Abstract
Triptolide, a major active constitute of Tripterygium wilfordii Hook. F, is prescribed for the treatment of autoimmune diseases in China. One of its most severe adverse effects observed in the clinical use is hepatotoxicity, but the mechanism is still unknown. Therefore, the present study applied an LC/MS-based metabolomic analysis to characterize the metabolomic changed in serum and liver induced by triptolide in mice. Mice were administered triptolide by gavage to establish the acute liver injury model, and serum biochemical and liver histological analysis were applied to assess the degree of toxicity. Multivariate data analyses were performed to investigate the metabolic alterations. Potential metabolites were identified using variable importance in the projection values and student’s t-test. A total of 30 metabolites were observed that were significantly changed by triptolide treatment and the abundance of 29 metabolites were correlated with the severity of toxicity. Pathway analysis indicated that the mechanism of triptolide-induced hepatotoxicity was related to alterations in multiple metabolic pathways, including glutathione metabolism, tricarboxylic acid cycle, purine metabolism, glycerophospholipid metabolism, taurine and hypotaurine metabolism, pantothenate and CoA biosynthesis, pyrimidine metabolism and amino acids metabolism. The current study provides new mechanistic insights into the metabolic alterations that lead to triptolide-induced hepatotoxicity.
Keywords: triptolide, hepatotoxicity, metabolomics, HILIC, UPLC-ESI-QTOFMS
1. Introduction
Triptolide is the principal active component extracted from Triptergium wilfordii Hook. F, which has been used for the treatment of rheumatoid arthritis and systemic lupus erythematosus (Han et al., 2012; Kong et al., 2013). Clinical trials also have been conducted in the United States to assess its safety and efficacy (Ziaei & Halaby, 2016). Recent studies have indicated that triptolide has multiple effects, including immunosuppression, anti-inflammatory, anti-cancer and antifertility activities (Meng et al., 2014; Ziaei & Halaby, 2016). However, the application of triptolide in the clinic is restricted by its narrow therapeutic window and potential for liver toxicity (Lu et al., 2017). Thus, it is important to elucidate the detailed mechanisms of triptolide-induced hepatotoxicity.
Metabolomics is an emerging ‘omics’ research field focusing on downstream alterations in low-molecular-weight metabolites resulting from changes in genomics, transcriptomics and proteomics (Sun, 2012; Wishart, 2016). In recent years, metabolomics has been widely applied in the identification of biomarkers related to drug toxicity and revealing the mechanisms of toxicity via changes of endogenous metabolites (Gonzalez et al., 2015). Since this systematic analysis strategy is highly consistent with the theory of traditional Chinese medicine (TCM), increasing studies have applied metabolomics to investigate TCM efficacy and toxicity (Cao et al., 2015; Dong et al., 2015). Some studies used metabolomic technologies to investigate the possible mechanisms of triptolide-induced hepatotoxicity. A GC-MS-based serum metabolomics study revealed that triptolide exposure could result in the perturbation of taurine, creatinine, free fatty acids (FFA), β-hydroxybutyrate, citric acid cycle intermediates and amino acids, which indicated impaired mitochondria function and β-oxidation of FFA (Aa et al., 2010). An 1H NMR-based serum and liver metabolomics study showed that treatment with triptolide lead to more severe hepatoxicity and nephrotoxicity in cytochrome P450 oxidoreductase knockout mice than in wild-type mice, revealing the molecular mechanisms of triptolide-induced toxicity under conditions of hepatic CYP inactivation (Liu et al., 2011). A UPLC-MS lipidomic analysis revealed that energy lipid modification, membrane remodeling, potential signaling lipid alterations and abnormal inflammation response were associated with TWHF-induced liver injury (Xie et al., 2016). Recently, a UPLC-ESI-QTOFMS urine metabolomic survey found that eight potential biomarkers associated with tryptophan, pantothenic acid and porphyrin were related to triptolide toxicity and licorice had potential protective therapeutic effects against such toxicity (Wang et al., 2017). Since many endogenous metabolites are highly polar molecular, the application of UPLC-ESI-QTOFMS using hydrophilic HILIC chromatography can improve the retention of polar metabolites (Joyce et al., 2016) and provide a relatively comprehensive separation method for the analysis of biological samples.
In the present study, UPLC-ESI-QTOFMS was applied with HILIC column chromatography to analyze the metabolic profiles of serum and liver tissues and to characterize the metabolic alterations between triptolide-treated mice and control mice by the identification of potential biomarkers and pathways, that lead to clues for the mechanisms of triptolide-induced hepatotoxicity.
2. Materials and Methods
2.1 Chemicals and reagents
Triptolide was purchased from Vicket Biological Technology Inc. (Chengdu, China). Chlorpropamide, aminopimelic acid, sulfadimethoxine, ammonium hydroxide and ammonium acetate were purchased from Sigma-Aldrich (Burlington, MA, USA). HPLC-grade methanol, acetonitrile and chloroform were purchased from Fisher Corporation (Pittsburgh, PA, USA). Ultrapure water was produced using the Milli-Q system (Millipore, Bedford, MA, USA). The alanine aminotransferase assay kit and aspartate aminotransferase assay kit were purchased from Catachem inc. (Oxford, UK).
2.2 Animal management
Male C57BL/6N mice weighing 20–25 g (6 to 8 weeks old) were obtained from the National Institutes of Health Contractor. Mouse experimental procedures were approved by the National Cancer Institute Animal Care and Use Committee. The mice were housed in a specific pathogen-free environment controlled for temperature and light (25°C,12-h light/dark cycle), and humidity (45–65%). The experiments were started after acclimatization for 1 week in the NCI vivarium.
2.3 Experimental design and sample collection
Mice were randomly divided into two groups with eight mice per group and given a single oral dose of triptolide (0.8 mg/kg) or the same volume of vehicle. Triptolide was dissolved at 0.08 mg/ml in 0.1% dimethyl sulfoxide (DMSO) in sterile saline. After triptolide exposure for 24 h, the mice were killed with CO2 asphyxiation and blood and liver tissues collected. Serum was obtained by centrifuging the blood for 10 min at 8,000 × g at 4°C. A portion of liver was fixed in 10% formalin solution for histological examination. Both serum and the rest of liver tissues were stored at −80°C for subsequent analysis.
2.4 Serum aminotransferase analysis
The levels of serum ALT and AST were quantified using commercially available kits. All procedures were under the manufacturer’s instructions.
2.5 Histological examination
To assess any histological changes in the liver after triptolide exposure, formalin-fixed liver tissues were embedded in paraffin, sectioned (5-μm-thick) and stained with hematoxylin-eosin followed by microscopic examination.
2.6 Sample preparation
Serum
Fifty μl of serum was mixed with 200 μl cold solution (chloroform/methanol, 2:1 v/v) containing chlorpropamide (20 μM) and aminopimelic acid (30 μM) as internal standards. After vortexing for 30 s at room temperature, the mixture was centrifuged at 13,000 × g for 5 min. The supernatant was transferred into a fresh tube and dried under vacuum and the resultant residue was dissolved in 200 μl acetonitrile/H2O/methanol (65:30:5 v/v/v), vortexed, and centrifuged at 18,000 × g at 4°C for 10 min. The supernatant was used for injection in UPLC-ESI-QTOFMS. The QC sample was pooled aliquots from all serum samples collected in the study.
Liver tissues
Liver tissues (~25 mg) were added to 700 μl solution (methanol/H2O, 4:3 v/v) containing chlorpropamide (20 μM) and aminopimelic acid (30 μM) as internal standards, homogenized using two 30 s pulses in a precellys 24 homogenizer (Bertin, France) at 5,500 × rpm. The homogenate was washed with 800 μl solution chloroform and incubated at 37°C for 20 min and centrifuged at 18,000 × g at 4°C for 15 min. Furthermore, supernatant was transferred to a fresh tube and dried under vacuum. The residue was dissolved in 300 μl solution (acetonitrile/H2O/methanol, 65:30:5 v/v/v), vortexed and centrifuged at 18,000 × g at 4°C for 10 min. The supernatant was used for injection in UPLC-ESI-QTOFMS. The QC sample was pooled aliquots from all liver samples collected in the study.
2.7 Mass spectrometry detection
MS analysis was performed using a Waters Acquity UPLC system (Waters, Milford, MA) equipped with a Waters Xevo G2 Quadrupole-Time of Flight (Q-TOF) mass spectrometer (Waters MS Technologies, Manchester, UK). The chromatographic separation was carried out at 40°C using a Waters Acquity BEH Amide column (2.1 mm × 50 mm, 1.7 μm) under basic conditions buffered with 10 mM ammonium acetate using the following composition: (A) 10% acetonitrile in water; (B) 90% acetonitrile in water. Both A and B were adjusted to pH 9.0 using ammonium hydroxide. The mobile phase consisted of a gradient system: 0–0.5 min, 99% B; 0.5–6.0 min, 99–60% B; 6.0–8.0 min, 60–20% B; 8.0–8.5 min, 20% B; 8.5–9.5 min, 20–99% B; 9.5–12.5 min, 99% B. The flow rate was 0.4 ml/min. The autosampler was maintained at 4°C, and all the samples were injected 5 μl for a run.
Mass spectrometry was performed on a Waters Xevo G2 Quadrupole-Time of Flight (Q-TOF) mass spectrometer (Waters MS Technologies, Manchester, UK) equipped with electrospray ionization. The optimal conditions were as follows: the mass range acquired was 50 – 1000 m/z at 0.2 second scans, the capillary voltage, and sample cone voltage was 2.8 kV and 40 V, the source temperature was set at 150°C, desolvation temperature was 550°C, cone and desolvation gas flow 50 and 950 L/h, respectively. Data were acquired in centroid mode in both positive and negative electrospray ionization modes, using sulfadimethoxine (m/z 311.0814+) as the Lock Mass. To eliminate complications due to artifacts related to injection order, all samples were analyzed in a randomized fashion.
2.8 Data processing and analysis
The original mass spectral data were generated using Masslynx software (Waters Corp.) for peak detection and alignment, and then normalized to the total ion intensity of each chromatogram to acquire a data matrix containing the m/z value, retention time, and the normalized peak area. The 80% rule (Bijlsma et al., 2006; Hodson et al., 2007) was applied to filter the ions. The data matrix obtained were imported to SIMCA-P+14 software (Umetrics, Umea, Sweden) for multivariate statistical analysis. Unsupervised principle component analysis (PCA) was used to show the distribution of all the samples. Partial least-squares discriminant analysis (PLS-DA) was applied to identify features contributed significantly to group separation. The statistical analysis was determined using a two-tailed student’s t-test in GraphPad Prism 7 (GraphPad Software, San Diego, CA) to compare the significant differences between two groups. All data in the figures were expressed as the means ± standard deviations. The value of p<0.05 was regarded as statistically significant in multivariable analysis and t-test.
3. Results
3.1 Toxic effect of triptolide treatment on liver
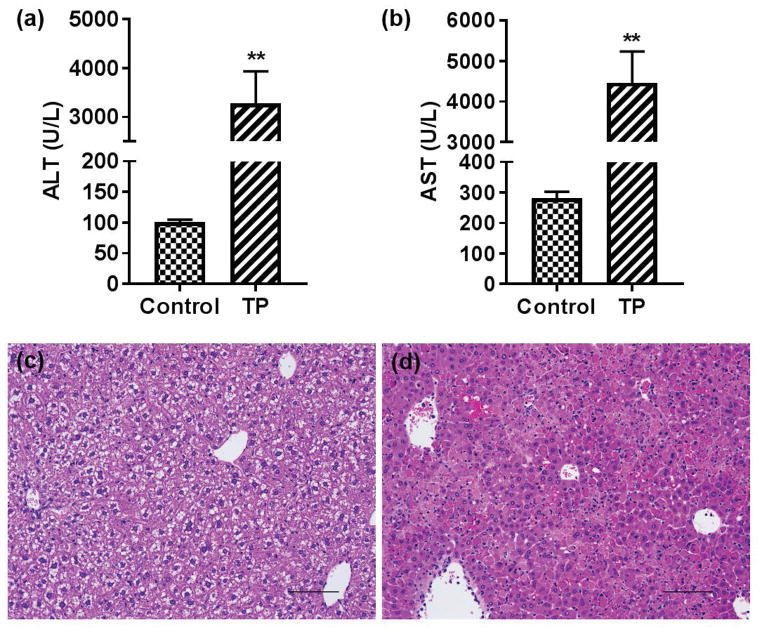
Compared to the control group, serum ALT and AST levels were significantly increased in the triptolide treatment group (Fig. 1a and b). Consistent with the serum ALT and AST levels, histological analysis showed severe necrosis with inflammatory cell infiltration in the livers of triptolide-treated mice (Fig. 1c and d). These data suggested that triptolide treatment at 0.8 mg/kg caused severe liver injury to the mice.
Fig. 1.
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels and the hematoxylin and eosin staining pictures of control and triptolide treatment group. (a) ALT levels of control and triptolide treatment group; (b) AST levels of control and triptolide treatment group; (c) histological examination of the control group (200×); (d) histological examination of the triptolide treatment group (200×). *p <0.05, between control and triptolide treatment group, **p<0.01, between control and triptolide treatment group
3.2 Metabolomics analysis
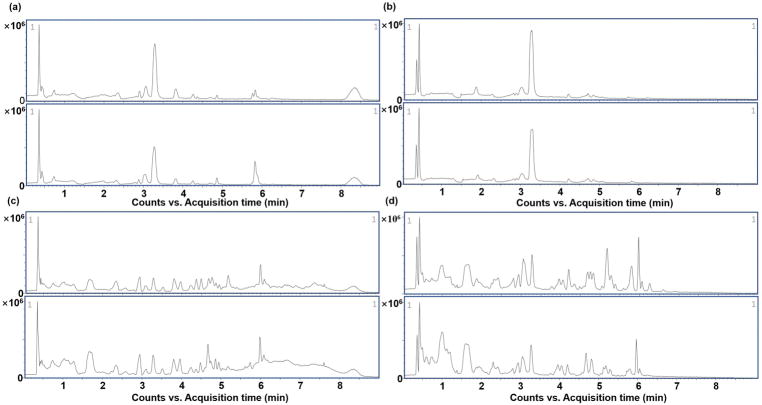
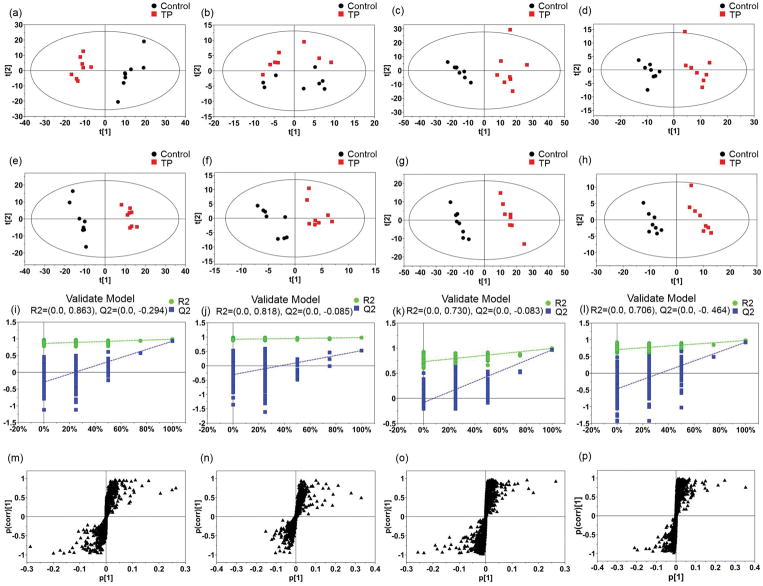
Total ion current (TIC) chromatograms of serum and liver samples from control and triptolide treatment group in both positive and negative modes were obtained (Fig. 2). During the acquisition process, quality control samples were used to validate the stability of the system. Scatter plots of PCA from the two groups (Fig. 3a–d) were applied to explore the metabolite changes and to filter the outliers. After showing a visualized illustration of the changes in metabolite profile induced by triptolide treatment, PLS-DA was applied to analyze the data sets from control and triptolide-treated mice. Both the control and triptolide treatment group clustered well and there was a clear separation between the two groups (Fig. 3e–h). In the positive mode from serum samples, the cumulative R2X was 0.491 and Q2 was 0.938. In the negative mode, the cumulative R2X was 0.576 and Q2 was 0.959. In the positive mode from liver samples, the cumulative R2X was 0.451 and Q2 was 0.965. In the negative mode, the cumulative R2X was 0.576 and Q2 was 0.959. Two rounds of cross validation were used in the PLS-DA analysis. Q2 > 0.4 was considered good for biological data (Lundstedt et al., 1998). Permutation testing results showed that there were no over-fitted in either the positive or negative mode from serum and liver samples (Fig. 3i–l). In the PLS-DA model, an S-plot was obtained to signal out the variable metabolites contributing to the group separation (Fig. 3m–p).
Fig. 2.
Representative of typical total ion chromatograms (TIC) of UPLC-ESI-QTOFMS. (a) TIC in positive mode of the serum samples; (b) TIC in negative mode of the serum samples; (c) TIC in positive mode of the liver samples; (d) TIC in negative mode of the liver samples. In each pair of images, the image above from the control group, and the image below from triptolide treatment group
Fig. 3.
Plots of multivariate statistical analysis based on the serum and liver metabolites. (a–d) PCA scores plots of serum and liver samples. (a, b) serum samples in positive mode and negative mode. (c, d) liver samples in positive mode and negative mode; (e–h) PLS-DA scores plots of serum and liver samples. (e, f) serum samples in positive mode and negative mode. (g, h) liver samples in positive mode and negative mode; (i–l) validation of PLS-DA model. (i, j) validation of serum samples in positive mode and negative mode. (k, l) validation of liver samples in positive mode and negative mode; (m–p) S-plots of serum and liver samples. (m, n) serum samples in positive mode and negative mode. (o, p) liver samples in positive mode and negative mode
3.3 Identification of differential metabolites and pathway analysis
The potential metabolites were screened based on the variable importance in the projection (VIP) values and a student’s t-test performed to validate the statistical significance. Only the features with VIP>1 and p<0.05 were considered as potential biomarkers for further investigation. In order to identify the potential metabolites, features with exact masses were matched on online metabolic databases including Metlin (http://metlin.scripps.edu), HMDB (http://www.hmdb.ca/) and PubChem (http://pubchem.ncbi.nlm.nih.gov) (the mass tolerance was ±10 ppm). All metabolites were confirmed by comparing the MS/MS fragment information in Metlin. A total of 30 metabolites were identified as potential biomarkers contributing to triptolide treatment. In serum samples, there were 5 and 4 changed metabolites identified in positive and negative mode, respectively. In addition, 11 and 10 changed metabolites were identified in positive and negative mode from liver samples. All identified metabolites are listed in Table 1.
Table 1.
Potential biomarkers related to triptolide-induced hepatotoxicity and their metabolic pathways
| No. | Retention time (min) | m/z | Ion | Formula | Metabolite | VIP | FC | P | Pathway |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.7148 | 130.051 | [M+H]+ | C5H7NO3 | N-Acryloylglycinea | 4.51 | 0.77 | 0.00009654 | - |
| 2 | 3.8153 | 162.113 | [M+H]+ | C7H15NO3 | Carnitinea | 20.08 | 0.78 | 0.00034823 | - |
| 3 | 3.0422 | 204.123 | [M+H]+ | C9H17NO4 | Acetylcarnitinea | 16.39 | 1.18 | 0.00575433 | - |
| 4 | 2.3531 | 235.167 | [M+NH4]+ | C10H19NO4 | Propionylcarnitinea | 1.53 | 0.07 | 0.00000263 | - |
| 5 | 4.6764 | 290.16 | [M+H]+ | C13H23NO6 | 3-Methylglutarylcarnitinea | 6.23 | 10.09 | 0.00000074 | - |
| 6 | 4.6199 | 101.025 | [M−H]− | C4H6O3 | Succinic acid semialdehydea | 1.07 | 2.25 | 0.000364 | Alanine, Aspartate and glutamate metabolism |
| 7 | 2.2837 | 130.087 | [M−H]− | C6H13NO2 | L-Leucinea | 2.46 | 1.64 | 0.00005611 | Valine, leucine and isoleucine biosynthesis |
| 8 | 4.2165 | 145.062 | [M−H]− | C5H10N2O3 | L-Glutaminea | 2.23 | 2.84 | 0.00000008 | Purine metabolism |
| 9 | 0.4156 | 281.248 | [M−H]− | C18H34O2 | Oleic Acida | 1.52 | 0.31 | 0.007414 | - |
| 10 | 2.5675 | 126.023 | [M+H]+ | C2H7NO3S | Taurineb | 2.83 | 0.85 | 0.0554102 | Taurine and hypotaurine metabolism |
| 11 | 4.385 | 136.063 | [M+H]+ | C5H5N5 | Adenineb | 1.19 | 2.30 | 0.00000069 | Purine metabolism |
| 12 | 1.069 | 137.047 | [M+H]+ | C5H4N4O | Hypoxanthineb | 8.74 | 2.23 | 0.00254027 | Purine metabolism |
| 13 | 6.2476 | 145.051 | [M+H]+ | C6H8O4 | 2-Methylglutaconic acidb | 1.12 | 0.39 | 0.00002626 | - |
| 14 | 2.3816 | 152.058 | [M+H]+ | C5H5N5O | Guanineb | 2.02 | 2.54 | 0.00000069 | Purine metabolism |
| 15 | 4.3541 | 184.075 | [M+H]+ | C5H14NO4P | Phosphocholineb | 1.23 | 0.29 | 0.00000000 | Glycerophospholipid metabolism |
| 16 | 1.6621 | 269.089 | [M+H]+ | C10H12N4O5 | Inosineb | 2.97 | 3.09 | 0.0000331 | Purine metabolism |
| 17 | 4.7667 | 308.091 | [M+H]+ | C10H17N3O6S | Glutathioneb | 9.47 | 0.29 | 0.00002435 | Glutathione metabolism |
| 18 | 4.7807 | 346.057 | [M+H]+ | C10H12N5O7P | cGMPb | 1.37 | 0.47 | 0.00000597 | Purine metabolism |
| 19 | 5.3021 | 405.008 | [M+H]+ | C9H14N2O12P2 | UDPb | 2.77 | 0.39 | 0.00002522 | Pyrimidine metabolism |
| 20 | 0.454 | 520.34 | [M+H]+ | C26H50NO7P | LysoPC (18:2)b | 1.04 | 1.89 | 0.00000073 | Glycerophospholipid metabolism |
| 21 | 0.9945 | 111.02 | [M−H]− | C4H4N2O2 | Uracilb | 1.64 | 2.54 | 0.00000002 | Pyrimidine metabolism |
| 22 | 3.0229 | 116.072 | [M−H]− | C5H11NO2 | L-Valineb | 1.88 | 1.94 | 0.00003953 | Valine, leucine and isoleucine biosynthesis |
| 23 | 4.8267 | 133.014 | [M−H]− | C4H6O5 | Malic acidb | 4.78 | 1.36 | 0.02799705 | Citric acid cycle |
| 24 | 2.3185 | 145.015 | [M−H]− | C5H6O5 | 2-Oxoglutaric acidb | 1.32 | 0.10 | 0.00000054 | Citric acid cycle |
| 25 | 4.6829 | 146.046 | [M−H]− | C5H9NO4 | L-Glutamateb | 6.34 | 1.71 | 0.00000061 | Glutathione metabolism |
| 26 | 1.98 | 151.026 | [M−H]− | C5H4N4O2 | Xanthineb | 8.97 | 1.66 | 0.00492014 | Purine metabolism |
| 27 | 5.866 | 191.02 | [M−H]− | C6H8O7 | Citric acidb | 6.2 | 9.98 | 0.00011684 | Citric acid cycle |
| 28 | 2.3163 | 282.082 | [M−H]− | C10H13N5O5 | Guanosineb | 1.25 | 2.97 | 0.00000001 | Purine metabolism |
| 29 | 4.3591 | 357.089 | [M−H]− | C11H23N2O7PS | 4′-Phosphopantetheneb | 1.03 | 0.28 | 0.00219127 | Pantothenate and CoA biosynthesis |
| 30 | 1.012 | 514.282 | [M−H]− | C26H45NO7S | Taurocholic acidb | 6.82 | 2.29 | 0.01677127 | Taurine and hypotaurine metabolism |
Metabolites detected in serum and
metabolites detected in liver tissues.
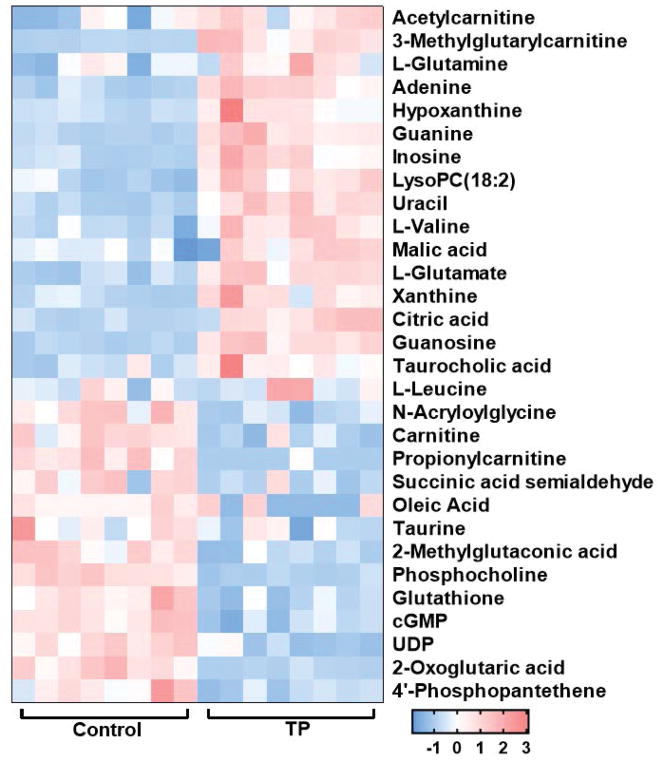
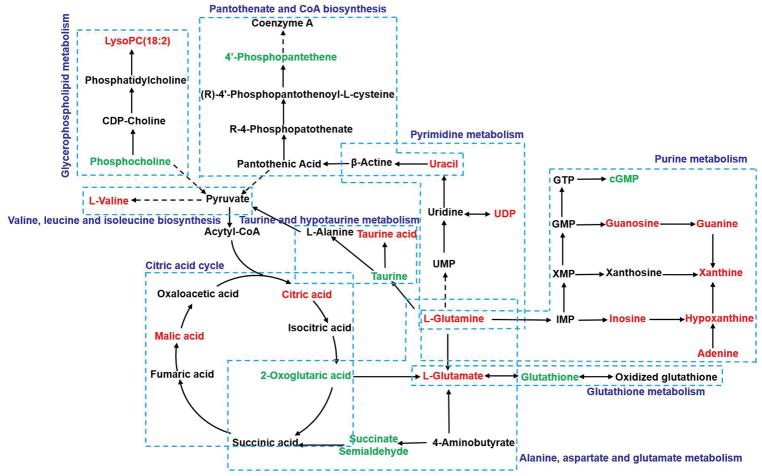
A heat map was generated to reveal the dynamic changes of the potential metabolites between the control and triptolide treatment group (Fig. 4). The correlation between the abundance of 30 metabolites and the levels of serum ALT and AST was assessed by using the Spearman’s rank correlation test. The abundance of 29 metabolites were correlated with the severe of toxicity (p<0.05) (Table 2). In order to obtain an integrated metabolic network, the changed metabolites subjected to MetaboAnalyst 4.0 (http://www.metaboanalyst.ca/) and KEGG database (http://www.genome.jp/kegg/). A metabolic network (Fig. 5) illustrated that these metabolites were found to be mainly associated with several pathways, including glutathione metabolism, tricarboxylic acid (TCA) cycle, purine metabolism, glycerophospholipid metabolism, taurine and hypotaurine metabolism, pantothenate and CoA biosynthesis, pyrimidine metabolism and amino acids metabolism including alanine, aspartate and glutamate metabolism and valine, leucine and isoleucine biosynthesis.
Fig. 4.
Heat map based on the 30 potential metabolites in serum and liver tissues. Each row represents a metabolite, and the columns showes the expression levels marked with different colors according to the sample type. The red color represents increased metabolites, while the blue color indicates decreased metabolites.
Table 2.
Correlation between the abundance of 30 metabolites and the levels of serum ALT and AST
| metabolite | ALT | AST | ||
|---|---|---|---|---|
| r | p | r | p | |
| Acetylcarnitine | 0.6794 | 0.0048 | 0.4647 | 0.0718 |
| 3-Methylglutarylcarnitine | 0.7765 | 0.0007 | 0.9641 | 0.0037 |
| L-leucine | 0.1558 | 0.5561 | 0.1059 | 0.6968 |
| L-Glutamine | 0.5441 | 0.0316 | 0.4059 | 0.1201 |
| Adenine | 0.85 | <0.0001 | 0.7412 | 0.0015 |
| Hypoxanthine | 0.7853 | 0.0005 | 0.7941 | 0.0004 |
| Guanine | 0.7471 | 0.0013 | 0.7441 | 0.0014 |
| Inosine | 0.6529 | 0.0074 | 0.8 | 0.0003 |
| LysoPC (18:2) | 0.6618 | 0.0065 | 0.8588 | <0.0001 |
| Uracil | 0.7088 | 0.0029 | 0.7088 | 0.0029 |
| L-Valine | 0.7941 | 0.0004 | 0.8206 | 0.0002 |
| Malic acid | 0.5088 | 0.0464 | 0.5941 | 0.0172 |
| L-Glutamate | 0.7382 | 0.0016 | 0.6088 | 0.0141 |
| Xanthine | 0.7188 | 0.0027 | 0.8794 | <0.0001 |
| Citric acid | 0.6882 | 0.0042 | 0.0044 | 0.6853 |
| Guanosine | 0.75 | 0.0012 | 0.7824 | 0.0006 |
| Taurocholic acid | 0.7353 | 0.0017 | 0.75 | 0.0012 |
| N-Acryloylglycine | −0.7324 | 0.0018 | −0.8441 | <0.0001 |
| Carnitine | −0.5176 | 0.0423 | −0.5588 | 0.0266 |
| Propionylcarnitine | −0.7768 | 0.0008 | −0.7983 | 0.0004 |
| Succinic acid semialdehyde | −0.644 | 0.0153 | −0.518 | 0.0418 |
| Oleic Acid | −0.4242 | 0.1324 | −0.5254 | 0.0386 |
| Taurine | −0.3147 | 0.2347 | −0.5529 | 0.0285 |
| 2-Methylglutaconic acid | −0.8265 | 0.0002 | −0.7353 | 0.0017 |
| Phosphocholine | −0.8176 | 0.0002 | −0.6647 | 0.0062 |
| Glutathione | −0.7 | 0.0034 | −0.8441 | <0.0001 |
| cGMP | −0.6735 | 0.0053 | −0.8088 | 0.0003 |
| UDP | −0.6676 | 0.0059 | −0.8059 | 0.0003 |
| 2-Oxoglutaric acid | −0.7794 | 0.0006 | −0.8029 | 0.0003 |
| 4′-Phosphopantethene | −0.6464 | 0.0110 | −0.825 | 0.0003 |
Fig. 5.
Overview of metabolic pathways based on the differential metabolites related to triptolide-induced acute hepatotoxicity. Metabolite with red color represents it is increased compared to control group, and metabolite with green color represents it is decreased compared to control group.
4. Discussion
Triptolide, a major active component of Triptergium wilfordii Hook. F, has therapeutic effects on many diseases as a result of its anti-inflammatory, immunosuppressive and anti-neoplastic properties. However, the clinical application of triptolide has been limited by its liver toxicity. Although it was reported that the mechanism of triptolide induced hepatotoxicity may be due to oxidative stress, apoptosis and autophagy (Cao et al., 2015; Chan et al., 2017), the main cause of liver damage has not been well characterized. In this study, an integrated metabolomic study was applied to analysis serum and liver samples in control and triptolide-treated mice. Totally 30 (9 serum samples and 21 liver samples) significantly changed metabolites were identified, and 31 metabolites were regarded as potential biomarkers related to triptolide-induced hepatotoxicity. Based on online database including MetaboAnalyst and KEGG databases, we obtained several metabolic pathways associated with these changed metabolites as we presented above.
Glutathione (GSH), a predominant low-molecular-weight thiol antioxidant, plays a critical role in protecting against oxidative damage (Forman et al., 2009). Accumulated studies revealed that disturbances of GSH homeostasis have been associated with xenobiotics-induced liver disease (Chen et al., 2013). GSH contains a cysteinyl residue that can provide a thiol (−SH) group to electrophilic toxic chemicals converting into conjugated compounds mediated by glutathione S-transferase (GSTs) (Chasseaud, 1979). In addition, through glutathione peroxidase (GPx), GSH can donate electrons to react with reactive oxygen species (ROS) inhibiting the oxidative damage, during which GSH is oxidized to oxidized glutathione (GSSG) (Aoyama & Nakaki, 2012). In turn, GSSG can be reduced back to GSH by glutathione reductase (Zhang & Forman, 2012). Previous studies found that triptolide caused oxidative stress by increasing the generation of ROS and decreasing the activities of some antioxidant enzymes (SOD, GPx and catalase) (Li et al., 2014). In the present study, the level of GSH was significantly decreased in mice from the triptolide treatment group. L-Glutamate, a substrate for GSH production, increased significantly. Thus, GSH depleted by increased ROS due to triptolide-induced oxidative stress, caused the increase in L-glutamate levels for GSH production. The decreased GSH can diminish the livers protective effect against oxidative damage.
The TCA cycle is the most important energy cycle metabolic pathway for carbohydrates, lipids and proteins, and plays a crucial role in connecting almost all the individual metabolic pathways (Akram, 2014). Some studies suggested that perturbation of TCA cycle was highly associated with drug-induced liver injury (Satapati et al., 2012). Citric acid, 2-oxoglutaric acid and malic acid are intermediates of the TCA cycle. One study reported that increased levels of citric acid and 2-oxoglutaric acid were observed in realgar-induced hepatotoxicity, indicating that energy metabolism is disturbed after the treatment of realgar (Huo et al., 2016). Other studies revealed that disturbance of the TCA cycle could lead to an increased in the content of malic acid in rats exposed to CCl4 (Gao et al., 2017). 2-Oxoglutaric acid is the key molecule in determining the overall rate of TCA cycle, and it is also a source of glutamate (Wu et al., 2016). In the present study, citric acid and malic acid contents were significantly increased, and the content of 2-oxoglutaric acid was decreased in the mice from the triptolide-treated mice, indicating alterations of the TCA cycle. The decrease in 2-oxoglutaric acid level could inhibit the TCA cycle to reduce the production of ROS under oxidative stress induced by triptolide treatment.
Purines are groups of essential cellular constituents which are involved in synthesis of RNA and DNA, metabolic regulation and energy transfer. In the present study, metabolites related to purine metabolism were observed markedly increased in mice after triptolide treatment, including guanosine, guanine, inosine, adenine, hypoxanthine, and xanthine, which suggested the enhancement of purine metabolism pathway. In addition, a previous study revealed that increased metabolism of purines could lead to accumulation of hepatic contents of hypoxanthine and xanthine in ethanol induced liver damage (Kato et al., 1990). As per the KEGG pathway map, xanthine can be synthesized in two ways: from guanine and from hypoxanthine. Xanthine oxidase (XO) is an enzyme that catalyzes the oxidation of hypoxanthine to xanthine with the generation of ROS (Ardan et al., 2004). The increased xanthine may be attributed to the abnormal XO activity which further aggravated the oxidative stress. Cyclic guanosine 3′,5′-monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP) and acts as a second messenger regulating foundational cellular process. Several studies reported that cGMP could suppress cell death by inhibiting activation of caspase via cGMP-dependent protein kinase in primary hepatocytes (Li et al., 2000). In the present study, compared to the control group, cGMP was significantly decreased in mice from triptolide treatment group. This reduction may further decrease anti-apoptosis effect of cGMP on liver cellular.
Glycerophospholipids (GPs), known as phospholipids, are the main constituents of membrane biolayers, and participate in regulating many cellular processes. Lysophosphatidylcholine (LysoPC) is a class of substance from GPs and plays a vital role in various liver disease. In the present study, the level of LysoPC was significantly increased in mice from the triptolide treatment group, which was consistent with another report (Xie et al., 2016). Previously studies revealed that LysoPC was a strong proinflammatory mediator, which could lead to the release of arachidonic acid (AA) by activating phospholipase A2 (PLA2) in two signaling pathways. Phosphocholine is an intermediate in the synthesis of phosphatidylcholine. A recent study demonstrated that choline, phosphocholine and phosphocholine-modified protein could efficiently inhibit the release of proinflammatory factor IL-1β via nicotinic acetylcholine receptors in human and rat monocytes (Hecker et al., 2015). In the present study, phosphocholine decreased in response to triptolide exposure, which was in accordance with increased LysoPC. These data suggested that changes occur in the LysoPC and phosphocholine contents were induced by the dysregulation of glycerophospholipid metabolism.
Taurine, an essential amino acid, participates in protecting numerous physiological functions including modulation of calcium levels, antioxidation and stabilization of membranes. Some studies have reported that taurine could ameliorate drug-induced chronic or acute liver injury via its anti-oxidative and anti-apoptotic activities (Heidari et al., 2016). In the present study, the decreased taurine may be attributed to the enhanced synthesis of taurocholic acid after triptolide exposure. This further reduced the protective effect of taurine against triptolide-induced oxidative stress and apoptosis. In addition, several amino acids were markedly increased including L-valine, L-glutamine and L-glutamate in the triptolide-treated group compared with the control group. Elevated levels of these amino acids suggested that disorders of protein biosynthesis and catabolism were involved in triptolide-induced liver injury.
5. Conclusion
In the present study, a metabolomic strategy based on UPLC-ESI-QTOFMS was applied to profile serum and liver metabolic alternations in mice with triptolide-induced liver injury. In total, 30 metabolites have been identified and 29 metabolites correlated with the severity of toxicity were selected as potential biomarkers for triptolide-induced hepatotoxicity. These changed metabolites were involved in several pathways, including glutathione metabolism, TCA cycle, purine metabolism, glycerophospholipid metabolism, taurine and hypotaurine metabolism, pantothenate and CoA biosynthesis, pyrimidine metabolism and amino acids metabolism. Present study provided an integral understanding of mechanism in toxic effect, and it may be useful for further prediction and diagnosis of liver injury at an early stage during triptolide’s clinical use.
Acknowledgments
This study was supported by the National Cancer Institute Intramural Research Program and by Hebei Science and Technology Department in China (17392501D). JZ was supported by a student fellowship from the China Scholarship Councils.
References
- Aa J, Shao F, Wang G, Huang Q, Zha W, Yan B, Zheng T, Liu L, Cao B, Shi J, Li M, Zhao C, Wang X, Wu Z. Gas chromatography time-of-flight mass spectrometry based metabolomic approach to evaluating toxicity of triptolide. Metabolomics. 2010;7:217–225. [Google Scholar]
- Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys. 2014;68:475–8. doi: 10.1007/s12013-013-9750-1. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Nakaki T. Inhibition of GTRAP3-18 may increase neuroprotective glutathione (GSH) synthesis. Int J Mol Sci. 2012;13:12017–35. doi: 10.3390/ijms130912017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardan T, Kovaceva J, Cejkova J. Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium. Acta Histochem. 2004;106:69–75. doi: 10.1016/j.acthis.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Bijlsma S, Bobeldijk I, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, van Ommen B, Smilde AK. Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem. 2006;78:567–74. doi: 10.1021/ac051495j. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang A, Zhang H, Sun H, Wang X. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother Res. 2015;29:159–66. doi: 10.1002/ptr.5240. [DOI] [PubMed] [Google Scholar]
- Chan SF, Chen YY, Lin JJ, Liao CL, Ko YC, Tang NY, Kuo CL, Liu KC, Chung JG. Triptolide induced cell death through apoptosis and autophagy in murine leukemia WEHI-3 cells in vitro and promoting immune responses in WEHI-3 generated leukemia mice in vivo. Environ Toxicol. 2017;32:550–568. doi: 10.1002/tox.22259. [DOI] [PubMed] [Google Scholar]
- Chasseaud LF. The Role of Glutathione and Glutathione S-Transferases in the Metabolism of Chemical Carcinogens and Other Electrophilic Agents. 1979 doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol. 2013;60:38–44. doi: 10.1016/j.fct.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yan G-L, Han Y, Sun H, Zhang A-H, Li X-N, Wang X-J. UPLC-Q-TOF/MS-based metabolomic studies on the toxicity mechanisms of traditional Chinese medicine Chuanwu and the detoxification mechanisms of Gancao, Baishao, and Ganjiang. Chinese Journal of Natural Medicines. 2015;13:687–698. doi: 10.1016/S1875-5364(15)30067-4. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Qin XJ, Jiang H, Chen JF, Wang T, Zhang T, Xu SZ, Song JM. Detecting serum and urine metabolic profile changes of CCl4-liver fibrosis in rats at 12 weeks based on gas chromatography-mass spectrometry. Exp Ther Med. 2017;14:1496–1504. doi: 10.3892/etm.2017.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ, Fang ZZ, Ma X. Transgenic mice and metabolomics for study of hepatic xenobiotic metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2015;11:869–81. doi: 10.1517/17425255.2015.1032245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74:424–36. doi: 10.1111/j.1365-2125.2012.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker A, Kullmar M, Wilker S, Richter K, Zakrzewicz A, Atanasova S, Mathes V, Timm T, Lerner S, Klein J, Kaufmann A, Bauer S, Padberg W, Kummer W, Janciauskiene S, Fronius M, Schweda EK, Lochnit G, Grau V. Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1beta Release. J Immunol. 2015;195:2325–34. doi: 10.4049/jimmunol.1400974. [DOI] [PubMed] [Google Scholar]
- Heidari R, Jamshidzadeh A, Niknahad H, Mardani E, Ommati MM, Azarpira N, Khodaei F, Zarei A, Ayarzadeh M, Mousavi S, Abdoli N, Yeganeh BS, Saeedi A, Najibi A. Effect of taurine on chronic and acute liver injury: Focus on blood and brain ammonia. Toxicol Rep. 2016;3:870–879. doi: 10.1016/j.toxrep.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson MP, Dear GJ, Roberts AD, Haylock CL, Ball RJ, Plumb RS, Stumpf CL, Griffin JL, Haselden JN. A gender-specific discriminator in Sprague-Dawley rat urine: the deployment of a metabolic profiling strategy for biomarker discovery and identification. Anal Biochem. 2007;362:182–92. doi: 10.1016/j.ab.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Huo T, Fang Y, Zhao L, Xiong Z, Zhang Y, Wang Y, Feng C, Yuan M, Wang S, Chen M, Jiang H. (1)HNMR-based metabonomic study of sub-chronic hepatotoxicity induced by realgar. J Ethnopharmacol. 2016;192:1–9. doi: 10.1016/j.jep.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Joyce R, Kuziene V, Zou X, Wang X, Pullen F, Loo RL. Development and validation of an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry method for rapid quantification of free amino acids in human urine. Amino Acids. 2016;48:219–34. doi: 10.1007/s00726-015-2076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kawase T, Alderman J, Inatomi N, Lieber CS. Role of xanthine oxidase in ethanol-induced lipid peroxidation in rats. Gastroenterology. 1990;98:203–10. doi: 10.1016/0016-5085(90)91311-s. [DOI] [PubMed] [Google Scholar]
- Kong X, Zhang Y, Liu C, Guo W, Li X, Su X, Wan H, Sun Y, Lin N. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS One. 2013;8:e77513. doi: 10.1371/journal.pone.0077513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shen F, Guan C, Wang W, Sun X, Fu X, Huang M, Jin J, Huang Z. Activation of Nrf2 protects against triptolide-induced hepatotoxicity. PLoS One. 2014;9:e100685. doi: 10.1371/journal.pone.0100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang S, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor alpha-mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. J Biol Chem. 2000;275:13026–34. doi: 10.1074/jbc.275.17.13026. [DOI] [PubMed] [Google Scholar]
- Liu X, Xue X, Gong L, Qi X, Wu Y, Xing G, Luan Y, Xiao Y, Wu X, Li Y, Chen M, Miao L, Yao J, Gu J, Lin D, Ren J. 1H NMR-based metabolomic analysis of triptolide-induced toxicity in liver-specific cytochrome P450 reductase knockout mice. Metabolomics. 2011;8:907–918. [Google Scholar]
- Lu Y, Xie T, Zhang Y, Zhou F, Ruan J, Zhu W, Zhu H, Feng Z, Zhou X. Triptolide Induces hepatotoxicity via inhibition of CYP450s in Rat liver microsomes. BMC Complement Altern Med. 2017;17:15. doi: 10.1186/s12906-016-1504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstedt T, Seifert E, Abramo L, Thelin B, Nystrom A, Pettersen J, Bergman R. experimental design and optimization. Chemometrics and Intelligent Laboratory Systems. 1998;42:3–40. [Google Scholar]
- Meng C, Zhu H, Song H, Wang Z, Huang G, Li D, Ma Z, Ma J, Qin Q, Sun X, Ma J. Targets and molecular mechanisms of triptolide in cancer therapy. Chin J Cancer Res. 2014;26:622–6. doi: 10.3978/j.issn.1000-9604.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–92. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. Metabolomics in Drug-induced Toxicity and Drug Metabolism. Journal of Drug Metabolism & Toxicology. 2012:03. [Google Scholar]
- Wang Z, Liu JQ, Xu JD, Zhu H, Kong M, Zhang GH, Duan SM, Li XY, Li GF, Liu LF, Li SL. UPLC/ESI-QTOF-MS-based metabolomics survey on the toxicity of triptolide and detoxication of licorice. Chin J Nat Med. 2017;15:474–480. doi: 10.1016/S1875-5364(17)30071-7. [DOI] [PubMed] [Google Scholar]
- Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016;15:473–84. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol Ther (Seoul) 2016;24:1–8. doi: 10.4062/biomolther.2015.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Zhou X, Wang S, Lu Y, Zhu H, Kang A, Deng H, Xu J, Shen C, Di L, Shan J. Development and application of a comprehensive lipidomic analysis to investigate Tripterygium wilfordii-induced liver injury. Anal Bioanal Chem. 2016;408:4341–55. doi: 10.1007/s00216-016-9533-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Glutathione synthesis and its role in redox signaling. Semin Cell Dev Biol. 2012;23:722–8. doi: 10.1016/j.semcdb.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei S, Halaby R. Immunosuppressive, anti-inflammatory and anti-cancer properties of triptolide: A mini review. Avicenna J Phytomed. 2016;6:149–64. [PMC free article] [PubMed] [Google Scholar]