Abstract
The objective of this article is to increase the awareness of gastroenterologists to the effects of cannabinoids on gastrointestinal motility, as gastroenterologists are likely to encounter patients who are taking cannabinoids, or those with dysmotility that may be associated with cannabinoid mechanisms. The non-selective cannabinoid agonist, dronabinol, retards gastric emptying and inhibits colonic tone and phasic pressure activity. In addition to the well-recognized manifestations of cannabinoid hyperemesis, cannabinoid mechanisms result in human and animal models of gastrointestinal and colonic dysmotility. Decreased enteric FAAH activity is associated with colonic inertia in slow transit constipation and, conversely, the orphan G-protein coupled receptor, GPR55, is overexpressed in streptozotocin-induced gastroparesis, suggesting it is involved in inhibition of antral motility. Experimental therapies in gastrointestinal motility and functional disorders are focused predominantly on pain relief mediated through cannabinoid 2 receptors or inhibition of DAGLα to normalize colonic transit. In summary, cannabinoid mechanisms and pharmacology are relevant to the current and future practice of clinical gastroenterology.
Keywords: anandamide, 2-AG, FAAH, DAGL
Graphical Abstract
Cannabinoid mechanisms result in human and animal models of gastrointestinal and colonic dysmotility.
Experimental therapies in gastrointestinal motility and functional disorders are focused predominantly on pain relief mediated through cannabinoid 2 receptors or inhibition of DAGLα to normalize colonic transit.
Cannabinoid mechanisms and pharmacology are relevant to the current and future practice of clinical gastroenterology.
Introduction to Cannabinoids
There are natural, endogenous and synthetic cannabinoids. The marijuana plant Cannabis sativa has been cultivated by humans for medicinal and other purposes for millennia, and it has also been a source of controversy throughout history. The main active ingredient in Cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), activates two Gi-coupled membrane receptors, CB1 and CB2 receptors. CB1 receptors are located throughout the gastrointestinal tract, predominantly in myenteric and submucosal neurons, as well as non-neuronal cells such as epithelial cells (reviewed in ref 1). Conversely, CB2 receptors are mainly located on inflammatory and epithelial cells, and to a lesser extent on myenteric and submucosal neurons.2,3 Cannabinoid ligands activate cannabinoid receptors; endogenous ligands identified in mammalian tissues include the endocannabinoids anandamide and 2-arachydonylglycerol (2-AG)].4 Endocannabinoids are biosynthesized ‘on demand’ from membrane phospholipids. Immediately after their production, they are released from cells, activate their target receptors activation to induce a biological response, and are then inactivated through a reuptake process facilitated by a putative endocannabinoid membrane transporter (EMT). Reuptake is followed by enzymatic degradation catalysed by the fatty acid amide hydrolase (FAAH, in the case of anandamide) or by monoacylglycerol lipase (MGL, and possibly FAAH, in the case of 2-AG).5–7 In addition to effects on cannabinoid receptors, the endocannabinoid anandamide may also activate the transient receptor potential (TRP) vanilloid type 1 (TRPV1), which is mainly expressed by primary afferent neurons and the orphan G-protein-coupled cannabinoid receptor GPR55.8
A common scenario encountered in clinical practice illustrates the frequent encounter with the effects of natural cannabinoid, used for recreational or “medical” purposes, that presents the potential negative effect on gut motility.
Case Study
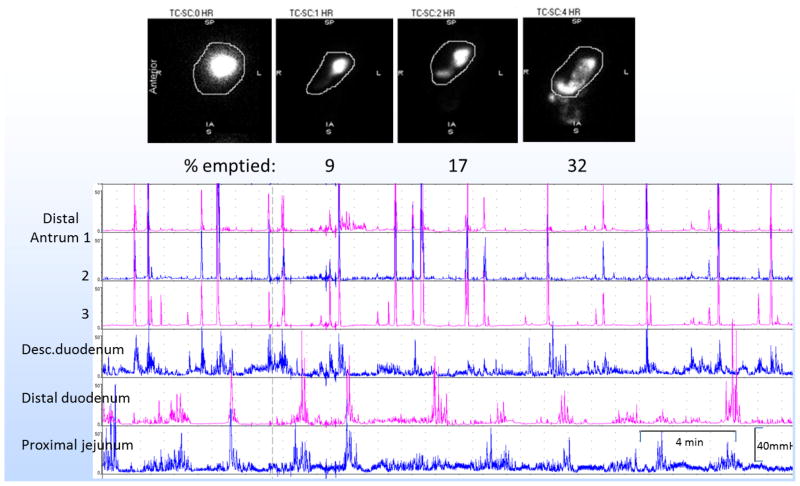
A 51 year-old female with prior history of cholecystectomy, hysterectomy, migraine and bronchial asthma presented with acute worsening of nausea, vomiting, and abdominal pain. She denied using showers to relieve her symptoms. She underwent removal of a residual 1cm common bile duct stone (which unfortunately did not relieve her symptoms). Upper gastrointestinal endoscopy, ultrasound, CT and MR of the abdomen were otherwise unremarkable. She was also treated for constipation and abdominal wall tenderness associated with a trigger point. She acknowledged prior use of marijuana. Gastric emptying of a 300kcal, 30% fat, solid-liquid meal was markedly delayed. On the other hand, gastroduodenal manometry showed essentially normal amplitude antral phasic pressure, reduced antral frequency, no evidence of pylorospasm and well-coordinated small bowel phasic pressure activity (Figure 1).
Figure 1.
Case study showing marked retardation of gastric emptying with unremarkable antroduodenal manometry.
Objectives
The current article demonstrates the role of cannabinoids in causing gastrointestinal dysmotility and summarizes the following themes focusing on the literature in humans: the biosynthesis of endocannabinoids, CB receptors, basic and clinical pharmacology of CB including effects of dronabinol (a nonselective CB receptor agonist) on human gastrointestinal and colonic motility, the association of genetic variations in FAAH and CBR1 genes with symptom phenotypes, colonic transit and pharmacogenomics in IBS patients, and a brief survey of experimental CB agents and their potential use in functional and motility disorders of the gut.
Current Use of Cannabinoids
There is moderate quality evidence to support the use of cannabinoids (CB) for the treatment of chronic pain and spasticity, and there is low quality evidence suggesting improvements in nausea and vomiting due to chemotherapy, weight gain in HIV infection, sleep disorders, and Tourette syndrome.9 There is also some analgesic benefit provided by selective CB in patients with chronic neuropathic pain.10 However, there is increased risk of short-term adverse events, the most frequent being tachycardia, agitation and nausea.11 Nevertheless, CB are increasingly used as medicinal or recreational agents, particularly in states having decriminalized medical and recreational cannabis, where unintentional cannabis ingestion by children,12 in utero effects on fetal neural development associated with cannabis use during pregnancy,13 and cannabinoid hyperemesis syndrome are increasingly recognized.14 Synthetic CB products have effects that are somewhat similar to those of natural cannabis, but are more potent and long lasting than Δ9-tetrahydrocannabinol (THC). Some of these compounds are potent and dangerous, having been linked to psychosis, mania, and suicidal ideation.15
Biosynthesis of Endocannabinoids
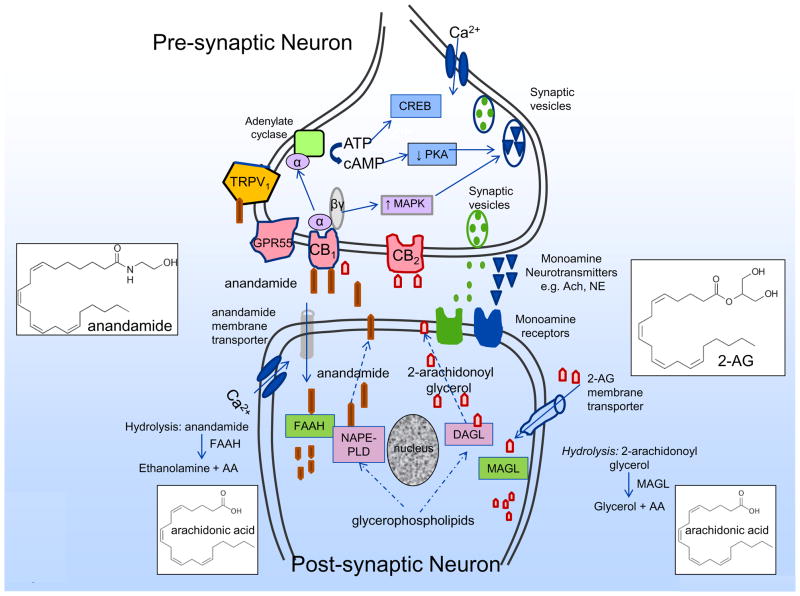
Endocannabinoids are biosynthesized ‘on demand’ from membrane phospholipids and they are released immediately after their production. Endocannabinoids [e.g. anandamide (AEA)] are synthesized in postsynaptic neurons: a synthetic enzyme is fatty acid amide hydrolase FAAH (Figure 2). AEA released into the synaptic cleft functions as a retrograde messenger binding to the presynaptic CB1 receptor through various effectors, reduced activity of protein kinases, modulation of ion channels and neurotransmitter release, e.g. acetylcholine.
Figure 2.
Synthesis, action and hydrolysis of endocannabinoids. Endocannabinoids (e.g. anandamide, 2-arachidonoylglycerol) are synthesized in postsynaptic neurons by synthetic enzymes such as NAPE-phospholipase D (NAPE-PLD) and diacyl glycerol lipase-α (DAGLα) respoectively. Endocannabinoids released into the synaptic cleft function as retrograde messengers binding to the presynaptic receptors including CB1, CB2, GPR55 and TRPV1, and through various effectors, reduce activity of protein kinases (e.g. PKA), increase activity of MAP kinases, and thereby modulate channels and monoamine neurotransmitter release e.g. acetyl choline, norepinephrine. Endocannabinoids undergo re-uptake into the post-synaptic neuron by membrane transporters and hydrolysed by enzymes such as fatty acid amide hydrolase (FAAH) and monoacyl glycerol lipase (MAGL).
Following receptor activation and induction of a biological response, endocannabinoids are inactivated (Figure 2) through reuptake by putative the endocannabinoid membrane transporters (EMT), followed by enzymatic degradation by the fatty acid amide hydrolase [(FAAH) in the case of anandamide] or by monoacylglycerol lipase [(MAGL) and, possibly FAAH in the case of 2–arachydonylglycerol (2–AG)].1,5,16 These catalytic enzymes have also been identified in the digestive tract.6,7 Apart from effects on CB receptors, the endocannabinoid, anandamide, may also activate the transient receptor potential (TRP) vanilloid type 1 (TRPV1), which is mainly expressed by primary afferent neurons and the orphan G-protein-coupled receptor GPR55.8 The enzymes involved in biosynthesis and degradation of endocannabinoids in the nervous system are extensively reviewed elsewhere.17
Cannabinoid Receptors and Endocannabinoid Expression in the Gastrointestinal Tract
CB1 receptors are located throughout the gastrointestinal tract, mainly in myenteric and submucosal neurons, but they are also expressed by non-neuronal [e.g. epithelial cells (reviewed in ref. 1)]. CB2 receptors are mainly located on inflammatory and epithelial cells, but they are also expressed in myenteric and submucosal neurons.2,3 Endogenous ligands that activate CB receptors (i.e. the endocannabinoids, anandamide and 2-AG)4,18 have been identified in mammalian tissues.
The major site of action of CB in the digestive tract is the enteric nervous system,19,20 on mammalian and human intestinal cholinergic nerves in the myenteric and submucosal plexuses and on nerve fibers in circular and longitudinal muscles,19,21,22 as well as substance P or vasoactive intestinal peptide neurons, and in non-neuronal cells including crypt epithelial cells and smooth muscle cells.19–22
Expression levels of the endocannabinoid system in intestinal diseases23 and the role of cannabis and CB modulation in digestive disorders are reviewed elsewhere, including effects on esophageal and lower esophageal sphincter function, appetite and inflammatory bowel disease;24 therefore, these topics are not reviewed here. Similarly, the effects of chronic stress on peripheral endocannabinoid pathways in visceral primary afferent neurons and the brain, including mechanisms involved in nausea and vomiting, as well as effects on epigenetic regulation of the gene encoding CB1 are reviewed elsewhere.17
The main properties of CB receptor agonists and antagonists to which the following discussion refers are listed in Table 1.25
Table 1.
Main properties of cannabinoid receptor agonists and antagonists. Reproduced with permission from ref. 25, Curr Gastroenterol Rep 2015;17:429.
| AGONISTS | |
|---|---|
| Plant Derived | |
| Δ9-THC | Main psychoactive cannabinoid in the marijuana plant |
| Δ8-THC | Slightly less potent than Δ9-THC |
| 11-OH-Δ9-THC | Bioactive compound formed when the body breaks down Δ9-THC |
| Animal Derived | |
| Anandamide | 2-AG |
| THC Analogs | |
| Dronabinol | Nabilone, CP-55,940, HU-210, levonantradol |
| Different Chemical Structure | |
| WIN-55,212 | Binds to both cannabinoid receptors |
| ANTAGONISTS | |
| SR-241716A | Synthetic CB1 antagonist |
| SR-144528 | Synthetic CB2 antagonist |
| AM841 | CB1 mega-agonist with negligible central effects at doses that potently reduce GI motor function |
| WIN-55,212-2 | A weaker CB1R/CB2R non-selective cannabinoid agonist |
Basic Pharmacology Demonstrating Effects of Cannabinoid Modulation on Gut Motility
The profound effects of CB on gut motility were demonstrated in vitro in studies involving human and animal tissues.
CB receptors significantly impact intestinal motility in rats, as shown through systematic studies using pharmacological agonists (WIN-55,212-2 and CP-55,940) and antagonists of the CB1 (SR141716A) and CB2 receptors (SR144528).26
In colonic longitudinal muscle strips from patients with diverticular disease, electrical field stimulation-induced, neutrally-medicated contractions were inhibited by the CB agonist, (+)WIN55212-2. Conversely, the cannabinoid antagonist, SR141716, potentiated EFS-induced twitch contractions of longitudinal colonic muscle strips from patients with diverticular disease and inhibited the relaxation of control muscle strips induced by the CB agonist, (+)WIN 55,212-2.27
Within the myenteric plexus, CB1 receptor agonists inhibit contractile activity and peristalsis in the intestine via inhibition of excitatory acetylcholine in the pre-synaptic neuron.28 CB1 receptors within the myenteric plexus modulate intestinal propulsion by attenuation of intestinal motor responses of the peristaltic reflex in mice. In addition, there was increased gastrointestinal transit measured by charcoal in CB1−/− knockout mice as compared to their wild-type littermates.29
A CB1 mega-agonist, AM841, has negligible central effects at doses that dose dependently reduce transit in rat stomach in vivo, small intestine, cecum and colorectum. In addition, effects on compliance or tone could be inferred from morphometric analysis of stomach and cecum size by radiographic imaging.30
Pharmacodynamic Effects of Cannabinoids on Human Gastrointestinal and Colonic Motility
In human studies, most of the observations on CB effects have emanated from studies of the approved pharmacological agent, dronabinol, a synthetic delta-9-tetrahydrocannabinol (Δ9-THC). This is a non-selective CB agonist that is 90–95% absorbed after a single oral dose, undergoes extensive first pass hepatic metabolism, and has high lipid solubility so that 10–20% of the administered oral dose reaches the systemic circulation. Its onset of action occurs at approximately 0.5–1 hour, and its peak effect occurs at 2–4 hours. Elimination follows a two-compartment model with initial half-life of ~4 hours and terminal half-life of 25–36 hours. It undergoes hepatic metabolism, primarily by microsomal hydroxylation, yielding both active and inactive metabolites, and biliary excretion is the major route of elimination.
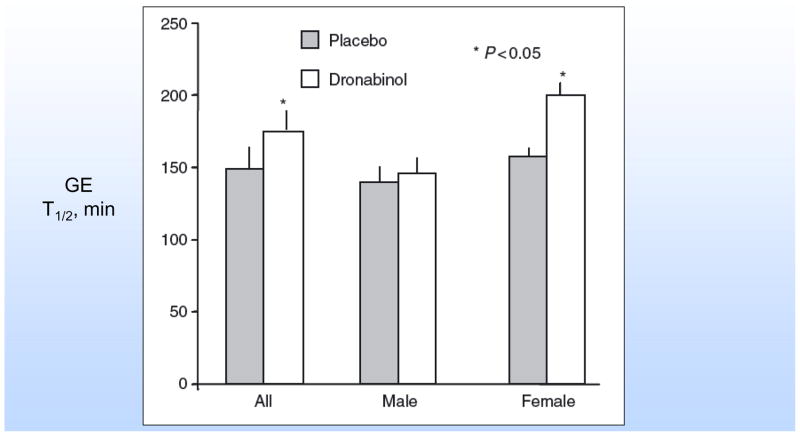
Effects on human gastric functions - Dronabinol delays gastric emptying of solids,31 and this effect was seen predominantly in females (Figure 3). There were no significant effects of dronabinol on postprandial gastric volumes (accommodation), satiation [maximum tolerated volume (MTV)], and postprandial symptoms during a nutrient drink test, but there were significantly more central effects (lightheadedness and drowsiness) and nausea on dronabinol compared to placebo. Fasting gastric volume was greater in males receiving dronabinol compared with placebo. The precise reason for the gender-related observations is unclear, and it may simply reflect dosing (since standard dose rather that weight-based dose was used). Overall, the inhibitory effects on gastric motor function in humans are consistent with the observations in animal studies.30
Effects on colonic transit were not statistically significant at 24 hours;31 however, there appeared to be delays in colonic transit in males at 24 hours; colonic transit at 48 hours was not tested. In patients with colonic inertia associated with slow transit constipation (STC), there was decreased enteric FAAH activity, suggesting that, with reduced enzymatic degradation of endogenous endocannabinoids by the decreased FAAH activity, there is higher expression of anandamide, 2-AG, and palmitoylethanolamide, and numerically higher CB1 receptor expression in myenteric nerve fibers (despite reduced ganglion cells) in patients with STC.32
Effects on colonic compliance, tone and phasic pressure activity were measured in the human descending or sigmoid colon by intraluminal barostat-manometry.33 Females treated with dronabinol demonstrated increased colonic compliance. Overall, both genders were shown to have profound inhibition of colonic postprandial tone and significant inhibition of fasting and postprandial phasic pressure activity (Figure 4).
Figure 3.
Effects of dronabinol on gastric emptying in healthy humans. Reproduced with permission from ref. 31, Neurogastroenterol Motil 2006;18:831–8.
Figure 4.
Effects of dronabinol on colonic tone (A) and phasic pressure activity (B) in healthy humans. Reproduced with permission from ref. 33, Am J Physiol 2007;293:137–45.
The relevance of CB receptor agonists to colonic motility is also illustrated by the experimental data from animal studies that demonstrated cannabinoid inhibition of colonic contractility, as detailed above.26–29 These observations in human and animal studies also lead to the suggestion that inhibiting endocannabinoid biosynthesis provides a novel approach to the treatment of constipation. Thus, the enzyme diacylglycerol lipase-α (DAGLα), which is involved in biosynthesis of the endocannabinoid 2–AG, is co-localized with the vesicular acetylcholine transporter in myenteric cholinergic nerves. Inhibition of DAGLα (e.g. with OMDM-188) reversed both scopolamine-reduced intestinal contractility and loperamide-prolonged whole gut transit in normal mice and normalized fecal output in C3H/HeJ mice, a genetically constipated strain.34
Pharmacogenomics Studies Support Modulation of Colonic Motility by Cannabinoids
Pharmacogenomics studies also support a potential role of CB mechanisms on colonic motility. Dronabinol showed treatment-by-genotype effects as well as IBS sub-phenotype by genotype interactions. One example is the association of CNR1 rs806378 in different IBS sub–phenotypes with dronabinol treatment. Thus, genotype CC in IBS-diarrhea/alternators was associated (borderline significant) with colonic compliance and tone and proximal left colon phasic pressure activity (motility index). Similarly, the IBS-constipation subgroup demonstrated CC genotype interaction with treatment on fasting and postprandial colon tone.35,36 A second example is the association of FAAH rs324420 CC genotype with reduced postprandial tone response during treatment with dronabinol, whereas the CA/AA genotype was associated with a borderline increase in postprandial tone. Differential treatment effects on left colon motility indices in the CC compared to the CA/AA genotypes were also observed.35
Abnormal Cannabinoid Mechanisms in Gastrointestinal Dysmotility
Cannabinoids are recognized as etiologic factors in adult patients presenting with cyclic vomiting syndrome (CVS) and cannabinoid hyperemesis. CVS is quite prevalent (10.8%) among patients presenting to outpatient gastroenterology clinics with intermittent episodes of nausea and vomiting. Identified associations of CVS include younger age, tobacco smoking, psychiatric comorbidity, and symptoms compatible with other FGIDs.37 Cannabinoid hyperemesis presents either during use or withdrawal from cannabis and is associated with a stereotypical craving for showers to relieve symptoms.38 In addition to the role of CB in cyclic vomiting syndrome and CB hyperemesis, there are two commonly encountered gastrointestinal dysmotilities in human or animal models that have been associated with altered CB expression or mechanisms. Decreased enteric FAAH activity is associated with colonic inertia in STC; conversely, CB1 expression appears to be non-significantly increased.32 The orphan G-protein coupled receptor, GPR55, is a novel receptor that is a target of anandamide and cannabidiol and is involved in regulation of rodent intestinal motility.39 It was shown that there was increased expression of GPR55 in a streptozotocin mouse model of gastroparesis, suggesting that CB mechanisms may result in inhibition of gastric motility.40
Association of Genetic Variations in FAAH and CBR1 with Phenotype and Colonic Transit
Further evidence supporting a role of CB mechanisms in gut dysmotility is provided by associations between genetic variations in CB pathways or receptors and gastrointestinal phenotypes, including intermediate phenotypes of motility and transit.
FAAH genotype is associated with symptom phenotypes and colonic transit in IBS-diarrhea.41
CNR1 rs806378 is associated with IBS-diarrhea,42 the CC genotype with symptoms, and, paradoxically, the TT genotype with faster colonic transit, given that the TT (not the CC) group had fastest colonic transit at 24 and 48 hours.
There are different allelic frequencies of AAT repeats in the CNR1 gene in healthy controls and IBS patients in Korea.43 In studies of colonic transit, there was a CNR1 (AAT)n gene by subgroup interaction, but the significant association was in healthy controls, not in IBS.42
Increased risk of cyclic vomiting syndrome (CVS) was observed among individuals with AG and GG genotypes of CNR1 rs806380 (intron) and decreased risk of CVS with CC genotype of CNR1 rs806368 (3′-UTR, exon).44
Current Therapeutic Applications of Cannabinoid Medication
Dronabinol is the only approved cannabinoid medication. Reviewing the information and published data on the role of cannabinoids, CB1 receptors and interactions with sensory neurotransmitters (e.g. substance P, CGRP and TRPV1), Sharkey and Wiley proposed that peripherally restricted CB and modulators of endocannabinoid synthesis and/or degradation might be effective for short-term treatment of symptoms in functional bowel disorders.17 Cannabinoids are commonly used in relief of chemotherapy-induced nausea and vomiting (CINV), although no good quality evidence to recommend the use of cannabinoids for CINV was identified in a systematic analysis of the literature.45 Given the inhibition of gastric emptying shown with dronabinol, especially among female participants, the use of dronabinol for nausea due to gastroparesis is unlikely to be efficacious.
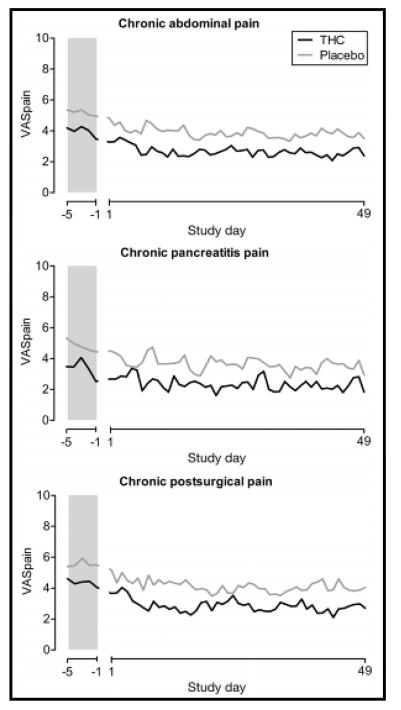
Recent studies have assessed the potential of CB in pain syndromes. In a study of 65 patients with chronic abdominal pain for at least 3 months after surgery or pain from chronic pancreatitis, THC administered 3 times daily did not reduce pain measures compared to placebo, although it appeared that THC was safe and well tolerated over ~7 weeks’ treatment46 (Figure 5). Similarly, THC did not reduce post-operative pain compared to placebo.47
Figure 5.
Randomized, controlled trial of THC and placebo in three forms of chronic pain showing mean VAS pain scores at baseline and during study treatment showing no significant effect of treatment with THC. Reproduced with permission from ref. 46, Clin Gastroenterol Hepatol 2017;15:1079–86.
Potential Therapeutic Effects with Novel or Experimental Cannabinoid Agents
The best characterized CB receptors, CB1 and CB2, are G-protein-coupled receptors with similar amino acid sequences. In addition to expression in the immune system, CB2 receptors are widely expressed in brain, peripheral nervous system, and gastrointestinal tract. Selective CB2 receptor agonists may be candidates for treating pain and other (e.g. inflammatory) disease states, as proposed from preclinical data.
LY3038404 HCl, which possesses analgesic properties without effects on higher brain function, attenuated pain in a rat model of pancreatitis.48 Orally administered PF-03550096 (3 and 10mg/kg) inhibited the 2,4,6-trinitrobenzene sulfonic acid-induced decrease in colonic pain threshold in a rat model of visceral hypersensitivity.49
APD371, an oral peripherally restricted, highly selective CB2 receptor agonist, is being tested for efficacy in relief of pain in patients with Crohn’s disease,50 although no results are yet available.
Finally, the DAGL inhibitors, orlistat and OMDM-188, accelerated colonic transit in mice, proving the concept that inhibition of synthesis of endocannabinoids may potentially provide a novel approach to relief of constipation.34
Case Resolution
The management of this patient included: first, reassurance that the delayed gastric emptying was likely the result of the inhibition of antral motility by cannabis; second, cessation of cannabis as a “treatment” for her symptoms; third, dietary recommendations (small particle or homogenized solids), a prokinetic medication (e.g. liquid formula or sublingual metoclopramide in 3–4 divided doses per day and a maximum total daily dose of 40mg), and antiemetics for symptom relief as needed (e.g. diphenhydramine, 12.5mg up to twice daily, or ondansetron, 4–8mg up to three times per day); and fourth, the patient was offered referral to a mental health provider to address potential consequences of addiction.
Conclusion
Cannabinoid mechanisms are involved in control of gastrointestinal and colonic motility. Studies to date have most clearly shown interaction through inhibition of intrinsic cholinergic mechanisms that result in inhibition of motility. These associations are manifested in humans by the use of CB for gastrointestinal symptoms, demonstrated pharmacological effects of agonists particularly on colonic tone and phasic pressure activity, as well as genotype associations with lower functional gastrointestinal phenotypes. As gastroenterologists, we will encounter these effects when patients present with symptoms of functional gastrointestinal disorders including the widely recognized cyclic vomiting syndrome and CB hyperemesis, but we also should be aware of less overt manifestations such as chronic constipation or symptoms associated with delayed gastric emptying. The observed effects of cannabinoids on human gastrointestinal and colonic motility are generally consistent with animal studies; as yet, there is no explanation for the greater retardation of dronabinol on gastric emptying observed in females.
With the development of novel, more specific agonists and antagonists, it is also possible that CB agents may be part of the pharmacological armamentarium to relieve symptoms, independent of possible effects on sensations such as nausea, anorexia or pain.
Acknowledgments
The author thanks Mrs. Cindy Stanislav for excellent secretarial support.
Funding: Dr. Camilleri is supported by NIH grants R01-DK67071 and R01-DK115950.
Disclosures: none
Author’s contributions: Michael Camilleri conceived, developed and wrote the entire manuscript
References
- 1.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- 2.Duncan M, Mouihate A, Mackie K, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander SP, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 2007;152:602–623. doi: 10.1038/sj.bjp.0707456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacher P, Bátkai S, Kunos G. The endocannabinoid systemas an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capasso R, Matias I, Lutz B, et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Duncan M, Thomas AD, Cluny NL, et al. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1255–G1265. doi: 10.1152/ajpgi.90500.2008. [DOI] [PubMed] [Google Scholar]
- 8.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 10.Meng H, Johnston B, Englesakis M, et al. Selective cannabinoids for chronic neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2017;125:1638–1652. doi: 10.1213/ANE.0000000000002110. [DOI] [PubMed] [Google Scholar]
- 11.Tait RJ, Caldicott D, Mountain D, et al. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila) 2016;54:1–13. doi: 10.3109/15563650.2015.1110590. [DOI] [PubMed] [Google Scholar]
- 12.Richards JR, Smith NE, Moulin AK. Unintentional cannabis ingestion in children: a systematic review. J Pediatr. 2017;190:142–152. doi: 10.1016/j.jpeds.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Grant KS, Petroff R, Isoherranen N, et al. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther. 2017 Aug 25; doi: 10.1016/j.pharmthera.2017.08.014. pii: S0163-7258(17)30224-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorensen CJ, DeSanto K, Borgelt L, et al. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment: a systematic review. J Med Toxicol. 2017;13:71–87. doi: 10.1007/s13181-016-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein AM, Rosca P, Fattore L, et al. Synthetic cathinone and cannabinoid designer drugs pose a major risk for public health. Front Psychiatr. 2017;8:156. doi: 10.3389/fpsyt.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology. 2016;151:252–266. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan M, Davison JS, Sharkey KA. Endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- 20.Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids. An update. Curr Opin Pharmacol. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB1) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol. 2006;148:191–199. doi: 10.1038/sj.bjp.0706710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y, Jo J, Chung HY, et al. Endocannabinoids in the gastrointestinal tract. Am J Physiol. 2016;311:G655–G666. doi: 10.1152/ajpgi.00294.2015. [DOI] [PubMed] [Google Scholar]
- 24.Goyal H, Singla U, Gupta U, et al. Role of cannabis in digestive disorders. Eur J Gastroenterol Hepatol. 2017;29:135–143. doi: 10.1097/MEG.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 25.Malik Z, Baik D, Schey R. The role of cannabinoids in regulation of nausea and vomiting, and visceral pain. Curr Gastroenterol Rep. 2015;17:429. doi: 10.1007/s11894-015-0429-1. [DOI] [PubMed] [Google Scholar]
- 26.Izzo AA, Mascolo N, Pinto L, et al. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- 27.Guagnini F, Valenti M, Mukenge S, et al. Neural contractions in colonic strips from patients with diverticular disease: role of endocannabinoids and substance P. Gut. 2006;55:946–953. doi: 10.1136/gut.2005.076372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol. 2004;141:1335–1345. doi: 10.1038/sj.bjp.0705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuece B, Sibaev A, Broedl UC, et al. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil. 2007;19:744–753. doi: 10.1111/j.1365-2982.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 30.Abalo R, Chen C, Vera G, et al. In vitro and non-invasive in vivo effects of the cannabinoid-1 receptor agonist AM841 on gastrointestinal motor function in the rat. Neurogastroenterol Motil. 2015;27:1721–1735. doi: 10.1111/nmo.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esfandyari T, Camilleri M, Ferber I, et al. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:831–838. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SC, Wang WL, Su PJ, et al. Decreased enteric fatty acid amide hydrolase activity is associated with colonic inertia in slow transit constipation. J Gastroenterol Hepatol. 2014;29:276–283. doi: 10.1111/jgh.12346. [DOI] [PubMed] [Google Scholar]
- 33.Esfandyari T, Camilleri M, Busciglio I, et al. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol. 2007;293:137–145. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- 34.Bashashati M, Nasser Y, Keenan CM, et al. Inhibiting endocannabinoid biosynthesis: a novel approach to the treatment of constipation. Br J Pharmacol. 2015;172:3099–3111. doi: 10.1111/bph.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong BS, Camilleri M, Eckert D, et al. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil. 2012;24:358–365. doi: 10.1111/j.1365-2982.2011.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong BS, Camilleri M, Busciglio I, et al. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141:1638–1647. doi: 10.1053/j.gastro.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagar RC, Sood R, Gracie DJ, et al. Cyclic vomiting syndrome is a prevalent and under-recognized condition in the gastroenterology outpatient clinic. Neurogastroenterol Motil. 2018 Jan;30(1) doi: 10.1111/nmo.13174. Epub 2017 Jul 26. [DOI] [PubMed] [Google Scholar]
- 38.Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin XH, Yuece B, Li YY, et al. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol Motil. 2011;23:862–e342. doi: 10.1111/j.1365-2982.2011.01742.x. [DOI] [PubMed] [Google Scholar]
- 40.Lin XH, Wei DD, Wang HC, et al. Role of orphan G protein-coupled receptor 55 in diabetic gastroparesis in mice. Sheng Li Xue Bao. 2014;66:332–340. [PubMed] [Google Scholar]
- 41.Camilleri M, Carlson P, McKinzie S, et al. Genetic variation in endocannabinoid metabolism, gastrointestinal motility and sensation. Am J Physiol. 2008;294:G13–G19. doi: 10.1152/ajpgi.00371.2007. [DOI] [PubMed] [Google Scholar]
- 42.Camilleri M, Kolar GJ, Vazquez-Roque MI, et al. Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits. Am J Physiol. 2013;304:G553–G560. doi: 10.1152/ajpgi.00376.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JM, Choi MG, Cho YK, et al. Cannabinoid receptor 1 gene polymorphism and irritable bowel syndrome in the Korean population: a hypothesis-generating study. J Clin Gastroenterol. 2011;45:45–49. doi: 10.1097/MCG.0b013e3181dd1573. [DOI] [PubMed] [Google Scholar]
- 44.Wasilewski A, Lewandowska U, Mosinska P, et al. Cannabinoid receptor type 1 and mu-opioid receptor polymorphisms are associated with cyclic vomiting syndrome. Am J Gastroenterol. 2017;112:933–939. doi: 10.1038/ajg.2017.73. [DOI] [PubMed] [Google Scholar]
- 45.Schussel V, Kenzo L, Santos A, et al. Cannabinoids for nausea and vomiting related to chemotherapy: Overview of systematic reviews. Phytother Res. 2017 Nov 23; doi: 10.1002/ptr.5975. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.de Vries M, van Rijckevorsel DCM, Vissers KCP, et al. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol. 2017;15:1079–1086. doi: 10.1016/j.cgh.2016.09.147. [DOI] [PubMed] [Google Scholar]
- 47.Buggy DJ, Toogood L, Maric S, et al. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106:169–172. doi: 10.1016/s0304-3959(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Kline RH, 4th, McNearney TA, et al. Cannabinoid receptor 2 agonist attenuates pain related behavior in rats with chronic alcohol/high fat diet induced pancreatitis. Mol Pain. 2014;10:66. doi: 10.1186/1744-8069-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kikuchi A, Ohashi K, Sugie Y, et al. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci. 2008;106:219–224. doi: 10.1254/jphs.fp0071599. [DOI] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov NCT #03155945. A randomized, open-label, parallel, phase 2a study to determine the tolerability, pharmacokinetics, and efficacy of APD371 in subjects with Crohn’s disease experiencing abdominal pain.