Abstract
Polarity is critical for development and tissue-specific function. However, the acquisition and maintenance of tissue polarity is context dependent. Thus, cell and tissue polarity depend upon cell adhesion which is regulated by the cytoskeleton and influenced by the biochemical composition of the extracellular microenvironment and modified by biomechanical cues within the tissue. These biomechanical cues include fluid flow induced shear stresses, cell-density and confinement-mediated compression, and cellular actomyosin tension intrinsic to the tissue or induced in response to morphogens or extracellular matrix stiffness. Here, we discuss how extracellular matrix stiffness and fluid flow influence cell-cell and cell-extracellular matrix adhesion and alter cytoskeletal organization to modulate cell and tissue polarity. We describe model systems that when combined with state of the art molecular screens and high resolution imaging can be used to investigate how force modulates cell and tissue polarity.
Introduction
Polarity, which is the asymmetric organization of cellular proteins, membranes, organelles and the cytoskeleton, is a key regulator of cell fate and is important for tissue development and homeostasis. The establishment of apical-basal tissue polarity, which first emerges when a polarized sheet of epithelial cells forms the trophectoderm, is arguably one of the most critical events in early embryonic development. As development progresses apical-basal polarity continues to play a major role by directing the organization and function of cell clusters that create the distinct interfacial tissue layers that comprise the endoderm, ecotoderm and mesoderm 1. When more complicated tissue-level structures develop, planar polarity emerges to modulate tissue orientation, as has been documented during wing morphogenesis and hair follicle formation 2,3. Planar polarity, which establishes cell and tissue orientation, is also critical for cell and tissue function in the adult organism. Back to front orientation is key for directed migration and facilitates neutrophil infiltration into injured tissues and orients the directed collective migration of keratinocytes during wound healing 4. Not surprisingly, during development and in the adult organism, both apical-basal and planar polarity are important for the organization and maintenance of the structure-function of cells and tissues. Indeed, polarity enables the afferent and efferent biochemical information flow in neurons, facilitates directed migration during gastrulation, and permits efficient nutrient exchange and polarized secretion in differentiated epithelial and endothelial sheets 5,6.
Cell and tissue polarity are regulated by the asymmetric targeting of proteins and membranes mediated by directed vesicle trafficking and cytoskeletal reorganization in response to soluble cues such as growth factors and morphogens 3,7–9. The establishment and maintenance of cell and tissue polarity are tightly regulated by cell-extracellular matrix (ECM) and cell-cell adhesion that are in turn influenced by biomechanical cues within the tissue microenvironment 10–12. For instance, the acquisition and maintenance of apical-basal and planar tissue polarity both depend upon adhesion to the ECM through specialized matrix adhesion receptors such as integrins (cell-ECM adhesions), and to other cells via adherens, tight, and scribble junctional complexes (cell-cell adhesions) 13,14. Cell-ECM and cell-cell adhesions and soluble factors such as growth factors and morphogens synergize to direct cellular and tissue polarity by modulating the activity of GTPases including Rac, Cdc42, and Rho, which are molecular switches that regulate actin cytoskeletal dynamics and organization 14,15. Apical-basal polarity in an epithelium requires the continuous apical and basolateral sorting of proteins through the trans-golgi network (TGN), and this protein trafficking is influenced by the actin cytoskeleton that is modulated by the activity of GTPases 16. Planar polarity is also regulated by GTPases that modulate protein trafficking and reorganize the actin cytoskeleton 15,17,18.
Cell-ECM and cell-cell adhesion assembly and strength as well as Rac and Rho GTPase activity are enhanced in response to biomechanical forces such as exposure to a shear force or ECM stiffening 19. Shear flow for instance stimulates Rac activity and modulates actin reorganization and integrin adhesion dynamics to modulate endothelial tissue integrity and orientation 20. Furthermore, a stiff ECM promotes integrin engagement and signaling and activates GEFs that stimulate Rho to induce mDia-dependent actin remodeling and ROCK-induced type-II myosin contractility that then reinforce integrin adhesion assembly 21. The elevated RhoGTPase-dependent stress fiber formation and ROCK-induced actomyosin contractility also perturb the polarized sorting of proteins through the transgolgi network (TGN) and destabilize tight junction and adherens junction integrity that compromise apical-basal polarity. In tumors chronically elevated cellular actomyosin tension induced by oncogenes such as mutant Ras or by enhanced integrin focal adhesion signaling in response to a stiffened fibrotic ECM, disrupt apical-basal polarity 22–26. Similarly, amplification of erbB2 (Her2) receptors hyperstimulate Ras to enhance ROCK-dependent cellular tension that disrupts PAR/scribble cell-cell complexes and redistributes scribble to ECM adhesions to promote mammary epithelial cell invasion22,27–30. Importantly however, biomechanical forces are also important for normal tissue development and for maintaining tissue homeostasis. For instance, flow can enhance planar polarity in cells and tissues by activating RhoGTPases that reorient the cytoskeleton and stimulate actomyosin tension to strengthen cell-cell versus cell-ECM adhesion 13,31–34. Thus, a stiff ECM that enhances integrin adhesion assembly and signaling also stimulates the relocalization of Scribble from apical-lateral adhesions, where the protein resides in a complex with Crumbs and PARs, to the basal plasma membrane, where it assembles with Rac1 via Rac1GEF βPix 35–37 , PTEN 30, and MCC 38 to direct polarized cell migration.
The molecular mechanisms by which cell-cell and cell-ECM adhesions regulate apical-basal and planar polarity have been clarified by studies that have employed two and three dimensional organotypic culture models and natural and synthetic biomaterials with defined biochemical and biophysical properties 39–47. Recent innovations in cell culture models using architecturally defined tissues with microfluidics that recapitulate flow dynamics in the vasculature and lymphatic systems are now being used to clarify how fluid flow and shear stress regulate cell and tissue polarity. In this review, we discuss cell and tissue polarity in the context of mechanical signals derived from cell contractility, ECM elasticity, and fluid flow. We outline tractable model systems that include mechanically-tuned biomimetic cell culture devices and fluid flow devices that are available to study how these biomechanical cues regulate cell and tissue polarity.
Cell-extracellular matrix adhesion: the physical foundation of cell polarity
Cell adhesion to the extracellular matrix (ECM) or to other cells via cell-cell adhesions establishes the physical context in which a cell orients its functional structures and intracellular proteins and chemical gradients. This is especially auspicious in the context of endothelial cells lining vascular and lymphatic networks and epithelial cells lining secretory ductal trees, as these cell types adhere to a basement membranes and orient their endo/exocytic machinery towards their fluid filled lumens 48,49. Epithelial and endothelial barriers orient their polarity within these anisotropic physical conditions by adhering to a basement membrane through a plethora of transmembrane ECM receptors including syndecans, discoidin receptors, and integrins. Of these ECM adhesion receptors, integrins are the best studied, and their role in cell and tissue polarity has been well-established. Integrins are a family of transmembrane adhesion receptors comprised of 24 αβ heterodimeric members that bind specific regions of large macromolecular ECM proteins. Upon binding to the ECM, activated integrins cluster to form focal complexes that associate with adhesion plaque proteins such as talin that in response to either an externally-applied force or intrinsic actomysosin tension unfold to recruit vinculin and assorted cytoskeletal binding and signaling molecules to drive the assembly of integrins into mature focal adhesions 50–52.
Cells engage outside-in and inside-out integrin signaling to develop apical-basal polarity in multi-cellular tissues. For example, when epithelial cells such as MDCK (Madin-Darby Canine Kidney Epithelial Cells) are cultured in suspension they depolarize, however, once they aggregate to form cystic structures they repolarize to form multi-cellular structures with the apical domain localized to the outside surface of the cyst 53. This inversed cell polarity is reverted towards the tissue lumen when the MDCK cysts are embedded within an isotropically soft collagen gel in a beta 1 integrin-Rac1 GTPase dependent manner 53–55. Perturbations in alpha 2 beta 1 integrin-collagen interactions compromise MDCK cyst polarization, emphasizing the key role of cell-ECM adhesion in apical-basal polarity regulation, possibly by regulating polarized protein trafficking 56,57.
Integrin adhesion assembly and signaling are exquisitely modulated by rigidity sensing of the viscoelasticity of the ECM and by intracellular actomyosin tension 22,58–60. Physical force alters the conformation and localization of integrins and their adhesion plaque proteins including talin and vinculin and to foster the assembly of mature focal adhesions. A stiff ECM can influence tissue polarity by modulating cell-ECM adhesions which can destabilize cell-cell adhesions and compromise tissue organization. Under extreme conditions a chronically stiffened ECM will collaborate with increased growth factor receptor signaling to promote cell invasion and may foster the malignant transformation of an epithelial tissue 61. Moreover, the speed and persistence of cell migration is also tuned by ECM substrate compliance suggesting a stiff ECM could foster the migration of transformed cells into the interstitial stroma 62,63. Indeed, a stiffened, fibrotic ECM also permits a TGFb-dependent epithelial to mesenchymal transition that has been implicated in tumor metastasis (EMT) 64. Nevertheless and importantly, ECM stiffening is also critical for normal tissue development and homeostasis. For example, a stiffened ECM permits the directed, coordinated, collective migration of keratinocytes and instructs neutrophil infiltration and monocyte differentiation into macrophages to facilitate proper wound healing. The wound-activated macrophages secrete MMPs that induce ECM remodeling and TGFβ that stimulate the expression of ECM proteins and the transdifferentiation of fibroblasts into contractile myofibroblasts that stiffen the wound stroma 65,66. The stiffened ECM in collaboration with macrophage and fibroblast secreted chemokines and the TGFb induce a normal physiological EMT in the keratinocytes and then foster their directed migration into the wound to repopulate and heal the injured tissue site 67–69.
In vitro studies using substrates with defined elasticity, cell adhesive peptides, and MMP-degradable materials revealed that the formation of polarized tissue structures is tuned by ECM stiffness and depends upon ECM remodeling 62,63,70–72. This phenomenon has also been observed during development where gradients of a stiffened ECM modulate integrin-vinculin-talin mediated mechanotransduction to direct the collective sheet migration critical for neural crest development 73. In fact directed migration of cells towards increasingly rigid adhesion substrates has been experimentally demonstrated using materials that are resistant to ECM degradation and has been termed “durotaxis” 42,74,75. Durotaxis is consistent with the findings that efficient cell migration is a dynamic balance between adhesive and protrusive forces supported by a spatiotemporally variable program which integrates type-II myosin activity, focal adhesion assembly/disassembly, and remodeling of actin cytoskeleton 76.
ECM stiffness can also modulate tissue polarity indirectly by altering the synthesis and secretion of soluble factors that regulate polarity through auto-, juxta-, and paracrine signaling. This paradigm was illustrated by Przybyla et al. who employed protein-functionalized polyacrylamide gels with tuned elasticity to demonstrate that substrate stiffness modulates human embryonic stem cell polarity and differentiation by regulating the expression and secretion of key wnts and their inhibitors 77. Secreted wnt gradients function as directional cues which alter cell polarity 78, which implicates secreted signals as another possible mode of polarity disruption caused by aberrantly stiff ECM. Indeed, endo and exocytosis are regulated by membrane tension, which is modulated by intrinsic and extrinsic physical force 79. These mechanically altered secretory responses may lead to the loss of polarity by disrupting the maintenance of the basement membrane. Substrate rigidity also alters the production and secretion matrix metalloproteinases (MMPs), which remodel the basement membrane 80 and are exocytosed in a polarized fashion to support Planar Cell Polarity (PCP) 81. These secreted or membrane tethered enzymes foster cell migration, ECM remodeling 11, and the cleavage of cell surface receptors and signaling molecules 82. Apical-basolateral polarity depends upon polarized localized secretion of MT-MMP (MMP14) 83. Not surprisingly, the synthesis and secretion of MMPs are responsive to ECM elasticity, such that ECM mechanics modulate MMP levels and activity, and control apical-basal tissue polarity by catalyzing ECM remodeling and releasing soluble factors that stimulate cell migration 71.
Given strong links between cell-integrin ECM adhesions and tissue polarity, it is not unreasonable to suggest that defining how force modulates integrin structure/function to alter tissue polarity could provide critical insight into the role of force in tissue development and homeostasis. Moreover, delineating links between force and polarity should clarify the molecular basis of various pathologies that compromise epi/endothelial barrier function or induce diseases linked to loss of tissue polarity including atherosclerosis and cancer 84. This objective would be well served through the use of defined cellular model systems embedded within materials that accurately mimic the composition and physical properties of the native tissue and that permit high resolution imaging of live cultures. These approaches have become increasingly prevalent as 3D embedded culture conditions, often referred to as “organoids”, have demonstrated improved phenotypic recapitulation of their in vivo tissue counterparts than standard in vitro monolayer culture formats 85. These cell/tissue-specific 3D-culture conditions have become increasingly sophisticated and can be well-defined in terms of chemical composition, soluble factor addition, and physical manipulations required to generate in vitro models that mimic healthy and diseased human-like organs using primary and immortalized human and murine cells 86–88. These advanced and defined cellular materials can be complemented with a toolbox of increasingly elaborate physical microenvironments that include tuneable hydrogels with defined ECM ligands, morphogens, and mechanical properties. These defined biomaterials permit high resolution imaging of live cells and are able to facilitate the systematic assessment of the contributions-of and synergy-between biochemical and biophysical cues in adhesion-regulated cell and tissue polarity 89–91. To fully recreate the “tissue-like” or “bio-mimetic” microenvironments of normal and diseased tissues researchers can also incorporate biomimetic microfluidic devices as well as incorporate compression and stretch setups that have been successfully adapted to conform to organotypic geometries and function 39. These reconstituted 3D organotypic models permit the systematic tuning of fluid shear force, compression, stretch and ECM elasticity and composition such that it is now possible to delineate the molecular mechanisms whereby cell ECM adhesion regulates tissue polarity 92. Complementing these sophisticated models are newly developed “mechanically active organ-on-a-chip” microdevices that permit rapid molecular and drug screening to examine mechanisms regulating the polarized uptake of molecules in an epithelium or endothelium 93.
Interplay between physical force and cell-cell adhesion
The collective morphogenesis of sheets of cells within a developing tissue depend upon PCP and is modulated by mechanical force. During tissue development polarized epithelial sheets integrate directional cues over extended distances to establish aligned tissue patterns through physical forces and chemical cues mediated predominantly by cell-cell adhesion interactions 2,94–96. For example, planar polarity in the murine keratinocyte epithelium is dictated by anisotropic physical force that is generated and transmitted through cell-cell junctions that function to align Celsr1, Cadherin EGF LAG Seven-Pass G-Type Receptor 1 97. Similarly, during fly embryogenesis, Celsr1, Vangl2, and Fz6 (core PCP proteins) become asymmetrically distributed to the anterior/posterior cell borders in the basal cells to define axial-vectorial asymmetry in response to cell-cell generated tension 25,26. Thereafter, the force-directed PCP core proteins redistribute into specific plasma membrane domains to form instructive “puncta” at cell-cell adherens junctions.
Adherens junctions (AJ) are composed of cadherin receptors that bridge adjacent plasma membranes of cells through homophilic interactions that are critical for the development of apical-basal polarity 13,98,99. Cadherins coordinate with cytoplasmic catenins to integrate adhesions to actin filaments and microtubule networks to mechanically couple the contractile cortices of the cell thereby distributing physical stresses across a cellular sheet. In vertebrate polarized epithelia, AJs are part of the tripartite junctional complex comprised of tight junction (zonula occludens), AJ (zonula adherens), and desmosome (macula adherens) that are localized to the juxtaluminal region 100. A major function of AJs is to maintain the physical association between cells, and disruption of these contacts releases cell–cell tension and compromises tissue organization. The transmission of tension to the cytoskeleton through cadherin-mediated adhesions is thus critical for sculpting the epithelium 13,101–104 and its dysregulation disrupts tissue integrity and can foster disease pathologies including malignancy 22,61,105. Interestingly, although the application of an external force on E-cadherin can induce cytoskeletal stiffening 99, how E-cadherin transduces tension to the actin cytoskeleton remains unclear. Surely force transmission across the AJ must support the engagement and recruitment of the actin binding proteins that assemble and maintain the AJ 106, favoring some form of dynamic collective mechanical stabilization to generate and maintain PCP.
RhoA plays a critical role in PCP by supporting cell-cell adhesions through actin remodeling and by triggering myosin-induced tension to generate the requisite forces required to reorient the cells in an α-catenin-dependent manner 107,108. This adherens-localized actomyosin tension appears to be absolutely critical for the establishment of PCP in an epithelium and for the maintenance of tissue integrity 109. In this regard, tissue integrity depends upon sustaining an optimal range of force across the junction as was illustrated by a series of elegant optical trap studies by Buckley et al. 110 which demonstrated that an optimal range physical force was required for cytoskeletal association with AJs. Physical forces exceeding the optimal range of tension led to AJ deterioration and resulted in a loss of tissue integrity 22,111,112. Yet, the assembly of branched actin networks is also force-dependent 113 and the restructuring of branched actin networks to catenin/cadherin associated bundles is also critical for AJ stability and is likely mediated through catenin actin ARP2/3 competitive binding 114. Thus, force-dependent destabilization of AJs could be mediated either by changes in actin remolding or by direct destabilization of the AJ protein complex. Indeed, several pathogens that compromise intestinal barrier function also hijack actin cytoskeletal dynamics 115 raising the possibility that they might destabilize AJ integrity through actin remodeling that dysregulates force distribution at cell-cell junctions 116.
When good forces go bad
Normal tissue development and homeostasis and the acquisition of apical-basal and planar tissue polarity depend upon a tightly regulated balance of exogenous and intrinsic cell tension. Not surprisingly, chronically elevated external physical stresses or intracellular actomyosin tension exerted at the sites of adhesion alter the distribution, composition, and subcellular signaling systems within integrin adhesions and at cell-cell adhesion. Chronically modified integrin signaling and cell-cell adhesion integrity/composition ultimately compromise tissue polarity to perturb tissue integrity and tensional homeostasis that may promote disease. For instance, tumor progression in solid tissues is frequently accompanied by fibrosis that progressively stiffen and reorganize the stromal ECM. The stiffened stromal ECM in turn enhances the assembly of focal adhesions that potentiate growth factor receptor signaling through PI3 kinase and destabilize cell-cell adhesions to promote cell invasion and malignant transformation and eventually foster metastasis 22,61,117. Similarly, oncogenes such as ErbB2 and Ras enhance tumor cell actomyosin tension that promote focal adhesion assembly and induce ECM remodeling and stiffening that also then also destabilize cell-cell adhesions and promotes cell invasion and malignant transformation by enhancing pro-growth, pro-invasion and pro-survival signaling such as elevated β-catenin, Myc and STAT3 activity 117–119. Consistently, inhibiting FAK activity or reducing RhoA or ROCK activity can phenotypically revert the phenotype of malignant mammary tumors in culture and will impede the malignant transformation of multiple tumor types including squamous cell carcinoma, mammary carcinomas, and pancreatic carcinomas in vivo 22,61,118–126. Importantly, repression of the malignant phenotype in all of these instances associates with either maintenance-of or restoration of cell-cell adhesions and apical-basal tissue polarity. Given that many molecules that modulate tissue polarity such as scribble and discs large are putative tumor suppressor these findings imply that these tissue polarity regulators repress malignancy by maintaining tissue architecture 29,36,127,128. Consistent with this prediction, cells engage basement membrane proteins via specific integrin heterodimers to establish and sustain cell polarity 129 and integrin-mediated adhesion to laminin 130, directs the localization of polarity mediators, such as Par3, to facilitate the assembly of differentiated acinar structures with a polarized lumen 131,132. Malignancy ensues when the integrity of this “differentiated and apical-basally polarized tissue” is compromised, as occurs in response to the increasingly fibrotic and stiffened ECM surrounding transformed tissues or following increased expression or activity of oncogenes that elevate actomyosin tension 61,105,117–119,133,134. Indeed, the levels and subcellular localization of Par3 are not only critical for the development of polarized tissues, but are necessary for the prevention of malignancy 29.
How do we model the tissue microenvironment to understand polarity regulation?
The physical context of a tissue including the type and organization of the cellular constituents, the composition and architecture and mechanical properties of the ECM together with chemical gradients and tissue level forces including flow, compression and tension cooperate to generate cell and tissue behavior 19,135,136. The challenge has been to clarify how these various environmental cues independently and collectively influence tissue level behaviors such as polarity. Arguably genetically engineered mouse models in which specific ECM components can be specifically knocked out or mutated and their posttranslational modification manipulated in a tissue specific manner and using inducible constructs has greatly facilitated studies to explore the impact of the microenvironment on tissue development, homeostasis and disease. Nevertheless, despite their elegance, these live model systems present a unique challenge when trying to identify direct causal relationships between ECM composition and organization and delineating the impact of specific stromal cellular components or physical forces and chemical gradients on cell and tissue behavior. To address such issues increasing effort has been exerted to develop tractable culture systems that can accurately deconvolve the impact of ECM composition, stiffness, architecture and even dimensionality on tissue phenotype. These newly developed systems have also been perfected to study the impact of compressive, stretch or tensile forces on cells embedded within collagen or hyaluronidase or synthetic hydrogels 39. Both synthetic and natural polymer scaffolds are also readily amenable to modulation of matrix compliance and ECM ligand bioavailability and have been used to study the impact of two and three dimensional ECMs on tissue behavior 137.
Hydrogels which are aqueous polymer networks that behave as viscoelastic solids, are a standard for biomimetic 3D encapsulated in vitro and in vivo material manipulation models. Hydrogels can be generated using naturally-derived biopolymers such as collagen, hyaluronic acid, fibrin, agarose, alginate, and cellulose. Synthetic polymers such as polyethylene glycol (PEG), poly(vinylalcohol) (PVA), poly-lactic-glycolic-acid (PLGA) are also effective 3D cell culture hydrogel models. Natural and synthetic polymers each have their respective benefits and limitations. Historically, collagen I gels and basement membrane-enriched hydrogels have proven to be instrumental for the study of tissue-specific differentiation and have critically illustrated the differences between normal and malignant or diseased tissues 45,126,138–150. Naturally derived materials, especially ECMs which exist in abundance in the tissue/structure or around the cell-type of interest, provide a biointerfacial cell scaffold that engages integrin or CD44 and RHAMM receptors within collagen and hyaluronic acid gels and that directly support the growth, viability, and tissue-like behavior of cells and tissue. These “natural” hydrogels have been used extensively to study tissue specific differentiation such as in mammary epithelial differentiation 151,152, kidney function 153,154 or endothelial network behavior 155–157 and when appropriately “tuned” to specific elasticities and biodegradability can generate important insight into tissue-specific behaviors including defining what factors control branching morphogenesis 70,158–160 and conceivably promote the malignant behavior (invasiveness) of a tissue 39,133. Nevertheless, these natural hydrogels are notoriously variable and do not always lend themselves to consistent modification 91,161. By contrast, synthetic polymers are amenable to precise modifications including controlled crosslinking arrangements and density and can be tuned to include a specific biochemical and chemical composition. However, synthetic polymers do not always recapitulate the architecture of native ECMs and are not easily remodeled. An optimal strategy for design and implementation of 3D-cell culture systems likely lies with a combinatorial approach where synthetic of naturally derived polymers are either be modified to accommodate more efficient crosslinking reactions or to optimize and define the presentation of adhesion ligands derived from biopolymers such as fibronectin, collagen, or laminin 91,137,162–164. These combinatorial materials are readily available from vendors or can be engineered and modified to present specific ECM-derived or ECM-mimetic ligands and are amenable to facile approaches to dynamically stiffen or soften the material 165. For example, RGD and laminin-111 conjugated PEG-based hydrogels with degradable peptides have been judiciously applied to understand gut development using a combination of purified intestinal organoids and mechanically-tuned ECMs and surprisingly have illustrated differential effects of laminin-derived peptides and full length laminin-11 on intestinal lumen formation 89.
While amorphous 3D-hydrogels provide an exciting platform to study cell polarity, 2D-surfaces and structurally defined 3D-surfaces arguably provide a more readily available and hence appropriate model system for the study of planar and apical basolateral polarity. The most easily adapted model system that can be used to study the impact of substrate elasticity on tissue polarity is the polyacrylamide gel (PA) surface 166. PA gels can be generated across a wide spectrum of elasticity and can be adapted to present a wide assortment of purified ECMs or modified ECMs or even synthetic adhesion ligands and are amenable to fluorescence-based imaging and protein and RNA harvesting 22. Although PA gels are not biodegradable and at least for short term culture are not easily fouled, the different concentration of bis-acrylamide crosslinkers used to vary the elasticity does modify the gel pore size and this can influence ligand binding and presentation to inappropriately modify cell behavior 167,168. Furthermore, traditional PA gels do not lend themselves to super-resolution imaging approaches such as Total Internal Reflection Fluorescence (TIRF) or Scanning Angle Interference Microscopy (SAIM). To this end, silicone gel coatings with suitable refractive indexes that permit high resolution imaging and whose elasticity can be modified across a wide range have been developed and are now readily available for general experimental applications 169.
Soft lithography, a technique borrowed from the microfabrication of electrical circuits, has enabled major advances to generate 3D patterned cell culture hydrogels. Rather than culturing cells within a stochastic assemblage of cells, polymers, solutes, and fluids, soft lithography can generate geometrically defined networks of channels, void spaces, elastic and selectively permeable membranes as well as ports or sensors to allow for real-time monitoring of metabolite production/consumption or addition of pharmacologic compounds. Soft lithography is executed either by fabricating or purchasing a mold that is inversely replicated by an elastic material such as polydimethylsiloxane (PDMS) to form embossed microstructures 170,171. The PDMS cast around the mold can then be readily bonded to glass to form, what is now typically described as, a microfluidic device. The experimental format afforded by soft lithography not only allows for the culture of biologically relevant cellular geometric organizations but the viscoelasticity of the PDMS hydrogel material can also be tuned across a range of stiffness to model different normal and diseased ECMs 172,173. Experiments to explore the impact of ECM compliance and topology on sheets of epithelial cells in two dimensions and on organized ductal tissues in a three dimensional matrix using ultrasoft lithography (0.1–100 kPa) have illustrated how ECM stiffness gradients are able to induce a durotactic migratory response towards tissue-like structures within a more physiologically-relevant context 172,173.
Integrating Flow
Cells and tissues are constantly experiencing a variable range of shear stresses generated by fluid flow and these shear stresses regulate development and when corrupted may also induce tissue pathologies. Shear stresses play critical role in the maintenance and development of cell polarity primarily through the dynamic regulation of Rac1 and RhoA 174. For instance, during embryogenesis, heart development is exquisitely regulated by directional fluid flow dynamics which critically induce the maturation of the vasculature through shear stress activation of RhoGTPases that promote tissue polarity and endothelial junction integrity 175–177. In the adult organism the mechanical forces generated by the dynamics of fluid flow within the lymphatic and vasculature (~0.1–50 dynes/cm2) 178–181 are absolutely critical for the assembly and maintenance of adherens junctions and tight junctions and control the development of vascular and lymphatic valves which mitigate retrograde fluid flow 182–185. Indeed, endothelial valve forming cells sense shear stresses associated with fluid flow and adjust their polarity 186 through adhesion dynamics that depend upon ROCK activity 183. Not surprisingly, compromised fluid flow, as occurs at blood vessel bifurcations can perturb tissue homeostasis to induce cardiovascular disease through the disruption of chemical gradients, altered mechanical signaling, and the regional accumulation of aggregates of insoluble material or cells that can stimulate inflammation and lead to lesion formation 187.
The study of the molecular mechanisms whereby fluid flow regulates cell polarity and tissue microstructure homeostasis has been greatly enabled by the use of microfluidic devices. Such devices have been adapted to support epi/endothelial cells assembled into tube-like columns with a central lumen that is capable of supporting dynamic fluid flows modulated by a microfluidic pump. Using these microfluidic devices and altering the rate of flow through the lumen to proportionally modulate shear stress flow was shown to regulate PCP by altering microtubule stability and activating GSK-3β. These studies further revealed that GSK-3β inhibition not only reversed endothelial PCP but also compromised the ability of the vasculature to elongate 174,188. These types of microfluidic device models may also be used to analyze whether or not the valve structures within the cardiac, venous, or lymphatic systems degenerate in response to shear stresses above or below a critical threshold 189,190 or if valve degeneration occurs in response to inflammatory cytokines. Combining these microfluidic devices with biochemically defined and elastically-tuned materials has permitted an analysis of the impact of physiological ranges of fluid shear stress and defined the role biochemical and morphological gradients on tissue polarity in a three dimensional tissue-like context 191. Indeed, the use of microfluidic device models that faithfully mimic the architectural geometry and mechanical forces tissues typically experience in vivo have strong potential to clarify factors that regulate tissue polarity including morphogen gradients, ECM stiffness gradients (durotaxis) and fluid shear stress 192,193. For instance, a collagen lined, soft lithography generated (1.5 kPa), 3D-endothelial lumen model revealed the importance of frictional force on the durotaxis-dependent migration and orientation of lymphatic and venous networks 72. Similar devices have been used to address the impact of the torturous and leaky vasculature on the tumor epithelium and reported that the reduced flow rates found in these vessels fosters high tumor cell proliferation whereas high shear stress (12 dynes/cm2) promotes G2/M cell cycle arrest 194–197 and the results of these studies have been used to imply flow dynamics could modulate tumor phenotype 198. Microfluidic devices have also been used to demonstrate that the oscillatory mechanical stresses produced by breathing motions as occurs in the lungs, play a critical role in regulating the growth of human non-small-cell lung cancer cells 199 and to assess barrier function of the endo/epithelium 200–202. Furthermore, a 3D microfluidics in vitro model of intestinal crypts illustrated the impact of the human specific pathogen norovirus on epithelial barrier function 203 and could constitute a tractable model system to assess the impact of the Listeria monocytogene or Shigella flexneri, pathogens on cytoskeletal organization and adherens junctions integrity 115,204,205. Clearly, impressive advancements in biomaterials, ultrasoft lithography and microfluidics combined with tissue organ cultures are now available and afford the research community with an unprecedented opportunity to use culture models to study how tissue polarity is molecularly regulated not only by morphogens but also by force.
Conclusion
Mechanical force generated or applied to cell and tissue structures can either enforce or compromise apical-basolateral and planer cell polarity and thereby plays a critical role in development, tissue homeostasis, and disease. Actomyosin tension exerted at cell-cell junctions reinforces these adhesions to promote apical-basal polarity while a stiffened ECM destabilizes cell-cell adhesions by enhancing cell-ECM adhesion and stimulating actin reorganization and receptor tyrosine kinase or G-protein coupled receptor signaling. Gradients of ECM stiffness and directed shear forces influence cell-cell or cell-ECM interactions which orient cytoskeletal organization and membrane receptor signaling that engage the planar polarity machinery to induce durotactic migration that is required for normal homeostatic processes or when corrupted can foster tumor cell migration towards the vasculature to promote metastatic dissemination of tumor cells. Deciphering how these forces operate to differentially modulate normal development and tissue behavior versus disease require sophisticated models that faithfully recapitulate the biochemical and the dynamic and three-dimensional biophysical microenvironment of tissues in vitro. Clearly, concerted effort to use these newly available model systems to study how force modulates cell and tissue polarity in context should help to clarify the molecular basis of tissue development, homeostasis and disease.
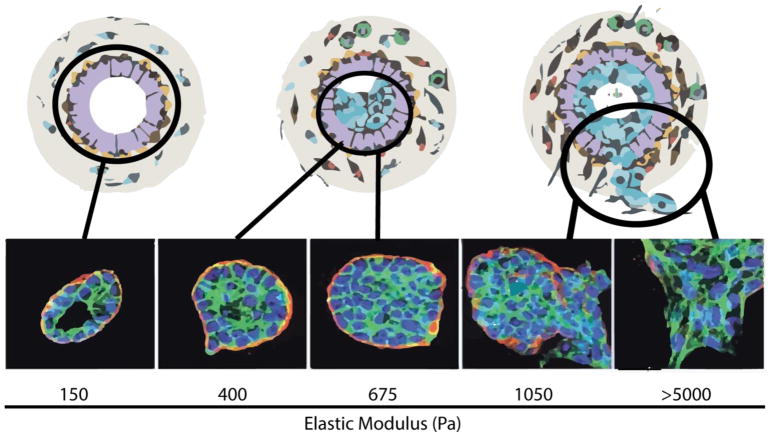
Figure 1. Polarity depends on a delicate balance of physical forces.
Increasing ECM stiffness causes the loss of apical basolateral polarity. Schematic depicting the effects of increasing mechanical stress on mammary epithelial cells. Chronic exposure to physical forces compromises the ductal structure and is accompanied by the loss of epithelial polarity. These effects are clearly observable with non-malignant MCF10A cell colonies cultured on a reconstituted basement membrane functionalized polyacrylamide gel surfaces of increasing stiffness (150–5,000 Pa). MCF10A cells cultured on surfaces with a biomimetic ECM stiffness similar to that measured in the normal murine mammary gland (150 Pa) form polarized acini organoids which model the terminal ductal lobular units of a differentiated breast. MCF10A organoids synthesize and localize an endogenous laminin 5 basement membrane (red) to the basolateral surface of the acini. These elastically tuned epithelial acini organoid models demonstrate that stiffening of the basement membrane causes a degeneration of polarity, breakdown of luminal structures, stable cell-cell junctions, and loss of the endogenous laminin 5 basement membrane. Nuclei (blue), F-Actin (green), and laminin 5 (red).
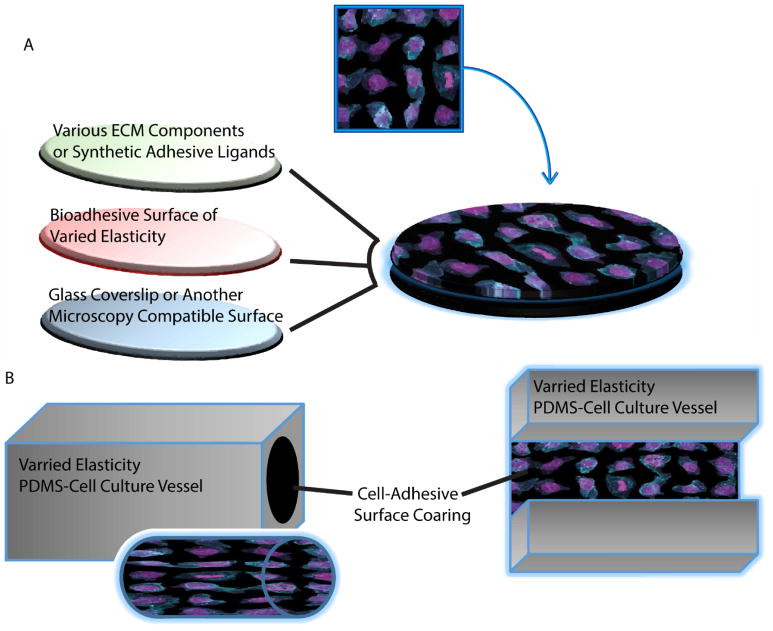
Figure 2. Methods to incorporate control of ECM stiffness into culture models of polarity.
A. Typical assembly of materials to generate a mechanically tuned 2D surface for cell culture models. Commonly, polyacrylamide gels are mechanically tuned via alterations in polymer and crosslinking density and bonded to γ-aminopropyltriethoxysilane (APTES) functionalized glass coverslips. The resulting gel surface can be functionalized with ECM proteins or bioadhesive ligands via carbodiimide-mediated crosslinking, N-hydroxysuccinimidyl acrylate (NHS-acrylate), N-succinimidyl ester of acrylaminohexanoic acid (N6), Hydrazine, or polydopamine films functioning as an adhesive interface between the polyacrylamide and the desired surface coating 206,207. B. Schematic representation of basic cell culture structures which can cast with elastically defined PDMS and are amenable to incorporate fluid flow induced shear stresses. Lumen mimetic tubes or channels may be then be associated with fluid pumps to control the volume and rate of fluid passing through the tube or channel to generate defined fluid flow across cell monolayers or through 3D-cell-lined-lumens.
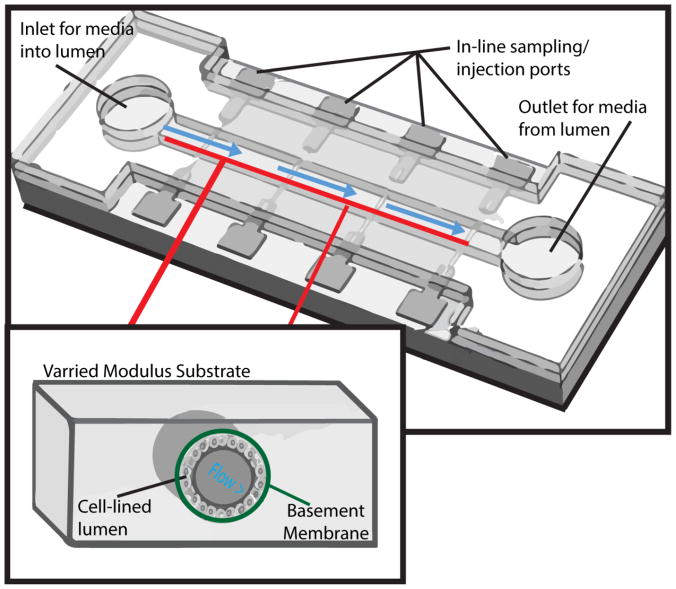
Figure 3. The utility of microfluidic devices.
Presented here is a microfluidic device cast of PDMS of varied stiffness and bonded to a glass surface to create a sealed chamber. The glass surface allows for the device to be monitored with microscopy techniques and the in-line ports allow for additions or removal of substances from the outer surface of the lumen. This device has media inlets and outlets at the terminal ends of the channel so that controlled fluid flow of culture media may be added and modulated to vary fluid flow shear stresses. The inner channel would be lined with a cell monolayer mediated via laminin or collagen coating to foster appropriate polarization. Importantly, these devices may be cast in multiple pieces or multiple devices could be interconnected via flexible hoses 208.
Box/figure defining basic concepts.
Mechanical forces are defined as physical forces which deform or accelerate matter in an opposing axis to the origin of the force. These physical forces are measured in Newtons (N) (SI system) and dynes (CGS system). When physical forces are defined across an area of measure, Pascals (Pa) are the unit of measure, which is defined newton per square meter (N/m2).
Mechanical forces pertinent to biology: tensile, compression, and shear.
When tensile and compressive force is applied to an object the resulting deformation will either increase or decrease the parallel or perpendicular axis of the object. The amount of deformation is defined by the physical characteristics of the object acted upon by force.
Shear stress occurs when forces act tangentially across a resisting object.
Viscoelasticity is the multifaceted properties of a materials or biological structures exhibiting viscous and elastic mechanical properties.
Elasticity (stiffness) is the ability of an object to resist deformation in response to a given force (~ solid phase).
Viscosity is measure of internal friction within a physical system (~ liquid phase).
Highlights of this review.
Cell and tissue polarity depend upon cell adhesion
Polarity instructing adhesions are affected by mechanical forces
Mechanical cues within the microenvironment can disrupt polarity6
Descriptions of model systems to study mechanical forces and polarity
Acknowledgments
We apologize to colleagues whose work could not be cited owing to space limitations. This work was supported by DOD grant BCRP BC122990, and NIH R01 grants CA222508-01, CA192914, CA174929, CA08592, U01 grant CA202241, U54 grant CA163155, and R33 grant CA183685 to V.M.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature reviews. Molecular cell biology. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annual review of genetics. 2008;42:517–540. doi: 10.1101/cshperspect.a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 5.Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 6.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nature reviews. Molecular cell biology. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nature reviews. Molecular cell biology. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 9.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken, NJ) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Developmental biology. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nature reviews. Molecular cell biology. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO reports. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai RA, Gao L, Raghavan S, Liu WF, Chen CS. Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci. 2009;122:905–911. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Molecular biology of the cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays : news and reviews in molecular, cellular and developmental biology. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007;178:323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter CG, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 18.Luxenburg C, Geiger B. Multiscale View of Cytoskeletal Mechanoregulation of Cell and Tissue Polarity. Handbook of experimental pharmacology. 2017;235:263–284. doi: 10.1007/164_2016_34. [DOI] [PubMed] [Google Scholar]
- 19.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. Journal of mammary gland biology and neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2130–2136. doi: 10.1161/atvbaha.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller HB, et al. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nature cell biology. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 22.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell motility and the cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 24.Dalous J, et al. Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation. Biophysical journal. 2008;94:1063–1074. doi: 10.1529/biophysj.107.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature reviews. Molecular cell biology. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nature reviews. Molecular cell biology. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsum IA, Martin C, Humbert PO. Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation. J Cell Sci. 2013;126:3990–3999. doi: 10.1242/jcs.129387. [DOI] [PubMed] [Google Scholar]
- 28.Zhan L, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaffrey LM, Montalbano J, Mihai C, Macara IG. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer cell. 2012;22:601–614. doi: 10.1016/j.ccr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feigin ME, et al. Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer research. 2014;74:3180–3194. doi: 10.1158/0008-5472.can-13-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehgal P, Kong X, Wu J, Sunyer R. Epidermal growth factor receptor and integrins control force-dependent vinculin recruitment to E-Cadherin junctions. 2018 doi: 10.1242/jcs.206656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andresen Eguiluz RC, Kaylan KB, Underhill GH, Leckband DE. Substrate stiffness and VE-cadherin mechano-transduction coordinate to regulate endothelial monolayer integrity. J Cell Sci. 2017;140:45–57. doi: 10.1242/jcs.206656. doi: 10.1242/jcs.206656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murrell M, Oakes PW, Lenz M, Gardel ML. Forcing cells into shape: the mechanics of actomyosin contractility. Nature reviews. Molecular cell biology. 2015;16:486–498. doi: 10.1016/j.biomaterials.2017.06.010. doi: 10.1016/j.biomaterials.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends in cell biology. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Dow LE, et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 37.Audebert S, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Current biology : CB. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 38.Pangon L, et al. The PDZ-binding motif of MCC is phosphorylated at position -1 and controls lamellipodia formation in colon epithelial cells. Biochim Biophys Acta. 2012;1823:1058–1067. doi: 10.1016/j.bbamcr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Cassereau L, Miroshnikova YA, Ou G, Lakins J, Weaver VM. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. Journal of biotechnology. 2015;193:66–69. doi: 10.1016/j.jbiotec.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbell JA. Biomaterials in tissue engineering. Bio/technology (Nature Publishing Company) 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 41.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nature materials. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 42.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A. 2012;109:10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill E. Scientific reports. doi: 10.1038/s41598-017-09686-0. [DOI] [Google Scholar]
- 44.Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nature reviews. Molecular cell biology. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and bioengineering. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta biomaterialia. 2013;9:4635–4644. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends in cell biology. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nature cell biology. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 50.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harbor perspectives in biology. 2011:3. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harbor perspectives in biology. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS computational biology. 2009;5:e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- 54.Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- 55.Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107(Pt 3):561–576. [PubMed] [Google Scholar]
- 56.Taddei I, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nature cell biology. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nature cell biology. 2013;15:17–27. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annual review of cell and developmental biology. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 59.Mekhdjian AH, et al. Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix. Molecular biology of the cell. 2017;28:1467–1488. doi: 10.1091/mbc.E16-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 61.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annual review of biomedical engineering. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 63.Nasrollahi S, et al. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials. 2017;146:146–155. doi: 10.1016/j.biomaterials.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei SC, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nature cell biology. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark RA. Fibrin and wound healing. Annals of the New York Academy of Sciences. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 66.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 67.Hadjipanayi E, Mudera V, Brown RA. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell motility and the cytoskeleton. 2009;66:121–128. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- 68.Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A. 2005;102:9133–9137. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophysical journal. 1999;77:2813–2826. doi: 10.1016/s0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jha AK, et al. Matrix metalloproteinase-13 mediated degradation of hyaluronic acid-based matrices orchestrates stem cell engraftment through vascular integration. Biomaterials. 2016;89:136–147. doi: 10.1016/j.biomaterials.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ehrbar M, et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophysical journal. 2011;100:284–293. doi: 10.1016/j.bpj.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HJ, et al. Fluid shear stress activates YAP1 to promote cancer cell motility. 2017;8:14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barriga EH, Franze K, Charras G, Mayor R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature. 2018;554:523–527. doi: 10.1038/nature25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophysical journal. 2000;79:144–152. doi: 10.1016/s0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 77.Przybyla L, Lakins JN, Weaver VM. Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation. Cell stem cell. 2016;19:462–475. doi: 10.1016/j.stem.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldstein B, Takeshita H, Mizumoto K, Sawa H. Wnt signals can function as positional cues in establishing cell polarity. Developmental cell. 2006;10:391–396. doi: 10.1016/j.devcel.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apodaca G. Modulation of membrane traffic by mechanical stimuli. American journal of physiology. Renal physiology. 2002;282:F179–190. doi: 10.1152/ajprenal.2002.282.2.F179. [DOI] [PubMed] [Google Scholar]
- 80.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:3589–3599. doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 81.Williams BB, et al. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J Cell Sci. 2012;125:2141–2147. doi: 10.1242/jcs.097964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer research. 2001;61:577–581. [PubMed] [Google Scholar]
- 83.Weaver SA, et al. Basal localization of MT1-MMP is essential for epithelial cell morphogenesis in 3D collagen matrix. J Cell Sci. 2014;127:1203–1213. doi: 10.1242/jcs.135236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finney AC, Stokes KY, Pattillo CB, Orr AW. Integrin signaling in atherosclerosis. 2017;74:2263–2282. doi: 10.1007/s00018-017-2490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 86.Dutta D, Heo I, Clevers H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends in molecular medicine. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nature cell biology. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 88.Baker LA, Tiriac H, Clevers H, Tuveson DA. Modeling pancreatic cancer with organoids. Trends in cancer. 2016;2:176–190. doi: 10.1016/j.trecan.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Cellular and molecular life sciences : CMLS. 2016;539:560–564. doi: 10.1007/s00018-017-2490-4. doi: 10.1007/s00018-017-2490-4. [DOI] [PubMed] [Google Scholar]
- 90.Thery M, et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Nature communications. 2006;103:19771–19776. doi: 10.1038/ncomms14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubashkin MG, Ou G, Weaver VM. Deconstructing signaling in three dimensions. Biochemistry. 2014;53:2078–2090. doi: 10.1021/bi401710d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 93.Huh D, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1038/nature20168. doi: 10.1038/nature20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harbor perspectives in biology. 2009;1:a002964. doi: 10.1242/dev.054080. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–179. doi: 10.1146/annurev.genet.42.110807.091432. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aw WY, Heck BW, Joyce B, Devenport D. Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Current biology : CB. 2016;26:2090–2100. doi: 10.1083/jcb.201408039. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1038/ncb2284. [DOI] [PubMed] [Google Scholar]
- 99.Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1242/jcs.028183. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 102.Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annual review of cell and developmental biology. 2014;30:291–315. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- 103.Mertz AF, et al. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc Natl Acad Sci U S A. 2013;110:842–847. doi: 10.1073/pnas.1217279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore KA, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 105.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature reviews. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nelson WJ, Weis WI. 25 Years of Tension over Actin Binding to the Cadherin Cell Adhesion Complex: The Devil is in the Details. Trends in cell biology. 2016;26:471–473. doi: 10.1126/science.aaa4559. doi: 10.1126/science.aaa4559. [DOI] [PubMed] [Google Scholar]
- 107.Ratheesh A, et al. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nature cell biology. 2012;14:818–828. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borghi N, et al. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luxenburg C, et al. Wdr1-mediated cell shape dynamics and cortical tension are essential for epidermal planar cell polarity. Nature cell biology. 2015;17:592–604. doi: 10.1038/ncb3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buckley CD, et al. Cell adhesion The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nature reviews. Molecular cell biology. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nature cell biology. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 113.Bieling P, et al. Force Feedback Controls Motor Activity and Mechanical Properties of Self-Assembling Branched Actin Networks. Cell. 2016;164:115–127. doi: 10.1016/j.cell.2015.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell host & microbe. 2013;14:242–255. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barrila J, et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nature reviews. Microbiology. 2010;8:791–801. doi: 10.1038/nrmicro2423. [DOI] [PubMed] [Google Scholar]
- 117.Mouw JK, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Samuel MS, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laklai H, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harbor perspectives in biology. 2011;3:a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. The American journal of pathology. 2008;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huck L, Pontier SM, Zuo DM, Muller WJ. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci U S A. 2010;107:15559–15564. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lahlou H, Muller WJ. beta1-integrins signaling and mammary tumor progression in transgenic mouse models: implications for human breast cancer. Breast cancer research : BCR. 2011;13:229. doi: 10.1186/bcr2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang H, Hegde S. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vennin C, Rath N, Pajic M. Targeting ROCK activity to disrupt and prime pancreatic cancer for chemotherapy. 2017:1–8. doi: 10.1080/21541248.2017.1345712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays : news and reviews in molecular, cellular and developmental biology. 2003;25:542–553. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- 128.Muthuswamy SK, Xue B. Cell polarity as a regulator of cancer cell behavior plasticity. Annual review of cell and developmental biology. 2012;28:599–625. doi: 10.1146/annurev-cellbio-092910-154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu W, et al. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO reports. 2008;9:923–929. doi: 10.1038/embor.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- 131.Zovein AC, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Developmental cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rasmussen JP, Reddy SS, Priess JR. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 2012;139:2050–2060. doi: 10.1242/dev.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miroshnikova YA, et al. alpha5beta1-Integrin promotes tension-dependent mammary epithelial cell invasion by engaging the fibronectin synergy site. Molecular biology of the cell. 2017;28:2958–2977. doi: 10.1091/mbc.E17-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Northey JJ, Przybyla L, Weaver VM. Tissue Force Programs Cell Fate and Tumor Aggression. Cancer discovery. 2017;7:1224–1237. doi: 10.1158/2159-8290.cd-16-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Northcott JM, Dean IS, Mouw JK, Weaver VM. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Frontiers in cell and developmental biology. 2018;6:17. doi: 10.3389/fcell.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang F, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iwamoto Y, et al. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 144.Weaver VM, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Simian M, Bissell MJ. Organoids: A historical perspective of thinking in three dimensions. 2017;216:31–40. doi: 10.1083/jcb.201610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin-Belmonte F, et al. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Current biology : CB. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends in cell biology. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 148.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 149.Drost J, Karthaus WR, Gao D. Organoid culture systems for prostate epithelial and cancer tissue. 2016;11:347–358. doi: 10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 151.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Streuli CH, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O'Brien LE, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nature cell biology. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 154.O'Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nature reviews. Molecular cell biology. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 155.Schnaper HW, et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. Journal of cellular physiology. 1993;156:235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- 156.Stevenson MD, et al. A self-assembling peptide matrix used to control stiffness and binding site density supports the formation of microvascular networks in three dimensions. Acta biomaterialia. 2013;9:7651–7661. doi: 10.1016/j.actbio.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 158.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast cancer research : BCR. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jha AK, et al. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tharp KM, Stahl A. Bioengineering Beige Adipose Tissue Therapeutics. Frontiers in endocrinology. 2015;6:164. doi: 10.3389/fendo.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 163.Griffith LG. Emerging design principles in biomaterials and scaffolds for tissue engineering. Annals of the New York Academy of Sciences. 2002;961:83–95. doi: 10.1111/j.1749-6632.2002.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 164.Rice JJ, et al. Engineering the regenerative microenvironment with biomaterials. Advanced healthcare materials. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- 165.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nature communications. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 166.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kremer M, et al. Pore-Size Distributions of Cationic Polyacrylamide Hydrogels Varying in Initial Monomer Concentration and Cross-Linker/Monomer Ratio. Macromolecules. 1994;27:2965–2973. doi: 10.1021/ma00089a012. [DOI] [Google Scholar]
- 168.Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 169.Ou G, et al. Visualizing mechanical modulation of nanoscale organization of cell-matrix adhesions. Integrative biology : quantitative biosciences from nano to macro. 2016;8:795–804. doi: 10.1039/c6ib00031b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annual review of biomedical engineering. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 171.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 172.Moraes C, Labuz JM, Shao Y, Fu J, Takayama S. Supersoft lithography: candy-based fabrication of soft silicone microstructures. Lab on a chip. 2015;15:3760–3765. doi: 10.1039/c5lc00722d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nature protocols. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 174.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. Cardiovascular medicine. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Davies PF. Overview: temporal and spatial relationships in shear stress-mediated endothelial signalling. Journal of vascular research. 1997;34:208–211. doi: 10.1159/000159224. [DOI] [PubMed] [Google Scholar]
- 177.Azuma N, et al. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol. 2001;280:H189–197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- 178.Davies PF. Flow-mediated endothelial mechanotransduction. Physiological reviews. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nature reviews. Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 180.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annual review of biomedical engineering. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 181.Topper JN, Gimbrone MA., Jr Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Molecular medicine today. 1999;5:40–46. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 182.Sacks MS, Yoganathan AP. Heart valve function: a biomechanical perspective. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2007;362:1369–1391. doi: 10.1098/rstb.2007.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 184.Sabine A, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Developmental cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 185.Bazigou E, Makinen T. Flow control in our vessels: vascular valves make sure there is no way back. Cellular and molecular life sciences : CMLS. 2013;70:1055–1066. doi: 10.1007/s00018-012-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. Journal of biomechanical engineering. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 187.Cecchi E, et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214:249–256. doi: 10.1016/j.atherosclerosis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 188.McCue S, et al. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circulation research. 2006;98:939–946. doi: 10.1161/01.res.0000216595.15868.55. [DOI] [PubMed] [Google Scholar]
- 189.Nagy E, et al. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123:1316–1325. doi: 10.1161/circulationaha.110.966846. [DOI] [PubMed] [Google Scholar]
- 190.Stewart BF, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 191.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab on a chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 192.Kohn JC, et al. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophysical journal. 2015;108:471–478. doi: 10.1016/j.bpj.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sunyer R, et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353:1157–1161. doi: 10.1126/science.aaf7119. [DOI] [PubMed] [Google Scholar]
- 194.Chang SF, et al. Tumor cell cycle arrest induced by shear stress: Roles of integrins and Smad. Nature communications. 2008;105:3927–3932. doi: 10.1038/ncomms14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bordeleau F, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc Natl Acad Sci U S A. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiological reviews. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hashizume H, et al. Openings between defective endothelial cells explain tumor vessel leakiness. The American journal of pathology. 2000;156:1363–1380. doi: 10.1016/s0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 199.Hassell BA, et al. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell reports. 2017;21:508–516. doi: 10.1016/j.celrep.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 200.Alimperti S, Mirabella T, Bajaj V, Polacheck W. Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell-endothelial cell-regulated barrier function. 2017;114:8758–8763. doi: 10.1073/pnas.1618333114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pirone DM, et al. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Proc Natl Acad Sci U S A. 2012;12:2165–2174. doi: 10.1073/pnas.1618333114. doi: 10.1073/pnas.1618333114. [DOI] [PubMed] [Google Scholar]
- 202.Shim KY, et al. Microfluidic gut-on-a-chip with three-dimensional villi structure. 2017;19:37. doi: 10.1007/s10544-017-0179-y. [DOI] [PubMed] [Google Scholar]
- 203.Straub TM, et al. In vitro cell culture infectivity assay for human noroviruses. Emerging infectious diseases. 2007;13:396–403. doi: 10.3201/eid1303.060549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - Current knowledge and future perspectives. Journal of Crohn's & colitis. 2008;2:279–290. doi: 10.1016/j.crohns.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 205.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. The Journal of allergy and clinical immunology. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kandow CE, Georges PC, Janmey PA, Beningo KA. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods in cell biology. 2007;83:29–46. doi: 10.1016/s0091-679x(07)83002-0. [DOI] [PubMed] [Google Scholar]
- 207.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Loskill P, Marcus SG, Mathur A, Reese WM, Healy KE. muOrgano: A Lego(R)-Like Plug & Play System for Modular Multi-Organ-Chips. PLoS One. 2015;10:e0139587. doi: 10.1371/journal.pone.0139587. [DOI] [PMC free article] [PubMed] [Google Scholar]