Abstract
Plant heterotrimeric G proteins modulate numerous developmental stress responses. Recently, receptor-like kinases (RLKs) have been implicated as functioning with G proteins, and may serve as plant G-protein-coupled-receptors (GPCRs). The RLK FERONIA (FER), in the Catharantus roseus RLK1-like subfamily, is activated by a family of polypeptides called Rapid Alkalinization Factors (RALFs). We previously showed that the Arabidopsis G protein β subunit, AGB1, physically interacts with FER, and that RALF1 regulation of stomatal movement through FER requires AGB1. Here, we investigated genetic interactions of AGB1 and FER in plant salinity response by comparing salt responses in the single and double mutants of agb1 and fer. We show that AGB1 and FER act additively or synergistically depending on the conditions of the NaCl treatments. We further show that the synergism likely occurs through salt-induced ROS production. In addition, we show that RALF1 enhances salt toxicity through increasing Na+ accumulation and decreasing K+ accumulation rather than by inducing ROS production, and that the RALF1 effect on salt response occurs in an AGB1-independent manner. Our results indicate that RLK epistatic relationships are not fixed, as AGB1 and FER display different genetic relationships to RALF1 in stomatal vs. salinity responses.
Keywords: AGB1, FERONIA, heterotrimeric G protein, receptor-like-kinase (RLK), RALF1, salinity, reactive oxygen species (ROS), Na+/K+ homeostasis
Introduction
Heterotrimeric guanine nucleotide-binding (G) proteins are composed of Gα, Gβ and Gγ subunits, and Gα subunits associate with or dissociate from Gβγ dimers following GDP or GTP binding (Perfus-Barbeoch et al., 2004; Temple and Jones 2007). The Arabidopsis G protein suite is composed of one canonical Gα (GPA1) (Ma et al., 1990), three extra-large Gα (XLG1, XLG2 and XLG3) (Lee and Assmann, 1999; Ding et al., 2008; Chakravorty et al., 2015), one Gβ (AGB1) (Weiss et al., 1994) and three Gγ (AGG1, AGG2 and AGG3) subunits (Mason and Botella, 2000 Mason and Botella, 2001; Chakravorty et al., 2011). Genetic studies have shown that these G protein subunits play numerous roles in plant development and in responses to biotic and abiotic stresses (Ullah et al., 2001; Ullah et al., 2003; Joo et al., 2005; Misra et al., 2007; Trusov et al., 2007; Fan et al., 2008; Zhu et al., 2009: Chakravorty et al., 2011; Yu and Assmann, 2015; Liang et al., 2016). In particular, we and others have shown that agb1 mutants are hypersensitive to salinity stress, exhibiting a leaf bleaching phenotype (Colaneri et al., 2014; Ma et al., 2014; Yu and Assmann, 2015). Under saline conditions, agb1 accumulates more Na+ and less K+ in both shoots and roots, and translocates more Na+ from roots to shoots, which can be attributed in part to higher transpiration and larger stomatal apertures in agb1 (Yu and Assmann, 2015).
FERONIA (FER) is a membrane-localized receptor-like kinase (RLK) in the Catharantus roseus RLK1-like (CrRLK1L) subfamily of RLKs in Arabidopsis (Lindner et al., 2012). The ligands for FER are a family of polypeptides called Rapid Alkalinization Factors (RALFs) (Haruta et al., 2014; Stegmann et al., 2017). The most-studied ligand, RALF1, elicits phosphorylation of FER, alkalinization of the apoplast, and inhibition of plant growth (Pearce et al., 2001; do Canto et al., 2014; Haruta et al., 2014). Similar to G proteins, FER also functions in numerous developmental and physiological responses, including in salinity response (Chen et al., 2016, Feng et al., 2018). FER is necessary for cell wall integrity and cell-specific Ca2+ induction in the root tip region under salinity conditions. FER may sense salinity-induced cell wall structural changes in root tips by physically interacting with pectic cell wall polysaccharides (Feng et al., 2018). Reactive Oxygen Species (ROS) act downstream of FER signaling. FER is required for ROS production in root hair growth (Duan et al., 2010) as well as at the filiform apparatus in the process of pollen tube reception (Duan et al., 2014). However, the fer mutant exhibits enhanced ROS accumulation in immunity response (Keinath et al., 2010), suggesting that FER may play different roles in ROS production in responses to different stimuli.
Salinity is one of the major agricultural problems that reduces plant growth and crop yield. Na+ competes with other mineral nutrients such as K+ and Ca2+ for acquisition (Lynch and Lauchli 1985; Maathuis and Amtmann 1999), and induces oxidative stress (Munns and Tester 2008). ROS can play dual roles in salinity response, as second messengers that trigger defense and protective responses (Jiang et al. 2012; Evans et al. 2016), and as toxins that deteriorate cellular macromolecules (Katiyar-Agarwal et al. 2006). Therefore, the balance between ROS production and scavenging is critical for normal cellular function (Mittler et al., 2004).
In our previous work, we identified FER in FLAG-AGB1 co-immunoprecipitated protein complexes, and confirmed direct interaction between FER and AGB1 by BiFC assays. We further showed that RALF1-mediated stomatal responses occur through FER and require AGB1 (Yu et al., 2018). Here, we investigated the genetic interactions between FER and AGB1 in salt response, and the effect of RALF1 on salt toxicity. Surprisingly, although AGB1 and FER show epistasis in RALF1 effects on stomatal apertures (Yu et al., 2018), agb1 and fer can act additively or synergistically in the salinity response, and the synergism may occur through salt-induced ROS production. In addition, we show that RALF1 enhances salt toxicity in Col and in agb1 by increasing Na+ content in the seedlings. We also show that RALF1 induces ROS production in the root elongation zone independent of salt stress. Our results indicate that epistatic relationships between AGB1, FER, and RALF1 differ depending on the system under study.
Materials and methods
Plant growth conditions and treatment
The agb1 and fer single mutants used in this study are in the Col background and have been described previously (Ullah et al., 2003; Deslauriers and Larsen, 2010; Duan et al., 2010). Double mutants of agb1-2 fer-2 were made using agb1-2 as the female parent. Except for the experiments with RALF1 treatment, plants were grown in a growth chamber with an 8 h light/16 h dark regime with light intensity of 150 μmol m−2 s−1 and temperature of 21 °C during the light period and 19 °C during the dark period. Seeds were initially sterilized, spread on agar plates and kept at 4 °C in the dark for 48 h. For plate assays, the seedlings were grown vertically on 1⁄2 Murashige and Skoog (MS) medium with 1% sucrose and 0.8% agar (A1296; Sigma, St. Louis, MO, USA) for 9 d and then transferred to treatment plates and grown vertically for the specified number of days. For assays involving hydroponically grown plants, the set-up for hydroponic growth was as described previously (Yu and Assmann, 2015), with the hydroponic medium containing ¼ strength Hoagland’s solution (0.25 mM KH2PO4, 3.71 μM FeNaEDTA, 0.5 mM MgSO4, 1.26 mM KNO3, 1.26 mM Ca(NO3)2, 11.56 μM H3BO3, 2.29 μM MnCl2, 0.20 μM ZnCl2, 0.073 μM CuCl2, 0.026 μM Na2MoO4).
Seedling growth conditions for RALF1 treatment were based on Haruta et al. (2014). Specifically, the seeds were germinated on ½ MS media with a low concentration of sucrose (0.5 mM) and 0.5% agar for 3 or 4 days under continuous light with light intensity of 125 μmol m−2 s−1 at room temperature, and then transferred to 500 μL growth solution (½ MS media with 0.5 mM sucrose) with 1 μM synthesized active RALF1 (72-120 amino acids) (Biomatik, Wilmington, DE, USA) in the absence or in the presence of NaCl treatment for indicated time periods. As is standard in the literature (Haruta et al. 2014; Li et al. 2015; Chen et al. 2016; Stegmann et al. 2017), growth media without RALF1 served as the negative control.
Ion content determination by inductively coupled plasma atomic emission spectrometry (ICP-AES)
Three-week-old seedlings grown hydroponically were treated with or without 100 mM NaCl for 3 d. For the experiments with RALF1 treatment, 4-day-old seedlings were treated with 75 mM NaCl and/or 1 μM RALF1 for 24 h. The same duration of incubation, but with no added RALF1, was used as the negative control (Haruta et al. 2014; Li et al. 2015; Chen et al. 2016; Stegmann et al. 2017). Separated shoot and root tissues or whole seedlings were rinsed briefly with 2 L of Milli-Q water at 18.2 MΩ resistance, dried for 3 d at 70 °C, weighed and placed in 15 mL plastic beakers (VWR, Bridgeport, NJ, USA). The samples were digested in 37% hydrochloric acid (GR-grade, EMD Millipore, Temecula, CA, USA) for 13 h at room temperature, and heated at 80 °C in a heat block for 30 min. The samples were then cooled to room temperature for 30 min and brought to 25 or 100 mL of Milli-Q water at 18.2 MΩ resistance. Ion contents were determined by ICP-AES using a Perkin Elmer Optima 5300DV (Waltham, MA, USA). Independent experiments were repeated 3 or 4 times.
ROS measurement
3,3′-Diaminobenzidine (DAB) staining to visualize H2O2 accumulation was modified from a previous method (Wohlgemuth et al., 2002). Briefly, 9-day-old seedlings were transferred to control or 150 mM NaCl plates and grown for 24 h. Seedlings were immersed in 8 mL staining solution (0.1% (w/v) DAB, 10 mM MES-tris, pH 5.8), vacuum infiltrated 3 times for 1 min, and kept under room light and room temperature for 90 min. Chlorophyll was removed by incubating seedlings in 95% ethanol at 37 °C overnight. Seedlings were washed with water, mounted on microscope slides and imaged using an MVX10 MacroView dissecting microscope equipped with an Olympus DP80 camera controlled by cellSens Dimension 1.11 software.
ROS production in living roots was quantified using the fluorescent dye 2′7′-dichlorofluorescein diacetate (H2DCFDA) (Sigma, St. Louis, MO, USA). To test NaCl effects on ROS production, 9-day-old seedlings grown on ½ MS, 1% sucrose and 0.8% agar were treated in the absence or presence of 150 mM NaCl with 10 μM H2DCFDA dye in ½ MS media in the dark for 15 min. To test effects of RALF1 and NaCl on ROS production, 4-day-old seedlings grown on ½ MS, 0.5 mM sucrose and 0.5% agar were treated in the absence or presence of 1 μM RALF1 and/or 75 mM NaCl with 10 μM H2DCFDA dye in ½ MS media in the dark for 15 min. Excess dye was removed by three washes with ½ MS media. An ~1.2 mm long region of the root tip was imaged with a Zeiss LSM 510 Meta laser scanning confocal microscope (Carl Zeiss, Thornwoood, NY, USA) with a Plan-Neofluar 10×/0.3 objective. H2DCFDA was excited by the 488-nm line of an argon laser with a power of 2%. Fluorescence was detected using a 500-550 nm bandpass emission filter. The images were analyzed using ImageJ software (NIH, Bethesda, MD, USA). Background fluorescence was subtracted, and average fluorescence intensity of the region of interest was quantified in ImageJ. For the 150 mM NaCl treatment, the root region including meristem and elongation zones was used for quantification. For the 1 μM RALF and 75 mM NaCl treatment, the elongation zone and meristematic zone were quantified separately in ImageJ. The experiments were repeated 4-6 times, and 10 to 14 samples in all experiments were measured per genotype per treatment.
Real-time qRT-PCR
Nine-day-old seedlings grown on ½ MS plates were treated with 150 mM NaCl for 3 or 27 h. Shoots and roots were separated and total RNA was isolated from each tissue using NucleoSpin RNA Plant kit (Macherey-Nagel) and treated with RQ1 RNase-Free DNase (Promega, Madison, WI, USA) to remove DNA contamination following the manufacturers’ instructions. Two μg RNA was reverse-transcribed into cDNA using the SuperScript® III Reverse Transcriptase kit (Invitrogen, NY, USA), and the cDNA was diluted 3-5 times for use as a template in qRT-PCR. qRT-PCR was performed using SYBR Green (Bio-Rad, Hercules, CA, USA) to detect synthesized double-stranded DNA in a IQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The cycling conditions comprised a 5 min denaturation at 95 °C, 40 cycles at 95 °C for 30 s, 62 °C for 30 s and 72 °C for 40 s, and a final extension cycle at 72 °C for 8 min. Actin2/8 was used as the reference to normalize gene expression. Three independent biological experiments with 3 technical replicates each were performed. The gene specific primers were as follows: Actin2/8 FP: 5′-GGTAACATTGTGCTCAGTGGTGG-3′; Actin2/8 RP: 5′-AACGACCTTAATCTTCATGCTGC-3′; RALF1 FP: 5′-CGACCTCGGTGGTGTAGCAT-3′; RALF1 RP: 5′- CTCCGCCCCGATACACTCTG-3′.
Measurement of external acidification
The acidification assay was performed as described previously (Haruta et al., 2014) with minor modifications. Specifically, 4-day-old seedlings grown under continuous light were transferred to a 24-well Falcon polystyrene microplate containing 500 μL ¼ MS, 0.5 mM sucrose, with or without 75 mM NaCl and/or 1 μM RALF1, pH adjusted to 6.6 with KOH, and grown under continuous light for 8 h. The pH of the media was determined by mixing 200 μL media solution with a pH indicator fluorescein-Dextran conjugate (MW 10,000) (Sigma, St. Louis, MO, USA) at a final concentration of 30 μg/mL. The fluorescence intensity was recorded in a 96-well microplate (Corning, Corning, NY, USA) using an FLx800 fluorescence reader (BioTek, Winooski, VT, USA) with filters capable of excitation at 488 nm and emission at 525 nm. pH was calculated using a standard curve generated with ¼ MS and 0.5 mM sucrose adjusted to pH 6.0, 6.2, 6.4 and 6.6. Each sample was measured in duplicate and each experiment was repeated 6 times.
Statistical analysis
Two/three/four-way ANOVA was used for analyzing genotype-environment interactions and genetic interactions between genotypes using R software (Brady et al., 2015). If the genotype-environment comparisons were significant (P < 0.05), meaning that the genotype interactions depended on treatment conditions, ANOVA was performed under individual treatment conditions to assess the genetic interactions between genotypes. Tukey’s HSD (honest significant difference) test was used for subsequent pairwise comparisons between samples. Different letters used in the figures indicate adjusted p value < 0.05 between pairwise comparisons.
Results
AGB1 and FER act synergistically in salt tolerance
We previously identified FER in AGB1-associated plasma membrane protein complexes and confirmed physical interaction between FER and AGB1 in BiFC assays (Yu et al., 2018). Both FER and G proteins are signaling modulators in multiple pathways, including salinity response: both agb1 and fer show hypersensitivity to salt stress (Yu and Assmann, 2015; Chen et al., 2016; Feng et al., 2018). By growing agb1 and fer under identical conditions, we found that fer exhibits a more severe phenotype of leaf bleaching than agb1 (Fig. 1a). In order to study the genetic relationship between these interacting proteins, an agb1-2 fer-2 double mutant was treated with different concentrations of NaCl in plate assays and compared with Col and single mutants. Under 100 mM and 150 mM NaCl treatments, agb1-2 fer-2 showed the same low survival rate as fer-2 single mutants, and the interaction effects between the mutations of AGB1 and FER were not significant (two-way ANOVA, p = 0.132 under 100 mM NaCl and 0.175 under 150 mM NaCl) (Fig. 1a, b). However, under 125 mM NaCl treatment, the survival rate of agb1-2 fer-2 was significantly lower than that of either agb1-2 or fer-2 (two-way ANOVA, p = 0.013), suggesting a synergistic interaction between AGB1 and FER under this condition (Fig. 1a, b).
Figure 1.
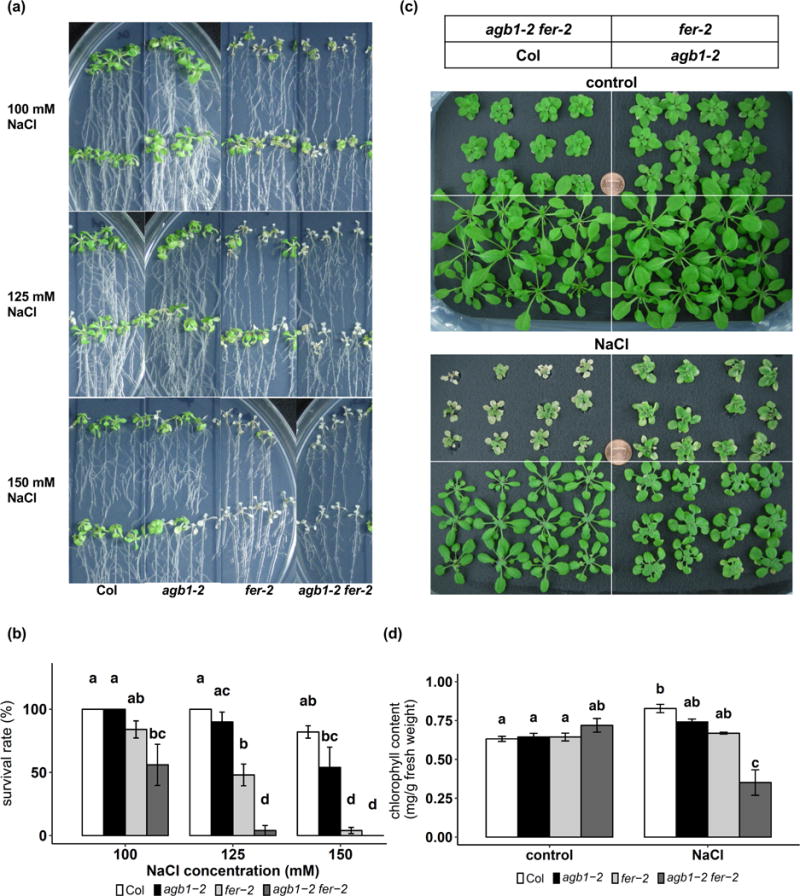
agb1 and fer have a synergistic effect on salt tolerance. (a) Representative photographs of 9-day old seedlings grown on ½ MS media, 1% sucrose and 0.8% agar treated with 100, 125 or 150 mM NaCl for 2 weeks. (b) Survival rates (defined as >50% cotyledon/leaf bleaching) (mean ± S.E., n = 3 independent experiments) of the seedlings as shown in (a). (c) Representative images of 3-week-old hydroponically grown seedlings treated with 100 mM NaCl for 10 days. (d) chlorophyll content of seedlings shown in (c). Data are means ± S.E. of 3 independent experiments.
The synergism was also observed in seedlings grown hydroponically under transpiring conditions (Fig. 1c, d). Three-week-old seedlings were treated with or without 100 mM NaCl for 10 days. Under control conditions, chlorophyll contents are comparable among genotypes (Fig. 1c, d), Under salt conditions, while both agb1-2 and fer-2 show slightly less chlorophyll content compared to Col, the differences are not statistically significant (Fig. 1c, d). By contrast, a significant interaction effect is detected in the double mutant (two-way ANOVA, p = 0.030). The agb1-2 fer-2 double mutant displays severe chlorosis and decreased chlorophyll content as compared to either agb1-2 or fer-2 single mutants (Fig. 1c, d), again supporting synergism between AGB1 and FER in response to salt stress.
agb1 and fer mutants show different responses in K+ and Na+ accumulation under salinity conditions
We have shown that agb1 mutants accumulate more Na+ and less K+ than Col when grown hydroponically under saline conditions (Yu and Assmann, 2015). The fer mutant is proposed to have a more hyperpolarized plasma membrane (PM), which could provide a driving force for cation uptake (Haruta et al., 2014), resulting in Na+ overaccumulation. However, Feng et al. (2018) demonstrated that the inhibitor of the PM H+-ATPase, vanadate, cannot rescue the root growth inhibition by salt stress, indicating that PM H+-ATPases may not play a role in the salt hypersensitivity of fer. To directly measure whether there is Na+ overaccumulation in fer, we determined ion contents by ICP-AES using 3-week-old seedlings grown hydroponically. After 3 days of 100 mM NaCl treatment, agb1-2 accumulated significantly more Na+ and less K+ compared to Col in both shoots and roots, resulting a higher Na+/K+ ratio in agb1 (Fig. 2), which is consistent with previous findings (Yu and Assmann, 2015). However, under this condition, although fer-2 exhibited hypersensitivity to salt treatment (Fig. 1c, d), fer-2 showed only slightly more accumulation of Na+ and less accumulation of K+ than Col, and the differences were not statistically significant (Fig. 2). In the agb1-2 fer-2 double mutants, the Na+ and K+ content and Na+/K+ ratio are comparable to those in agb1-2, and significantly different from those of Col (Fig. 2), even though the double mutant showed a much more severe phenotype compared to agb1 or fer single mutants (Fig. 1c, d). The lack of synergism observed for Na+/K+ homeostasis implies that altered ion homeostasis is probably not the major cause of the synergistic hypersensitivity to salt observed in the double mutant survival and chlorophyll content phenotypes (Fig. 1).
Figure 2.
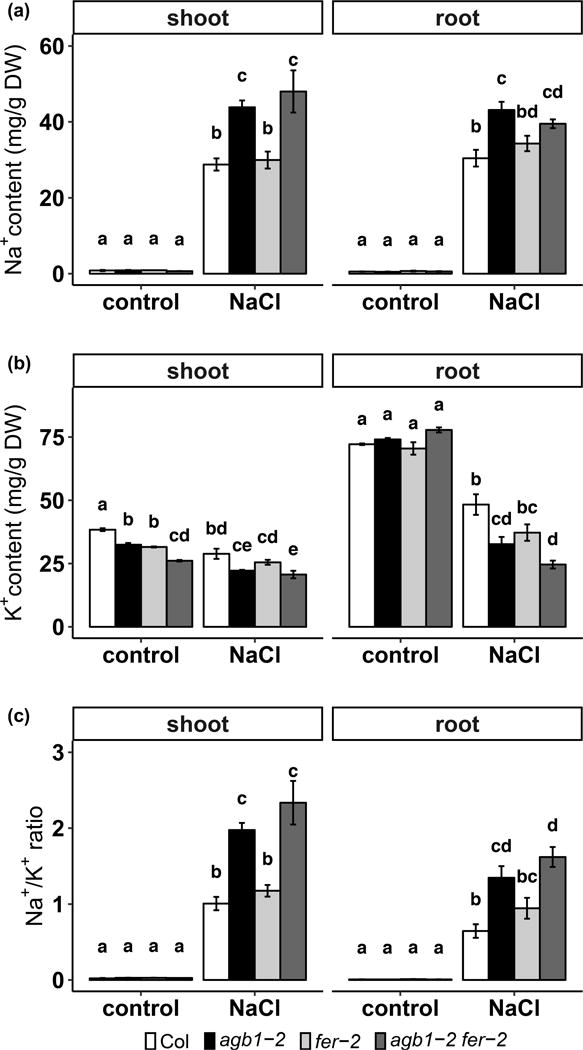
agb1-2 and agb1-2 fer-2 have elevated Na+ content and reduced K+ content in both shoots and roots. (a) Na+ content, (b) K+ content, and (c) Na+/K+ ratio for 3-week-old hydroponically grown plants treated with or without 100 mM NaCl for 3 days. Data are means ± S.E. of 3 independent experiments.
AGB1 and FER are both involved in salt-induced ROS generation
ROS production is required for salt stress-induced signaling activation, while excessive ROS also results in oxidization and damage of proteins and nucleotides (Mittler et al., 2004). Both FER (Duan et al., 2010; Duan et al., 2014) and AGB1 (Joo et al., 2005; Lorek et al., 2013; Torres et al., 2013; Liang et al., 2016) are implicated in ROS production in various responses. We therefore examined whether FER and/or AGB1 are involved in NaCl-induced ROS generation. First, in order to measure ROS production at an early stage of salt response, plate grown seedlings were treated with 150 mM NaCl for 15 min, and ROS production in the root, including meristem and elongation zones, was quantified by H2DCFDA staining. In the absence of salt, fer showed less ROS production than Col (Fig. 3a, b), which is consistent with a previous report (Duan et al., 2010). The agb1-2 fer-2 double mutants produced comparable levels of ROS in roots as fer-2 (Fig. 3a, b). In the presence of salt treatment, both agb1-2 and fer-2 showed less ROS accumulation in the roots as compared to Col. The ROS accumulation of the double mutants appeared to be due to the additive effects of the single mutants (two-way ANOVA, p = 0.868) (Fig. 3a, b).
Figure 3.
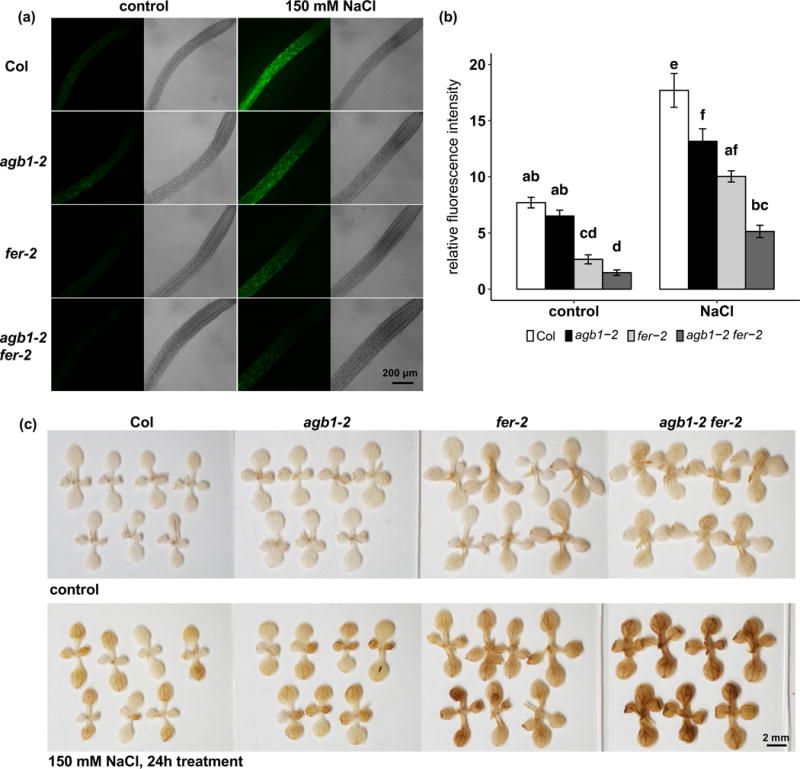
AGB1 and FER are both involved in salt-induced ROS production. Seedlings were grown on ½ MS media, 1% sucrose and 0.8% agar for 9 days and treated with 150 mM NaCl for 15 min or 24 h. (a) Representative photographs and (b) quantification of H2DCFDA staining of roots after 15 min of 150 mM NaCl treatment. Data are means ± S.E. of 23-26 seedlings per genotype per treatment. (c) Representative photographs of DAB staining with or without 24 h of 150 mM NaCl treatment.
Next, ROS production in shoots was visualized by DAB staining after long-term (24 h) NaCl treatment. Without NaCl treatment, fer-2 and agb1-2 fer-2 produced slightly greater amounts of ROS compared to Col and agb1-2 in the cotyledons and first true leaves (Fig. 3c). After 24 h of 150 mM NaCl treatment, agb1-2 and fer-2 each produced more ROS in the shoot as compared to Col (Fig. 3c). The NaCl-induced ROS production in agb1-2 was more pronounced in true leaves than cotyledons as compared to Col. By contrast, fer-2 exhibited more ROS production than Col in both true leaves and cotyledons (Fig. 3c). The ROS production staining in agb1-2 fer-2 double mutants was darker than in both of the single mutants (Figure 3c), consistent with the lower survival rates of the double mutants (Fig. 1a, b). However, no obvious differences in root production of ROS were observed after 24 h of NaCl treatment among genotypes by either H2DCFDA or DAB staining (Supplemental Fig. 1).
In summary, agb1, fer and their double mutant are impaired in salt-induced ROS signaling in the root during the initial salt response, which may in turn result in ROS overaccumulation and accompanying salt hypersensitivity at later response stages.
The ligand of FER, RALF1, enhances salt toxicity
FER is an RLK that is activated by a family of secreted peptide ligands, RALFs, which induce phosphorylation of FER, increase external pH and inhibit plant growth (Pearce et al., 2001; Haruta et al., 2014; Murphy and De Smet, 2014). ralf1 mutants have wild-type salt response in root growth inhibition, while overexpression of RALF1 results in a relative increase in salt resistance, implying that RALFs may play positive roles in salt tolerance (Feng et al., 2018). There is always a caveat that exogenous application of a signaling molecule may not faithfully mimic its endogenous role; nevertheless, exogenous application of RALF has been seminal in elucidating the role of this peptide ligand in signaling and growth responses (Pearce et al., 2001; Haruta et al., 2008; Haruta et al., 2014; Li et al., 2015; Stegmann et al., 2017). We therefore directly applied active RALF1 peptide to the seedlings to investigate the role of RALFs in salinity response and whether AGB1 is involved in the FER-RALF pathway under these conditions. In these experiments, 3-day old seedlings were treated with 75 mM NaCl, 1 μM RALF1 or a combination of the two. In the absence of salt, 1 μM RALF1 treatment for 4 days inhibited root growth by an average of 70.0% in Col (Fig. 4), which is consistent with the growth inhibition role of RALF1 reported in previous studies (Haruta et al., 2014; Li et al. 2015). agb1 exhibited RALF1 inhibition of root growth, but the inhibition percentage is smaller than in Col (Fig. 4, Supplemental Fig. 2), suggesting that AGB1 may be partially responsible for mediating RALF1 inhibition of root growth. As expected, no effects of RALF1 were observed in genotypes lacking FER (fer-2, fer-4 and agb1-2 fer-2) (Fig. 4, Supplemental Fig. 3a, b). RALF1 treatment alone, i.e. in the absence of NaCl, did not cause any visible shoot phenotype under these conditions (Fig. 4a, Supplemental Figs. 2a, 3a).
Figure 4.
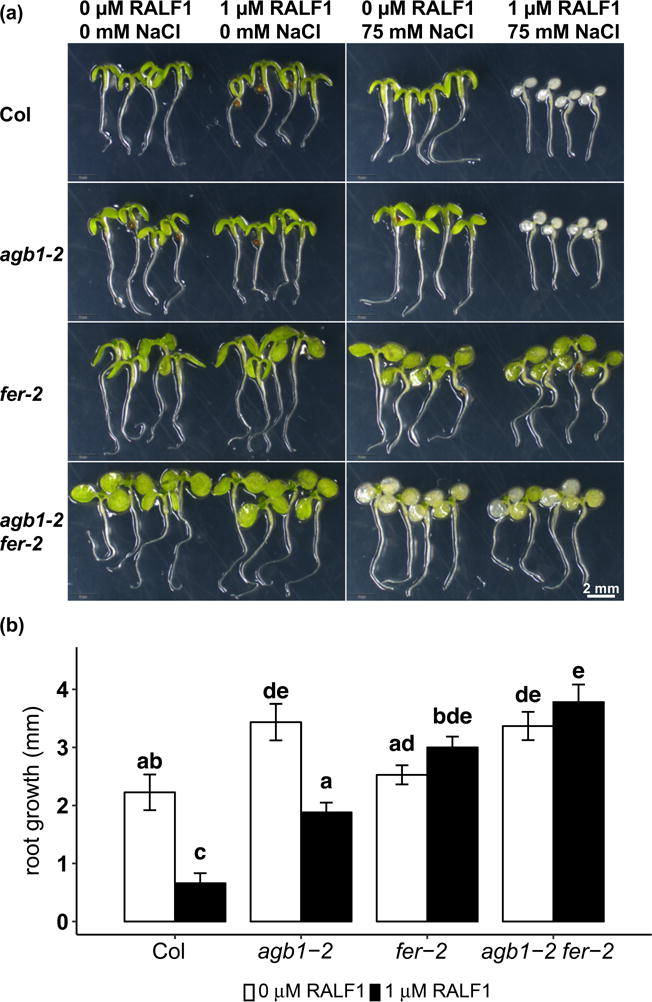
RALF1 causes salt hypersensitivity and inhibits root growth. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 days and treated with 1 μM RALF1 and/or 75 mM NaCl for 4 days. (a) Representative photographs of Col, agb1-2, fer-2 and agb1-2 fer-2 with different treatments. (b) Root elongation with or without 1 μM RALF1 treatment for 4 days. Data are means ± S.E. of 5 independent replicates, and each replicate contains 3-5 seedlings.
Salt treatment at this concentration and duration (75 mM NaCl treatment for 4 days) did not cause any visible bleaching in the shoots of Col, agb1-2 or fer-2. However, the agb1-2 fer-2 double mutants showed bleaching in the cotyledons (Fig. 4a), consistent with our other results showing a synergistic effect of agb1 and fer on salt sensitivity (Fig. 1). The combination of 1 μM RALF1 and 75 mM NaCl treatment caused salt hypersensitivity in both Col and agb1, but not in fer-2, fer-4 or agb1-2 fer-2 as compared to NaCl treatment alone (Fig. 4a, Supplemental Figs. 2a, 3a). Both Col and agb1 were completely bleached, showing an enhancement of salt toxicity by RALF1 (Fig. 4a, Supplemental Figs. 2a, 3a). Consistent with a negative role of RALF1 in salt tolerance, the expression of RALF1 is decreased in the root after 27 h of salt treatment in Col (Supplemental Fig. 4).
Salt toxicity from RALF1 treatment is attributable to Na+ overaccumulation
RALF1 promotes alkalinization of the apoplast, which is proposed to occur through inhibition of the PM H+-ATPase AHA2 (Haruta et al., 2014). Active AHAs promote PM hyperpolarization, which would promote cation uptake, so one would expect RALF1-based inhibition of AHA2 to oppose uptake of cations, including Na+. However, saline conditions also activate the PM H+-ATPase (Yang et al., 2010), which is thought in turn to oppose Na+ accumulation by promoting the activity of PM Na+/H+ antiporters (Zhu, 2003). In order to test how these contrasting effects influence Na+ accumulation, ion contents were measured by ICP-AES using 4-day-old seedlings treated with 75 mM NaCl in the absence or presence of 1 μM RALF1 for 24 h. Under these conditions, transpiration is negligible because seedlings were kept in parafilm sealed plates. In the presence of NaCl and the absence of RALF1, agb1-2 showed comparable Na+ content to Col, while fer and agb1-2 fer-2 showed significantly greater Na+ accumulation compared to Col (Fig. 5a). The K+ content was on average 93.6% in agb1-2 and 90.6% in fer-2 as compared to Col. However, agb1-2 fer-2 double mutants had only 56.8% of the K+ accumulation of Col (Fig. 5b), resulting in a much higher Na+/K+ ratio than in all of the other genotypes (Fig. 5c). AGB1 and FER mutations show a significant interaction effect on both K+ content and Na+/K+ ratio (three-way ANOVA, p < 0.001), as reflected in the synergistic phenotypical hypersensitivity of the double mutant to salt under this growth condition (Fig. 4a).
Figure 5.
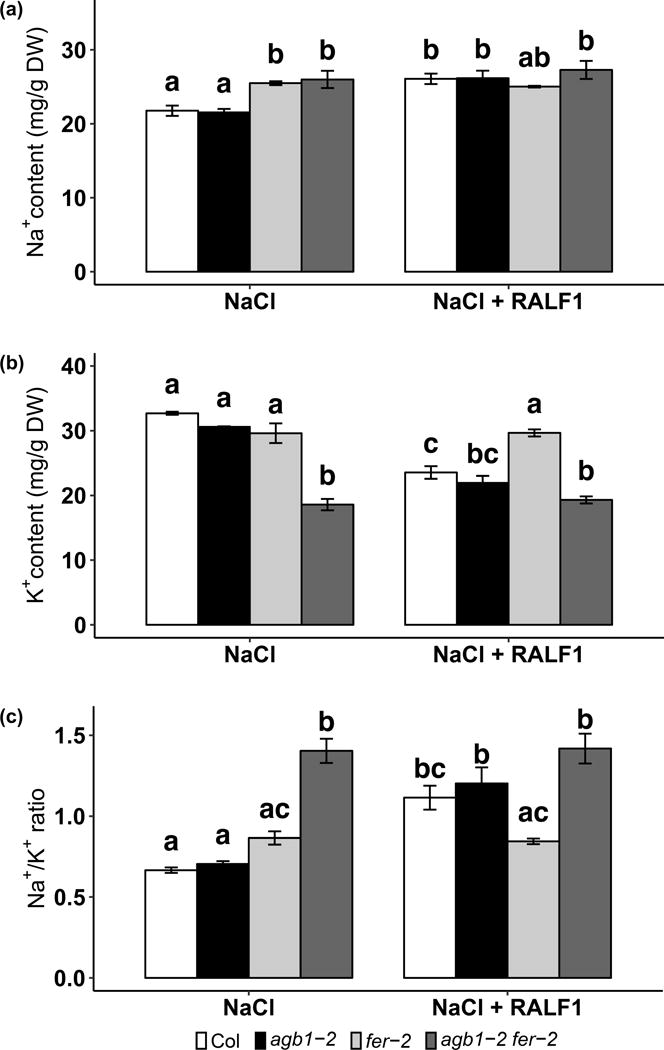
fer null mutation and RALF1 treatment both result in Na+ overaccumulation. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 4 days and treated with 75 mM NaCl in the absence or presence of 1 μM RALF1 for 24 h. (a) Na+ content, (b) K+ content, and (c) Na+/K+ ratio of the seedlings treated with or without 1 μM RALF1 in the presence of 75 mM NaCl for 24 h. Data are means ± S.E. of 3 independent experiments.
In the presence of both RALF1 and NaCl, Col and agb1-2 each responded to RALF1 with increased Na+ content, decreased K+ content and increased Na+/K+ ratio, whereas fer-2 and agb1-2 fer-2 retained the same levels of Na+, K+ and Na+/K+ ratio as compared to NaCl treatment alone, as would be expected for a receptor mutant that cannot sense RALF1 (Fig. 5). Together, these results suggest that RALF1 enhances salt toxicity through regulation of ion homeostasis regulation.
Both RALF1 and NaCl result in alkalinization of the apoplast
Since salt stress activates both H+-ATPases (Yang et al., 2010) which pump out protons, and Na+/H+ antiporters which uptake protons, we next directly measured external pH in response to RALF1 and NaCl treatment. Four-day-old seedlings were treated with 1 μM RALF1, 75 mM NaCl or their combination for 8 h, and the media pH was determined using the pH indicator fluorescein. In our hands, media acidification under the control condition by both agb1-2 and fer-2 is comparable to acidification caused by Col (Fig. 6, Supplemental Fig. 3c), providing no evidence for a constitutively active PM H+-ATPase in either mutant. Both RALF1 and salt suppressed media acidification in Col (Fig. 6, Supplemental Fig. 3c). In the absence of NaCl, agb1-2 displayed similar trend of media pH increase as Col in response to RALF1, but the difference of media pH in the absence and in the presence of RALF1 is not statistically significant in agb1, although very close to the significant level (p = 0.054), which is consistent with the milder response of RALF1 inhibition of root growth compared to Col (Fig. 4b, Supplemental Fig. 2b). agb1-2 also displayed wild-type responses in media pH change in response to NaCl or the combination of RALF1 and NaCl (Fig. 6). As expected, RALF1 did not cause external pH change in fer or agb1-2 fer-2 mutants (Fig. 6, Supplemental Fig. 3c). There is also no interaction effect observed in the agb1-2 fer-2 double mutants (four-way ANOVA, p = 0.556). Interestingly, the NaCl-suppression of media acidification observed in Col and agb1-2 was greatly reduced in the fer and agb1-2 fer-2 mutants (Fig. 6, Supplemental Fig. 3c). A similar response was observed in the Na+/H+ antiporter mutant, sos1 (Supplemental Fig. 5), suggesting that FER and SOS1 may be in the same pathway in salt-induced apoplastic alkalinization response.
Figure 6.
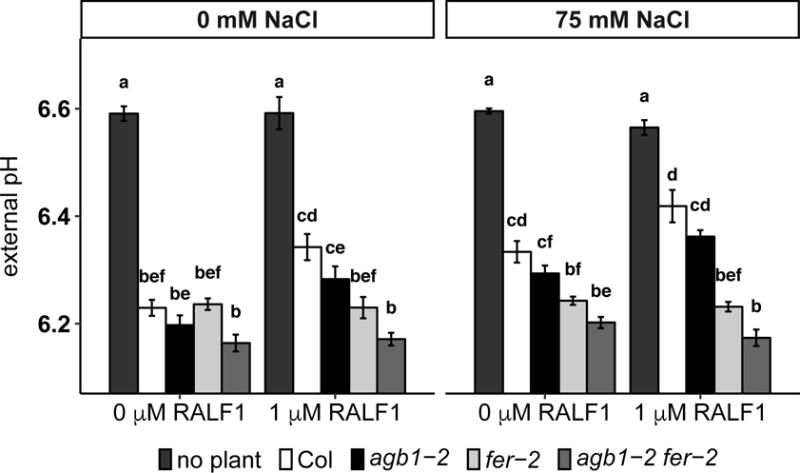
Both RALF1 and NaCl treatment suppress media acidification. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 4 days and treated with 1 μM RALF1 and/or 75 mM NaCl for 8 h. The media pH was measured in a plate reader using the fluorescent pH indicator fluorescein. Data are means ± S.E. of 6 independent replicates, and each replicate contains 8 seedlings.
RALF1 induces ROS generation in the root elongation zone independent of salt stress
RALF1 treatment induces a FER-dependent rapid cytosolic Ca2+ increase in Arabidopsis seedlings, with a more dramatic increase in the root than in the shoot (Haruta et al., 2008; Haruta et al., 2014). ROS production is required for salt-induced systematic Ca2+ wave transmission (Evans et al., 2016). We therefore tested whether ROS plays a role in RALF1 signaling and whether there is a synergistic effect of RALF1 and NaCl in ROS production. First, we used H2DCFDA dye to visualize ROS generation in the roots of four-day-old seedlings after 15 min of RALF1 treatment in the absence of NaCl. RALF1 itself induced ROS generation in both Col and agb1-2, but not in fer-2 or agb1-2 fer-2 (Fig. 7). Different from the localization of ROS induction following treatment with 150 mM NaCl, in which ROS was detected in the meristem and elongation zones of the root (Fig. 3a), the RALF1-induced ROS was predominantly localized in the elongation zone (Fig. 7, Supplemental Fig. 6), suggesting that plant roots respond differently to RALF1 vs. NaCl.
Figure 7.
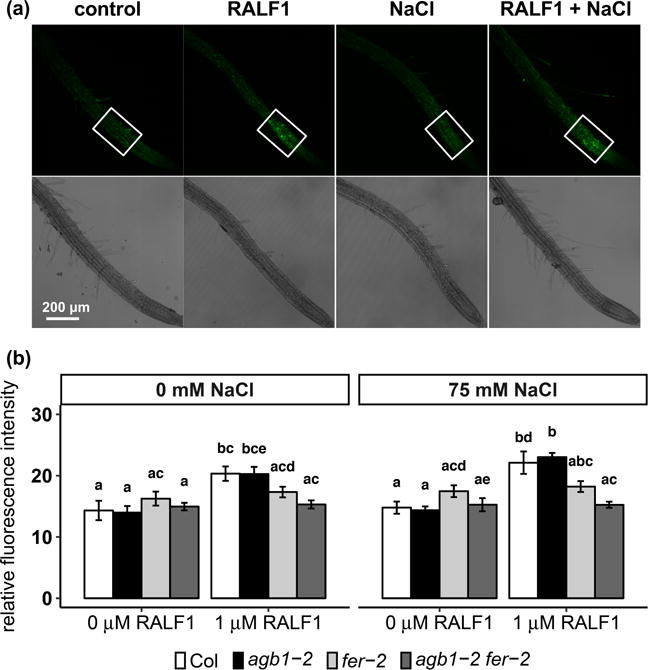
RALF1 induces ROS production in the root elongation zone equally in the absence and presence of NaCl. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 4 days and treated with or without 1 μM RALF1 and/or 75 mM NaCl for 15 min. (a) Representative photographs of Col seedlings with different treatments. The average fluorescence intensity in elongation zones (white frames) was quantified. (b) Quantification of the average fluorescence intensity. Data are means ± S.E. of 10-12 seedlings per genotype per treatment.
We next identified a low concentration of NaCl (75 mM) that was not enough to induce observable ROS generation in any of the genotypes (Fig. 7). Also, no obvious cell death observed in the roots as assessed by FDA staining in any genotypes (Supplemental Fig. 7). We then tested the impact of the combination of 1 μM RALF1 and 75 mM NaCl. This combined treatment did not result in enhanced ROS production as compared to RALF1 treatment alone, and the ROS was still restricted to the elongation zone, despite the fact that RALF1 greatly enhanced salt toxicity as indicated by leaf bleaching (Fig. 4a, Supplemental Figs. 2a, 3a, 5a). This suggests that RALF1 enhancement of salt toxicity may not be through ROS generation.
Discussion
AGB1 and FER display different genetic relationships in different responses
Mutants of plant G proteins display a large number of phenotypes (Chakravorty et al. 2015; Urano et al. 2016 ref; Trusov and Botella, 2016), and most phenotypes are moderate, suggesting that G protein signaling plays modulating but not essential roles in these responses (Trusov and Botella, 2016). In recent years, RLKs have been implicated as functioning together with G proteins in plant development and defense responses, and may serve as plant GPCRs distinct from the classical 7TM-domain GPCRs of animals. For example, in Arabidopsis, AGB1 physically interacts with RECEPTOR-LIKE PROTEIN KINASE 2 (RPK2) but not CLAVATA 1 (CLV1) or CLV2, and acts synergistically with RPK2 in shoot apical meristem (SAM) maintenance (Ishida et al., 2014). In maize, the Gα subunit COMPACT PLANTS2 (CT2) and a CLAVATA RLK FASCIATED EAR2 (FEA2) appeared in an immunoprecipitate with CT2, and showed epistasis in the control of SAM diameter (Bommert et al., 2013; Je et al., 2018). In plant defense response, both AGB1 and XLG2 bind and stabilize BOTRYTIS-INDUCED KINASE 1 (BIK1) for immune response activation (Liang et al., 2016). agb1 and xlg2 null mutants partially rescue the seedling lethal phenotypes of another defense RLK, bir1 (brassinosteroid insensitive-associated receptor kinase1-interacting receptor-like kinase1) (Maruta et al., 2015). Another study showed that GPA1, AGG1 and AGG2 but not AGB1 physically interact with BIR1, BRI1-ASSOCIATED RECEPTOR KINASE (BAK1) and CHITIN ELICITOR RECEPTOR KINASE 1 (CERK1) in split ubiquitin and BiFC assays (Aranda-Sicilia et al., 2015). In soybean, Nod factor RLKs NFR1α and NFR1β physically interact with Gαs, although the GTP-binding and hydrolysis activities of Gαs are not affected by NFRs (Choudhury and Pandey, 2015).
Despite the increasing evidence for a relationship between G proteins and RLKs, the extent to which RLK signaling functions through G proteins is still unclear. We previously showed that AGB1 and FER physically interact with each other, that AGB1 participates in RALF1 inhibition of stomatal opening and promotion of stomatal closure, and that AGB1 and FER function epistatically in RALF control of stomatal movement (Yu et al., 2018). Here, given that agb1 and fer mutants each exhibit salt hypersensitivity (Yu and Assmann, 2015; Chen et al., 2016), we evaluated the relationship between AGB1 and FER in salt response. We confirmed that both agb1 and fer show hypersensitivity to salt stress (Fig. 1), The phenotype of agb1 is more moderate as compared to that of fer (Fig. 1), which is consistent with other phenotypes where G protein mutants show less severe phenotypes as compared to rlk mutants (Bommert et al., 2013; Ishida et al., 2014; Maruta et al., 2015). Moreover, we found that AGB1 and FER act additively or synergistically in salt response, suggesting that the AGB1 and FER pathways overlap but are not in a linear relationship. A synergistic relationship has also been observed for AGB1 and the RLK RPK2 in SAM maintenance (Ishida et al., 2014). These results suggest that RLK signaling has both G protein-dependent and AGB1-independent aspects, and that epistatic relationships between AGB1 and FER differ across different phenotypes.
agb1 and fer exhibit different patterns in salt-induced Na+ overaccumulation
Sodium accumulation is influenced by the rate of Na+ influx and efflux at the PM of root cells, the capacity of Na+ compartmentation in the vacuole, and the translocation of Na+ from root to shoot via the transpiration stream (Munns and Tester 2008). Na+ influx may occur through passive uptake mediated by non-selective cation channels (NSCC) (Maathuis and Sanders 2001; Demidchik and Tester 2002), while Na+ efflux is an active process mediated by PM Na+/H+ antiporters (Qiu et al. 2002). We previously showed that the whole plant salt hypersensitivity of agb1 is partially attributable to a higher stomatal conductance (Yu and Assmann, 2015), which provides a driving force for Na+ translocation from root to shoot via transpiration stream. Therefore, it is not surprising that the overaccumulation of Na+ in agb1 is only observed in seedlings grown under transpiring conditions (Fig. 2a).
However, the patterns of Na+/K+ homeostasis in the fer mutants differed from those of agb1 (Figs. 2 and 5). Under transpiring conditions, 3-week-old fer seedlings treated with 100 mM NaCl for 3 days failed to show significant overaccumulation of Na+ compared to Col (Fig. 2a), while under non-transpiring conditions, 4-day-old fer-2 and agb1-2 fer-2 showed significantly greater Na+ content compared to Col and agb1 (Fig. 5a). This difference may be due to the age of seedlings used, or the transpiration conditions. Both our group and others observed constitutively smaller stomatal apertures in the fer mutants (Keinath et al., 2010; Yu et al., 2018), and fer is hypersensitive to ABA-induced stomatal closure (Yu et al., 2012; Chen et al. 2016). We previously showed that salt stress increases the ABA content in leaves (Yu and Assmann, 2015). Therefore, it is likely that under saline conditions, the more closed stomata in fer inhibit transpiration and thus Na+ uptake, which prevents the overaccumulation of Na+ in fer and results in comparable Na+ and K+ content between fer and Col when grown under transpiring conditions (Fig. 2).
Under non-transpiring conditions (Fig. 5), the net Na+ content is controlled by Na+ influx through NSCC and efflux through Na+/H+ antiporters. Haruta et al. (2014) proposed that the hypersensitivity of fer to Li+ toxicity is due to a hyperpolarized PM of fer, which promotes Li+ uptake through NSCC, although the membrane potential of fer was not directly compared with Col, but inferred from a faster media acidification by fer mutants, suggesting a more active PM H+-ATPase. However, under saline conditions, the activity of the PM H+-ATPase is enhanced (Yang et al., 2010), which is presumed to promote Na+ exclusion by PM Na+/H+ antiporters. Therefore, an active PM H+-ATPase promotes salt tolerance. In our external pH measurement, we found that 75 mM NaCl treatment suppresses media acidification by Col (Fig. 6, Supplemental Fig. 3c), and this suppression is abolished in the Na+/H+ antiporter mutant, sos1 (Supplemental Fig. 5), suggesting that the apoplastic alkalinization is due to the activation of Na+/H+ antiporters. In our hands, we did not observe that fer acidifies the media more than Col under our control conditions (Fig. 6, Supplemental Fig. 3c), suggesting that fer may not have a constitutively active PM H+-ATPase under our experimental conditions.
The fer mutants accumulate greater Na+ content and similar K+ content compared to Col under non-transpiring conditions (Fig. 5). Overaccumulation of Na+ in fer may be partially attributable to symplastic uptake of Na+ due to the loss of cell integrity of the epidermal and cortex cells in roots (Feng et al., 2018). In our external pH measurement, we found that fer fails to exhibit significant salt-suppression of media acidification compared to Col (Fig. 6), implying that FER might be required for activating Na+/H+ antiporters to inhibit Na+ overaccumulation. agb1 exhibits WT response under 75 mM NaCl treatment (Figs. 5a, 6), which is consistent with the mild response of G protein mutants. The agb1-2 fer-2 double mutants did not show synergism in terms of Na+ content and external pH changes under these conditions, but resemble the trends of the fer-2 single mutants (Figs. 5a, 6). Interestingly, the agb1-2 fer-2 double mutants show significantly less K+ content than agb1 and fer single mutants under the non-transpiring salt conditions (Fig. 5b), which may be a contributing factor for the observed synergism between agb1 and fer in salt hypersensitivity (Figs. 1, 4a).
Based on comparison of the results from transpiring vs. non-transpiring conditions, we conclude that FER plays opposite roles in Na+ uptake into roots vs. translocation of Na+ from root to shoot via transpiration. Additionally, since the Na+ overaccumulation in agb1 is observed under transpiring conditions while that in fer is observed under non-transpiring conditions, and the double mutants resemble agb1 under transpiring conditions and fer under non-transpiring conditions, we conclude that the synergism between agb1 and fer in salt sensitivity is not due to Na+ accumulation, but could be impacted by effects of the mutations on K+ accumulation (Fig. 8a).
Figure 8.
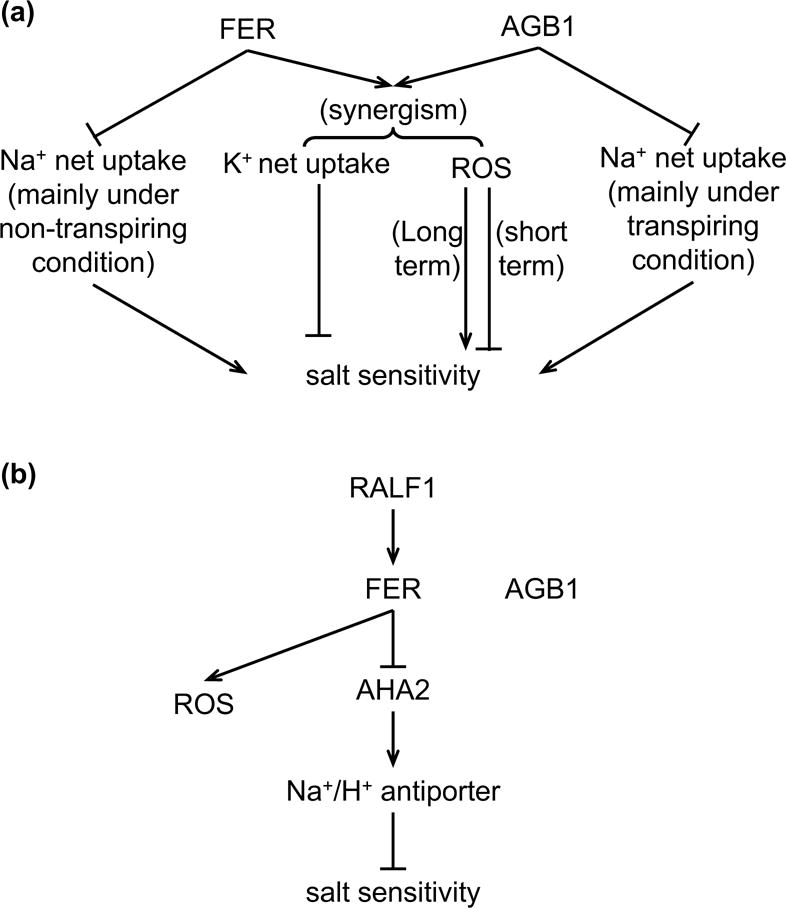
Schematic models showing the roles of AGB1 and FER in salinity response and in RALF1 effect on the salinity response. (a) AGB1 and FER may act synergistically in salt response by regulating ROS production and K+ net uptake. AGB1 and FER inhibit Na+ net uptake through different mechanisms. AGB1 is mainly involved in inhibiting Na+ translocation from root to shoot under transpiring condition, while FER is mainly involved in inhibiting Na+ net uptake under non-transpiring condition. (b) RALF1 enhances salt toxicity by increasing Na+ accumulation, and is independent of AGB1 and ROS production. RALF1 is likely to inhibit the activity of AHA2 through FER, which suppresses the activity of Na+/H+ antiporter and cause salt hypersensitivity. AGB1 and RALF1-induced ROS generation are not involved in this process.
ROS imbalance may be involved in the synergism of agb1 and fer in response to salt stress
ROS are often oxidative stressors that accompany longer-term salt stress, but also act as secondary messengers in the initial responsive phase. For example, the Na+/H+ antiporter mutant, sos1, and a transcriptional regulator of abiotic stress rcd1 (radical-induced cell death1), both show salt hypersensitivity and enhanced ROS production in the roots compared to the wild-type after long term (24 h) 200 mM NaCl treatment (Katiyar-Agarwal et al., 2006). Conversely, mutants of PHOSPHOINOSITIDE 3-KINASE (PI3K), PtdIn 5-PHOSPHATASE 7 and the NADPH oxidases RBOHD/F show compromised ROS production after 15 min - 1 h (short term) salt stress, yet again show salt hypersensitivity (Leshem et al., 2007; Kaye et al., 2011; Ma et al., 2011). Mutants of the ROS-producing NADPH oxidase AtrbohD, but not AtrbohF, fail to show obvious ROS induction in the roots after 20 min of 150 mM NaCl or 90 min of 100 mM NaCl treatment as revealed by staining with the ROS-sensitive H2DCFDA fluorescent dye (Leshem et al., 2007; Achard et al., 2008). Loss-of-function of AtRbohD or treatment with ROS scavenger or NADPH oxidase inhibitor DPI results in reduced systemic Ca2+ wave transmission following 100 mM NaCl treatment (Evans et al., 2016), suggesting that ROS acts upstream of Ca2+ in cell to cell communication of the salt response.
In our assays, we found that NaCl-induced ROS production depends on the concentration of NaCl and duration of treatment. We found that 150 mM but not 75 mM NaCl for 15 min is able to trigger ROS production in the root meristem and elongation zones in Col (Figs. 3a, 3b, 7). Under 150 mM NaCl, ROS production is less in agb1 and in fer compared to Col (Fig. 3a, b). However, after 24 h of 150 mM NaCl treatment, both agb1 and fer produce more ROS in the shoots (Fig. 3c), but not in the roots (Supplemental Fig. 1), compared to Col. These results suggest that the ROS production at different time points after stress treatment can be different and that the balance of ROS production in response to salt is impaired in both agb1 and fer (Fig. 8a). Consistent with the whole-plant salt phenotypes (Fig. 1), the agb1 fer double mutants exhibit more severe ROS imbalance than the single mutants – i.e., more ROS production in the leaves after 24 h treatment and less ROS in the roots after 15 min treatment compared to the single mutants (Fig. 3).
Both AGB1 and FER have been shown to participate in ROS production in other responses. agb1 mutants exhibit reduced ROS burst and less phosphorylation of RBOHD in immunity response (Liu et al., 2013; Lorek et al., 2013; Liang et al., 2016). agb1 and gpa1 are also impaired in O3-induced oxidative burst (Joo et al., 2005). fer produces less ROS in roots in both the absence and presence of auxin (Duan et al., 2010) as well as at the filiform apparatus in the process of pollen tube reception (Duan et al., 2014). However, fer exhibits enhanced ROS accumulation in immunity response (Keinath et al., 2010). A downstream effector of FER in root hair development, ROP2 (RHO-RELATED GTPASE OF PLANTS 2), has been shown to be associated with RBOHD in pull-down assays (Li et al., 2015). These results indicate that ROS produced by RBOHD may be a shared downstream component in the synergism between AGB1 and FER in the salt response.
RALF1-induced salt hypersensitivity is attributable to Na+ overaccumulation, and is independent of AGB1 and ROS production
We previously showed that AGB1 is required for RALF1 regulation of stomatal movement through FER, which is likely mediated by downstream ABA signaling components, ABI1 and OST1 (Yu et al., 2018). Here we found that the agb1 mutants exhibit reduced sensitivity to RALF1 inhibition of root elongation (Fig. 4b, Supplemental Fig. 2b), but show a wild-type response to RALF1 enhancement of salt hypersensitivity (Fig. 4a, Supplemental Fig. 2a), suggesting that AGB1 involvement in RALF1 signaling is phenotype dependent.
Our results show that RALF1 treatment causes an increase in Na+ content in both Col and agb1-2 (Fig. 5a). RALF1 can reduce root acidification of the medium (Fig. 6), possibly through inhibiting the activity of PM H+-ATPases. Under saline conditions, the activity of the PM H+-ATPase is enhanced to presumably promote Na+ exclusion through Na+/H+ antiporters (Yang et al., 2010). Therefore, it is likely that RALF1 enhancement of salt hypersensitivity is through inhibiting the activity of PM H+-ATPases and thus indirectly inhibiting Na+ exclusion (Fig. 8b).
We also investigated whether the RALF1 effect on salt response is also through ROS signaling. We found that RALF1 treatment itself induces ROS production (Fig. 7). Different from 150 mM NaCl-induced ROS production, which is present in root meristematic and elongation zones, RALF-induced ROS production is predominantly limited to the elongation zone (Fig. 7, Supplemental Fig. 6). Furthermore, although RALF1 dramatically enhanced the bleaching phenotype associated with salt toxicity (Fig. 4), the combination of RALF1 and NaCl treatment did not enhance ROS production as compared to treatment with RALF1 alone (Fig. 7), suggesting that RALF1 may not enhance salt toxicity through regulating ROS production.
We noticed that in the experiments where we measured RALF1-induced ROS production, the fer mutants did not display a lower basal level of ROS production than Col (Fig. 7, Supplemental Fig. 6), in contrast to the results of Fig. 3b and Duan et al. (2010). This discrepancy is likely attributable to the different growth conditions, particularly sucrose concentrations used in the growth media in different experiments. The fer mutant is hypersensitive to growth inhibition by external sucrose and shows decreased cellulose content when grown under high sucrose concentrations (Yeats et al., 2016) that may contribute to loss of cell wall integrity. We observed that at the low sucrose concentration used in our assays (0.5 mM sucrose), the fer mutant is able to grow some elongated root hairs (Supplemental Fig. 7) while at 1% (29.2 mM) sucrose, only burst root hairs were observed in fer (Supplemental Fig. 1). Moreover, there was no sucrose present in our hydroponic conditions, yet fer mutants still exhibited salt hypersensitivity.
Both RALF1 application and loss of FER function result in salt hypersensitivity
RALF1 is a ligand of FER and thus logically acts through the activation of FER. Indeed, both the ralf1 and fer mutants have longer roots and are hypersensitive to ABA inhibition of root growth (Bergonci et al., 2014; Haruta et al., 2014; Chen et al., 2016). In the RALF1 effect on ABA inhibition of root growth, the fer mutant is hypersensitive to ABA inhibition, and RALF1 treatment suppresses the ABA-induced inhibition (Chen et al. 2016), which is also consistent with RALF1 activation of FER. However, we and others also show that RALF1 and FER may play opposite functions in certain responses. For example, RALF1 RNAi lines exhibit longer hypocotyls and larger rosette sizes, while fer exhibits the opposite phenotypes (Deslauriers and Larsen, 2010; Duan et al., 2010; Bergonci et al., 2014). Our previous results showed that the loss of FER function results in constitutively smaller stomatal apertures, and RALF1 treatment also reduces aperture sizes (Yu et al., 2018). RALF23, which is another ligand of FER, inhibits elf18-induced ROS production. And yet the fer mutants also exhibit reduced ROS production in response to elf18 (Stegmann et al., 2017). Here, we again observed the opposite effects of RALF1 and FER: although the null mutation of FER causes salt hypersensitivity (Fig. 1) and Na+ overaccumulation (Fig. 5a), RALF1 treatment causes the same phenotypes (Figs. 4, 5a). The ralf1 mutants have wild-type response in salt-inhibition of root growth (Feng et al., 2018), although we cannot rule out the possibility of functional redundancy of the RALF family in FER-mediated responses. Overexpression of RALF1 may actually result in relative resistance to salt-inhibition of root growth, which would be consistent with the protective role of FER in salt response. However, one potential caveat is that the roots of the RALF1 overexpression lines are much shorter even without salt treatment (Feng et al., 2018). One way to reconcile such results is to hypothesize that RALF1 may activate FER in some responses, and inhibit FER in others. For example, if RALF1 treatment causes FER internalization under some conditions, as occurs with some mammalian GPCRs following agonist exposure (Smith and Rajagopal 2016), treatment with RALF1 could ultimately terminate FER signaling. The opposite effects of exogenous application of the RALF1 peptide and constitutively expression of the gene under salt conditions could also be due to different effects of RALF1 under short term and constitutive exposure or to non-physiological effects of the exogenous treatment, although both methods are limited in their ability to fully reflect native expression of RALF1 under physiological conditions. Alternatively, RALF1 might act as an antagonist of FER signaling in the salinity response, thus phenocopying the fer null mutation, while other peptides encoded by the ~35 RALF family genes (Murphy and De Smet, 2014) might act as agonists and play positive roles (Lee and De Smet, 2016), a duality that has been observed in regulation of pollen tube growth (Ge et al. 2017; Mecchia et al. 2017). Another possibility is that FER may function independently from RALF1 in some phenotypes, which would be consistent with the WT-response to salt-inhibition of root growth in the ralf1 mutants (Feng et al., 2018). Our results also showed that ROS plays a role in FER response to salt stress, while RALF1-induced salt hypersensitivity is independent of ROS, consistent with the hypothesis that FER could function independently of RALF1 in some aspects of the salinity response.
In conclusion, we have shown that AGB1 and FER act synergistically in chlorophyll content and K+ accumulation under saline conditions, and that salt-induced ROS production appears to play roles in mediating the synergism (Fig. 8a). By contrast, RALF1 enhances salt toxicity by regulating Na+ and K+ accumulation in a FER-dependent but AGB1-independent manner (Fig. 8b).
Supplementary Material
Supplemental Figure 1. ROS production in the roots after 24 h of 150 mM NaCl treatment is comparable among genotypes. Seedlings were grown on ½ MS media, 1% sucrose and 0.8% agar for 9 days and treated with 150 mM NaCl for 24 h. (a) Representative photographs and (b) quantification of H2DCFDA staining after 24h of 150 mM NaCl treatment. Data are means ± S.E. of 14-15 seedlings per genotype per treatment. (c) Representative photographs of DAB staining with or without 24 h of 150 mM NaCl treatment.
Supplemental Figure 2. agb1 shows wild-type response to RALF1-induced salt hypersensitivity but is less sensitive to RALF1 inhibition of root growth. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 days and treated with 1 μM RALF1 and/or 75 mM NaCl for 4 days. (a) Representative photographs of 3-day-old seedlings treated with RALF1 and/or NaCl for 4 days. (b) root elongation with or without 1 μM RALF1 treatment for 4 days. Data are means ± S.E. of 5 independent replicates, and each replicate contains 3-5 seedlings.
Supplemental Figure 3. fer-4 is not responsive to RALF1 in various seedling assays. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 or 4 days and treated with 1 μM RALF1 and/or 75 mM NaCl for various time. (a) Representative photographs of 3-day-old seedlings treated with RALF1 and/or NaCl for 4 days. (b) Root elongation with or without 1 μM RALF1 treatment for 4 days. Data are means ± S.E. of 5 independent replicates, and each replicate contains 3-5 seedlings. (c) External pH of 4-day-old seedlings treated with 1 μM RALF1 and/or 75 mM NaCl for 8 h. The media pH was measured in a plate reader using the fluorescent pH indicator fluorescein. Data are means ± S.E. of 6 independent replicates, and each replicate contains 8 seedlings.
Supplemental Figure 4. RALF1 is down-regulated in root tissue by 27 h of 150 mM NaCl treatment. Col seedlings were grown on ½ MS media, 1% sucrose and 0.8% agar for 9 days and treated with 150 mM NaCl for 3 h or 27 h. Shoots and roots were separated and RNA was extracted. Data are means ± S.E. of 3 independent replicates.
Supplemental Figure 5. Salt suppression of media acidification requires the Na+/H+ antiporter SOS1. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 or 4 days and treated with 1 μM RALF1 and/or NaCl for various times. (a) Representative photographs of 3-day-old seedlings treated with RALF1 and/or 50 mM, 75 mM and 100 mM NaCl for 4 days. (b) External pH of 4-day-old seedlings treated with 1 μM RALF1 and/or 75 mM NaCl for 8 h. The media pH was measured in a plate reader using the fluorescent pH indicator fluorescein. Data are means ± S.E. of 6 independent replicates, and each replicate contains 8 seedlings.
Supplemental Figure 6. RALF1 does not induce ROS production in the root meristematic zone in either the absence or presence of NaCl. ROS production was analyzed in the root meristematic zones using the same images as used for quantifying ROS in the elongation zone in Figure 7.
Supplemental Figure 7. Viability is not affected after 15 min of 1 μM RALF1 or 75 mM NaCl treatment. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 4 days and treated with or without 1 μM RALF1 and/or 75 mM NaCl for 15 min. Seedlings were stained with fluorescein diacetate (FDA) and visualized by confocal microscopy. The root hair burst.
Summary statement.
Receptor-like kinases (RLKs) have been implicated as functioning with heterotrimeric G proteins, and may serve as plant G-protein-coupled-receptors. We investigated genetic interactions of the Arabidopsis G protein β subunit, AGB1, and the RLK FERONIA (FER) in plant salinity response, comparing salt-influenced phenotypes in the single and double mutants of agb1 and fer. We show that AGB1 and FER act additively or synergistically depending on the phenotype assayed. Our results indicate that epistatic relationships between FER and G proteins are not fixed, and support FER and G protein interaction in aspects of salinity tolerance.
Acknowledgments
We thank Prof. Gabriele Monshausen (Pennsylvania State University) for providing seeds of the fer-2 and fer-4 mutants. This research was supported by NSF-MCB grant 1121612 to S.M.A., with additional support from NSF-MCB grant 1715826 and from the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM126079. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Current Biology. 2008;18:656–660. doi: 10.1016/j.cub.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Bergonci T, Ribeiro B, Ceciliato PH, Guerrero-Abad JC, Silva-Filho MC, Moura DS. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. Journal of Experimental Botany. 2014;65:2219–2230. doi: 10.1093/jxb/eru099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Burow M, Busch W, Carlborg Ö, Denby KJ, Glazebrook J, Maloof JN. Reassess the t test: interact with all your data via ANOVA. The Plant Cell. 2015;27:2088–2094. doi: 10.1105/tpc.15.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D, Gookin TE, Milner M, Yu Y, Assmann SM. Extra-large G proteins (XLGs) expand the repertoire of subunits in arabidopsis heterotrimeric G protein signaling. Plant Physiology. 2015:00251.02015. doi: 10.1104/pp.15.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Botella JR. An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. The Plant Journal. 2011;67:840–851. doi: 10.1111/j.1365-313X.2011.04638.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Yu F, Liu Y, Du C, Li X, Zhu S, Liu X. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proceedings of the National Academy of Sciences. 2016;113:E5519–E5527. doi: 10.1073/pnas.1608449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Pandey S. Phosphorylation-dependent regulation of G-protein cycle during nodule formation in soybean. The Plant Cell. 2015;27:3260–3276. doi: 10.1105/tpc.15.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaneri AC, Tunc-Ozdemir M, Huang JP, Jones AM. Growth attenuation under saline stress is mediated by the heterotrimeric G protein complex. BMC Plant Biology. 2014;14:129. doi: 10.1186/1471-2229-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Tester M. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis root. Plant Physiology. 2002;128:379–387. doi: 10.1104/pp.010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Molecular Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- Ding L, Pandey S, Assmann SM. Arabidopsis extra‐large G proteins (XLGs) regulate root morphogenesis. The Plant Journal. 2008;53:248–263. doi: 10.1111/j.1365-313X.2007.03335.x. [DOI] [PubMed] [Google Scholar]
- do Canto AM, Ceciliato PH, Ribeiro B, Morea FAO, Garcia AAF, Silva-Filho MC, Moura DS. Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis. Plant Physiology and Biochemistry. 2014;75:45–54. doi: 10.1016/j.plaphy.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nature Communications. 2014;5:3129. doi: 10.1038/ncomms4129. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proceedings of the National Academy of Sciences. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Choi WG, Gilroy S, Morris RJ. A ROS-assisted Calcium Wave Dependent on AtRBOHD and TPC1 Propagates the Systemic Response to Salt Stress in Arabidopsis Roots. Plant Physiology. 2016:00215.02016. doi: 10.1104/pp.16.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LM, Zhang W, Chen JG, Taylor JP, Jones AM, Assmann SM. Abscisic acid regulation of guard-cell K+ and anion channels in G -and RGS-deficient Arabidopsis lines. Proceedings of the National Academy of Sciences. 2008;105:8476. doi: 10.1073/pnas.0800980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Dinneny R. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Current Biology. 2018;28:666–675.e5. doi: 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, Zhong S. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science. 2017;358:1596–1600. doi: 10.1126/science.aao3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gévaudant F, Duby G, von Stedingk E, Zhao R, Morsomme P, Boutry M. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt toleranc. Plant Physiology. 2007;144:1763–1776. doi: 10.1104/pp.107.103762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Monshausen G, Gilroy S, Sussman MR. A Cytoplasmic Ca2+ Functional Assay for Identifying and Purifying Endogenous Cell Signaling Peptides in Arabidopsis Seedlings: Identification of AtRALF1 Peptide. Biochemistry. 2008;47:6311–6321. doi: 10.1021/bi8001488. [DOI] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annual Review of Plant Biology. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Jiang C, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Harberd NP. ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. The EMBO Journal. 2012;31:4359–4370. doi: 10.1038/emboj.2012.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je BI, Xu F, Wu Q, Liu L, Meeley R, Gallagher JP, Jackson D. The CLAVATA receptor FASCIATED EAR2 responds to distinct CLE peptides by signaling through two downstream effectors. Elife. 2018;7:e35673. doi: 10.7554/eLife.35673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. The Plant Cell. 2005;17:957–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Zhu J, Kim K, Agarwal M, Fu X, Huang A, Zhu JK. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proceedings of the National Academy of Sciences. 2006;103:18816–18821. doi: 10.1073/pnas.0604711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye Y, Golani Y, Singer Y, Leshem Y, Cohen G, Ercetin M, Levine A. Inositol polyphosphate 5-phosphatase7 regulates the production of reactive oxygen species and salt tolerance in Arabidopsi. Plant Physiology. 2011;157:229–241. doi: 10.1104/pp.111.176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Panstruga R. PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. Journal of Biological Chemistry. 2010;285:39140–39149. doi: 10.1074/jbc.M110.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SA, Lindner H, Jones DS, Grossniklaus U. (EMBO Reports: e201438801).Functional analysis of related CrRLK1L receptor-like kinases in pollen tube reception. 2014 doi: 10.15252/embr.201438801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- Lee JS, De Smet I. Fine-Tuning Development Through Antagonistic Peptides: An Emerging Theme. Trends in Plant Science. 2016;21:991–993. doi: 10.1016/j.tplants.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Lee YRJ, Assmann SM. Arabidopsis thaliana “extra-large GTP-binding protein” (AtXLG1): a new class of G-protein. Plant Molecular Biology. 1999;40:55–64. doi: 10.1023/a:1026483823176. [DOI] [PubMed] [Google Scholar]
- Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol 3‐kinase‐mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt toleranc. The Plant Journal. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- Li C, Yeh FL, Cheung AY, Duan Q, Kita D, Liu MC, Gates L. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:e06587. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ding P, Lian K, Wang J, Ma M, Li L, Chen S. Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 recepto. eLife. 2016;5:e13568. doi: 10.7554/eLife.13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H, Müller LM, Boisson-Dernier A, Grossniklaus U. CrRLK1L receptor-like kinases: not just another brick in the wall. Current Opinion in Plant Biology. 2012;15:659–669. doi: 10.1016/j.pbi.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Liu J, Ding P, Sun T, Nitta Y, Dong O, Huang X, Zhang Y. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinase. Plant Physiology. 2013;161:2146–2158. doi: 10.1104/pp.112.212431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorek J, Griebel T, Jones AM, Kuhn H, Panstruga R. The role of Arabidopsis heterotrimeric G-protein subunits in MLO2 function and MAMP-triggered immunity. Molecular Plant-Microbe Interactions. 2013;26:991–1003. doi: 10.1094/MPMI-03-13-0077-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Lauchli A. Salt stress disturbs the calcium nutrition of barley (Hordeum vulgare L) New Phytologist. 1985;99:345–354. [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proceedings of the National Academy of Sciences. 1990;87:3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stres. Journal of Experimental Botany. 2011;63:305–317. doi: 10.1093/jxb/err280. [DOI] [PubMed] [Google Scholar]
- Ma Y-n, Chen M, Xu D-b, Fang G-n, Wang E-h, Gao S-q, Min D-h. G Protein β Subunit AGB1 Positively Regulates Salt Stress Tolerance in Arabidopsis. Journal of Integrative Agriculture. 2014;14:314–325. [Google Scholar]
- Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of botany. 1999;84:123–133. [Google Scholar]
- Maathuis FJM, Sanders D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiology. 2001;127:1617–1625. [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Pandey GK, Tuteja N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Archives of Biochemistry and Biophysics. 2008;471:146–158. doi: 10.1016/j.abb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Mason MG, Botella JR. Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proceedings of the National Academy of Sciences. 2000;97:14784–14788. doi: 10.1073/pnas.97.26.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR. Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression. 2001;1520:147–153. doi: 10.1016/s0167-4781(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Muschietti JP. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science. 2017;358:1600–1603. doi: 10.1126/science.aao5467. [DOI] [PubMed] [Google Scholar]
- Misra S, Wu Y, Venkataraman G, Sopory SK, Tuteja N. Heterotrimeric G protein complex and G protein coupled receptor from a legume (Pisum sativum): role in salinity and heat stress and cross talk with phospholipase C. The Plant Journal. 2007;51:656–669. doi: 10.1111/j.1365-313X.2007.03169.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plant. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Monshausen G, Bibikova T, Messerli M, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hair. Proceedings of the National Academy of Sciences. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity toleranc. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Murphy E, De Smet I. Understanding the RALF family: a tale of many specie. Trends in Plant Science. 2014;19:664–671. doi: 10.1016/j.tplants.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Pearce G, Moura DS, Stratmann J, Ryan CA. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proceedings of the National Academy of Sciences. 2001;98:12843–12847. doi: 10.1073/pnas.201416998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Jones AM, Assmann SM. Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Current Opinion in Plant Biology. 2004;7:719–731. doi: 10.1016/j.pbi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Pitman MG, Läuchli A. Salinity: environment-plants-molecules. Springer; Netherlands: 2002. Global impact of salinity and agricultural ecosystems; pp. 3–20. [Google Scholar]
- Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB. The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Current Biology. 2014;24:1887–1892. doi: 10.1016/j.cub.2014.06.064. [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proceedings of the National Academy of Sciences. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Rajagopal S. The β-arrestins: multifunctional regulators of G protein-coupled receptors. Journal of Biological Chemistry. 2016;291:8969–8977. doi: 10.1074/jbc.R115.713313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Zipfel C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signalin. Science. 2017;355:287–289. doi: 10.1126/science.aal2541. [DOI] [PubMed] [Google Scholar]
- Temple BRS, Jones AM. The plant heterotrimeric G-protein complex. Annual Review of Plant Biology. 2007;58:249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- Torres MA, Morales J, Sánchez-Rodríguez C, Molina A, Dangl JL. Functional interplay between Arabidopsis NADPH oxidases and heterotrimeric G protein. Molecular Plant-Microbe Interactions. 2013;26:686–694. doi: 10.1094/MPMI-10-12-0236-R. [DOI] [PubMed] [Google Scholar]
- Trusov Y, Botella JR. Plant G-proteins come of age: breaking the bond with animal models. Frontiers in chemistry. 2016;4:24. doi: 10.3389/fchem.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Botella JR. Heterotrimeric G protein γ subunits provide functional selectivity in G βγ dimer signaling in Arabidopsis. The Plant Cell. 2007;19:1235–1250. doi: 10.1105/tpc.107.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR, Jones AM. The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. The Plant Cell. 2003;15:393–409. doi: 10.1105/tpc.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science. 2001;292:2066–2069. doi: 10.1126/science.1059040. [DOI] [PubMed] [Google Scholar]
- Vitart V, Baxter I, Doerner P, Harper JF. Evidence for a role in growth and salt resistance of a plasma membrane H+‐ATPase in the root endodermis. The Plant Journal. 2001;27:191–201. doi: 10.1046/j.1365-313x.2001.01081.x. [DOI] [PubMed] [Google Scholar]
- Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H. Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1) Proceedings of the National Academy of Sciences. 1994;91:9554–9558. doi: 10.1073/pnas.91.20.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Langebartels C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell and Environment. 2002;25:717–726. [Google Scholar]
- Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Schumaker KS. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. The Plant Cell. 2010;22:1313–1332. doi: 10.1105/tpc.109.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Sorek H, Wemmer DE, Somerville CR. Cellulose Deficiency Is Enhanced on Hyper Accumulation of Sucrose by a H+-Coupled Sucrose Symporter. Plant Physiology. 2016;171:110–124. doi: 10.1104/pp.16.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Qian L, Nibau C, Duan Q, Kita D, Levasseur K, Hou C. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatas. Proceedings of the National Academy of Sciences. 2012;109:14693–14698. doi: 10.1073/pnas.1212547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Assmann SM. The heterotrimeric G-protein β subunit, AGB1, plays multiple roles in the Arabidopsis salinity response. Plant, Cell and Environment. 2015;38:2143–2156. doi: 10.1111/pce.12542. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chakravorty D, Assmann SM. The G protein β subunit, AGB1, interacts with FERONIA in RALF1-regulated stomatal movement. Plant Physiology. 2018:17.01277. doi: 10.1104/pp.17.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Li GJ, Ding L, Cui X, Berg H, Assmann SM, Xia Y. Arabidopsis extra large G-protein 2 (XLG2) interacts with the Gβ subunit of heterotrimeric G protein and functions in disease resistance. Molecular Plant. 2009;2:513–25. doi: 10.1093/mp/ssp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. ROS production in the roots after 24 h of 150 mM NaCl treatment is comparable among genotypes. Seedlings were grown on ½ MS media, 1% sucrose and 0.8% agar for 9 days and treated with 150 mM NaCl for 24 h. (a) Representative photographs and (b) quantification of H2DCFDA staining after 24h of 150 mM NaCl treatment. Data are means ± S.E. of 14-15 seedlings per genotype per treatment. (c) Representative photographs of DAB staining with or without 24 h of 150 mM NaCl treatment.
Supplemental Figure 2. agb1 shows wild-type response to RALF1-induced salt hypersensitivity but is less sensitive to RALF1 inhibition of root growth. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 days and treated with 1 μM RALF1 and/or 75 mM NaCl for 4 days. (a) Representative photographs of 3-day-old seedlings treated with RALF1 and/or NaCl for 4 days. (b) root elongation with or without 1 μM RALF1 treatment for 4 days. Data are means ± S.E. of 5 independent replicates, and each replicate contains 3-5 seedlings.
Supplemental Figure 3. fer-4 is not responsive to RALF1 in various seedling assays. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 or 4 days and treated with 1 μM RALF1 and/or 75 mM NaCl for various time. (a) Representative photographs of 3-day-old seedlings treated with RALF1 and/or NaCl for 4 days. (b) Root elongation with or without 1 μM RALF1 treatment for 4 days. Data are means ± S.E. of 5 independent replicates, and each replicate contains 3-5 seedlings. (c) External pH of 4-day-old seedlings treated with 1 μM RALF1 and/or 75 mM NaCl for 8 h. The media pH was measured in a plate reader using the fluorescent pH indicator fluorescein. Data are means ± S.E. of 6 independent replicates, and each replicate contains 8 seedlings.
Supplemental Figure 4. RALF1 is down-regulated in root tissue by 27 h of 150 mM NaCl treatment. Col seedlings were grown on ½ MS media, 1% sucrose and 0.8% agar for 9 days and treated with 150 mM NaCl for 3 h or 27 h. Shoots and roots were separated and RNA was extracted. Data are means ± S.E. of 3 independent replicates.
Supplemental Figure 5. Salt suppression of media acidification requires the Na+/H+ antiporter SOS1. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 3 or 4 days and treated with 1 μM RALF1 and/or NaCl for various times. (a) Representative photographs of 3-day-old seedlings treated with RALF1 and/or 50 mM, 75 mM and 100 mM NaCl for 4 days. (b) External pH of 4-day-old seedlings treated with 1 μM RALF1 and/or 75 mM NaCl for 8 h. The media pH was measured in a plate reader using the fluorescent pH indicator fluorescein. Data are means ± S.E. of 6 independent replicates, and each replicate contains 8 seedlings.
Supplemental Figure 6. RALF1 does not induce ROS production in the root meristematic zone in either the absence or presence of NaCl. ROS production was analyzed in the root meristematic zones using the same images as used for quantifying ROS in the elongation zone in Figure 7.
Supplemental Figure 7. Viability is not affected after 15 min of 1 μM RALF1 or 75 mM NaCl treatment. Seedlings were grown on ½ MS media, 0.5 mM sucrose and 0.5% agar for 4 days and treated with or without 1 μM RALF1 and/or 75 mM NaCl for 15 min. Seedlings were stained with fluorescein diacetate (FDA) and visualized by confocal microscopy. The root hair burst.


