Abstract
TAS2R38 gene variants, which confer sensitivity to specific bitter tastants (e.g., 6-n-propylthiouracil), have been repeatedly associated with lower alcohol use via greater bitterness perception, but research exploring TAS2R38 variation in relation to smoking shows mixed results. In both, the working hypothesis is that 1 or more copies of the functional allele increases bitterness and may provide a barrier to early use. Such a barrier to initiation may, conceivably, manifest as differential rates of current use across diplotypes. Here, an age-diverse convenience sample (n = 886) of Denver Museum of Nature and Science guests was used to explore cross-sectional relationships between TAS2R38 diplotype, self-reported tobacco use (current, former, never smokers), and a rapid measure of 6-n-propylthiouracil phenotype (bitterness of filter paper discs). TAS2R38 diplotypes were determined by Sanger sequencing. After excluding rare diplotypes, data from 814 participants were analyzed. A mix of current (~10%), former (25%), and never smokers (65%) were included. As expected, there was a relationship between TAS2R38 diplotype and 6-n-propylthiouracil bitterness. However, contrary to our hypothesis, there was no evidence of a relationship between diplotype and smoker status among participants with common TAS2R38 diplotypes. Notably, we observed a relationship between of 6-n-propylthiouracil bitterness and smoking status, but the effect was opposite of what was expected: current smokers perceived higher (not lower) bitterness than never smokers. When all the various factors (diplotype, age, sex, and smoking status) were included in ANOVA, all remained predictive of 6-n-propylthiouracil bitterness. Reasons for greater phenotypic bitterness among current smokers are unknown and merit further study.
Keywords: bitterness, citizen science, crowdsourcing, TAS2R38, tobacco
Introduction
Smoking is a major public health concern and the leading cause of preventable death (Department of Health and Human Services 2014). Each day, thousands of young people take up smoking (Department of Health and Human Services 2014), despite decades of public health efforts intended to prevent initiation. Identifying novel risk factors for smoking initiation and downstream nicotine dependence may help break this cycle. Some evidence suggests that genetic variability in taste perception may be such a risk factor.
Bitterness is widely claimed to be an evolutionarily important indicator of toxicity (Drewnowski and Gomez-Carneros 2000; Behrens and Meyerhof 2016), although more precisely, it probably serves instead as a marker of pharmacological activity (see Fischer and Griffin 1964; Garcia and Hankins 1975). That is, not all bitter stimuli are toxic (Glendinning 1994), and recent analysis suggests only a little over half (60%) of the ligands in the BitterDB database are toxic (Nissim et al. 2017). The stimuli humans describe as bitter are detected when one or more TAS2R receptors are activated (Adler et al. 2000; Chandrashekar et al. 2000; Behrens et al. 2007). Humans have 25 different TAS2R genes that code for these receptors, and approximately 5 of these contain polymorphisms that associate with differences in perceived bitterness (Kim et al. 2003; Duffy et al. 2004; Soranzo et al. 2005; Hayes et al. 2015). The most studied is TAS2R38. It contains 3 single nucleotide polymorphisms (SNPs), which result in 2 common haplotypes that are named for their amino acid substitutions: PAV (proline, alanine, and valine) and AVI (alanine, valine, and isoleucine) (Kim et al. 2003; Kim et al. 2005). TAS2R38 diplotype influences the ability to taste a family of bitter compounds including phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP). PAV homozygotes and heterozygotes perceive greater bitterness than AVI homozygotes that perceive little or no bitterness (Kim et al. 2003; Duffy et al. 2004; Hayes et al. 2008; Garneau et al. 2014; Boxer and Garneau 2015; Hayes et al. 2015).
Numerous bitter compounds are found in tobacco products (Hummel et al. 1992), and it is thought that individuals with greater sensitivity and aversion to bitter taste may be less likely to smoke (Enoch et al. 2001). Several studies have investigated the influence of TAS2R38 variants on smoking habits, although results are mixed. For example, Mangold et al. (2008) reported an association between the AVI haplotype and smoking quantity; however, this effect was restricted to African-American individuals, as it was not observed in European Americans. Conversely, Cannon et al. (2005) found no evidence of an association between common TAS2R38 haplotypes and smoking; however, the rare AAV haplotype (which confers greater functionality than AVI but less that PAV; see Boxer and Garneau 2015) was associated with reduced smoking prevalence. More recently, a large study of genetic variation and tobacco use by Risso et al. (2016) reported that the nontaster haplotype (i.e., the AVI homozygotes) was more frequent among European-American smokers, whereas no difference in haplotype frequency was observed in African Americans, suggesting that TAS2R38 haplotype associations with smoking status may differ with ancestry. Separately, Oncken et al. (2015) reported an association between the PAV haplotype and menthol cigarette smoking in a convenience sample of Hispanic and non-Hispanic women who were pregnant; whether this finding generalizes to other groups remains unknown. Unfortunately, such conflicting reports have become quite common for numerous gene association studies over the past 15 years.
Many initially promising findings associating disease states or intermediate phenotypes with specific gene variants have failed to replicate (Cavalleri et al. 2005; Hart et al. 2013). One partial solution to this crisis is to leverage large existing data sets to help ensure that any failure to replicate is not merely a matter of power (see Gorroochurn et al. 2007); this replication crisis also highlights a need to revisit findings in new populations to document the robustness and increase the generalizability of the findings. Toward this goal, we used an approach known as crowdsourcing. Broadly speaking, crowdsourcing simply refers to the outsourcing of tasks to a large group of people. Within the scientific enterprise, this approach can take on 2 distinct aspects: 1) data contributed by community members (as has been done for decades in ornithology and ecology), as well as 2) community/citizen science, which directly engages nonprofessional researchers in data collection, processing and analysis. This engagement is believed to foster trust for science and scientists and increase public support of scientific research (Garneau et al. 2017). Critically, this approach may also provide access to populations that are otherwise not typically engaged in research studies, toward a goal of increased generalizability beyond traditional convenience samples such as those obtained on college campuses (see Anonymous 2010). Notably, crowdsourced data collection often occurs online (e.g., Primrose et al. 2016; Kraemer et al. 2017) but crowdsourcing should not be considered synonymous with online data collection, as it can also occur in person (e.g., Garneau et al. 2014; Garneau et al. 2017). Here, we use existing data from the later (i.e., a large community-based cohort with in-person data collection) to revisit the question of whether TAS2R38 haplotype associates with smoking.
In this study, we used both aspects of scientific crowdsourcing mentioned previously (i.e., community-contributed data and community/citizen scientists) to explore whether smoking was associated with TAS2R38 genotype and PROP phenotype using rapid screening measures. To do so, we tested 3 specific questions. First, as a confirmatory step, we asked whether PROP bitterness varied by TAS2R38 diplotype, after controlling for both sex and age. Second, we tested whether smoking status, assessed using a brief self-report measure, varied by TAS2R38 diplotype. Third, we tested whether PROP bitterness varied by smoking status, after controlling for sex.
Materials and methods
Participants
Participants were guests of the Denver Museum of Nature and Science (DMNS) that elected to participate as research subjects in the Bitter Taste Study hosted by the Genetics of Taste Lab between 2009 and 2013. A subset of this database consisted of 886 healthy participants with complete data for age, sex, PROP intensity score, TAS2R38 diplotype, and self-reported smoking status (current, former, or never). Of this group, 72 participants were found to have rare haplotypes for the TAS2R38 gene and were excluded from further analysis. The final analysis was completed on 814 participants (313 men and 501 women, aged 18–86). All procedures were approved by the Western Institutional Review Board (Study No. 1109386, Protocol No. 2009 1028) and the study complied with the Declaration of Helsinki for medical research involving human subjects. Participants volunteered their time and gave written consent.
Community/citizen scientists
Nonprofessional research volunteers (e.g., community scientists) were trained to collect and process the DNA samples (details below) and to collect phenotypic data from the study participants. Before doing so, they completed an intensive 12-week certification program that included trainings on internal quality control for data collection, the online ethics course “Protecting Human Research Participants” from the Office of Extramural Research at the National Institutes of Health, and visitor experience instructional sessions for educational facilitation. Community scientists who successfully completed the program received final certification and approval to enroll visitors over the age of 18 in this study. Certified community scientists were also trained on data processing and analysis protocols, including extraction and purification of DNA, and preparation and analysis of the gene sequencing reactions. All procedures were supervised by the museum’s professional scientific staff. For clarity, we note citizen scientist is the standard phrasing used by the National Institutes of Health and other federal agencies (e.g., www.citizenscience.gov); for precision, we use the term community scientist for the remainder of this document, as an individual’s ability to engage in participatory research is not related to immigration status.
TAS2R38 SNP analysis
DNA was extracted from Epicentre buccal swabs using the Maxwell 16 Buccal Swab LEV DNA Purification Kit and the Promega Maxwell. TAS2R38 was amplified using PCR primers (forward ACCAATGCCTTCGTTTTCTTGGTGA, reverse TCACAGCTCTCCTCAACTTGGCA; Invitrogen) and sequenced using the forward primer (High Throughput Genomics Center; www.htseq.org).
TAS2R38 (HGNC:9584) sequences were analyzed using the software Geneious to determine the first two SNPs of the gene at nucleotide positions 145 and 785 (rs713598 and rs1726866; NCBI Accession AY258598). A 3-step process for genetic analysis was used to ensure accurate recording of TAS2R38 diplotypes. First, professional staff imported all sequences into Geneious and aligned them to the reference sequence (AY258598). Second, professional staff used the program to locate and highlight both homozygous and heterozygous variations in the aligned sequences. Third, after sequence preparation, pairs of community members who had been trained in chromatographic analysis (i.e., community scientists) recorded the diplotype for each sample and ensured the chromatograph matched the computer program reading. Any samples that showed either a discrepancy between the chromatograph and the computer assignment or a potential rare diplotype were flagged for retesting. Flagged samples were resequenced in both the forward and reverse directions to include the third SNP of this gene at nucleotide 886 (rs10246939); common and rare diplotypes were confirmed in this way. As noted earlier, only those individuals with 1 of the 3 major diplotypes for TAS2R38 (PAV/PAV, AVI/AVI, and PAV/AVI) were included in the analysis for this report.
Bitter taste phenotype
Paper filter discs (Zhao et al. 2003; Khataan et al. 2009) were saturated with a solution of 0.453 M PROP and allowed to air-dry. Participants were trained to use a general Labeled Magnitude Scale (Green et al. 1996; Bartoshuk et al. 2004), by rating several imagined sensations (e.g., weight of a feather and sourness of a lemon). Once they understood the scale, participants were given the filter paper disc to taste and asked to rate the intensity of the bitterness they experienced. The rating on the scale was then converted to a score in millimeters for statistical analysis.
Statistical analysis
All statistical analyses were performed using SAS 9.4. Relationships between diplotype and smoker status were tested via chi-square test, Fisher’s Exact test, and Mantel–Haenszel chi-square test via proc freq. Relationships between PROP bitterness, diplotype, sex, age, and smoker status were tested via ANOVA using proc glm. Following significant F-tests in ANOVA, effects were decomposed using Tukey–Kramer test.
Results
The cohort described here is diverse in terms of self-reported smoking status, TAS2R38 diplotype, PROP phenotype, gender, and age (see summary in Table 1). On the basis of data from the Behavioral Risk Factor Surveillance System, the proportion of current smokers in the study cohort was lower than might be expected compared with state-level data: 9.7% in the study cohort versus a crude prevalence of 17.7% for Colorado in 2012 and 2013. Regarding former smokers, the proportion in the study cohort was similar to state-level data: 25.1% of the study cohort versus a crude prevalence of 26.3%. Despite the lower numbers of current smokers compared with state-level data over a similar time period, we nonetheless observed substantial variation in smoking status.
Table 1.
Summary of participant characteristics
| n (out of 814) | % | ||
|---|---|---|---|
| Sex (self-reported) | |||
| Men | 313 | 38.5 | |
| Women | 501 | 61.5 | |
| Smoking status (self-reported) | |||
| Never | 531 | 65.2 | |
| Previous | 204 | 25.1 | |
| Current | 79 | 9.7 | |
| TAS2R38 diplotype | |||
| AVI/AVI | 277 | 34.0 | |
| PAV/AVI | 359 | 44.1 | |
| PAV/PAV | 178 | 21.9 | |
| Age | Mean (SD) | 34.9 (15.4) | |
| Range | 18–86 | ||
| PROP bitterness (gLMS) | Mean (SD) | 33.2 (26.9) | |
| Range | 0–100 | ||
SD, standard deviation.
The Genetics of Taste Lab at the DMNS does not collect information on educational attainment or household income, so we are unable to characterize our participants in terms of socioeconomic status. Also, data on race and ethnicity are not available for this specific cohort. However, general patterns can be roughly inferred from typical demographics of individuals who have chosen to participate in subsequent experiments collected via the Genetics of Taste exhibit. Using the same recruitment approach used here, the self-identified racial and ethnic background of more recent studies is predominantly white (76.6%), with nonreporters (13.9%) and those who identify as more than one race (7.11%), and Asians (4.8%) representing the 3 next largest groups; proportions of African Americans, American Indians/Alaskan Natives, and Native Hawaiian/Pacific Islanders are each less than 1.7%. Regarding ethnicity, 13.9% identify as Hispanic or Latino.
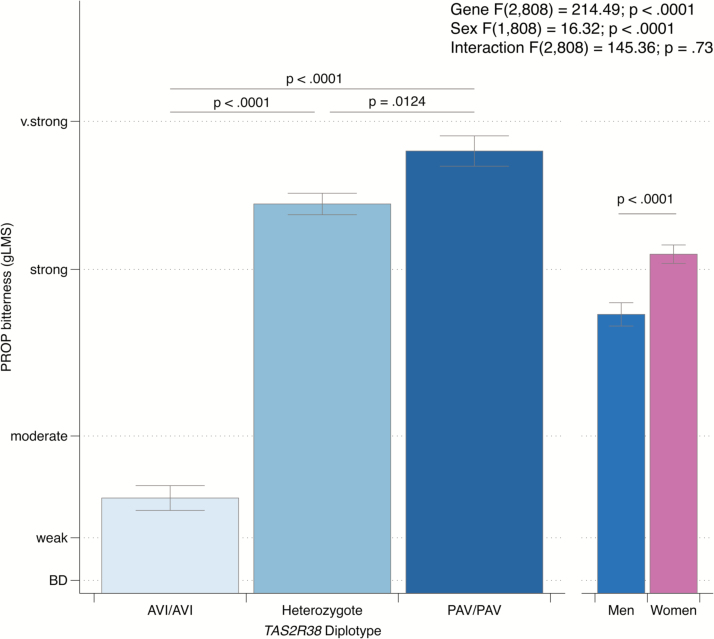
First, we confirmed suprathreshold bitterness of PROP associated with the common TAS2R38 diplotypes across all 814 participants (313 men and 501 women). This served as a quality check of the data collected by our community scientists. In a mixed model ANOVA, main effects of gene (TAS2R38 diplotype) [F(2,808) = 214.49; P < 0.0001] and sex [F(1,808) = 16.32; P < 0.0001] were significant, whereas the sex by gene interaction was not [F(2,808) = 0.32; P = 0.73]. As shown in Figure 1, men reported less bitterness than women. As was expected, the AVI/AVI homozygotes reported substantially lower bitterness than did PAV/AVI heterozygotes (Tukey–Kramer P < 0.0001) or PAV/PAV homozygotes (P < 0.001); the magnitude of difference between the PAV/AVI heterozygotes and PAV/PAV homozygotes was much smaller, but still significant (P = 0.012). In a Fisher’s Exact test, diplotype did not vary by sex (P = 0.51).
Figure 1.
The bitterness of PROP delivered via filter paper discs varied as a function of TAS2R38 diplotype, in a model including sex; men also reported less bitterness than women.
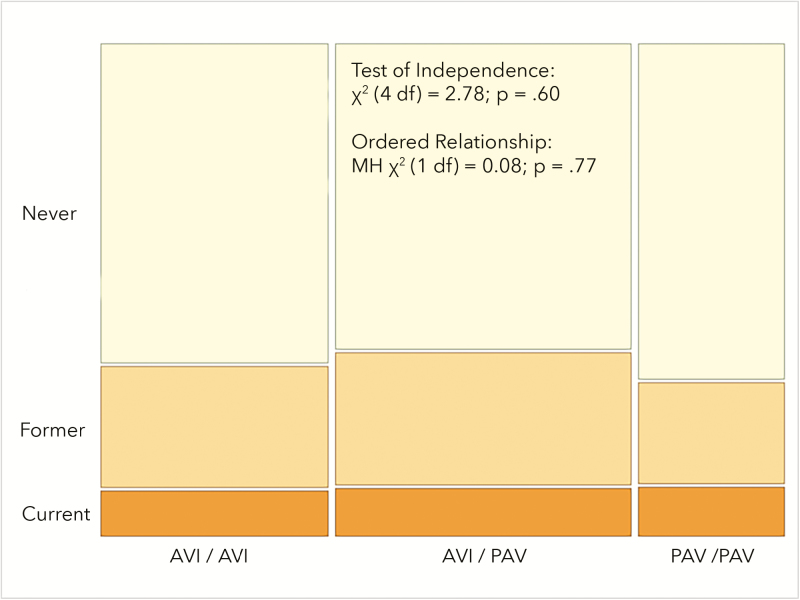
After obtaining expected results for TAS2R38 diplotype and PROP bitterness in this data set, we explored whether self-reported smoking status (current, former, and never) varied by TAS2R38 diplotype. A priori, we hypothesized carriers of the PAV allele would be protected against prior smoking initiation (due to increased bitterness from tobacco products), and this presumed barrier to prior initiation would manifest as differences in current smoking status. On the basis of a chi-square test of independence, however, we failed to observe any evidence of a relationship (χ2 (4 df) = 2.78; P = 0.60) between current smoking status and diplotype. We also tested for an ordered relationship via a Mantel–Haenszel chi-square test (given the ordered relationship across TAS2R38 diplotypes), and again, no evidence of a relationship was observed (Mantel–Haenszel χ2 (1 df) = 0.08; P = 0.77). The counts of the 3 × 3 matrix (smoking status by diplotype) are shown proportionally in Figure 2. Additional exploratory models in which former smokers were pooled with either current or never smokers were tested, and the absence of an association did not change (data not shown here).
Figure 2.
Chi square test for association or an ordered relationship failed to show any evidence that current smoking status (assessed via self-report) was associated with TAS3R38 diplotype.
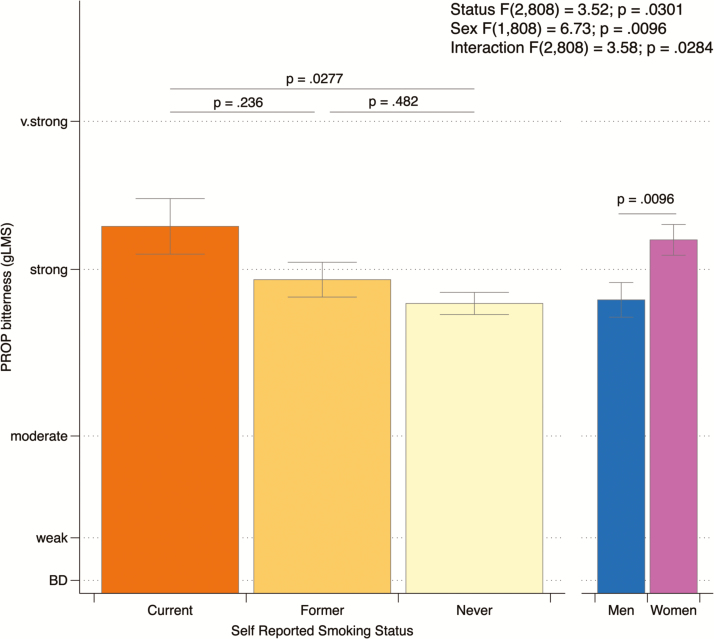
Third, we explored whether the bitterness of PROP varied with self-reported smoking status, while accounting for the sex differences previously reported in Figure 1. This analysis (PROP bitterness as a function of smoking status, accounting for sex) is summarized in Figure 3. In mixed model ANOVA, sex [F(1,808) = 6.73; P =0.0096] was a significant predictor of bitterness: women reported more bitterness than men, which is consistent the gene by sex analysis reported earlier. Smoking status was a significant predictor of bitterness as well [F(2,808) = 3.52; P = 0.0301]. Unexpectedly, however, the effect was in the opposite direction of smoking initiation/bitterness protection hypothesis. That is, the current smokers in our cohort showed higher (not lower) bitterness intensity ratings than never smokers (Tukey–Kramer P = 0.0277). As depicted in the figure, mean bitterness for the former smokers fell between current and former smokers and was not different from either extreme (Tukey–Kramer Ps > 0.2). The interaction term was also significant [F(2,808) = 3.58; P = 0.028]. Inspection of the pairwise comparisons for the interaction revealed elevated bitterness in women (but not men) who were former smokers (Tukey–Kramer P = 0.003) (data not shown).
Figure 3.
Contrary to our hypothesis that greater bitterness would serve as barrier to the initiation of smoking and thus influence current smoking rates, PROP bitterness was significantly higher in current smokers than in never smokers, not lower.
Given that the relatively unique recruitment context for this cohort (a community-based sample of museum attendees) differs substantially from traditional laboratory-based studies on university campuses (Anonymous 2010), Internet-based crowdsourced research (Kraemer et al. 2017), or disease-focused longitudinal studies, we explored the age distribution of participants to ensure that our unexpected results were not merely due to confounding with age. This is especially relevant here, given both the potential for changes in taste perception with aging and a major shift in public health messaging and health promotion efforts regarding tobacco over time. In spite of the very wide range of ages in this cohort (18–86 years), we observed a noticeable skew toward younger ages among participants. Thus, we split our sample at the median (29 years), resulting in groups of younger (n = 416) and older (n = 398) participants. Diplotype (Fisher’s Exact test, P = 0.48) and sex (Fisher’s Exact test, P = 0.89) did not differ by age group (younger vs. older). However, self-reported smoking status did vary by age group (Fisher’s Exact test, P = 0.004). The older group had fewer current and fewer never smokers, and more former smokers than expected, whereas the younger group had more current smokers, more never smokers, and fewer former smokers than expected. Accordingly, we repeated the analyses shown in Figures 1 and 3, while including age group (young vs. old, based on median split) in the analyses.
In a 3-way (diplotype by sex by age group) ANOVA model, PROP bitterness was associated with TAS2R38 diplotype [F(2,802) = 216.74; P < 0.0001] and sex [F(1,802) = 17.52; P < 0.0001], as mentioned earlier, even when age was included in the model. Age was also significant [F(1,802) = 18.33; P < 0.0001], with older individuals reporting less bitterness (Tukey–Kramer P < 0.0001). Evidence of an age group by diplotype interaction was weak [F(2,802) = 2.72; P = 0.067]. In pairwise comparisons between the younger and older groups, the AVI/AVI homozygotes did not differ (P = 0.74) across age (both low); the PAV/PAV homozygotes did not differ (P = 0.57) across age (both high); however differences were observed in the PAV/AVI heterozygotes (P < 0.001), with the younger heterozygotes reporting more bitterness than older heterozygotes. None of the other interactions showed any evidence of an association.
In a 3-way (smoking status × sex by age group) ANOVA model, sex [F(1,802) = 6.29; P = 0.0123] and age group [F(1,802) = 6.75; P = 0.0095] each explained variability in PROP bitterness, as expected. Critically, smoking status continued to be associated with bitterness [F(2,802) = 3.73; P = 0.024], even after controlling for age and sex effects. All effects were the same as those mentioned earlier: i.e., more bitterness for current smokers, women, and the younger group. Weak evidence of a smoking status by sex interaction [F(2,802) = 2.72; P = 0.067] was observed. In pairwise comparisons across men and women, bitterness was lower in never smokers regardless of sex (P = 0.98) and higher in current smokers regardless of sex (P = 0.99). However, differences of sex were observed in the former smokers, as women reported more bitterness than men (P = 0.003). None of the other interactions showed any evidence of an association.
Finally, given these complicated relationships, we took advantage of the large sample size afforded by crowdsourcing to test an omnibus 4-way ANOVA model predicting PROP bitterness while simultaneously accounting for age group, smoking status, TAS2R38 diplotype, and sex. As described previously, age group [F(1,802) = 4.72; P = 0.030], sex [F(1,802) = 16.17; P < 0.0001], and diplotype [F(2,802) = 135.94; P < 0.0001] all accounted for some variability in PROP bitterness. Critically, smoking status was also associated with bitterness [F(2,802) = 7.11; P = 0.0009], even when age, sex, and diplotype were all retained in the model. All effects were the same as those mentioned earlier: i.e., more bitterness in current smokers, PAV carriers, women, and the younger group. Also, we observed evidence of an age group by diplotype interaction [F(2,802) = 4.51; P = 0.0113]. In pairwise comparisons of PROP bitterness between the younger and older groups, the AVI/AVI homozygotes did not differ (P = 0.38) across age (both lower); the PAV/PAV homozygotes did not differ (P = 0.90) across age (both higher); however differences were observed in the PAV/AVI heterozygotes (P = 0.0034), with the younger heterozygotes reporting more bitterness than older heterozygotes. There was also evidence of 3-way interaction of diplotype by age group by self-reported smoking status [F(4,802) = 5.99; P < 0.0001], but we did not interpret this interaction due to the low numbers of observations (>10) in some of the cells.
Collectively, these data suggest that smoking status and TAS2R38 diplotype each influence the bitterness of the widely used taste probe PROP, even when controlling for age and sex.
Discussion
Via the Genetics of Taste Lab at the DMNS, we used a crowdsourced approach to test assess relationships between self-reported smoking status, TAS2R38 diplotype, and PROP phenotype. Assuming that increased bitterness would be a protective factor against smoking initiation, we had predicted a priori that PROP phenotype and TAS2R38 genotype would each be related to smoking status—specifically, lower bitterness ratings would be seen in current smokers, and fewer PAV/PAV homozygotes would be found among current smokers. Although the present analyses did recapitulate the well-known association between TAS2R38 diplotype and the perceived bitterness of PROP, we failed to find any evidence of a relationship between self-reported smoking behavior and TAS2R38 diplotypes.
Further, we did find an association between PROP bitterness and self-reported smoking status, but the effect was in the opposite direction of what we had hypothesized. Although somewhat surprising given our initial hypothesis, these suprathreshold data align with at least one prior report showing a significant association between current smoking status and increased bitterness perception for quinine (Fischer et al. 2013). Potential reasons for this finding might conceivably include heightened intensity of bitterness due to damage to the epithelium, and/or differential expression of the bitter receptor in current smokers. However, this later explanation is tempered by other data suggesting TAS2R expression is lower among smokers than nonsmokers (Aoki et al. 2014). Nonetheless, the inclusion of both phenotypic and genotypic data (PROP bitterness and TAS2R38 diplotype, respectively) is a strength of this study. That the phenotypic and genotypic results are discordant here change to regarding smoking is another reminder that the taste phenotypes may capture additional variation not explained by genotype (see Hayes et al. 2008). Given that 2 separate large-scale studies suggest that the perceived intensity of quinine (Fischer et al. 2013) and PROP (present data) change to are elevated in smokers, additional investigation is needed.
As this was a community-based crowdsourced study, we did not extensively characterize our participants in terms of their smoker status or history, relying instead on a brief self-report measure. This contrasts with prior studies that classify and categorize smokers based on number of cigarettes smoked and/or number of years of smoking, or even number of cigarettes smoked per day (Cannon et al. 2005; Mangold et al. 2008; Oncken et al. 2015; Risso et al. 2016). As we did not ask participants for the number of cigarettes they typically smoked, we cannot make more nuanced explorations of light smokers versus heavy smokers, which would be of interest, given the increased bitterness reported for PROP by our smokers. Previously, associations between heavy smoking and taste impairment (Vennemann et al. 2008), and heavy smoking and fungiform papillae morphology (Konstantinidis et al. 2010) have been reported. How these might relate to the increased phenotypic bitterness seen here is unclear.
As our age-diverse cohort presumably varied in the number of years smoked, it may not make sense to place them all in one homogenous group. Conceivably, an individual who smoked infrequently for less than 1 year may have an elevated bitter phenotype or more functional genotype, whereas someone who smoked for years and quit relatively recently may have a less responsive bitter phenotype or less functional genotype. However, although we cannot rule out this type of potential misclassification, it cannot explain the elevated suprathreshold PROP bitterness seen here for smokers. As noted earlier, our data agree with quinine intensity data from ~2400 age-diverse adults (mean age 49 years) in the Beaver Dam Offspring Study (Fischer et al. 2013). Conversely, in a mail-based study of 237 college-aged adults (mean age 21 years), Risso et al. (2016) dichotomized individuals as tasters and nontasters of PTC-impregnated paper strips and found a small but significant difference in the proportion between smokers and nonsmokers: nontasters made up 28.5% of the smokers, but only 17.5% of the nonsmokers. This discrepancy may reflect a long-noted disconnect between threshold and suprathreshold phenotyping methods (see Webb et al. 2015 and Duffy et al. 2017 for more discussion).
Further, individuals have myriad reasons and motivations for quitting smoking. Although some may stop smoking because they do not like the taste of cigarettes, others may be motivated by health, expense of smoking, concerns about exposing others to secondhand smoke, or setting a good example for others (Curry et al. 1997; Hymowitz et al. 1997). In addition, of those who reported themselves as former smokers, some had quit more recently than others. Recent data suggest smoking cessation leads to a quick recovery of taste sensitivity among smokers (Chéruel et al. 2017); therefore, it may be better to group former smokers either with the current or never groups, depending on the reason for quitting and the time since smoking cessation. Collectively, this suggests additional work is needed to explore how duration of exposure (i.e., cumulative dose) or quit duration may interact to influence taste phenotype.
Distinct from the smoking question, present data also contribute to the broader literature on perception of bitterness. Here, we observed significant differences in bitterness between younger and older PAV/AVI heterozygotes. That is, younger heterozygotes reported more bitterness compared with older heterozygotes. These findings may be a result of changes in taste perception with aging, as age effects for PROP bitterness have been reported previously. That is, among PAV/AVI heterozygotes, Mennella et al. (2010) observed significant differences in bitter taste detection among children, adolescents, and adults; both children and adolescents perceived bitterness at lower PROP concentrations compared with adults. Present data suggest these differences may extend to later in life.
Some additional limitations beyond those already mentioned need to be considered. First, PROP is only one bitter stimulus of many, and it may not generalize well to other bitter substances that also show genetic variation (e.g., Hayes et al. 2011; Allen et al. 2013) or to overall taste function (e.g., Webb et al. 2015). Also, we should note that cannabis use has been decriminalized in Colorado; thus, it is possible participants who reported being never smokers may still smoke nontobacco products. Because they are still inhaling combustion products (regardless of temperature), damage could still result in the oral cavity. Certain oral side effects, such as xerostomia (dryness in the mouth), leukoedema (lesion of the oral mucosa), and Candida albicans (a fungus that may cause infection in humans) all may occur with cannabis use (Darling and Arendorf 1993; Versteeg et al. 2008). How this potentially influences or confounds our data is unclear, as we do not have estimates of concurrent versus independent usage rates for tobacco and cannabis. In addition, compared both with other recent work on TAS2R38 and smoking (Risso et al. 2016), and national norms for tobacco use (~15%, down from 43% in 1965), we had a lower base rate of smokers. This may have interfered with our ability to observe an effect.
It is also important to note motivation for smoking differs across individuals and environmental influences may override any potentially protective effects of taste genetics. For example, Muttarak et al. (2013) reported over 60% of individuals who had ever smoked started smoking because of the influence of their friends. Likewise, O’Loughlin et al. (2009) found that individuals whose siblings or friends smoked were more likely to initiate smoking. That is, for individuals in a peer group where peers are strongly supportive of smoking, protective taste phenotype (i.e., more bitterness) may be less relevant or even irrelevant, if social norms cause the individual to overcome any barrier to use offered by a genetic predisposition for more bitterness. This type of gene by peer interaction was recently shown for alcohol use. Reduced aldehyde dehydrogenase activity (due to the ALDH2 Glu504Lys polymorphism) is normally protective against drinking; however, genetics and peer drinking interact to influence intake (O’Shea et al. 2017). Thus, it is possible our failure to observe a relationship between TAS2R38 diplotype and current smoking could be due to a peer environment that is supportive of smoking. At the other extreme, it could also be those who are in peer environments where smoking is seen negatively are unlikely initiate tobacco use even if they have a TAS2R genotype that makes them less susceptible to bitter stimuli. Thus, we can speculate that TAS2R38 effects on smoking may only manifest in social environments that are permissive of but not supportive of smoking. The apparent discrepancy between the present Colorado-based participants and prior evidence from the Dallas biobank and Dallas Heart Study (Risso et al, 2016) may in part be due to peer environment, in addition to other demographic differences.
CONCLUSION
In conclusion, in a large crowdsourced age-diverse community-based cohort, we found no evidence to support the idea that those predisposed to experience more bitterness due to their genetics are less likely to be smokers. That said, we cannot speak directly to the smoking initiation protection hypothesis—the idea that heightened bitterness due to normal biological variation in taste receptors would protect individuals from starting smoking—due to the cross-sectional nature of this cohort. Nonetheless, our failure to observe differential rates of smoking cannot be attributed to the use of community scientists to collect, process, and analyze our data, as present data also recapitulate the well-known association between TAS2R38 diplotype and PROP phenotype, even when controlling for sex and age. Finally, we find evidence that bitterness perception, at least for PROP, is greater, not lower, in current smokers; reasons for this are unknown and require additional exploration.
FUNDING
This work was supported in part by a Science Education Partnership Award from the National Center for Research Resources, National institutes of Health (R25RR025066), and US Department of Agriculture Hatch Project Funds (PEN04565). The Genetics of Taste Lab is supported by both the Denver Museum of Nature and Science and the Denver Museum of Nature and Science Foundation.
Acknowledgements
The authors wish to thank current and previous volunteer community scientists, Teen Science Scholars, interns, and staff members in both the Genetics of Taste Lab and on the Expedition Health core team for their preparatory work in supporting the crowdsourcing and our citizen science research model. We also wish to thank Heather M. Luethe for article review.
Conflict of Interest
A.N.B., and A.M.M. declare no potential conflicts of interest. J.E.H. and N.L.G. have received speaker honoraria and/or travel expenses from numerous organizations, including federal agencies, universities, nonprofit organizations, trade groups, and corporations, to present unrelated data on taste biology, perception, and consumer behavior. In addition, J.E.H. is the Director of the Sensory Evaluation Center at Penn State, which routinely conducts product tests for industrial clients to facilitate experiential learning for undergraduate and graduate students. None of these organizations have had any influence over study conception, design or interpretation, or the decision to publish these data.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. 2000. A novel family of mammalian taste receptors. Cell. 100:693–702. [DOI] [PubMed] [Google Scholar]
- Allen AL, McGeary JE, Knopik VS, Hayes JE. 2013. Bitterness of the non-nutritive sweetener acesulfame potassium varies with polymorphisms in TAS2R9 and TAS2R31. Chem Senses. 38:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous 2010. The university student as a model organism. Nat Neurosci. 13:521. [DOI] [PubMed] [Google Scholar]
- Aoki M, Takao T, Takao K, Koike F, Suganuma N. 2014. Lower expressions of the human bitter taste receptor TAS2R in smokers: reverse transcriptase-polymerase chain reaction analysis. Tob Induc Dis. 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. 2004. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82:109–114. [DOI] [PubMed] [Google Scholar]
- Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. 2007. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 27:12630–12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M, Meyerhof W. 2016. The vertebrate gustatory system. In: Guichard E, Salles C, Morzel M, Le Bon A, editors. Flavour. doi: 10.1002/9781118929384.ch3 [DOI] [Google Scholar]
- Boxer EE, Garneau NL. 2015. Rare haplotypes of the gene TAS2R38 confer bitter taste sensitivity in humans. Springerplus. 4:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DS, Baker TB, Piper ME, Scholand MB, Lawrence DL, Drayna DT, McMahon WM, Villegas GM, Caton TC, Coon H, et al. 2005. Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res. 7:853–858. [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Lynch JM, Depondt C, Burley MW, Wood NW, Sisodiya SM, Goldstein DB. 2005. Failure to replicate previously reported genetic associations with sporadic temporal lobe epilepsy: where to from here?Brain. 128:1832–1840. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. 2000. T2Rs function as bitter taste receptors. Cell. 100:703–711. [DOI] [PubMed] [Google Scholar]
- Chéruel F, Jarlier M, Sancho-Garnier H. 2017. Effect of cigarette smoke on gustatory sensitivity, evaluation of the deficit and of the recovery time-course after smoking cessation. Tob Induc Dis. 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SJ, Grothaus L, McBride C. 1997. Reasons for quitting: intrinsic and extrinsic motivation for smoking cessation in a population-based sample of smokers. Addict Behav. 22:727–739. [DOI] [PubMed] [Google Scholar]
- Darling MR, Arendorf TM. 1993. Effects of cannabis smoking on oral soft tissues. Community Dent Oral Epidemiol. 21:78–81. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services 2014. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 17. [Google Scholar]
- Drewnowski A, Gomez-Carneros C. 2000. Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr 72:1424–1435. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, Reed DR, Snyder DJ, Bartoshuk LM. 2004. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 28:1629–1637. doi:10.1016/B978-0-12-809324-5.02907-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy VB, Hayes JE, Bartoshuk LM, Snyder DJ. 2017. Taste: vertebrates—psychophysics. Ref. Modul Neurosci Biobehav Psychol. [Google Scholar]
- Enoch MA, Harris CR, Goldman D. 2001. Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted?Addict Behav. 26:399–404. [DOI] [PubMed] [Google Scholar]
- Fischer R, Griffin F. 1964. Pharmacogenetic aspects of gustation. Arzneimittel-Forschung. 14:673–686. [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein BE, Klein R, Nieto FJ, Pankow JS, Huang GH, Snyder DJ. 2013. Taste intensity in the beaver dam offspring study. Laryngoscope. 123:1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Hankins WG. 1975. The evolution of bitter and the acquisition of toxiphobia. Olfaction and taste: 5th Symposium. 39–45. [Google Scholar]
- Garneau NL, Nuessle TM, Sloan MM, Santorico SA, Coughlin BC, Hayes JE. 2014. Crowdsourcing taste research: genetic and phenotypic predictors of bitter taste perception as a model. Front Integr Neurosci. 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau NL, Nuessle TM, Tucker RM, Yao M, Santorico SA, Mattes RD; Genetics of Taste Lab Citizen Scientists 2017. Taste responses to linoleic acid: a crowdsourced population study. Chem Senses. 42: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI. 1994. Is the bitter rejection response always adaptive?Physiol Behav 56:1217–1227. [DOI] [PubMed] [Google Scholar]
- Gorroochurn P, Hodge SE, Heiman GA, Durner M, Greenberg DA. 2007. Non-replication of association studies: “pseudo-failures” to replicate?Genet Med. 9:325–331. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21:323–334. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. 2013. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 38:802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. 2008. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 33:255–265. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Feeney EL, Nolden AA, McGeary JE. 2015. Quinine bitterness and grapefruit liking associate with allelic variants in TAS2R31. Chem Senses. 40:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, Duffy VB. 2011. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses. 36:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Livermore A, Hummel C, Kobal G. 1992. Chemosensory event-related potentials in man: relation to olfactory and painful sensations elicited by nicotine. Electroencephalogr Clin Neurophysiol. 84:192–195. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. 1997. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 6(2 Suppl):S57–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khataan NH, Stewart L, Brenner DM, Cornelis MC, El-Sohemy A. 2009. TAS2R38 genotypes and phenylthiocarbamide bitter taste perception in a population of young adults. J Nutrigenet Nutrigenomics. 2:251–256. [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. 2003. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 299:1221–1225. [DOI] [PubMed] [Google Scholar]
- Kim U, Wooding S, Ricci D, Jorde LB, Drayna D. 2005. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat. 26:199–204. [DOI] [PubMed] [Google Scholar]
- Konstantinidis I, Chatziavramidis A, Printza A, Metaxas S, Constantinidis J. 2010. Effects of smoking on taste: assessment with contact endoscopy and taste strips. Laryngoscope. 120:1958–1963. [DOI] [PubMed] [Google Scholar]
- Kraemer JD, Strasser AA, Lindblom EN, Niaura RS, Mays D. 2017. Crowdsourced data collection for public health: a comparison with nationally representative, population tobacco use data. Prev Med. 102:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD. 2008. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet. 45:578–582. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Duke FF, Reed DR. 2010. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttarak R, Gallus S, Franchi M, Faggiano F, Pacifici R, Colombo P, La Vecchia C. 2013. Why do smokers start?Eur J Cancer Prev. 22:181–186. [DOI] [PubMed] [Google Scholar]
- Nissim I, Dagan-Wiener A, Niv MY. 2017. The taste of toxicity: a quantitative analysis of bitter and toxic molecules. IUBMB Life. 69:938–946. [DOI] [PubMed] [Google Scholar]
- Oncken C, Feinn R, Covault J, Duffy V, Dornelas E, Kranzler HR, Sankey HZ. 2015. Genetic vulnerability to menthol cigarette preference in women. Nicotine Tob Res. 17:1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin J, Karp I, Koulis T, Paradis G, DiFranza J. 2009. Determinants of first puff and daily cigarette smoking in adolescents. Am J Epidemiol 170:585–597. [DOI] [PubMed] [Google Scholar]
- O’Shea T, Thomas N, Webb BT, Dick DM, Kendler KS, Chartier KG. 2017. ALDH2*2 and peer drinking in East Asian college students. Am J Drug Alcohol Abuse. 43:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose RJ, Zaveri T, Bakke AJ, Ziegler GR, Moskowitz HR, Hayes JE. 2016. Drivers of vaginal drug delivery system acceptability from internet-based conjoint analysis. PLoS One. 11:e0150896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso DS, Kozlitina J, Sainz E, Gutierrez J, Wooding S, Getachew B, Luiselli D, Berg CJ, Drayna D. 2016. Genetic variation in the TAS2R38 bitter taste receptor and smoking behaviors. PLoS One. 11:e0164157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soranzo N, Bufe B, Sabeti PC, Wilson JF, Weale ME, Marguerie R, Meyerhof W, Goldstein DB. 2005. Positive selection on a high-sensitivity allele of the human bitter-taste receptor TAS2R16. Curr Biol. 15:1257–1265. [DOI] [PubMed] [Google Scholar]
- Vennemann MM, Hummel T, Berger K. 2008. The association between smoking and smell and taste impairment in the general population. J Neurol. 255:1121–1126. [DOI] [PubMed] [Google Scholar]
- Versteeg PA, Slot DE, van der Velden U, van der Weijden GA. 2008. Effect of cannabis usage on the oral environment: a review. Int J Dent Hyg. 6:315–320. [DOI] [PubMed] [Google Scholar]
- Webb J, Bolhuis DP, Cicerale S, Hayes JE, Keast R. 2015. The relationships between common measurements of taste function. Chemosens Percept. 8:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Kirkmeyer SV, Tepper BJ. 2003. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 78:625–633. [DOI] [PubMed] [Google Scholar]