Abstract
The emulsification properties of carboxymethyl chitosan (CMChi) and hydrophobically modified carboxymethyl chitosan (h-CMChi) were studied as a function of pH and dodecane/water ratio. The pH was varied between 6—10, and the oil/water ratio between 0.1—2.0. In CMChi solution, the emulsion stability increased as the pH was lowered from 10 to 7, and the phase inversion was shifted from oil/water ratio 1.0 to 1.8, respectively. The system behaved differently in pH 6 due to the aggregation of CMChi and the formation of nanoparticles (∼200—300 nm). No phase inversion was observed and the maximum amount of emulsified oil was reached at oil/water ratio 1.2. The h-CMChi showed similar behavior as a function of pH but, due to hydrophobic modification, the phase inversion was shifted to higher values in pH 7—10. In pH 6, the behavior was similar, but the maximum amount of emulsified oil was higher compared to CMChi. The amount of adsorbed particles correlated with the emulsified amount of oil. Reversible emulsification of dodecane was demonstrated by pH adjustment using CMChi and h-CMChi solutions. The formed emulsions were gel-like, suggesting particle–particle interaction.
Introduction
Chitosan is an abundant polycationic biopolymer that is derived mainly from chitin in crustacean shells by deacetylation of the amino groups. Typically, the deacetylation degree is over 50%. The deacetylated amino groups allow chitosan to dissolve in dilute acids at pH < 6.5 due to the protonation of amino groups (pKa ≈ 6.5). Chitosan is also nontoxic, biodegradable, and renewable, and the reactive amino and hydroxyl groups can be exploited to modify chitosan and its properties. These desirable features make chitosan a versatile material for a variety of applications, such as adsorption, food materials, biomedicine, and drug delivery. Recently, production and stabilization of emulsions by chitosan have received increasing attention.1−6 However, most of the applications are limited to acidic pH due to the insolubility of chitosan in alkaline pH.
The solubility of chitosan can be modified by introducing additional functional groups into the polymer. Solubility in alkaline media can be achieved, for example, by introducing carboxyl groups into the chitosan polymer.7 Carboxyl groups have a pKa value of ca. 4.5, indicating that all the carboxylic groups are expected to be deprotonated in pH ≥ 7, granting the carboxymethyl chitosan (CMChi) water solubility in neutral and alkaline pH. The simultaneous presence of amino and carboxyl groups along the polymer chain results in insolubility at pH near the isoelectric point (pI) which is ca. 5.5. Chitosan as a polysaccharide is fairly hydrophilic with only weak surface activity,8,9 but it can be enhanced by introducing hydrophobic characteristics to chitosan, for example by reacting the amino groups with hydrophobic aldehydes, and reducing the formed imine.10 These carboxyl and hydrophobic groups containing hydrophobically modified carboxymethyl chitosans (h-CMChi) are amphiphilic and water-soluble.11
Native1−3,12 and modified chitosans11,13−17 have been applied to produce and stabilize conventional and Pickering emulsions. In conventional emulsions, the dissolved native chitosan provides a mainly steric barrier against the coalescence of the oil droplets due to its weak surface activity.8 But in Pickering emulsions, small particles can adsorb “irreversibly” on the liquid–liquid interface and stabilize emulsions.18 Chitosan particles can be formed with or without cross-linking agents. Without cross-linking agents, the particles are formed near pH 6.5 due to the aggregation of the chitosan as the amino groups are neutralized.12 The particles can also be formed in lower pH by addition of cross-linking agents, such as sodium tripolyphosphate or glutaraldehyde. Ionic cross-linking agents are typically multivalent salts (i.e., sodium tripolyphosphate) that are fairly environmentally friendly and nonharmful, but due to the reversible ionic bonding, the leeching of the cross-linking agent may degrade the particles. Covalent cross-linking agents (i.e., glutaraldehyde) may provide better stability, but the compounds are often harmful which restricts the possible applications. The drawbacks of cross-linking agents can be solved by introducing oppositely charged functional groups into the polymer, such as carboxylic and amino groups in CMChi. The self-aggregation of CMChi and h-CMChi has been studied in the literature but the studies are often conducted only in neutral pH19,20 or with the assistance of sonication.21,22 Also, phosphate buffers sometimes used for the pH adjustment may affect the aggregation since it is well-known that multivalent phosphate ions are good cross-linking agents for chitosan.
Our previous study showed that CMChi forms nanoparticles without cross-linking agents at pH (5.5–7) near the pI.23 To our knowledge, self-assembled CMChi and h-CMChi particles have not been studied for the emulsification of oil. In this paper, we studied the emulsion formation properties of CMChi and h-CMChi nanoparticles formed by pH adjustment (6—10) without the addition of cross-linking agents or the assistance of sonication. The reversibility of the emulsification by pH adjustment was also studied. Similar kind of systems presented here could have use in oil–water separation, food, or environmental applications.
Experimental Section
Syntheses and Characterizations
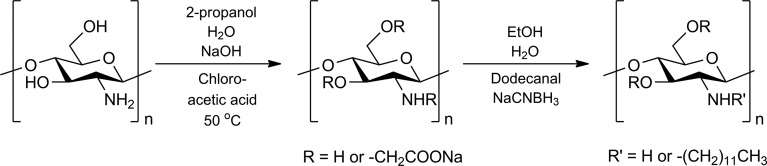
The synthesis of CMChi in sodium salt form was conducted according to a previously reported method,16,23 and the following synthesis of h-CMChi was based on methods reported in literature10,11 but modified to be suitable for the purpose of this study. The reaction scheme is presented in Figure 1.
Figure 1.
Synthesis of CMChi from native chitosan using chloroacetic acid, and the following synthesis of h-CMChi using dodecanal.
Figure 1 shows the syntheses of CMChi and h-CMChi. The details of the syntheses are presented in the Supporting Information. The CMChi and h-CMChi were characterized by FTIR (Figure S1) and NMR measurements (Figures S2 and S3). The carboxylic groups in CMChi and h-CMChi were detected with FTIR-spectroscope type Nicolet Nexus 8700. For NMR measurement, samples of CMChi and h-CMChi were dissolved in D2O containing 0.7% DCl and placed in 5 mm NMR tubes. The 1H NMR spectra were recorded using a Bruker Ascend 400 MHz spectrometer and standard proton parameters with a delay time (d1) of 6 s at 70 °C. The FTIR and NMR spectra are presented in the Supporting Information. The particle sizes and zeta potentials were measured using ZetaSizer Nano ZS apparatus (Malvern Instruments Ltd.). For zeta potential measurements, a 0.5 mg mL–1 (50 mL) solution of CMChi was prepared and the pH of the solution was adjusted with HCl (0.01 M). Small samples (∼0.5 mL) were taken from the solution/slurry and the zeta potential was measured with Zetasizer. The nanoprecipitates were also imaged via transmission electron microscopy (TEM; Hitachi 7700), and the sizes and size distributions were determined based on the obtained images. One drop of CMChi (or h-CMChi) solution was placed on the sample holder grid and allowed to dry at room temperature before imaging.
Emulsion Formation and Stability
The emulsification properties of CMChi and h-CMChi were studied by mixing 3 mL (0.5 mg mL–1) of CMChi or h-CMChi solution with varying amounts of dodecane (0.3–6.0 mL), resulting in a series of samples where the oil/water ratio varied between 0.1 and 2.0. These series of samples were prepared in varying pH between 6 and 10. The mixing was conducted in a glass vial (diameter 25 mm, height 95 mm) with a vortex mixer (VWR) at 2500 rpm for 10 s. The mixture was poured gently into a centrifuge tube (15 mL) and centrifuged at 1000 rpm (RCF 201) for 2 min. The amount of separated dodecane was measured to determine the stability of the emulsion. The amount of separated dodecane correlates with the emulsion stability.
Adsorption of CMChi and h-CMChi Nanoparticles on Dodecane–Water Interface
The opalescence of the CMChi and h-CMChi solutions at pH < 7 was exploited to determine the amount of nanoparticles in the solution after the emulsification of varying amount of dodecane. Due to the opalescence of the solutions, a UV–vis spectrophotometer could be used to construct a standard curve by diluting 0.5 mg mL–1 solution of CMChi (pH 6.0) or h-CMChi (pH 6.2) and measuring the absorbance of the solutions at 250 nm.
Reversible Emulsification of Dodecane by Adjusting pH
A volume of 6 mL of CMC-solution (0.5 mg/mL) at pH 6 and 1.2 mL of dodecane (oil/water ratio 0.2) were added to a vial, and the emulsion was formed by vortex mixing at 2500 rpm for 10 s. De-emulsification was conducted by adding NaOH (20 μL of 0.1 M and 10 μL of 1 M) followed by shaking by hand. The reformation of the particles was conducted by adding HCl (10 μL of 1 M and 17.5 μL of 0.1 M) and stirring the water phase with a magnetic stirrer at 150 rpm. The re-emulsification was conducted by vortex-mixing at 2500 rpm for 10 s. For h-CMC, the process was similar, except the de-emulsification and particle reformation happened simultaneously, and the original pH was 6.2.
Results and Discussion
Precipitation of CMChi and h-CMChi as a Function of pH
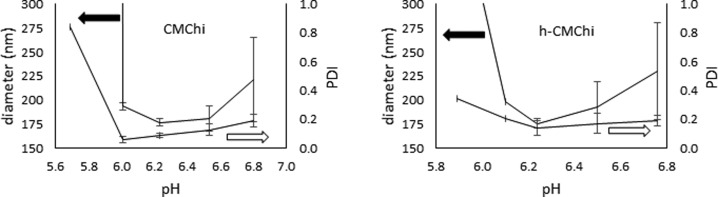
The CMChi and h-CMChi are soluble at pH 7–10 but start to aggregate at pH < 7 and precipitate out of the solution if the pH is low enough. The CMChi and h-CMChi particles were formed by carefully adjusting the pH of the solutions. The aggregation into colloidal particles was due to the interaction between carboxyl and amino groups. Figure 2 shows the aggregation of CMChi and h-CMChi as a function of pH detected by DLS measurements.
Figure 2.
Aggregation and precipitation of CMChi and h-CMChi detected by DLS measurements. Upper graph represents the hydrodynamic diameter of the aggregates and the lower graph presents the PDI. Average values until precipitation with standard deviations are presented based on two measurements.
Figure 2 shows that the CMChi and h-CMChi aggregate fairly similarly as the pH is decreased from 6.8 to about 5.8. The minimum diameter is detected at around pH 6.2 for both CMChi and h-CMChi. The minimum diameters are 170 nm, and 180 nm for CMChi and h-CMChi, respectively. The diameter increases rapidly in CMChi solution with a decrease of pH when pH < 6, indicating the precipitation of the polymer. The h-CMChi precipitates at slightly higher pH (6.1). This is probably due to the interaction between hydrophobic alkyl chains and lower solubility. In CMChi solution, the PDI value decreases almost linearly until pH 6 and then increases rapidly due to the precipitation. The minimum PDI value detected at pH 6 is around 0.06, indicating that the particle size distribution is narrow. In h-CMChi solution, the PDI value is almost constant (0.2) until pH 6.2, and increases slightly at pH < 6.2. The h-CMChi did not form stable colloidal particles below pH 6.2. These findings suggest that the hydrophobic modification of h-CMChi disturbs the aggregation and formation of particles. This could be explained by the competing interactions between oppositely charged amino and carboxyl groups, and the hydrophobic groups. The positively charged amino groups are attracted to the negatively charged carboxyl groups, and the hydrophobic alkyl chains are attracted to one another. Due to the random distribution of all these groups along the polymer chain, the conformations of the polymer chains are expected to be less uniform compared to the polymer chains without the hydrophobic groups. Figure 3. shows the determination of pI for CMChi and h-CMChi.
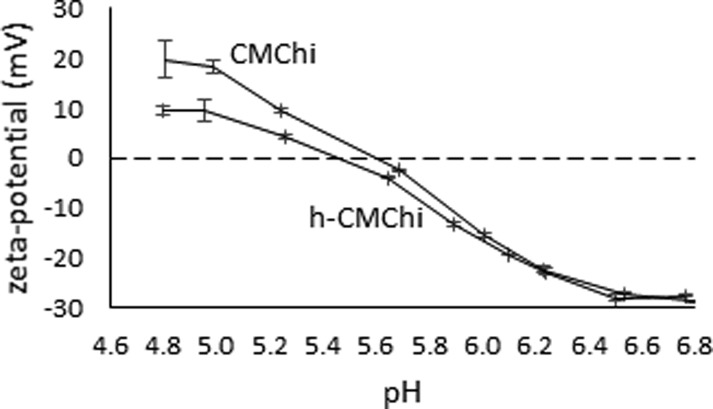
Figure 3.
Determination of pI for CMChi and h-CMChi by measuring the zeta potential of the CMChi and h-CMChi precipitates as a function of pH. Average values with standard deviations are presented based on three measurements.
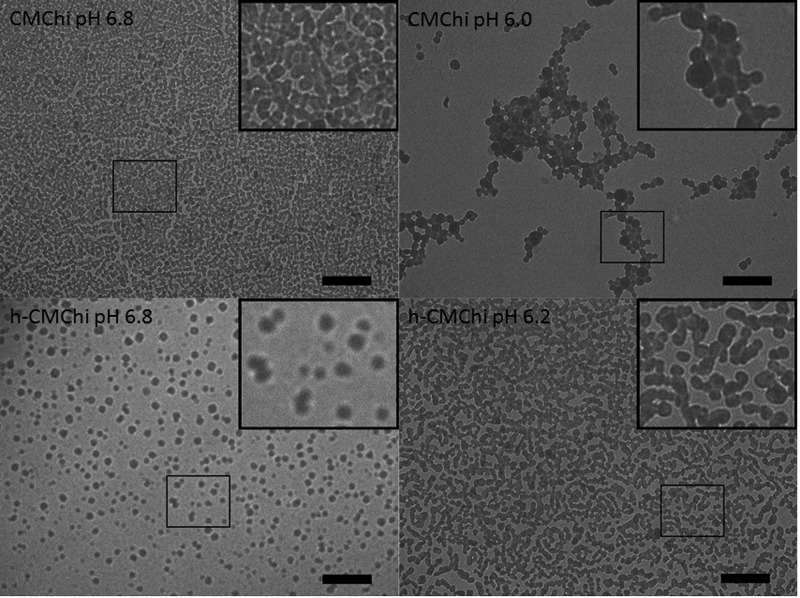
Figure 3 shows that the zeta potentials of CMChi and h-CMChi aggregates behave similarly as a function of pH. At pH 6.8, the zeta potentials are highly negative (−30 mV) due to the excess amount of negatively charged deprotonated carboxyl groups. The zeta potential increases as the pH is decreased due to the increasing amount of protonated amino groups. The pI values are 5.6 and 5.5 for CMChi and h-CMChi, respectively. At these pH values, the net charge of the precipitates is zero due to the protonation of the amino groups. The difference between the values is small and may be due to the conformation of the polymer and the size of precipitates as the polymers are precipitated near pI. The precipitates were studied with TEM and the images are shown in Figure 4. For both CMChi and h-CMChi, the aggregation starts at around pH 6.8, and therefore, this pH was chosen for TEM analysis. The other pH values (CMChi pH 6.0 and h-CMChi pH 6.2) were selected based on the lowest pH where the colloids were stable.
Figure 4.
TEM images of CMChi and h-CMChi precipitates in a dried state prepared by drying the sample at the presented pH (6.0–6.8). A magnification of the particles is shown at the corner of each image. The scale bar equals 2000 nm.
Figure 4 shows that CMChi and h-CMChi form spherical particles on aggregation at pH 6.0–6.8. The particle diameters were calculated based on the TEM images. The average diameters of CMChi particles were 200 and 301 nm, and the standard deviations were 40 and 85 nm in pH 6.8 and 6.0, respectively. The average diameter of h-CMChi particles were 233 and 267 nm, and the standard deviations were 47 and 63 nm, in pH 6.8 and 6.2, respectively. The detected diameters were in the same range as detected by DLS measurements. However, the results are not completely comparable, mainly because in DLS measurements the detected diameter is the hydrodynamic diameter of the particle, and in TEM images the particles were in a dried state. The drying of polymeric precipitates is expected to alter the size and shape of the particles. Also, the number of particles that can be practically measured from TEM images is limited (∼300 in this study).
Emulsification of Dodecane by CMChi and h-CMChi as a Function of pH
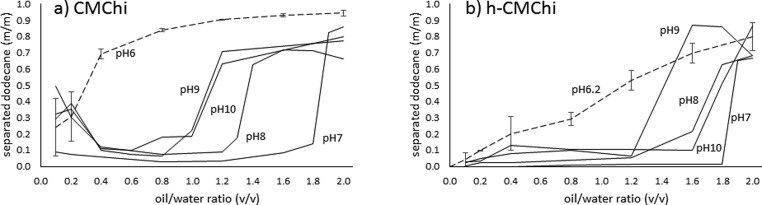
The stability of dodecane in water emulsions stabilized by CMChi as a function of pH is presented in Figure 5.
Figure 5.
Emulsion stability using (a) CMChi solution and (b) h-CMChi solutions. Emulsion stability measured as a fraction of separated dodecane after centrifuging. Lower dodecane fraction indicates a more stable emulsion. The emulsion stability is presented in varying pH as a function of dodecane to CMChi or h-CMChi solution volume ratio. Average values with standard deviations are presented based on two measurements for CMChi and h-CMChi particles at pH 6.0 and 6.2, respectively.
Figure 5a shows that the oil-in-water emulsion stability increases as pH is decreased from 10 to 7 indicated by the lower amount of separated dodecane in a wider oil/water ratio region. As the oil/water ratio is increased, a sudden increase in the separated amount of dodecane is observed which is attributed to phase inversion. When the oil/water ratio is large enough, a water-in-oil emulsion becomes more favorable rather than oil-in-water emulsion, but due to the instability of the water-in-oil emulsion, it breaks immediately after formation. This was evidenced by violent bursting of droplets right after the mixing of the two phases. The instability of the water-in-oil emulsion is due to the lack of surface activity in CMChi which is a water-soluble polymer and provides a mainly steric barrier between oil droplets to prevent coalescence. Since CMChi provides a mainly steric barrier against coalescence, the charge and conformation of the polymer affects the emulsion stability. The conformation of the CMChi is dictated by the pH of the aqueous solution, since CMChi has oppositely charged carboxyl and amino groups in the polymer chain. In the studied pH region (6–10), all the carboxyl groups are deprotonated (and negatively charged), while the amount of protonated amino groups (positively charged) varies. At pH ≥ 9, all the amino groups are deprotonated and neutral, so there is no intrachain or interchain interaction between the carboxyl and amino groups, all the carboxyl groups are negatively charged, there is repulsion between polymer chains, and the CMChi adopts an extended open-chain conformation. As the pH of the solution is decreased from 9 to 7, the amino groups start to protonate, and the conformation of the CMChi collapses to a coil-like conformation due to the increased intrachain interaction between the oppositely charged carboxyl and amino groups. The collapsed conformation and interchain interactions of the CMChi provides a more effective steric barrier against the coalescence of the oil droplets, and the phase inversion is shifted to higher oil/water ratio values as the pH is decreased from 9 to 7. At low oil/water ratios (0.1–0.4) in pH 7–10, the emulsions appear less stable than at slightly higher oil/water ratios. This could be explained by the low amount of oil compared to water. When the amount of oil is low, the number of oil droplets is low and most of the oil is on the surface of the water in contact with the air. Therefore, large portion of the droplets surface is not in contact with the polymer solution which could prevent the coalescence of droplets. It therefore appears that the emulsion stability increases as the oil/water ratio increases because a larger portion of the droplets are surrounded by the CMChi solution and more stable against coalescence. In pH 6, the system behaves differently due to excessive interchain cross-linking by the interaction between carboxyl and amino groups resulting in the precipitation of the CMChi and formation of colloidal particles. The oil-in-water emulsions are more stable at low oil/water ratios and the amount of separated dodecane increases gradually at higher oil/water ratios, in contrast to the sharp increase induced by phase inversion as observed in pH 7—10. The results suggest that the soluble CMChi (pH 7–10) stabilizes the emulsions by different mechanism compared to the CMChi particles (pH 6). The soluble CMChi provides a steric barrier to prevent droplet coalescence, whereas CMChi particles adsorb at the liquid–liquid interface. Long alkyl chains were introduced into the CMChi to produce h-CMChi, in order to study its effect on emulsion stabilization.
Figure 5b shows the stability of emulsions stabilized by h-CMChi as a function of pH. It shows that the oil-in-water emulsion stability decreases slightly as the pH is increased but not as significantly as in the emulsions stabilized by CMChi. This is attributed to the increased surface activity of the h-CMChi. The long alkyl chains grafted into the polymer-chain allow the h-CMChi to attach to the liquid–liquid interface. At pH 7, the emulsions are more stable compared to emulsions stabilized at pH 8–10. Similarly to CMChi, this observation can be attributed to the conformation and charge of the polymer. The charge of the h-CMChi is higher at pH 9–10, resulting in repulsion between the polymer chains. The intrachain repulsion favors an extended open-chain conformation and the interchain repulsion inhibits the close-packing of the h-CMChi at the liquid–liquid interface. As the pH is decreased, there is more attractive interchain interaction in h-CMChi, and the conformation of h-CMChi is more collapsed due to intrachain interactions favoring a denser packing of the h-CMChi at the liquid–liquid interface, resulting in more effective stabilization against coalescence. Also, at low oil/water ratios, the emulsions appear more stable compared to CMChi due to the increased surface activity of h-CMChi. An illustration is presented in Figure 6. that summarizes the differences between CMChi and h-CMChi.
Figure 6.
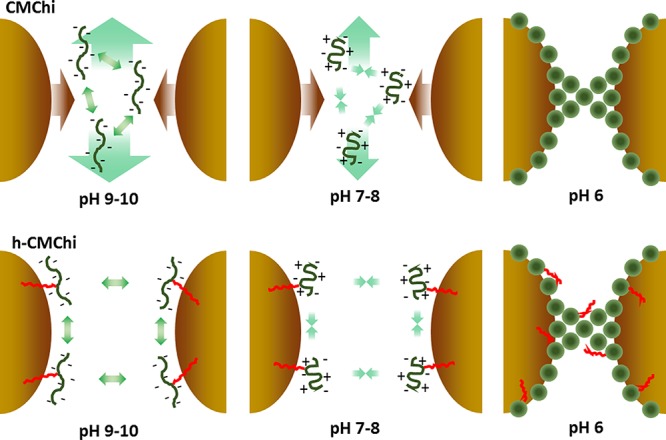
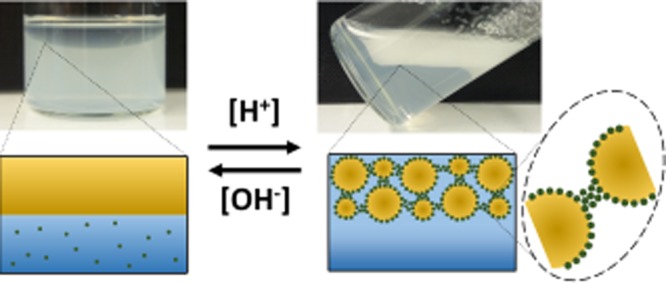
Effect of pH (6–10) on CMChi and h-CMChi in preventing the coalescence of oil droplets and stabilizing emulsions.
In Figure 6, the effect of pH on CMChi and h-CMChi is shown. In pH 9–10, the polymer chains adopt an open-chain conformation due to the repulsion between deprotonated carboxyl groups. CMChi remains free in the solution while h-CMChi is able to adsorb at the liquid–liquid interface. In pH 7–8, the polymer chain adopts a collapsed conformation due to the intrachain attraction between deprotonated carboxyl groups and protonated amino groups. Also, interchain attraction is possible between the oppositely charged functional groups. The stability against coalescence is increased due to the more collapsed conformation and weaker interchain repulsion in CMChi. The long alkyl chains in h-CMChi allow it to adsorb at the liquid–liquid interface, providing additional stabilization. In pH 6, both CMChi and h-CMChi are precipitated into colloidal particles that adsorb at the liquid–liquid interface and onto already adsorbed particles binding the droplets together.
Adsorption of CMChi and h-CMChi Nanoparticles on Dodecane–Water Interface
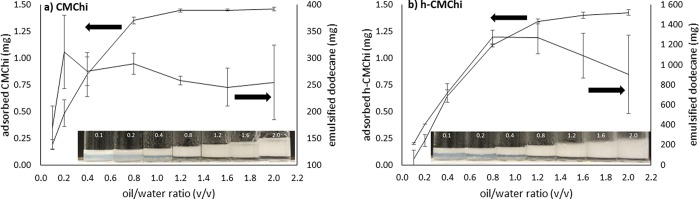
The amounts of emulsified dodecane and the adsorbed CMC and h-CMC are presented in Figure 7.
Figure 7.
Amount of adsorbed CMChi and h-CMChi particles, and the amount of emulsified dodecane after centrifuging as a function of oil/water ratio. Average values with standard deviations are presented based on two measurements. Photographs show the decrease in opalescence of the water phase as a function of oil/water ratio in the prepared emulsions before centrifuging.
Figure 7 shows that the amount of adsorbed particles saturates at around oil/water ratio 1.2 and 1.6 for CMChi and h-CMChi, respectively, indicating that almost all of the particles have been adsorbed at the liquid–liquid interface. The amount of emulsified dodecane correlates with the adsorbed particles. The maximum amount of emulsified oil is reached at an oil/water ratio of 0.4 for CMChi and 0.8 for h-CMChi. The maximum amount of dodecane emulsified using CMChi particles in these experimental conditions is approximately 300 mg (200 mg dodecane/mg CMChi), while the maximum amount of dodecane emulsified using h-CMChi particles is approximately 1300 mg (867 mg dodecane/mg h-CMChi). Thus, a very low degree of hydrophobic modification on the CMChi improves the emulsification ability of the material in the particle form as well.
Reversible Emulsification of Dodecane by pH Adjustment
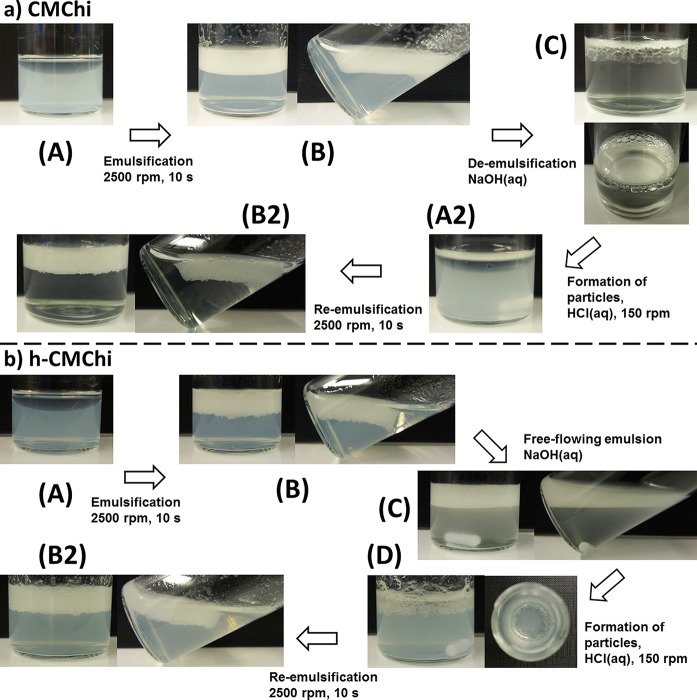
Figure 8 shows the scheme for pH-switchable and reversible emulsification of dodecane using CMChi and h-CMChi.
Figure 8.
(a) pH-switchable emulsification of dodecane by using CMChi (0.5 mg/mL pH 6.0) solution and varying the pH using aqueous NaOH and HCl solutions. (b) pH-switchable emulsification of dodecane using h-CMChi (0.5 mg/mL pH 6.2) solution and varying the pH using aqueous NaOH and HCl solutions.
In Figure 8a, the emulsification process started with the two separate phases (oil/water ratio 0.2) (A) and the emulsion was formed by vortex mixing. The emulsion was gel-like and floated on the water phase (B). De-emulsification could be achieved by adding a few drops of NaOH (20 μL of 0.1 M and 10 μL of 1 M) followed by shaking by hand (C). After a while, most of the emulsion had coalesced into large unstable droplets and the water was no longer opalescent due to the dissolution of CMChi. A small magnetic stirring bar was added and the water phase was stirred gently at 150 rpm without disturbing the oil phase. The slow movement of the water phase is enough to break the remaining oil droplets (A2). The reformation of particles was achieved by adding a few drops of HCl (10 μL of 1 M and 17.5 μL of 0.1 M). The water phase turned opalescent and the oil phase could be re-emulsified by vortex-mixing (B2). The gel-like emulsion suggests significant particle–particle interaction and bridging of particles between droplets. Particle–particle interactions are likely due to the simultaneous presence of positively charged amino groups and negatively charged carboxylic groups allowing both intra- and interparticle electrostatic interaction. In solution, the particles are dispersed, but at the oil–water interface the electrical double-layer around the particle is distorted allowing increased attractive interaction. This kind of behavior was not reported for native chitosan particle.2,12 This could be due to lack of oppositely charged functional groups in native chitosan. Particles based on native chitosan are held together by weaker forces such as hydrophobic interactions.
In Figure 8b, the emulsification process using h-CMChi started similarly to CMChi (A, B), but the addition of NaOH (aq) did not cause de-emulsification despite shaking and stirring (C). The emulsion transformed from a gel-like to free-flowing emulsion. This is attributed to the hydrophobic functionality and demonstrated the stability of the emulsion in varying pH. The addition of HCl (aq) aggregated the h-CMChi back into colloidal particles which caused the emulsion to degrade mostly, but some dodecane was trapped inside the aggregated h-CMChi (D) despite shaking and stirring. The mixture could be re-emulsified as previously (B2). The h-CMChi shows stronger interaction with the oil–water interface due to the hydrophobic modification as evidenced by the persistence of the emulsion, even when the h-CMChi was dissolved. In literature, h-CMChi has been employed for reversible gelation of vesicles and nanoparticles.11 In that study, the reversibility was achieved by the addition of α-cyclodextrin that sequesters the hydrophobic alkyl chains resulting in the degelation.
Summary and Conclusions
The emulsification properties of water-soluble CMChi and h-CMChi, and their nanoparticles were studied using dodecane as a model oil phase. The emulsification properties could be adjusted by adjusting the pH of the water phase. As the pH was decreased, the CMChi adopted a more collapsed conformation increasing the emulsification ability. The emulsion stability increased as the pH was decreased from 10 to 7, evidenced by the shifting of phase inversion from oil/water ratio 1 to 1.8, respectively. At pH < 7, the CMChi aggregated and formed colloidal particles (∼200 nm) and the emulsification mechanism changed. At pH 6, the emulsions were stabilized by the adsorption of the particle into the liquid–liquid interface, the emulsions were gel-like, and no phase inversion was observed. This result was supported by the amount of particles left in the water phase after emulsification which correlates with the amount of emulsified oil. The h-CMChi behaved similarly to CMChi, but the effect of pH on the emulsification was hindered by hydrophobic modification. The stability of the emulsions increased as the pH was decreased from 10 to 7 and the phase inversion was shifted to higher oil/water ratio values (1.6–1.8) compared to CMChi. The h-CMChi also formed small particles at pH < 7 that can stabilize gel-like emulsions similarly to CMChi. Overall, the hydrophobic modification of h-CMChi increased the emulsification ability compared to CMChi. The gel-like emulsion suggests that particle–particle interactions contribute significantly to the emulsification. The particle–particle interactions are attributed to oppositely charged functional groups in both CMChi and h-CMChi. The pH-dependent emulsification of CMChi and h-CMChi could be applied to the pH-switchable emulsification of oil. The emulsion could be formed at low pH (6) and then de-emulsified by adjusting the pH with NaOH (and HCl for h-CMC) resulting in destabilization of the emulsion due to charge and conformation changes in the polymers. The oil could then be re-emulsified after adjusting the pH back to the original value.
Acknowledgments
This study was funded by the Academy of Finland (decision number 283200).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.langmuir.7b03959.
Syntheses and characterizations details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu H.; Wei Z.; Hu M.; Deng Y.; Tong Z.; Wang C. Fabrication of degradable polymer microspheres via pH-responsive chitosan-based Pickering emulsion photopolymerization. RSC Adv. 2014, 4, 29344–29351. 10.1039/C4RA01660B. [DOI] [Google Scholar]
- Wang X.-Y.; Heuzey M.-C. Chitosan-Based Conventional and Pickering Emulsions with Long-Term Stability. Langmuir 2016, 32, 929–936. 10.1021/acs.langmuir.5b03556. [DOI] [PubMed] [Google Scholar]
- Wei Z.; Wang C.; Zou S.; Liu H.; Tong Z. Chitosan nanoparticles as particular emulsifier for preparation of novel pH-responsive Pickering emulsions and PLGA microcapsules. Polymer 2012, 53, 1229–1235. 10.1016/j.polymer.2012.02.015. [DOI] [Google Scholar]
- Mwangi W.; Ho K.-W.; Ooi C.-W.; Tey B.-T.; Chan E.-S. Facile method for forming ionically cross-linked chitosan microcapsules from Pickering emulsion templates. Food Hydrocolloids 2016, 55, 26–33. 10.1016/j.foodhyd.2015.10.022. [DOI] [Google Scholar]
- Ho K.; Ooi C.-W.; Mwangi W.; Leong W.; Tey B.-T.; Chan E.-S. Comparison of self-aggregated chitosan particles prepared with and without ultrasonication pretreatment as Pickering emulsifier. Food Hydrocolloids 2016, 52, 827–837. 10.1016/j.foodhyd.2015.08.019. [DOI] [Google Scholar]
- Shah B.; Li Y.; Jin W.; An Y.; He L.; Li Z.; Xu W.; Li B. Preparation and optimization of Pickering emulsion stabilized by chitosan-tripolyphosphate nanoparticles for curcumin encapsulation. Food Hydrocolloids 2016, 52, 369–377. 10.1016/j.foodhyd.2015.07.015. [DOI] [Google Scholar]
- Chen X.; Park H. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–259. 10.1016/S0144-8617(03)00051-1. [DOI] [Google Scholar]
- Payet L.; Terentjev E. Emulsification and Stabilization Mechanisms of O/W Emulsions in the Presence of Chitosan. Langmuir 2008, 24, 12247–12252. 10.1021/la8019217. [DOI] [PubMed] [Google Scholar]
- Klinkesorn U. The Role of Chitosan in Emulsion Formation and Stabilization. Food Rev. Int. 2013, 29, 371–393. 10.1080/87559129.2013.818013. [DOI] [Google Scholar]
- Desbriéres J.; Martinez C.; Rinaudo M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. 10.1016/0141-8130(96)01095-1. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Javvaji V.; MacIntire I.; Raghavan S. Gelation of Vesicles and Nanoparticles Using Water-Soluble Hydrophobically Modified Chitosan. Langmuir 2013, 29, 15302–15308. 10.1021/la4037343. [DOI] [PubMed] [Google Scholar]
- Liu H.; Wang C.; Zou S.; Wei Z.; Tong Z. Simple, Reversible Emulsion System Switched by pH on the Basis of Chitosan without Any Hydrophobic Modification. Langmuir 2012, 28, 11017–11024. 10.1021/la3021113. [DOI] [PubMed] [Google Scholar]
- Benner S.; John V. T.; Hall C. K. Simulation Study of Hydrophobically Modified Chitosan as an Oil Dispersant Additive. J. Phys. Chem. B 2015, 119, 6979–6990. 10.1021/acs.jpcb.5b01092. [DOI] [PubMed] [Google Scholar]
- Bratskaya S.; Avramenko V.; Schwarz S.; Philippova I. Enhanced flocculation of oil-in-water emulsions by hydrophobically modified chitosan derivatives. Colloids Surf., A 2006, 275, 168–176. 10.1016/j.colsurfa.2005.09.036. [DOI] [Google Scholar]
- Venkataraman P.; Tang J.; Frenkel E.; McPherson G.; He J.; Raghavan S.; kolesnichenko V.; Bose A.; John V. Attachment of Hydrophobically Modified Biopolymer at the Oil-Water Interface in the Treatment of Oil Spills. ACS Appl. Mater. Interfaces 2013, 5 (9), 3572–3580. 10.1021/am303000v. [DOI] [PubMed] [Google Scholar]
- Kalliola S.; Repo E.; Sillanpää M.; Arora J.; He J.; John V. T. The stability of green nanoparticles in increased pH and salinity for applications in oil spill-treatment. Colloids Surf., A 2016, 493, 99–107. 10.1016/j.colsurfa.2016.01.011. [DOI] [Google Scholar]
- Doshi B.; Repo E.; Heiskanen J. P.; Sirviö J. A.; Sillanpää M. Effectiveness of N,O-carboxymethyl chitosan on destabilization of Marine Diesel, Diesel and Marine-2T oil for oil spill treatment. Carbohydr. Polym. 2017, 167, 326–336. 10.1016/j.carbpol.2017.03.064. [DOI] [PubMed] [Google Scholar]
- Binks B. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. 10.1016/S1359-0294(02)00008-0. [DOI] [Google Scholar]
- Zhu A.; Chan-Park M.; Dai S.; Li L. The aggregation behavior of O-carboxymethylchitosan in dilute aqueous solution. Colloids Surf., B 2005, 43, 143–149. 10.1016/j.colsurfb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Chen L.; Du Y.; Tian Z.; Sun L. Effect of the Degree of Deacetylation and the Substitution of Carboxymethyl Chitosan on Its Aggregation Behavior. J. Polym. Sci., Part B: Polym. Phys. 2005, 43, 296–305. 10.1002/polb.20212. [DOI] [Google Scholar]
- Yinsong W.; Lingrong L.; Jian W.; Zhang Q. Preparation and characterization of self-aggregated nanoparticles of cholesterol-modified O-carboxymethyl chitosan conjugates. Carbohydr. Polym. 2007, 69, 597–606. 10.1016/j.carbpol.2007.01.016. [DOI] [Google Scholar]
- Tan Y.-L.; Liu C.-G. Self-aggregated nanoparticles from linoleic acid modified carboxymethyl chitosan: Synthesis, characterization and application in vitro. Colloids Surf., B 2009, 69, 178–182. 10.1016/j.colsurfb.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Kalliola S.; Repo E.; Srivastava V.; Heiskanen J. P.; Sirviö J. A.; Liimatainen H.; Sillanpää M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloids Surf., B 2017, 153, 229–236. 10.1016/j.colsurfb.2017.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.