Abstract
Background and aim
The distinction of intestinal fibrosis from inflammation in Crohn’s disease (CD) associated strictures has important therapeutic implications. Ultrasound elastography is useful in evaluating the degree of fibrosis in liver, but there is little evidence whether it can assess fibrosis in the bowel. We determined whether shear-wave elastography (SWE), a novel modification of elastography, quantifying tissue stiffness, could differentiate between inflammatory and fibrotic components in strictures of patients with CD.
Methods
Consecutive CD patients with ileal/ileocolonic strictures who underwent SWE within 1 week to surgical resection were enrolled. The SWE value of the stenotic bowel wall was compared to the grade and severity of fibrosis and inflammation, respectively, in the resected bowel specimen.
Results
Thirty-five patients were enrolled. The mean SWE value of stenotic bowel wall was significantly higher in severe fibrosis (23.0 ± 6.3 Kpa) than that in moderate (17.4 ± 3.8 Kpa) and mild fibrosis (14.4 ± 2.1 Kpa)(P = 0.008). Using 22.55 KPa as the cutoff value in discriminating between mild/moderate and severe fibrosis, the sensitivity and specificity was 69.6 % and 91.7% with an area under the curve (AUC) of 0.822 (P = 0.002). However, no significant difference regarding mean SWE existed among different grades of inflammation. The sensitivity and specificity of bowel vascularization score on conventional ultrasound in differentiating severe inflammation from mild/moderate was 87.5 % and 57.9% with AUC of 0.811 (P = 0.002). Combining SWE and conventional ultrasound (bowel vascularization score), we propose a bowel ultrasound classification of intestinal strictures. A moderate agreement between ultrasound and pathological classification was observed (κ = 0.536, P<0.001).
Conclusions
This pilot study suggests that SWE is feasible and accurate in detecting intestinal fibrosis in patients with CD. After validation, combing SWE and bowel vascularization on conventional ultrasound might be applied to guide a management strategy in CD patients through defining the type of intestinal stricture.
Keywords: stricture, Crohn’s disease, elastography
INTRODUCTION
Crohn’s disease (CD) is a chronic gastrointestinal inflammatory disease characterized by segmental and transmural bowel wall inflammation that often progresses to fibrosis with intestinal strictures and obstruction, fistulas, and abscesses.1 Although modern medical therapies such as biologic agents have revolutionized treatment of CD, the cumulative rate of surgery for CD remains high.2–4 Nearly half of the indications for surgery are due to bowel obstruction caused by intestinal strictures.2, 5 Based on histologic assessment, intestinal strictures can be classified according to components of inflammation and fibrosis. Inflammation may be relieved by anti-inflammatory medical treatment, whereas fibrosis may require endoscopic or surgical treatment.6 Therefore, precisely defining the type of CD strictures is crucial for deciding on the best management strategy.
Computed tomography enterography (CTE) has a limited role in assessing the degree of fibrosis on bowel stenosis, whereas magnetic resonance enterography (MRE) has potential tools for better assessing the degree of fibrosis.7, 8 However, radiation exposure of CTE and cost of MRE limit their use. As a noninvasive method without radiation exposure, conventional bowel ultrasound has been used for detecting disease activity and complications in CD with reasonable sensitivity and specificity.9 However, information obtained from conventional ultrasound is mostly insufficient for an accurate differentiation between inflammatory and fibrotic strictures.9
Ultrasound elastography has been proposed for differentiating different degrees of intestinal fibrosis in CD, but this was mostly tested in animal models or ex vivo and human studies are lacking.10–12 Shear-wave elastography (SWE) is a novel technique, which estimates the speed of a shear wave, providing a quantitative estimate of tissue stiffness. It has the advantage of being able to image tissue stiffness in real time. This method could lead to a more accurate grading of fibrosis based on the image guidance.13 SWE has been used to assess breast tumors, thyroid nodules, and liver fibrosis.13–15 Recently, SWE was reported to be used in CD animal models and ex vivo human bowel specimens with CD.11, 12 Lu et al16 reported that SWE could reflect intestinal muscular hypertrophy in patients with CD. However, whether SWE can distinguish inflamed from fibrotic bowel segments has seldom been investigated. The purpose of this study was to evaluate the role of SWE for the characterization of intestinal fibrosis compared with conventional bowel ultrasound, and the capability of SWE in differentiating fibrotic from inflammatory strictures in patients with CD using surgical histopathology as the reference standard.
MATERIAL AND METHODS
Design and Patients
This is a prospective cross-sectional study. From April 2015 to May 2017, consecutive patients with ileal/ileocolonic CD scheduled for surgery because of symptomatic stenosis who consented to SWE examination were enrolled to the study. Inclusion criteria were: (1) age 18–75 years; (2) the interval between SWE and intended selective surgery less than 1 week, and (3) stenotic bowel with or without proximal dilation could be clearly demonstrated by conventional ultrasound. The exclusion criteria included inadequate SWE image quality. The study protocol was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University.
Conventional Bowel Ultrasound
All bowel ultrasound examinations and real-time SWE were conducted by an experienced ultrasonographist (Xiao-yan Xie ), who has more than 5 years of experience in bowel ultrasound. Aixplorer US system (SuperSonic Imagine S.A., Aix-en-Provence, France) with a convex broadband probe (SC6-1) and linear array probe (SL 15-4) was used. The examiner was blinded to clinical, laboratory, and radiological findings. All patients underwent US examinations within 1 week before surgery.
Firstly, conventional ultrasound mode was used to evaluate bowel wall thickness (mm), presence of stratified echo pattern, and proximal dilation. In addition, a previously reported Limberg score of bowel vascularization in the stenotic bowel was recorded.17 Briefly, Limberg score of bowel vascularization was graded as I, II, III, and IV by color Doppler ultrasound: grade I, intestine wall thickening (>3 mm) without discovered vascularization; grade II, intestine wall thickening with spot vascular signals; grade III, intestine wall thickening with longer vascular signals; and grade IV, intestinal wall thickening with longer vascular signals extending from the mesentery.
Real-time SWE
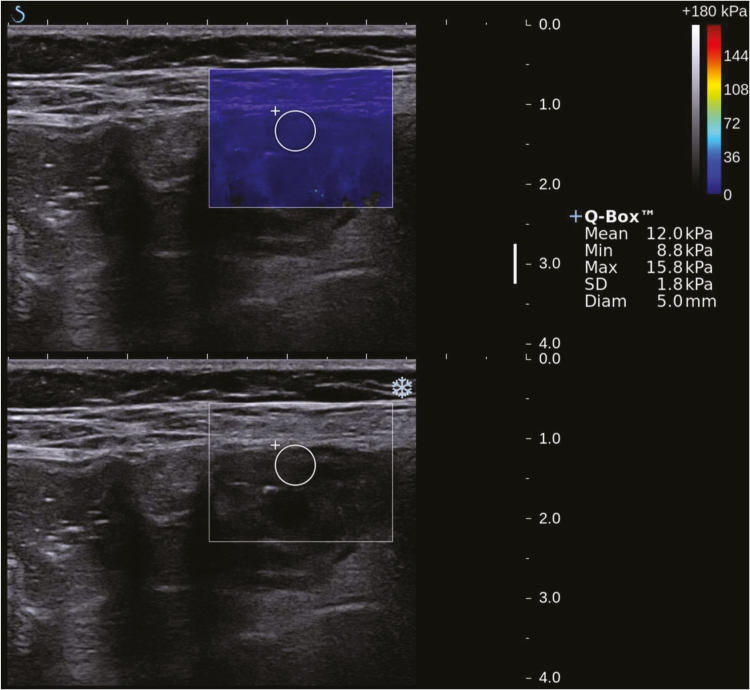
Real-time SWE was used to detect the tissue stiffness of stenotic bowel and an adjacent loop of unaffected bowel (a nonthickened bowel wall used as control). The mechanism of real-time SWE was reported previously.18, 19 Briefly, SWE was performed in the patient with supine position, using a high linear transducer SL15-4(4~15MHz) in axial or longitudinal view. Elasticity estimates were color coded to generate a quantitative SWE image (kPa) at the point of maximal stricture with exclusion of surrounding tissue and luminal content, which is displayed overlaying a conventional image. By placing a circular region of interest (ROI) in the SWE image to include the full thickness of bowel wall, the mean and standard deviation (SD) of the elasticity within the ROI can be displayed (Fig. 1). The mean value of 3 times of consecutive measurements in the same stenotic bowel wall area was used for statistical analyses.19 The minimum thickness for SWE was 1mm. Measurements were determined as technical failure when no or weak signals were obtained in the SWE box for all acquisitions.
FIGURE 1.
Real-time SWE image of stenotic bowel wall overlaying conventional ultrasound grayscale images in a 21-year-old CD patient. Circular ROI are depicted. The color scale indicates the distribution of the measured elasticity within the circular ROI.
CD, Crohn’s disease, Min, minimum, Max, maximum, ROI, regions of interest, SD, standard deviation, SWE, shear-wave elastography, and Diam,diameter.
Bowel Segments Selection for Matched SWE Evaluation and Histologic Assessment
Matched evaluation between SWE and resected specimens was performed by a radiologist (Yu-jun Chen ) with more than 5 years of experience in interpretation of bowel ultrasound and a pathologist (Qing-hua Cao ) with 9 years of experience in digestive tract pathology. The anatomic location of sectioned areas was documented with respect to defined anatomic landmarks (eg, ileocecal valve, appendix, or surgical resection margin). The most stenotic area was chosen more matched analysis.
Histopathologic Evaluation
After tissue fixation in formalin, a full thickness sample of the resected bowel segment was embedded in paraffin and sliced into several 4-µm-thick sections. One section was stained using hematoxylin and eosin (H & E) for histologic inflammation score and another section was stained with Masson trichrome for histologic fibrosis score. The histologic sections were graded by the pathologist (Qing-hua Cao ) who was blinded to the results of clinical, radiological, and real-time SWE data. Pathological analysis was performed using a semiquantitative scoring system modified from the previously described methods (Table 1).7
TABLE 1:
Histologic Score for Inflammatory and Fibrotic CD
| Score | Inflammation | Fibrosis |
|---|---|---|
| 0 | No inflammation or distortion | No fibrosis |
| 1 | Lamina propria inflammation only | Minimal fibrosis in submucosa or subserosa |
| 2 | Submucosal foci of inflammation and/or foci of transmural inflammation | Increased submucosal fibrosis, septa into muscularis propria and/or septa through muscularis propria, increase in subserosal collage |
| 3 | Significant, dissecting, confluent transmural inflammation | Significant transmural scar, marked subserosal collagen |
CD, Crohn’s disease
Statistical Analysis
As this was a preliminary pilot study, the formal sample size was not calculated. Statistical analysis was performed using SPSS 16.0 software (SPSS Inc, Chicago, Illinois, USA). Demographic and clinical parameters were compiled and summary statistics were calculated. Data were described using medians with interquartile range (IQR) for continuous data and percentages for discrete data. Comparison of ratios was tested by Fisher’s exact test. Kruskal-Wallis test was used to analyze differences between groups. The area under receiver operating characteristic (AUROC) was calculated to evaluate SWE in differentiating different grade of inflammation and fibrosis. The optimal cutoff value of real-time SWE in differentiating grade of fibrosis was determined using ROC curve analysis followed by the study of Youden index. The degree of agreement between ultrasound analysis and pathological analysis was assessed by Cohen’s kappa. P values < 0.05 were considered to be statistically significant.
RESULTS
Demographic and Clinical Data
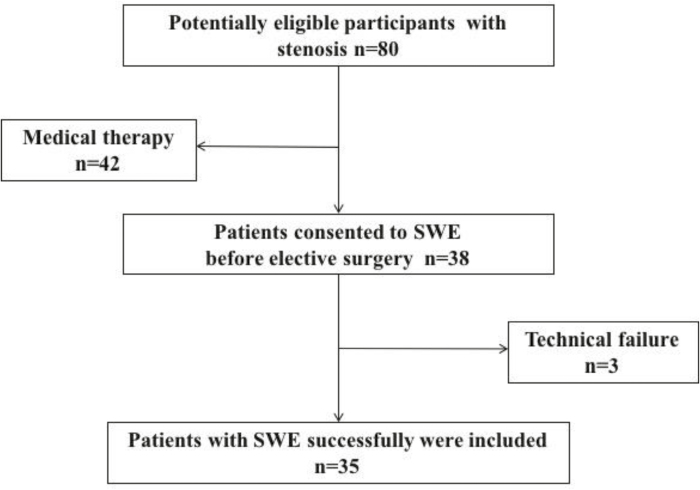
Figure 2 shows the flow of patients through the study. Of the 80 CD patients with symptomatic stricture from April 2015 to May 2017, 42 patients received medical therapy, and the other 38 patients consented to undergo SWE before elective surgery. After excluding 3 patients with failed real-time SWE measurements because of thick subcutaneous fat, 35 patients were included in the final analysis. Of these 35 patients, 26 had a stricture in the terminal ileum and 9 had stricture in the colon (5 in the ascending colon, 1 in the descending colon, and 3 in sigmoid). The patients’ demographic and clinical characteristics are presented in Table 2. The entire real-time SWE examination lasted a median of 8 minutes (IQR, 5–12).
FIGURE 2.
Flow diagram of the study.
TABLE 2:
Demographic Characteristics of 35 CD Patients Operated for Intestinal Stricture
| N | |
|---|---|
| Gender (M:F) | 26:9 |
| Age, mean (SD), years | 34.8(11.3) |
| Disease duration, mean (SD), month | 32.9(34.4) |
| Disease location, no. (%) | |
| L1 Terminal ileal | 17(48.6) |
| L2 Colonic | 9 (25.7) |
| L3 Ilecolon | 9 (25.7) |
| Perianal disease | 10(28.6) |
| Smokers, no. (%) | 8(22.9) |
| CRP, mean±SD, mg/dL | 29.5 ± 35.2 |
| ESR, mean ±SD, mm/h | 47.3 ± 33.3 |
| CDAI score, mean±SD | 280.1 ± 77.2 |
CD, Crohn’s disease; CDAI, Crohn's Disease Activity Index; CRP, C-reactive protein, ESR, erythrocyte sedimentation rate ; IQR, interquartile range; SD, standard deviation
Bowel Wall Histologic Evaluation
As shown in Table 3, 5 bowel segments were classified as having mild inflammation, 11 as moderate, and 19 as severe according to histology analysis. Fibrosis grading on Masson trichrome stain was mild (n = 3), moderate (n = 12), or severe (n = 20). The inflammation score positively correlated with the fibrosis score (r = 0.561, P < 0.001), which necessitates controlling 1 factor when performing analysis with the other.
TABLE 3:
Conventional Ultrasound and Shear-Wave Elastography Findings in Different Grades of Intestinal Inflammation and Fibrosis
| Grade of Inflammation | Grade of Fibrosis | |||||||
|---|---|---|---|---|---|---|---|---|
| mild (no. = 5) |
moderate (no. = 11) |
severe (no. = 19) |
P | mild (no. = 3) |
moderate (no. = 12) |
severe (no. = 20) |
P | |
| Bowel thickness | 11.5 ± 2.7 | 10.0 ± 3.9 | 10.7 ± 3.1 | 0.699 | 7.39 ± 2.8 | 9.9 ± 2.4 | 10.9 ± 3.7 | 0.392 |
| Proximal bowel dilation | 1 | 4 | 6 | 0.808 | 1 | 4 | 6 | 0.451 |
| Limberg classificationa | 0.001 | 0.373 | ||||||
| I | 1 | 0 | 0 | 0 | 0 | 1 | ||
| II | 4 | 3 | 1 | 2 | 1 | 5 | ||
| III | 0 | 6 | 7 | 3 | 5 | 5 | ||
| IV | 0 | 2 | 11 | 4 | 6 | 3 | ||
| Stratified echo pattern | 1 | 8 | 7 | 0.106 | 2 | 6 | 8 | 0.290 |
| SWE value (mean) | 19.3 ± 1.6 | 20.9 ± 9.0 | 20.3 ± 4.6 | 0.380 | 14.4 ± 2.1 | 17.4 ± 3.8 | 23.0 ± 6.3 | 0.008 |
a Limberg score of bowel vascularization was graded as I, II, III, and IV by color Doppler ultrasound: grade I, intestine wall thickening (>3 mm) without discovered vascularization; grade II, intestine wall thickening with spot vascular signals; grade III, intestine wall thickening with longer vascular signals; and grade IV, intestinal wall thickening with longer vascular signals extending from the mesentery.
Conventional Ultrasound and SWE in Characterization of Bowel Inflammation
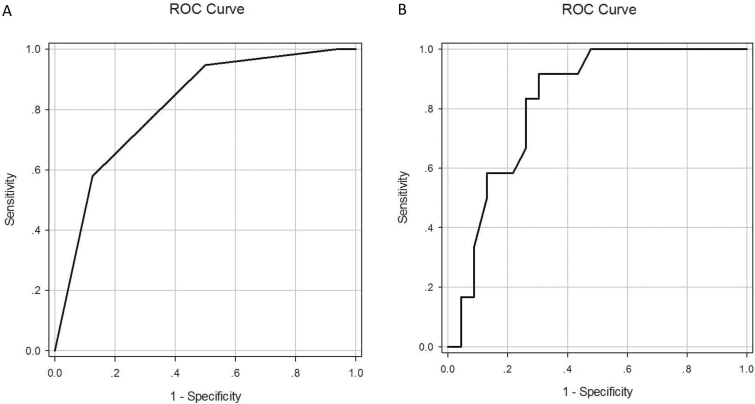
Group difference analysis was performed to evaluate which parameter differs between mild, moderate, and severe inflammation groups. As shown in Table 3, there was a significant difference regarding the Limberg score of bowel vascularization with different grades of inflammation (P = 0.001). However, there was no significant difference in SWE value between mild (19.3 ± 1.6 Kpa), moderate (20.9 ± 9.0 Kpa), and severe (20.3 ± 4.6 Kpa) of inflammation (P = 0.380). Other variables including bowel thickness, rate of proximal dilation, and stratified echo pattern did not differ significantly according to degree of inflammation. Using Limberg III as the cutoff differentiating between mild/moderate and severe inflammation, the sensitivity and specificity of conventional ultrasound was 87.5 % (95% confidence interval,CI, 60.4%~97.8%) and 57.9% (95% CI, 34.0%~78.9%) with AUC of 0.811(95% CI, 0.664~0.958) (P = 0.002) (Fig. 3A)
FIGURE 3.
A, ROC curves for Limberg classification (score of bowel vascularization) in differentiating grades of intestinal inflammation. B, ROC curves for real-time SWE in differentiating grades of fibrosis.
ROC, receiver operating characteristic and SWE, shear-wave elastography
Begin
Conventional Ultrasound and SWE in Characterization of Bowel Fibrosis
Group difference analysis was performed to evaluate which parameter differs between mild, moderate, and severe fibrosis groups. As shown in Table 3, there was a significant difference between mean SWE value and different grades of fibrosis (P = 0.008) (Fig. 4). Using 22.55 kPa as the cutoff differentiating between mild/moderate and severe fibrosis, the sensitivity and specificity was 69.6 % (95% CI, 47.0% ~ 85.9%) and 91.7% (95% CI, 59.8 % ~ 99.6 %) with AUC of 0.822 (95% CI, 0.685 ~ 0.960) (P = 0.002) (Fig. 3B).
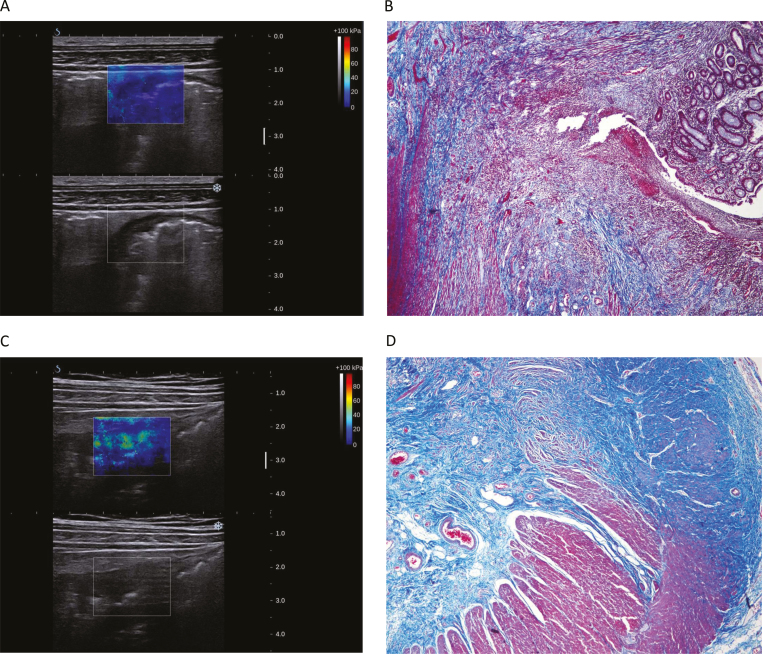
FIGURE 4.
Examples of SWE of different grades of fibrosis. A, The mean SWE value of stenotic bowel wall in a 44-year-old female patient was 14.2 kpa. B, Histology showed severe inflammation and mild fibrosis. C, The mean SWE value of stenoctic bowel wall in a 54-year-old male patient was 25.6 kpa. D, Histology showed mild inflammation and severe fibrosis.
SWE, shear-wave elastography
Classification of Intestinal Stricture Based on Conventional Bowel Ultrasound and SWE : Correlation with Pathological Classification
On the basis of the predictors identified in this study for severe inflammation and fibrosis of CD lesions, we categorized CD strictures into 4 groups considering both the degree of inflammation and fibrosis. In this new ultrasound classification method, CD lesions with Limberg III/IV on conventional ultrasound were considered severe inflammation, and CD lesions with high SWE value (> 22.55 kPa) were considered severe fibrosis. According to this ultrasound classification method, among the 35 specimens analyzed, 2 showed low inflammation and low fibrosis, 16 showed severe inflammation and low fibrosis, 7 segments showed low inflammation and marked fibrosis, and the remaining 10 showed both marked inflammation and fibrosis (Table 4). A moderate agreement between ultrasound and pathological classification was observed (Cohen’s kappa; P<0.001,κ = 0.536).
TABLE 4:
Stricture Classification Based on Grade of Inflammation and Fibrosis on Bowel Ultrasound
| SWE value <22.55 Kpa | SWE value ≥22.55 Kpa | |
|---|---|---|
| Limberga I/II | Low inflammation Low fibrosis (n = 2) |
Low inflammation Marked fibrosis (n = 7) |
| Limbergb III/IV | Low fibrosis Severe inflammation (n = 16) |
Severe inflammation Marked fibrosis (n = 10) |
aLimberg score of bowel vascularization was graded as I, II, III ,and IV by color Doppler ultrasound: grade I, intestine wall thickening (>3 mm) without discovered vascularization; grade II, intestine wall thickening with spot vascular signals; grade III, intestine wall thickening with longer vascular signals; and grade IV, intestinal wall thickening with longer vascular signals extending from the mesentery.
SWE, shear-wave elastography
DISCUSSION
Although several studies have investigated strain elastography11 and real-time elastography20, 21 in the detection of intestinal fibrosis in patients with CD, these techniques are limited by several factors including lack of quantitative information, operator dependence, poor reproducibility, and selection bias.22 SWE is a novel real-time elastography technique that provides a map of the elasticity within a bowel region and allows quantitative analysis of tissue stiffness expressed as Young’s modulus (kPa).23 Because of its advantages including high reproducibility and quantitative elasticity measurement, SWE has been used to detect breast tumors, thyroid nodules, and liver fibrosis.13–15
Dillman et al12 used SWE to detect intestinal fibrosis in ex vivo human intestinal specimens of CD. Lu et al16 reported that SWE could reflect intestinal muscular hypertrophy in patients with CD. In our study, human bowel segments with transmural severe fibrosis had significantly higher mean SWE values than those with mild or moderate fibrosis scores. Receiver operating characteristic (ROC) curve analysis for discriminating bowel segments with high and low fibrosis scores shows promising diagnostic performance, with area under the ROC curve of greater than 0.8. As the pathology classification we used do not include muscular hypertrophy, the correlation analysis between SWE and muscular hypertrophy was not performed in the present study.
Intestinal strictures usually have a mixed pattern, with both inflammatory and fibrotic components.6 Our study confirmed the findings that intestinal strictures present with an overlap of different degrees of fibrosis and inflammation. Indeed, this overlap represents an important challenge for detecting and quantifying fibrosis deposition in the bowel wall. The present findings showed that SWE was not able to differentiate different grades of inflammation, which is consistent with previous studies.12 However, the Limberg score of bowel vascularization on conventional bowel ultrasound reflecting bowel vascularization was able to differentiate different grade of inflammation. When using Limberg III as the cutoff, ROC curve analysis for discriminating bowel segments with high and low inflammation grade showed promising diagnostic performance, with area under the ROC curve of greater than 0.8. Interestingly, the overall accuracy for distinguishing fibrosis grade by SWE was not different from the overall accuracy of the established Limberg score to differentiate between inflammation grades, thereby further supporting the validity and clinical relevance of SWE as novel tool for assessing intestinal fibrosis in CD patients.
Similar to the study reported by Rimola et al,8 we also introduced the classification based on the combination of both histological inflammation and fibrosis. This classification of intestinal stricture was in accordance with clinical practice maintaining the coexistence of both inflammation and fibrosis components in a majority of CD lesions. Another novelty of our study is that Limberg score of bowel vascularization and SWE were combined together to produce an ultrasound classification. Again, as previously shown, a moderate agreement between ultrasound and pathological classification system was observed in our study.
Compared with CTE and MRE, SWE has the following advantages. First, contrast agents are not needed, and adverse reactions to the contrast agent are thereby avoided. Furthermore, SWE is easily carried out and can be conducted as a bedside procedure for some severe CD patients with bowel obstruction. This is not possible with CTE and MRE. Our study showed that the time required for SWE was less than 10 minutes. Finally, SWE has no radiation and can be performed as a repeated follow-up modality for intestinal assessment. This is especially important for patients with CD who often require serial examinations during the course of their disease.
Contrast-enhanced ultrasound (CEUS) was reported to be accurate in quantitative assessment of the bowel wall vascularization24, 25 and, therefore, may help in differentiating inflammatory from fibrostenotic lesions. Ripolles et al24 reported that substantial agreement (kappa = 0.632) was found between CEUS and histopathological findings. Girlich et al25 found a negative correlation (kappa = -0.677) between vascularisation by CEUS and histological fibrosis. Our study showed a similar agreement between ultrasound and pathological classification (kappa = 0.536).
Our study has certain limitations. First, one may argue that SWE of stenotic bowel is operator independent. However, our study used the mean value of 3 times of consecutive measurements in the same stenotic bowel wall area, which might reduce the bias. Secondly, strictures always have a mixed pattern with both inflammatory and fibrotic components. It is noteworthy that only a few strictures were classified as “purely inflammatory” or “purely fibrotic” at histopathology. Hence, we did not compare the differences in SWE among purely fibrotic, purely inflammatory, and mixed strictures in CD for the lack of relevant specimens. Thirdly, an important weakness of this pilot study of diagnostic accuracy is the absence of a control group to identify the right cutoff value of SWE for the presence or absence of fibrosis. Future study with a control group (absence of fibrosis) is needed. Last but not least, the analysis was carried out in a relatively small sample size and larger studies are required to validate these results.
In conclusion, this pilot study suggests that SWE is feasible and accurate in detecting intestinal fibrosis in patients with CD, using surgical resection specimens as a reference standard. By combining the Limberg score of bowel vascularization and SWE value for defining the type of strictures, bowel ultrasound could be applied to help guide management strategy in CD patients with intestinal strictures.
Conflicts of Interest: Ren Mao has received speaker fees from Janssen, Falk, Takeda, and Ipson. Min-hu Chen has received speaker fees from Janssen, Falk, Takeda, AbbVie, and Ipson. Shomron Ben-Horin has received consultancy and/or advisory board fees from Abbvie, Novartis, Schering-Plough, Janssen, Celltrion, and Takeda, and research support from Abbvie, Janssen, Celltrion, and Takeda. Florian Rieder received speaker fees from Boehringer-Ingelheim, Celgene, Helmsley, Pliant, Roche, Samsung, Thetis, and UCB. The other authors declared no conflict of interest.
Supported by: This work was supported by National Natural Science Foundation of China (NSFC grant No. 81601514, 81600508 ,and 81500501).
REFERENCES
- 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 2. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 3. Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of crohn’s disease? Surgical rates and medical management in a population-based inception cohort from western hungary between 1977-2009. Am J Gastroenterol. 2012;107:579–88. [DOI] [PubMed] [Google Scholar]
- 4. Ramadas AV, Gunesh S, Thomas GA, et al. Natural history of crohn’s disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 5. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, et al. Surgery in a population-based cohort of crohn’s disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. 2012;107:1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012;18:849–56. [DOI] [PubMed] [Google Scholar]
- 8. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432–40. [DOI] [PubMed] [Google Scholar]
- 9. Maconi G, Sampietro GM, Parente F, et al. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in crohn’s disease: a prospective comparative study. Am J Gastroenterol. 2003;98:1545–55. [DOI] [PubMed] [Google Scholar]
- 10. Dillman JR, Stidham RW, Higgins PD, et al. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a crohn disease animal model. Radiology. 2013;267:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with crohn’s disease. Gastroenterology. 2011;141:819–26.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014;33:2115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferraioli G, Tinelli C, Dal Bello B, et al. ; Liver Fibrosis Study Group Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125–33. [DOI] [PubMed] [Google Scholar]
- 14. Berg WA, Cosgrove DO, Doré CJ, et al. ; BE1 Investigators Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435–49. [DOI] [PubMed] [Google Scholar]
- 15. Dobruch-Sobczak K, Zalewska EB, Gumińska A, et al. Diagnostic performance of shear wave elastography parameters alone and in combination with conventional B-mode ultrasound parameters for the characterization of thyroid nodules: A prospective, dual-center study. Ultrasound Med Biol. 2016;42:2803–11. [DOI] [PubMed] [Google Scholar]
- 16. Lu C, Gui X, Chen W, et al. Ultrasound shear wave elastography and contrast enhancement: effective biomarkers in crohn’s disease strictures. Inflamm Bowel Dis. 2017;23:421–30. [DOI] [PubMed] [Google Scholar]
- 17. Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol. 1999;37:495–508. [PubMed] [Google Scholar]
- 18. Thiele M, Detlefsen S, Sevelsted Møller L, et al. Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150:123–33. [DOI] [PubMed] [Google Scholar]
- 19. Liu B, Liang J, Zhou L, et al. Shear wave elastography in the diagnosis of thyroid nodules with coexistent chronic autoimmune hashimoto’s thyroiditis. Otolaryngol Head Neck Surg. 2015;153:779–85. [DOI] [PubMed] [Google Scholar]
- 20. Baumgart DC, Müller HP, Grittner U, et al. US-based real-time elastography for the detection of fibrotic gut tissue in patients with stricturing crohn disease. Radiology. 2015;275:889–99. [DOI] [PubMed] [Google Scholar]
- 21. Fraquelli M, Branchi F, Cribiù FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in crohn’s disease patients. Inflamm Bowel Dis. 2015;21:2605–12. [DOI] [PubMed] [Google Scholar]
- 22. Veyrieres JB, Albarel F, Lombard JV, et al. A threshold value in shear wave elastography to rule out malignant thyroid nodules: a reality?Eur J Radiol. 2012;81:3965–72. [DOI] [PubMed] [Google Scholar]
- 23. Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med. 2013;34:169–84. [DOI] [PubMed] [Google Scholar]
- 24. Ripollés T, Rausell N, Paredes JM, et al. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis. 2013;7:120–8. [DOI] [PubMed] [Google Scholar]
- 25. Girlich C, Jung EM, Huber E, et al. Comparison between preoperative quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and operative macroscopic findings and results of histopathological scoring in Crohn’s disease. Ultraschall Med. 2011;32:154–59. [DOI] [PubMed] [Google Scholar]