycemia ↓ |
| [11] | 140 SNPs | HDL-C ↑ | T2D ↓ |
| [13] | 140 SNPs | HDL-C ↑ | T2D ↓ |
| [12] | 41 SNPs | HDL-C ↑ | T2D ↓ |
| [38] | 9 SNPs | HDL-C ↓ | ↔ |
| [35] | CETP | HDL-C ↑ | Glucose ↓ |
| [13] | 140 SNPs | TG ↑ | ↔ or T2D ↓ |
| [11] | 140 SNPs | TG ↑ | ↔ or T2D ↑ |
| [12] | 25 SNPs | TG ↑ | T2D ↑ |
| [39] | 10 SNPs | TG ↑ | ↔ |
| [14] | LPL | TG ↑ | T2D ↑ |
| [14] | ANGPTL4 | TG ↑ | T2D ↑ |
HbAlc, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SNPs, single nucleotide polymorphisms; T2D, type 2 diabetes; TG, triglycerides.
Recent evidence confirming the link between LDL-cholesterol and diabetes
Over the last 2 years, significant progress has been made, particularly through the use of genetics. Mendelian randomization (based on 130 SNPs) demonstrated that genetic risk for increased LDL-cholesterol increases coronary artery disease risk and lowers type 2 diabetes risk [13]. Likewise, there is an inverse correlation between the effects of 113 coding variants on LDL-cholesterol and type 2 diabetes [14]. However, the substantial heterogeneity in this effect highlights that associations between LDL-cholesterol and diabetes may be mechanism-specific.
Individual genes associated with LDL-cholesterol have been queried (Table 1). In addition to HMGCR, at least seven other loci are associated with both lower LDL-cholesterol and higher diabetes risk. This includes NPC1L1, HNF4A, and GCKR [10▪▪,14], and multiple variants in or near APOE [14–16]. The same holds for variants at PCSK9 [9,10▪▪,14,17]. It is worth noting that individual studies of PCSK9 inhibitors have not shown an effect of these drugs on glycemia or diabetes diagnosis [18–24]. However, the follow-up periods in these studies were all 2.2 years or less, and a meta-analysis across 20 PCSK9 inhibitor trials did identify increases in plasma glucose and HbA1c, in proportion to LDL-cholesterol-lowering [25▪▪]. Two additional gene variants, in TM6SF2 and PNPLA3, were recently found to be associated with low LDL-cholesterol and increased type 2 diabetes in a study of more than 300 000 individuals [14]. The small effect size may explain why multiple smaller prior studies did not find any effect of these variants on insulin sensitivity [26]. Supporting the idea of mechanism-specific effects is the finding that an LDL-cholesterol-modulating allele in APOB showed no relationship with diabetes risk [27].
Importantly, in addition to LDL-cholesterol, some of these variants are associated – sometimes more strongly – with other metabolic traits. For example, GCKR, TM6SF2, and PNPLA3 are all associated with high liver fat [14]. Given the well established correlation between liver fat and insulin resistance, it would be reasonable to predict that for these variants, high liver fat – rather than LDL-cholesterol – is the driver of increased diabetes risk. However, for statins in particular, at least one analysis implicates LDL-cholesterol-lowering as the key mediator [28]. Ultimately, mechanistic investigations are required to establish the causal links.
Do other lipoproteins contribute to diabetes risk?
Several studies have used multivariate regression analyses using simple clinical measurements to identify that low HDL-cholesterol and high triglycerides can significantly predict diabetes [29–34]. New publications move beyond these biomarker analyses to ask whether HDL-cholesterol and triglycerides could be causative factors in diabetes development.
The enzyme cholesteryl ester transfer protein (CETP) transfers cholesterol from HDL particles onto triglyceride-rich lipoproteins. Therefore, inhibitors of CETP raise HDL-cholesterol and lower LDL-cholesterol. Based on the effects of low LDL-cholesterol described above, one might predict CETP inhibitors would worsen glycemia. On the contrary, two different inhibitors of CETP, as well as genetic CETP deficiency, are associated with improved glycemia [35]. Moreover, recent data from the REVEAL trial – which had 4.1 years of follow-up – showed that Anacetrapib raised HDL-cholesterol and lowered LDL-cholesterol while reducing new-onset diabetes and HbA1c [36▪▪]. These findings suggest the possibility that CETP inhibition, or raising HDL-cholesterol more generally, has a beneficial effect on glycemia that is independent of, or counteracts against, the effects of LDL-cholesterol-lowering.
In support of this, three independent studies used genetic instruments of SNPs associated with high HDL-cholesterol and found them to be associated with lower diabetes risk [11–13]. Furthermore, genetically determined increases in cholesterol specifically on large and extra-large HDL particles is associated with lower fasting glucose [37]. In contrast, an independent study showed no effect of HDL-cholesterol on diabetes risk [38].
Data on the effects of plasma triglycerides on glycemia are more varied. One study of more than 300 000 people found that variants in LPL and ANGPTL4 that lower triglycerides also lower diabetes [14]. Two others also found that genetically high triglycerides associate with increased diabetes risk [12,37]. However, three studies did not find conclusive effects [11,13,39]. Experiments in preclinical models suggest an important role for the metabolism of triglyceride-rich lipoproteins by lipoprotein lipase (LPL) in the regulation of body weight and glycemia. Several years ago it was shown that LPL promotes uptake of triglycerides from chylomicrons into the hypothalamus, and that deficiency of neuronal LPL causes obesity [40]. Recent studies have extended this finding, showing that LPL deficiency specifically in the mediobasal hypothalamus [41], or in astrocytes [42], or in microglia [43], all cause increased obesity and worse glycemia.
Causative mechanisms
Plausible mechanisms linking low lipoprotein-cholesterol to worsening glycemia are limited. A few potential cellular mechanisms have been proposed [44]. One invokes cell membrane fluidity: perhaps low circulating cholesterol would impact plasma membrane composition or microdomains and impair the localization or function of glucose transporters or signaling receptors. A second possibility indicts intracellular cholesterol metabolism. Inhibition of HMGCR decreases production of not only cholesterol, but also other products of the mevalonate pathway such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate. These two lipids help form membrane anchors, and dampening their production may impair plasma membrane protein localization. However, the cell-types and the plasma membrane proteins that would potentially be involved have not yet been defined.
Recent publications report new roles for hepatocellular cholesterol homeostasis on insulin-regulated pathways, although none implicate lipoprotein-cholesterol per se. One suggests – in conceptual contrast to the prediction cited above – that hepatocyte accumulation of mevalonate pathway intermediates, caused by impaired cholesterol catabolism, is linked to increased glucose production [45]. However, the direct molecular mediators of this link are not yet defined. Moreover, one of the proposed players – the cholesterol-catabolizing enzyme CYP7A1 – was shown to have the opposite effect on glucose metabolism in vivo [46]. Thus, the role of this pathway in linking cholesterol with glycemia remains uncertain. Ablating the sinusoidal hepatocyte cholesterol efflux transporter Abca1 impairs insulin signaling [47]. However, this impairment was specific for insulin-regulated lipogenesis, without affecting gluconeogenesis. Thus, these studies of hepatocellular cholesterol catabolism and efflux have not yet yielded clear hypotheses linking low lipoprotein-cholesterol and glucose homeostasis.
Perhaps other, nonhepatocyte cell types are involved. One candidate would be macrophages, as they take up LDL particles. However, data do not yet support this prediction. Reducing cholesterol content in myeloid cells, by ablating the enzyme fatty acid synthase, reduces inflammatory signaling and actually improves insulin sensitivity during high fat feeding [48]. Macrophages also efflux cholesterol onto HDL; could this reveal a link? Transplanting wild-type mice with bone marrow from mice lacking the cholesterol efflux transporter Abca1 worsens high fat diet-induced insulin resistance [49]. However, the opposite conclusion was reached after transplanting bone marrow transplant from mice lacking both efflux transporters Abca1 and Abcg1; these mice showed improved glucose parameters [50]. Thus, macrophage cholesterol handling is not a clear link between lipoproteins and glycemia.
Another candidate cell-type would be pancreatic β-cells, as a substantial portion of diabetes risk is attributed to β-cell function or survival. Multiple lines of evidence suggest that increased β-cell cholesterol accumulation impairs insulin secretion [51,52,53]. Notably, humans with mutations in ABCA1 also have impaired insulin secretion [54]. This might suggest a mechanism whereby low HDL-cholesterol is a marker for impaired cholesterol efflux causing β-cell cholesterol accumulation and defective insulin secretion. However, this would not explain the benefit of CETP inhibitors or CETP deficiency, as these affect circulating lipoprotein particles, nor would it explain effects of LDL-cholesterol.
Overall, studies over the last few years have solidly demonstrated that LDL-cholesterol lowering by pharmacotherapy or genetic variation causes increased risk of type 2 diabetes, whereas HDL-cholesterol raising may be protective. It is important to note the much greater benefit of lowering LDL-cholesterol for reducing cardiovascular events is considered to outweigh the small risk of diabetes [55]. However, the molecular mechanisms of this effect are almost completely obscure, and understanding these biological underpinnings may aid in the design of future drugs or in the identification of the minority of patients who are at risk and should be given alternative cardioprotective therapies.
IMPACT OF DIABETES ON LIPOPROTEIN METABOLISM
The pro-atherogenic lipid profile associated with diabetes is evident even before the diagnosis of diabetes, in the states of insulin resistance or impaired fasting glucose [56,57]. However, the metabolic and molecular mechanisms responsible for these phenotypes remain under debate. New studies shed light on the effects of diabetes and diabetes therapies on lipoproteins, and these are summarized here (Table 2).
Table 2.
Recent data on the effects of diabetes or diabetes treatments on lipoproteins
| References | Condition/treatment | TG | LDL | HDL |
|---|---|---|---|---|
| [57] | T2D, insulin resistance | VLDL-TG ↑ | Small dense LDL↑ | HDL-C ↓ |
| [97] | T2D, obese vs. healthy, lean | VLDL-TG secretion ↑, LDL-TG clearance↓ | ||
| [59▪,60] | T2D, obese vs. bese, no diabetes | VLDL-TG secretion ↔, VLDL-TG clearance↔ | Insulin suppression of apoB-100 secretion ↓ | |
| [66–68] | Weight loss | TG↓ | HDL-C↑ | |
| [70–73] | Bariatric surgeries | TG↓ | LDL-C↔ or ↓ | |
| [74] | Sleeve gastrectomy | HDL cholesterol efflux capacity↑ | ||
| [67] | Weight regain after loss | TG↑ | HDL-C↓ | |
| [77] | GLP-1 receptor agonist | TG↓ | LDL-C↓ | |
| [75,76] | DPP-4 inhibitor | TG↓ | LDL particle size↑ | |
| [89,92,93] | SGLT2 inhibitor | TG↔ or ↓ | LDL-C↑ | HDL-C↑ |
DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes; TG, triglycerides; VLDL, very low-density lipoprotein.
Effects on lipoproteins in the natural history of diabetes
New kinetic studies have added to our understanding of VLDL-triglycerides, which are influenced by both secretion and clearance [58]. Nielsen et al. assessed VLDL kinetics in male type 2 diabetes patients and healthy controls [59▪,60,61]. In contrast to prior studies, the two groups were matched for body mass index. Surprisingly, VLDL-triglyceride secretion, clearance, and oxidation were the same between the two groups [59▪,60]. The effects of insulin or a mixed meal to suppress VLDL-triglyceride secretion were also comparable. Moreover, there were no differences in the ability of VLDL-triglyceride to be stored in adipose tissue [60]. These findings suggest that VLDL-triglyceride kinetics may be affected more strongly by obesity or insulin resistance than by diabetes per se. One of the only differences observed in diabetes patients was impaired suppression of apoB-100 secretion by insulin [59▪]. Perhaps this suggests a contributor to the accumulation of small, dense LDL particles.
Hypotheses to explain the overproduction and impaired clearance of VLDL-triglycerides during insulin resistance are summarized in references [57,62,63]. These include increased flux of free fatty acids from adipose to liver, as a consequence of poor insulin suppression of lipolysis, or increased hepatic lipogenesis. A new suggested mechanism for impaired clearance of triglyceride-rich lipoproteins in diabetes implicates the membrane raft protein flotillin-1, which is required for syndecan-1-mediated lipoproitein endocytosis, and which is strongly reduced in a mouse model of type 2 diabetes [64▪▪].
Effects of diabetes therapies on lipoproteins
Weight loss is a known method for improving diabetes and dyslipidemia [65], and new publications support this. Body weight loss of even 5–7% is sufficient to lower triglycerides, and further weight loss causes greater improvements [66–68]. Bariatric surgeries also lower triglycerides [69], as highlighted by recent publications [70–73]. Most weight loss interventions are also associated with increased HDL-cholesterol [67,68,70–73], and in the case of sleeve gastrectomy, this may be consequent to enhanced HDL cholesterol efflux capacity [74]. However, weight re-gain reverses the improvements in lipoproteins [67]. Changes in LDL-cholesterol in response to weight loss were minor or absent in these studies, except a study of gastric bypass patients, 12 years after surgery [71].
Incretin-based antidiabetes therapies – including agonists of the glucagon-like peptide-1 (GLP-1) receptor and inhibitors of DPP-4, the enzyme responsible for degradation of GLP-1 and other incretins – have been scrutinized for their effects on cardiovascular risk. These drugs have been found to modestly improve lipoprotein profiles, by reducing chylomicron production, without affecting lipoprotein clearance [75–77]. The molecular mechanisms are unclear, but may be pharmacologic, as endogenous GLP-1 and GLP-2 appear to be minor contributors to postprandial chylomicron production [78]. Moreover, the potential lipid-lowering benefits of these drugs do not necessarily correlate with improvements in cardiovascular outcomes: two have successfully lowered major adverse cardiovascular events [79,80], but five have not [81–85], though all are confirmed to be safe [86]. It is possible that future drugs targeting these pathways can be designed to have greater lipid-lowering effects. Indeed, a dual agonist for both GLP-1 receptor and the glucose-dependent insulinotropic peptide (GIP) receptor lowers total cholesterol, potentially through the GIP signaling pathway [87].
Inhibitors of sodium-glucose transporter 2 (SGLT2) have also been assessed in recent years, and two have shown cardioprotective effects [88,89]. Though the mechanisms of this protection are unlikely to involve lipoproteins [90,91], it is still interesting to inspect the impact of these drugs on lipoproteins. Triglycerides tend to be reduced with SGLT2 inhibition, although differences are not reported in all studies [92,93]. More consistently reported are increases in HDL-cholesterol and LDL-cholesterol [89,92,93]. What are the causes of these effects? Decreases in triglycerides and increases in HDL-cholesterol could be attributable to the weight loss observed after SGLT2 inhibition. However, the cause of increased LDL-cholesterol is unknown. It could involve the effect of SGLT2 inhibitors to switch hepatic fuel selection towards fatty acid oxidation and ketogenesis [94,95], as a recent study suggests that ketogenic flux secondarily increases hepatocyte cholesterol synthesis, thus lowering LDL receptor and impairing LDL particle clearance [96].
CONCLUSION
The interrelationships between diabetes and lipid metabolism are complex. Basic biomedical investigations into these pathways are essential to fully understand these links and their consequences on the substantial portion of adults that are at risk for metabolic diseases.
KEY POINTS.
Low levels of LDL-cholesterol are associated with increased type 2 diabetes risk
HDL-cholesterol may also be inversely associated with diabetes risk
The biological underpinnings of these effects are unknown
Lipoprotein metabolism is altered in the natural history of diabetes, and in response to diabetes therapies
Acknowledgements
We acknowledge Samuel X. Lee for helpful discussions.
Financial support and sponsorship
This work was supported by the National Institutes of Health R01HLHL125649 and R56DK115825 to R.A.H. and from the American Diabetes Association 1-17-PMF-017 to M.C.I. and the Russell Berrie Foundation.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375: 735–742. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 4.▪.Crandall JP, Mather K, Rajpathak SN, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care 2017; 5:e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study confirms the inverse association of LDL-cholesterol-lowering by statins and increased diabetes risk, in a trial with diabetes as the primary endpoint.
- 5.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011; 305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 6.Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia 2014; 57:2444–2452. [DOI] [PubMed] [Google Scholar]
- 7.Livingstone SJ, Looker HC, Akbar T, et al. Effect of atorvastatin on glycaemia progression in patients with diabetes: an analysis from the Collaborative Atorvastatin in Diabetes Trial (CARDS). Diabetologia 2016; 59:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015; 385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016; 375: 2144–2153. [DOI] [PubMed] [Google Scholar]
- 10.▪▪.Lotta LA, Sharp SJ, Burgess S, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA 2016; 316:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was one of the first to show that LDL-cholesterol-modulating alleles other than HMGCR are inversely linked to diabetes risk.
- 11.Fall T, Xie W, Poon W, et al. Using genetic variants to assess the relationship between circulating lipids and type 2 diabetes. Diabetes 2015; 64:2676–2684. [DOI] [PubMed] [Google Scholar]
- 12.Qi Q, Liang L, Doria A, et al. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes 2012; 61:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White J, Swerdlow DI, Preiss D, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol 2016; 1:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu DJ, Peloso GM, Yu H, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nature genetics 2017; 49:1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W, Rasheed A, Tikkanen E, et al. Identification of new susceptibility loci for type 2 diabetes and shared etiological pathways with coronary heart disease. Nature genetics 2017; 49:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook JP, Morris AP. Multiethnic genome-wide association study identifies novel locus for type 2 diabetes susceptibility. Eur J Hum Genet 2016; 24:1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt AF, Swerdlow DI, Holmes MV, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 2017; 5:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5:941–950. [DOI] [PubMed] [Google Scholar]
- 19.Sattar N, Toth PP, Blom DJ, et al. Effect of the proprotein convertase subtilisin/ kexin type 9 inhibitor evolocumab on glycemia, body weight, and new-onset diabetes mellitus. Am J Cardiol 2017; 120:1521–1527. [DOI] [PubMed] [Google Scholar]
- 20.Leiter LA, Cariou B, Muller-Wieland D, et al. Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM-INSULIN randomized trial. Diabetes Obes Metab 2017; 19:1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J 2016; 37:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom DJ, Koren MJ, Roth E, et al. Evaluation of the efficacy, safety and glycaemic effects of evolocumab (AMG 145) in hypercholesterolaemic patients stratified by glycaemic status and metabolic syndrome. Diabetes Obes Metab 2017; 19:98–107. [DOI] [PubMed] [Google Scholar]
- 23.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017; 376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 25.▪▪.de Carvalho LSF, Campos AM, Sposito AC. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and incident type 2 diabetes mellitus: a systematic review and meta-analysis with over 96,000 patient-years. Diabetes Care 2018; 41:364–367. [DOI] [PubMed] [Google Scholar]; This analysis is one of the first to indicate that LDL-cholesterol-lowering drugs other than statins (namely, PCSK9 inhibitors) negatively impact diabetes and glycemia.
- 26.Petaja EM, Yki-Jarvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD – a systematic review. Int J Mol Sci 2016; 17:pii: E633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Ryan KA, Jaworek TJ, et al. Familial hypercholesterolemia and type 2 diabetes in the old order Amish. Diabetes 2017; 66:2054–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labos C, Brophy JM, Smith GD, et al. Evaluation of the pleiotropic effects of statins: a reanalysis of the randomized trial evidence using egger regression. Arterioscler Thromb Vasc Biol 2018;38:262–265. [DOI] [PubMed] [Google Scholar]
- 29.D’Agostino RB, Hamman RF, Karter AJ, et al. , The Insulin Resistance Atherosclerosis Study. Cardiovascular disease risk factors predict the development of type 2 diabetes. Diabetes Care 2004; 27:2234–2240. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt MI, Duncan BB, Bang H, et al. , The Atherosclerosis Risk In Communities Study. Identifying individuals at high risk for diabetes. Diabetes Care 2005; 28:2013–2018. [DOI] [PubMed] [Google Scholar]
- 31.Wilson PF, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham offspring study. Arch Intern Med 2007; 167:1068–1074. [DOI] [PubMed] [Google Scholar]
- 32.Gupta AK, Dahlof B, Dobson J, et al. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the anglo-scandinavian cardiac outcomes trial – blood pressure lowering arm and the relative influence of antihypertensive medication. Diabetes Care 2008; 31: 982–988. [DOI] [PubMed] [Google Scholar]
- 33.Chien K, Cai T, Hsu H, et al. A prediction model for type 2 diabetes risk among Chinese people. Diabetologia 2008; 52:443–450. [DOI] [PubMed] [Google Scholar]
- 34.Kahn HS, Cheng YJ, Thompson TJ, et al. TWo risk-scoring systems for predicting incident diabetes mellitus in U.S. adults age 45 to 64 years. Ann Intern Med 2009; 150:741–751. [DOI] [PubMed] [Google Scholar]
- 35.Tall AR, Rader DJ. The trials and tribulations of CETP inhibitors. Circ Res 2018; 122:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.▪▪.HPS3/TIMI55–REVEAL Collaborative Group. Bowman L, Hopewell JC, Chen F, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017; 377:1217–1227. [DOI] [PubMed] [Google Scholar]; The REVEAL trial had the longest follow-up (4.1 years) of any CETP inhibitor trial, and it demonstrated a protective effect on type 2 diabetes and HbA1c.
- 37.Liu J, van Klinken JB, Semiz S, et al. A mendelian randomization study of metabolite profiles, fasting glucose and type 2 diabetes. Diabetes 2017; 66:2915–2926. [DOI] [PubMed] [Google Scholar]
- 38.Haase CL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL cholesterol and risk of type 2 diabetes: a mendelian randomization study. Diabetes 2015; 64:3328–3333. [DOI] [PubMed] [Google Scholar]
- 39.De Silva NM, Freathy RM, Palmer TM, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes 2011; 60: 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Astarita G, Taussig MD, et al. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab 2011; 13:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laperrousaz E, Moulle VS, Denis RG, et al. Lipoprotein lipase in hypothalamus is a key regulator of body weight gain and glucose homeostasis in mice. Diabetologia 2017; 60:1314–1324. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y, Layritz C, Legutko B, et al. Disruption of lipid uptake in astroglia exacerbates diet-induced obesity. Diabetes 2017; 66:2555–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Y, Vidal-Itriago A, Kalsbeek MJ, et al. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep 2017; 20:3034–3042. [DOI] [PubMed] [Google Scholar]
- 44.Brault M, Ray J,Gomez YH, et al. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism 2014; 63: 735–745. [DOI] [PubMed] [Google Scholar]
- 45.Okin D, Medzhitov R. The effect of sustained inflammation on hepatic mevalonate pathway results in hyperglycemia. Cell 2016; 165:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrell JM, Boehme S, Li F, Chiang JY. Cholesterol 7alpha-hydroxylasedeficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders. J Lipid Res 2016; 57:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Key CC, Liu M, Kurtz CL, et al. Hepatocyte ABCA1 deletion impairs liver insulin signaling and lipogenesis. Cell Rep 2017; 19:2116–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei X, Song H, Yin L, et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature 2016; 539:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang C, Liu Y, Yang W, et al. Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice. J Lipid Res 2016; 57:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautier EL, Westerterp M, Bhagwat N, et al. HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J Exp Med 2013; 210:339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunham LR, Kruit JK, Pape TD, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007; 13:340–347. [DOI] [PubMed] [Google Scholar]
- 52.Kruit JK, Wijesekara N, Fox JE, et al. Islet cholesterol accumulation due to loss of ABCA1 leads to impaired exocytosis of insulin granules. Diabetes 2011; 60:3186–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee AK, Yeung-Yam-Wah V, Tse FW, Tse A. Cholesterol elevation impairs glucose-stimulated Ca(2+) signaling in mouse pancreatic beta-cells. Endocrinology 2011; 152:3351–3361. [DOI] [PubMed] [Google Scholar]
- 54.Vergeer M, Brunham LR, Koetsveld J, et al. Carriers of loss-of-function mutations in ABCA1 display pancreatic β-cell dysfunction. Diabetes Care 2010; 33:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegele RA, Gidding SS, Ginsberg HN, et al. Nonstatin low-density lipoprotein-lowering therapy and cardiovascular risk reduction-statement from ATVB council. Arterioscler Thromb Vasc Biol 2015; 35:2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JE, Min SH, Lee DH, et al. Comprehensive assessment of lipoprotein subfraction profiles according to glucose metabolism status, and association with insulin resistance in subjects with early-stage impaired glucose metabolism. Int J Cardiol 2016; 225:327–331. [DOI] [PubMed] [Google Scholar]
- 57.Taskinen MR, Bore´n J New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015; 239:483–495. [DOI] [PubMed] [Google Scholar]
- 58.Mittendorfer B, Yoshino M, Patterson BW, Klein S. VLDL triglyceride kinetics in lean, overweight, and obese men and women. J Clin Endocrinol Metab 2016; 101:4151–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.▪.Johansen RF, Sondergaard E, Sorensen LP, et al. Basal and insulin-regulated VLDL1 and VLDL2 kinetics in men with type 2 diabetes. Diabetologia 2016. 59:833–843. [DOI] [PubMed] [Google Scholar]; This human kinetic study demonstrated that diabetes per se does not affect VLDL triglyceride secretion or clearance.
- 60.Søndergaard E, Johansen RF, Jensen MD, Nielsen S. Postprandial VLDL-TG metabolism in type 2 diabetes. Metabolism 2017; 75:25–35. [DOI] [PubMed] [Google Scholar]
- 61.Andersen IR, Sondergaard E, Sorensen LP, et al. Increased VLDL-TG fatty acid storage in skeletal muscle in men with type 2 diabetes. J Clin Endocrinol Metab 2017; 102:831–839. [DOI] [PubMed] [Google Scholar]
- 62.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011; 22:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otero YF, Stafford JM, McGuinness OP. Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux. J Biol Chem 2014; 289:20462–20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.▪▪.Chen K, Wu Q, Hu K, et al. Suppression of hepatic FLOT1 (Flotillin-1) by type 2 diabetes mellitus impairs the disposal of remnant lipoproteins via syndecan-1. Arterioscler Thromb Vasc Biol 2018; 38:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work suggests an intriguing new mechanism to explain impaired triglyceride clearance in diabetes.
- 65.Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res 2016; 118:1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016; 23:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Action for Health in Diabetes (Look AHEAD) Study Group. Association of weight loss maintenance and weight regain on 4-year changes in CVD risk factors: the Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care 2016; 39:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajjo T, Almasri J, Al Nofal A, et al. The association of weight loss and cardiometabolic outcomes in obese children: systematic review and metaregression. J Clin Endocrinol Metab 2017; 102:758–762. [DOI] [PubMed] [Google Scholar]
- 69.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 70.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med 2017; 376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 2017; 377:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ooi GJ, Doyle L, Tie T, et al. Weight loss after laparoscopic adjustable gastric band and resolution of the metabolic syndrome and its components. Int J Obes (Lond) 2017; 41:902–908. [DOI] [PubMed] [Google Scholar]
- 73.Ooi GJ, Earnest A, Doyle L, et al. Detailed description of change in serum cholesterol profile with incremental weight loss after restrictive bariatric surgery. Obes Surg 2017; 10.1007/s11695-017-3015-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 74.Heffron SP, Lin B, Parikh M, et al. Changes in high-density lipoprotein cholesterol efflux capacity after bariatric surgery are procedure dependent. Arterioscler Thromb Vasc Biol 2018; 38:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev 2014; 35:992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahren B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia 2016; 59:907–917. [DOI] [PubMed] [Google Scholar]
- 77.Blonde L, Pencek R, MacConell L. Association among weight change, glycemic control, and markers of cardiovascular risk with exenatide once weekly: a pooled analysis of patients with type 2 diabetes. Cardiovasc Diabetol 2015; 14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matikainen N, Bjo¨ rnson E, So¨ derlund S, et al. Minor contribution of endogenous GLP-1 and GLP-2 to postprandial lipemia in obese men. PLoS One 2016; 11:e0145890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 80.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 82.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242. [DOI] [PubMed] [Google Scholar]
- 83.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 84.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 85.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schnell O, Ryden L, Standl E, Ceriello A. Updates on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol 2017; 16:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frias JP, Bastyr EJ 3rd, Vignati L, et al. The sustained effects of a dual GIP/ GLP-1 receptor agonist, NNC0090–2746, in patients with type 2 diabetes. Cell Metab 2017; 26:343, e342–352.e342. [DOI] [PubMed] [Google Scholar]
- 88.Zinman B, Wanner C, Lachin JM, et al. , EMPA-REGOUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 89.Neal B, Perkovic V, Mahaffey KW, et al. , CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377:644–657. [DOI] [PubMed] [Google Scholar]
- 90.Ferrannini E Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017; 26:27–38. [DOI] [PubMed] [Google Scholar]
- 91.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017; 13: 11–26. [DOI] [PubMed] [Google Scholar]
- 92.Stenloöf K, Cefalu WT, Kim K-A, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin 2014; 30:163–175. [DOI] [PubMed] [Google Scholar]
- 93.Bode B, Stenloöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab 2015; 17:294–303. [DOI] [PubMed] [Google Scholar]
- 94.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodiumglucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Briand F, Mayoux E, Brousseau E, et al. Empagliflozin, via switching metabolism toward lipid utilization, moderately increases LDL cholesterol levels through reduced LDL catabolism. Diabetes 2016; 65:2032–2038. [DOI] [PubMed] [Google Scholar]
- 97.Søndergaard E, Sørensen LP, Rahbek I, et al. Postprandial VLDL-triacylglycerol secretion is not suppressed in obese type 2 diabetic men. Diabetologia 2012; 55:2733–2740. [DOI] [PubMed] [Google Scholar]


