Abstract
Hyaluronan, a major extracellular matrix component, is an active participant in many disease states, including inflammatory bowel disease (IBD). The synthesis of this dynamic polymer is increased at sites of inflammation. Hyaluronan together with the enzymes responsible for its synthesis, degradation, and its binding proteins, directly modulates the promotion and resolution of disease by controlling recruitment of immune cells, by release of inflammatory cytokines, and by balancing hemostasis. This review discusses the functional significance of hyaluronan in the cells and tissues involved in inflammatory bowel disease pathobiology.
Keywords: Inflammation, hyaluronan, inflammatory bowel disease, platelet, megakaryocyte, coagulation
Introduction
Extracellular matrices (ECM) have emerged as a key mediator capable of influencing cell behavior in homeostasis and disease. The ECM regulates cellular function by providing physical and biochemical cues to cells in the local environment through several mechanisms, including gas and nutrient exchange, regulating the bioavailability of growth factors, and modulating cell adhesion. During inflammation, the biochemical composition of the ECM becomes altered due to the activation of resident cells within the tissue and by extravasation of immune cells and is an active participant in both the progression and resolution of inflammatory disease. The increased deposition and turnover of the ECM molecule hyaluronan (HA) is associated with several inflammatory disease states, including inflammatory bowel disease (IBD). Consisting of two closely related disorders, Crohn’s disease and ulcerative colitis, IBD usually manifests in the first three decades of life and leads to relentless inflammatory destruction of the gastrointestinal tract in susceptible individuals [1, 2]. The chronic inflammation which characterizes IBD results in dramatic deposition of HA within affected tissues which both precedes and promotes immune cell infiltrate, tissue destruction, and coagulation [3–5].
Inflammatory Bowel Disease
IBD consists of two closely related disorders: Crohn’s disease (CD) and ulcerative colitis (UC), whose overall prevalence is increasing in both industrialized and developing countries worldwide [6]. In genetically susceptible individuals a combination of environmental factors, gut microbiota, the immune response together contributes to the onset of IBD [7]. These diseases usually manifest in the first three decades of life and lead to relentless inflammatory destruction of the gastrointestinal tract in susceptible individuals [1, 2]. Systemic manifestations can present years after the onset of disease and can affect almost all organs including the cardiovascular and pulmonary systems [8, 9]. This progressive, chronic and relapsing disease is thought to be a consequence of defects in the intestinal epithelial barrier and an excessive mucosal immune response leading to inflammation-associated tissue damage [7, 10]. Tissue damage is a consistent feature of IBD progression and severity throughout the structure of the intestine, and remodeling of ECM molecules, such as HA, are strongly implicated in IBD pathogenesis.
HA regulates inflammatory cell recruitment in IBD
HA is a glycosaminoglycan (GAG) of high average molecular mass (>1000 kDa) comprised solely of repeating disaccharides of glucuronic acid and N-acetyl-glucosamine without a protein core, unlike other GAGs. A dynamic, glycan-rich glycocalyx comprised of HA and other membrane-bound proteoglycans and glycoproteins lines the vascular endothelium, extending 30–100 nm from the plasma membrane and restricting inflammatory cell access under normal conditions [11, 12]. However, increased biosynthesis of HA matrices in the tissue are associated with a number of inflammatory disease states. In response to pro-inflammatory signals such as TNF-α or viral infection, HA synthesis is amplified, and the product is organized into a highly adhesive HA matrix [13]. The biosynthesis of this matrix requires modification of HA by tumor necrosis factor-stimulated gene 6 (TSG6), whose product encodes an enzyme induced during inflammation that transfers heavy chains (HCs) from a serum proteoglycan complex, inter-alpha-inhibitor, onto HA to form a covalent, crosslinked HA-HC matrix [14, 15]. This biologically distinct form of HA is observed as a cable-like structure capable of spanning multiple cell lengths [15, 16]. Thus, in response to inflammation the enzymatic activity of TSG6 converts homeostatic HA into unique, pathological HA cables capable of recruiting immune cells in many inflammatory disease states, including arthritis, lung injury, and IBD. Resting leukocytes and platelets do not bind to the HA pericellular matrix under normal conditions [17], but instead bind strongly to HA-HC [16]. Importantly, this HA-HC matrix also plays other critical roles in non-pathological processes such as ovulation and development outside the scope of this review [18–20].
These observations were first described in cells and tissues from IBD patients, where HA is present at elevated levels in inflamed IBD colon tissue (Figure 1) compared to non-inflamed IBD tissue or non-IBD controls [13]. Several cell types relevant to IBD pathobiology including human mucosal smooth muscle cells, intestinal microvascular endothelial cells, and porcine intestinal epithelial cells have all been demonstrated to enhance monocyte adhesion by producing HA cables in response to inflammatory stimuli, indicating that crosslinked HA potentially contributes to many inflammatory disease states [3, 4, 21]. Recently, a rat model of intestinal inflammation has demonstrated that HA surrounding myenteric neurons becomes significantly altered, suggesting that HAS2-derived HA may participate in neuromuscular dysfunction in colitis [22]. Studies using murine models of colitis further demonstrate that HA synthesis precedes the influx of inflammatory cells and thereby promotes inflammation, and therefore control of cell-surface HA levels may play a regulatory role in IBD [3]. These studies demonstrated that knock-out of hyaluronan synthase 3 (HAS3) protects mice from experimental models of colitis by reducing leukocyte infiltration and dramatically attenuating disease progression [5]. Both HAS2 and HAS3 mRNA levels are increased in mouse colon tissue during DSS colitis [23], but HAS3 appears to be important for increasing HA on microvascular endothelium under inflammatory conditions as knock-out of HAS1 did not improve disease activity [5]. Together, these data suggest that inflammation-induced HA synthesis and subsequent crosslinking of HA enhances recruitment of immune cells to inflamed tissues and promotes and sustains the chronic cycle of inflammation in IBD.
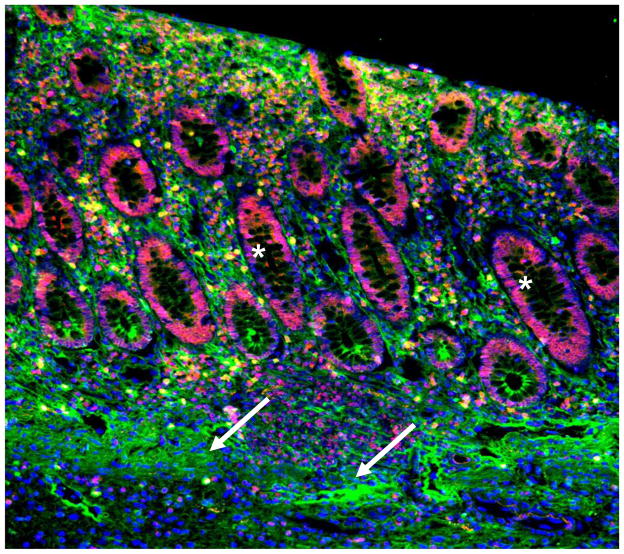
Figure 1. HA accumulates within human IBD colon tissue.
Human colon tissue from an IBD patient is stained for detection of HA (green), the HA receptor CD44 (red), and nuclei (blue). HA is abundant around smooth muscle cell layers, surrounding microvessels, the connective tissue between crypts, and in the epithelial cell layer. The letter L indicates the intestinal lumen, arrows indicate the smooth muscle layer, and the asterisks indicate the intestinal crypts.
At present, our understanding of how HA cables are regulated in the progression and resolution of inflammation is incomplete. It is likely that the balance of synthesis and degradation of HA, and interactions with associated binding proteins, may directly control the ability of this unique HA matrix to recruit inflammatory cells to sites of inflammation. Interestingly, in vitro studies of TSG6 have indicated that this enzyme can function “in reverse” and transfer HCs from HC-modified HA onto other HA oligosaccharides in a size-limiting fashion [24]. Whether this is a potential mechanism which could lead to the resolution of HA cables by transfer of HCs onto small HA fragments in vivo is not known. Several studies have demonstrated that HA plays a role in toll-like receptor signaling either by acting as a ligand or by regulating receptor activation and further studies are needed to determine whether purified HA and HC-modified HA exhibit similar or differing signaling properties [25–29].
Dysregulated HA catabolism in IBD
Deposition of HA appears to be an early event in the progression of IBD and may be the result of imbalanced HA synthesis and degradation. While many studies have described the accumulation of HA in inflammatory disease states, demonstrating activated HAS expression and/or activity, relatively few have examined how inflammation regulates the enzymes responsible for HA turnover. The main pathway of HA degradation requires the activity of hyaluronidase (HYAL) 1 and 2 enzymes and the cell surface HA receptor CD44 [30–32]. At the cell surface, Hyal-2 and CD44 interact with HA to generate fragments which may then be internalized and transported to the lysosome, where highly active Hyal-1 degrades HA into small oligosaccharides [30, 33]. Examination of the tissue distribution of Hyal-1 and 2 within a murine model of colitis indicated that Hyal-1 is primarily expressed in smooth muscle tissue and nucleated infiltrating leukocytes, while Hyal-2 is restricted to the endothelium and platelets [4]. Hyal-2 is expressed by many somatic tissues in mice but the cellular localization of Hyal-2 appears to be restricted to epithelial and endothelial cells within these tissues [34]. This cell-type specific expression suggests that HA turnover likely plays an important physiological role at the luminal interface on epithelial and endothelial cells. Indeed, significant depositions of HA are observed within the inflamed colon microvasculature from IBD patients and in mice subject to experimental colitis [3].
Microvascular endothelial cells isolated from colon tissue of IBD patients produce a leukocyte adhesive HA matrix in vitro, and cells from non-IBD patients exhibit the same response upon stimulation with TNF-α [4]. Several studies support the notion that in IBD, the endothelium is activated in response to inflammatory stimuli and exhibits increased adhesion of immune cells [35]. Further, in vitro studies demonstrate that platelets can degrade the HA matrix on the surface of TNF-α stimulated endothelial cells in a Hyal-2 dependent fashion [4]. The discovery that platelets could degrade cell-surface HA led to the observation that Hyal-2 is packaged within alpha-granules of platelets where it is translocated to the platelet cell-surface in an activation-dependent mechanism, indicating that platelet-mediated HA degradation is a regulated process [36].
Platelet-derived HA degradation fragments from TNF-α induced endothelial cells are capable of activating naïve human peripheral blood monocytes to produce IL-6 and IL-8. This suggests that while platelets can degrade an inflammatory HA matrix, the components of HA cables released as HA fragments contain signaling properties themselves and may be pro-inflammatory and pro-angiogenic [4, 37]. Collectively, these data suggest that turnover of HA by Hyal-2 on vascular surfaces is likely to be a regulated process that may become disrupted during inflammatory disease states such as IBD. Surprisingly, platelets from IBD patients carry less Hyal-2 protein (on average 45% less) than controls, suggesting that this deficiency may contribute to the accumulation of HA within the microvasculature of the colon and may thereby promote inflammatory disease [36].
While the HYAL family members are regarded as the primary enzymes involved in HA depolymerization, other proteins have emerged as additional players in HA catabolism. The cell migration-inducing and hyaluronan-degrading protein (CEMIP, also known as KIAA1199) has HA binding and degrading activities [38]. In contrast to HYAL2, KIAA1199 was found to be upregulated in cells and tissue isolated from Crohn’s disease patients [39]. While Hyal-2 is present at the platelet surface upon activation and may be important for maintenance of vascular HA in IBD, KIAA1199 participates in HA degradation in intestinal fibroblasts, where it is secreted from cultured cells and deposited within the ECM [39]. Intestinal fibroblasts are capable of degrading exogenous HA in vitro, but cells lacking KIAA1199 lose the ability to cleave exogenous HA into smaller sized fragments, similar to reports on KIAA1199 activity in skin fibroblasts [38–40]. The increased expression of KIAA1199 in Crohn’s disease fibroblasts appears to be dependent on IL-6 production, as addition of IL-6 to control fibroblasts induced expression of KIAA1199 while TNF-α treatment resulted in no effect on gene expression. Further, fibroblasts isolated from Crohn’s disease patients secrete high levels of IL-6 into their culture medium, and addition of a neutralizing IL-6 antibody to these cultures led to a reduction in KIAA1199 mRNA and protein levels [39]. The consequence of increased matrix deposition of KIAA1199 in IBD is still largely unknown, but HA and KIAA1199 are both present at increased levels in the submucosal regions of colon tissue from IBD patients compared to non-IBD controls. A plausible scenario is that KIAA1199 generates HA fragments in vivo within the ECM where they may act as endogenous danger signals, perpetuating and promoting inflammation and fibrosis in IBD. Recently, transmembrane protein 2 (TMEM2), a protein which contains sequence similarity to KIAA1199, has been reported to have HA degradation activities [41]. At present, little is known about TMEM2 in mammals and its contribution to disease remains an open area for investigation.
HA as a molecular interface between inflammation and coagulation in IBD
Microvascular occlusion is frequently observed in the mucosa of IBD patients where both while these clots are not present in healthy subjects [42]. The blood of IBD patients exists in a hypercoagulable state where patients are at increased risk of venous and arterial thrombosis, and perturbations in both the coagulation cascade and platelet reactivity are consistent features of IBD [42, 43]. The risk of thrombosis is increased even during latent phases of disease activity and further elevated during active disease, supporting the notion that IBD is a thromboinflammatory disease state. Vascular integrity is critical for preventing thrombus formation, and homeostatic HA regulates endothelial function through multiple mechanisms [44–46].
Many of the inflammatory mediators that initiate coagulation (e.g. TNF-α, IL-6) [47] are also known to activate synthesis of HA [48], which is present at high levels during initiation of coagulation and clot formation [44]. Fibrinogen, an essential coagulation factor, is also a HA-binding glycoprotein and interactions with HA have been implicated in thrombus formation [49–51]. Binding of HA to fibrinogen facilitates the assembly of a three-dimensional, hydrated, provisional matrix, and promotes migration of cells into a clot [52, 53]. In fact, streptokinase, the first thrombolytic drug to reach the market also contained hyaluronidase [54], and unidentified components of the coagulation cascade have been shown to inhibit hyaluronidase activity [55]. Thrombin, which catalyzes many coagulation-related activities including cleaving soluble fibrinogen to form insoluble fibrin networks [56], also interacts specifically with HA-HC complexes generated by inflammatory insult. The proteolytic activity of thrombin also cleaves HC1 from these HA-HC matrices and negatively regulates leukocyte adhesion to colon smooth muscle cells stimulated with viral mimetic (e.g. poly(I:C)), suggesting it can exert both pro- and anti-inflammatory effects [57]. Anti-thrombin is a naturally occurring endothelial regulator which is decreased in response to IBD. Anti-thrombin inhibits thrombin production at multiple steps in the coagulation cascade and also inhibits thrombin directly. However, HA, which is present at high levels in the serum of IBD patients [58], has been shown to be an in vitro inhibitor of anti-thrombin [59] and may thereby promote coagulation and leukocyte recruitment.
Exogenous, purified HA has been used to coat endovascular devices in many studies to protect against hemostasis, and has been shown to inhibit platelet adhesion, platelet aggregation, and reduce thrombus formation in in vitro assays of platelet function [60, 61]. However, studies of platelet interactions with endothelial cells stimulated with TNF-α demonstrate that platelets appear to bind specifically to crosslinked HA-HC cables rather than “coat” HA [4]. These data suggest that platelets may discriminate between HA cables and homeostatic HA, possibly on the basis of inflammation or platelet activation. Whether interaction with inflammation-induced HA-HC directly contributes to platelet activation and aggregation requires further study.
Regulation of HA in platelet biosynthesis
A number of chronic inflammatory diseases including IBD, arthritis [62], coronary artery disease [63], and some cancers [64] are associated with increased platelet production, altered platelet function, increased platelet reactivity, and thrombosis. Quantitative increases in inflammatory cytokines, adhesion molecules, mRNAs, and micro-RNAs have been reported in platelets from patients with chronic inflammatory disease relative to platelets from healthy individuals [65, 66]. These findings indicate that platelets can become dysregulated in response to inflammatory disease and altered platelet function can further exacerbate disease activity. Platelet dysfunction undoubtedly contributes to frequent systemic thromboembolic events and the blood of IBD patients existing in a hypercoagulable state [42, 43].
Platelet production requires the differentiation of hematopoietic stem cells within the bone marrow into megakaryocytes (MKs), the platelet progenitor cell [67]. HA is a crucial element in the bone marrow ECM and regulates stem cell proliferation, maturation, homing, and engraftment in bone marrow transplant [68–71]. In fact, depletion of bone marrow HA reduces the ability of the hematopoietic environment to support stem cell proliferation and stimulates release of cytokines such as IL-1β and IL-6 from bone marrow macrophages, both of which contribute to platelet production by MKs [72]. During differentiation, MKs synthesize platelet cellular material (including HA) and migrate from the endosteal niche to the vascular marrow sinusoids where they produce long proplatelet extensions to shed platelets into circulation [73]. This tightly regulated process is controlled by signals from circulating cytokines [74] and the bone marrow microenvironment [75, 76]. Because platelets have a short lifespan and receive their cellular material from MKs, changes in the bone marrow milieu which alter MK development, differentiation, and transcription can have a direct effect on platelet function [65].
Inflammation is known to alter platelet function and biosynthesis through: 1) direct interactions of cytokines with platelets and their progenitor cells, 2) increasing hepatic thrombopoietin (TPO) production [77], and 3) by altering the biochemical composition of the bone marrow ECM [76, 78]. Platelet synthesis is primarily regulated by TPO, which directly controls proliferation and differentiation of MK progenitors, and TPO is frequently elevated in inflammatory disease states, such as IBD [79]. Circulating TPO levels are believed to be primarily regulated by platelet number, but paradoxically platelet levels are significantly increased in IBD [80, 81]. Increased TPO production in response to inflammation is in part due to interleukin-6 (IL-6), another cytokine intimately involved in IBD pathogenesis [82], and IL-6 also regulates platelet production itself. Thus, the inflammatory environment of IBD involves several potent factors which may have direct consequences on gene expression in platelets and their progenitor cells.
As previously mentioned, platelets isolated from IBD patients are deficient in Hyal-2, but the mechanism underlying reduced Hyal-2 levels is not known [36]. While platelets deficient in Hyal-2 may contribute to dysregulated HA turnover in IBD, it may also reflect aberrant platelet production and aggregation. In a study of platelet aggregation using population based cohort data, whole exosome sequencing found associations of rare variants of Hyal-2 with enhanced platelet aggregation responses to pro-thrombotic stimuli [83]. Evidence within the literature suggests that Hyal-2 may modulate platelet reactivity, as well as platelet synthesis. Unlike many other cell types, MKs contain intracellular HA (Figure 2), and HA depolymerization by Hyal-2 by MKs is required at the critical stage of proplatelet synthesis [84]. Mice deficient in Hyal-2 exhibit mild anemia and thrombocytopenia, and a detailed study of megakaryopoiesis in mouse and human models definitively identified a requirement for HA depolymerization during platelet formation [84–86]. During MK biogenesis, HA synthesis likely increases to support maturation. Platelets and MKs contain only Hyal-2, and do not express Hyal-1 protein or mRNA [4, 84]. Ultimately, depolymerization by Hyal-2 must occur to support platelet synthesis, and although the specific size is not known, the resulting HA fragments become packaged within circulating platelets [4].
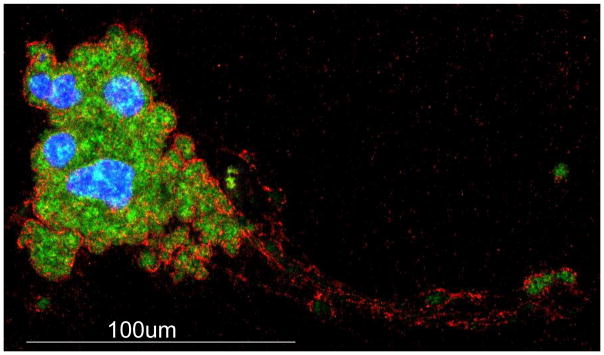
Figure 2. Megakaryocytes contain intracellular HA.
Murine bone marrow derived megakaryocytes were cytospun and stained for HA (green), von Willebrand factor (red), and nuclei (blue). HA is synthesized and packaged within the complex internal membrane network of mature, polyploid MKs and is contained within long proplatelet extensions during platelet synthesis.
Within the bone marrow, HA may play additional roles during MK maturation in both homeostasis and disease. MKs express multiple HA receptors demonstrated to have signaling properties including CD44, RHAMM, and members of the TLR family [75, 87, 88]. Although HA is reported to be increased in many tissues and cell types during inflammation, at present it is not known whether bone marrow HA levels increase during inflammatory disease or if it is capable of modification with HCs as in other tissues. Given the short lifespan of platelets, and that platelet dysfunction is associated with many inflammatory disease states, it is possible that in disorders such as IBD platelets may become dysregulated as a result of the disease process. Understanding whether altered HA synthesis or HA cable formation within the bone marrow microenvironment contributes to the platelet abnormalities in IBD, such as increased reactivity, platelet number and Hyal-2 deficiency, will provide key insights generalizable to other thromboinflammatory diseases.
Conclusion
Inflammation and coagulation are two connected processes which reinforce and support one another, ultimately resulting in the resolution of damage. However, chronic inflammatory conditions lead to dysregulation of these processes in many disease states. Molecules at the interface between inflammation and coagulation, such as HA can contribute to resolution or progression of disease in specific contexts. Cross-linking of HA into HA-HC cables can further amplify immune cell recruitment and promote inflammation in IBD, where evidence indicates that turnover of this polymer is deficient. This results in a sustained inflammatory insult which likely occurs in many tissues due to systemic inflammation. Left unresolved, this contributes to the endothelial dysfunction, thrombus generation, and tissue damage observed in IBD (Figure 3). At present, our understanding of how HA-HC matrices can modulate both the promotion and resolution of inflammation in specific contexts is incomplete. The current literature suggests that, at least in IBD, HA-HC complexes exacerbate disease activity. Further studies directed at disrupting the interactions between immune cells and HA-HC, directly antagonizing synthesis of HA-HC matrices, and selective degradation of HA-HC complexes in disease models are necessary to dissect the molecular pathways by which this novel polymer regulates thromboinflammatory disease.
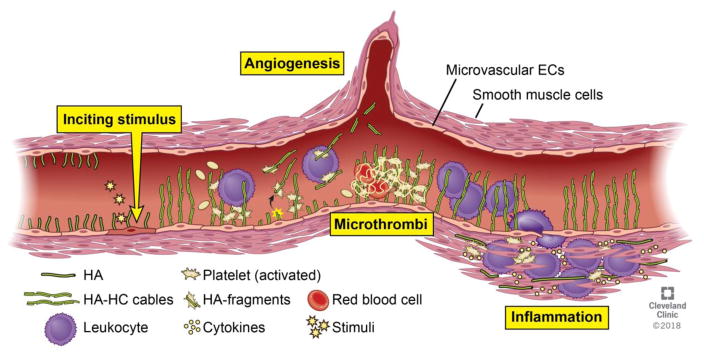
Figure 3. Hyaluronan is a novel regulator of the immune response in IBD.
In response to an inciting stimulus, HA synthesis is increased on the surface of microvascular endothelial cells, smooth muscle cells, and epithelial cells. Under normal conditions, HA restricts inflammatory cell access, but in inflammatory disorders such as IBD, HA is cross-linked to form distinct HA-HC matrices. HA-HC promotes immune cell recruitment and may be recognized as an endogenous danger signal by leukocytes and platelets. Fragments of HA, possibly containing HCs, promote angiogenesis and can drive immune cell responses. Dysregulation of HA clearance leads to accumulation of adhesive HA matrices which bind coagulation factors, enhance platelet recruitment, and increase leukocyte recruitment, together promoting and sustaining inflammation in IBD.
Highlights.
Hyaluronan deposition is increased in IBD tissues and regulates immune cell recruitment
Enzymatic cross-linking of hyaluronan contributes to thromboinflammatory disease
Regulation of hyaluronan-degrading enzymes is disrupted in IBD
Hyaluronan regulates megakaryocyte and platelet maturation
Acknowledgments
Funding sources
This work was financially supported by the National Institutes of Health [K99HL135265] to A.C.P] and the Programs of Excellence in Glycosciences [HL107147] from the National Heart, Lung, and Blood Institute (C.A.d.l.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBDGC, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–53. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 3.Kessler S, Rho H, West G, Fiocchi C, Drazba J, de la Motte C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin Transl Sci. 2008;1(1):57–61. doi: 10.1111/j.1752-8062.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Motte C, Nigro J, Vasanji A, Rho H, Kessler S, Bandyopadhyay S, Danese S, Fiocchi C, Stern R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. The American journal of pathology. 2009;174(6):2254–64. doi: 10.2353/ajpath.2009.080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler SP, Obery DR, de la Motte C. Hyaluronan Synthase 3 Null Mice Exhibit Decreased Intestinal Inflammation and Tissue Damage in the DSS-Induced Colitis Model. Int J Cell Biol. 2015;2015:745237. doi: 10.1155/2015/745237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 7.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11(46):7227–36. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twig G, Zandman-Goddard G, Szyper-Kravitz M, Shoenfeld Y. Systemic thromboembolism in inflammatory bowel disease: mechanisms and clinical applications. Ann N Y Acad Sci. 2005;1051:166–73. doi: 10.1196/annals.1361.058. [DOI] [PubMed] [Google Scholar]
- 10.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. The American journal of physiology. 1997;273(4 Pt 1):G769–75. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- 11.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv: European journal of physiology. 2007;454(3):345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wight TN. Provisional matrix: A role for versican and hyaluronan. Matrix biology: journal of the International Society for Matrix Biology. 2017;60–61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) The Journal of biological chemistry. 1999;274(43):30747–55. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- 14.Rugg MS, Willis AC, Mukhopadhyay D, Hascall VC, Fries E, Fulop C, Milner CM, Day AJ. Characterization of complexes formed between TSG-6 and inter-alpha-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. The Journal of biological chemistry. 2005;280(27):25674–86. doi: 10.1074/jbc.M501332200. [DOI] [PubMed] [Google Scholar]
- 15.Milner CM, Tongsoongnoen W, Rugg MS, Day AJ. The molecular basis of inter-alpha-inhibitor heavy chain transfer on to hyaluronan. Biochemical Society transactions. 2007;35(Pt 4):672–6. doi: 10.1042/BST0350672. [DOI] [PubMed] [Google Scholar]
- 16.de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function. The American journal of pathology. 2003;163(1):121–33. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culty M, O’Mara TE, Underhill CB, Yeager H, Jr, Swartz RP. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. Journal of leukocyte biology. 1994;56(5):605–11. doi: 10.1002/jlb.56.5.605. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. The Journal of biological chemistry. 2001;276(11):7693–6. doi: 10.1074/jbc.C000899200. [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, Kakinuma C, Kato K, Morishita H, Niwa H, Miyazaki J. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochemical and biophysical research communications. 2001;281(5):1154–60. doi: 10.1006/bbrc.2001.4475. [DOI] [PubMed] [Google Scholar]
- 20.Jessen TE, Odum L. Role of tumour necrosis factor stimulated gene 6 (TSG-6) in the coupling of inter-alpha-trypsin inhibitor to hyaluronan in human follicular fluid. Reproduction. 2003;125(1):27–31. doi: 10.1530/rep.0.1250027. [DOI] [PubMed] [Google Scholar]
- 21.Docampo MJ, Cabrera J, Bassols A. Hyaluronan mediates the adhesion of porcine peripheral blood mononuclear cells to poly (I:C)-treated intestinal cells and modulates their cytokine production. Vet Immunol Immunopathol. 2017;184:8–17. doi: 10.1016/j.vetimm.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Filpa V, Bistoletti M, Caon I, Moro E, Grimaldi A, Moretto P, Baj A, Giron MC, Karousou E, Viola M, Crema F, Frigo G, Passi A, Giaroni C, Vigetti D. Changes in hyaluronan deposition in the rat myenteric plexus after experimentally-induced colitis. Sci Rep. 2017;7(1):17644. doi: 10.1038/s41598-017-18020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Riehl TE, Stenson WF. Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology. 2009;137(6):2041–51. doi: 10.1053/j.gastro.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer ME, Glant TT, Mikecz K, DeAngelis PL, Haller FM, Husni ME, Hascall VC, Calabro A. Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. The Journal of biological chemistry. 2013;288(1):205–14. doi: 10.1074/jbc.M112.403998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature medicine. 2005;11(11):1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 26.Ebid R, Lichtnekert J, Anders HJ. Hyaluronan is not a ligand but a regulator of toll-like receptor signaling in mesangial cells: role of extracellular matrix in innate immunity. ISRN Nephrol. 2014;2014:714081. doi: 10.1155/2014/714081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saikia P, Roychowdhury S, Bellos D, Pollard KA, McMullen MR, McCullough RL, McCullough AJ, Gholam P, de la Motte C, Nagy LE. Hyaluronic acid 35 normalizes TLR4 signaling in Kupffer cells from ethanol-fed rats via regulation of microRNA291b and its target Tollip. Sci Rep. 2017;7(1):15671. doi: 10.1038/s41598-017-15760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. The Journal of biological chemistry. 2007;282(25):18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 29.Gariboldi S, Palazzo M, Zanobbio L, Selleri S, Sommariva M, Sfondrini L, Cavicchini S, Balsari A, Rumio C. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. Journal of immunology. 2008;181(3):2103–10. doi: 10.4049/jimmunol.181.3.2103. [DOI] [PubMed] [Google Scholar]
- 30.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. The Journal of biological chemistry. 2007;282(8):5597–607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 31.Jadin L, Bookbinder LH, Frost GI. A comprehensive model of hyaluronan turnover in the mouse. Matrix biology: journal of the International Society for Matrix Biology. 2012;31(2):81–9. doi: 10.1016/j.matbio.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Hida D, Danielson BT, Knudson CB, Knudson W. CD44 knock-down in bovine and human chondrocytes results in release of bound HYAL2. Matrix biology: journal of the International Society for Matrix Biology. 2015;48:42–54. doi: 10.1016/j.matbio.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13(12):105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury B, Hemming R, Faiyaz S, Triggs-Raine B. Hyaluronidase 2 (HYAL2) is expressed in endothelial cells, as well as some specialized epithelial cells, and is required for normal hyaluronan catabolism. Histochem Cell Biol. 2016;145(1):53–66. doi: 10.1007/s00418-015-1373-8. [DOI] [PubMed] [Google Scholar]
- 35.Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. The American journal of pathology. 2008;172(6):1457–66. doi: 10.2353/ajpath.2008.070593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albeiroti S, Ayasoufi K, Hill DR, Shen B, de la Motte CA. Platelet hyaluronidase-2: an enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood. 2015;125(9):1460–9. doi: 10.1182/blood-2014-07-590513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix biology: journal of the International Society for Matrix Biology. 2002;21(1):25–9. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(14):5612–7. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soroosh A, Albeiroti S, West GA, Willard B, Fiocchi C, de la Motte CA. Crohn’s Disease Fibroblasts Overproduce the Novel Protein KIAA1199 to Create Proinflammatory Hyaluronan Fragments. Cell Mol Gastroenterol Hepatol. 2016;2(3):358–368 e4. doi: 10.1016/j.jcmgh.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida H, Nagaoka A, Nakamura S, Sugiyama Y, Okada Y, Inoue S. Murine homologue of the human KIAA1199 is implicated in hyaluronan binding and depolymerization. FEBS Open Bio. 2013;3:352–6. doi: 10.1016/j.fob.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. The Journal of biological chemistry. 2017;292(18):7304–7313. doi: 10.1074/jbc.M116.770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamouard P, Grunebaum L, Wiesel ML, Frey PL, Wittersheim C, Sapin R, Baumann R, Cazenave JP. Prothrombin fragment 1 + 2 and thrombin-antithrombin III complex as markers of activation of blood coagulation in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 1995;7(12):1183–8. doi: 10.1097/00042737-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Kume K, Yamasaki M, Tashiro M, Yoshikawa I, Otsuki M. Activations of coagulation and fibrinolysis secondary to bowel inflammation in patients with ulcerative colitis. Intern Med. 2007;46(17):1323–9. doi: 10.2169/internalmedicine.46.0237. [DOI] [PubMed] [Google Scholar]
- 44.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix biology: journal of the International Society for Matrix Biology. 2007;26(1):58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 45.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40(4):828–39. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34(1):93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64(11):4733–8. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC, Passi A. Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB) pathway. The Journal of biological chemistry. 2010;285(32):24639–45. doi: 10.1074/jbc.M110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolodgie FD, Burke AP, Wight TN, Virmani R. The accumulation of specific types of proteoglycans in eroded plaques: a role in coronary thrombosis in the absence of rupture. Curr Opin Lipidol. 2004;15(5):575–82. doi: 10.1097/00041433-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol. 2002;22(10):1642–8. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- 51.Bergmeier W, Hynes RO. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb Perspect Biol. 2012;4(2) doi: 10.1101/cshperspect.a005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986;119(2):219–34. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- 53.Chester D, Brown AC. The role of biophysical properties of provisional matrix proteins in wound repair. Matrix biology: journal of the International Society for Matrix Biology. 2017;60–61:124–140. doi: 10.1016/j.matbio.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Wall CA, Hanlon CR. Experimental enzymatic dissolution of soft tissue hematomas. I. Streptokinase-streptodornase and hyaluronidase. Surgery. 1957;42(3):536–40. [PubMed] [Google Scholar]
- 55.Roth KL. Inhibition of hyaluronidase activity by factors associated with blood clotting. Proc Soc Exp Biol Med. 1954;85(4):533–7. doi: 10.3181/00379727-85-20942. [DOI] [PubMed] [Google Scholar]
- 56.Crawley JT, Zanardelli S, Chion CK, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007;5(Suppl 1):95–101. doi: 10.1111/j.1538-7836.2007.02500.x. [DOI] [PubMed] [Google Scholar]
- 57.Petrey AC, de la Motte CA. Thrombin Cleavage of Inter-alpha-inhibitor Heavy Chain 1 Regulates Leukocyte Binding to an Inflammatory Hyaluronan Matrix. The Journal of biological chemistry. 2016;291(47):24324–24334. doi: 10.1074/jbc.M116.755660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi Y, Noda H, Okaniwa N, Adachi K, Shinmura T, Nakagawa S, Ebi M, Ogasawara N, Funaki Y, Zhuo L, Kimata K, Sasaki M, Kasugai K. Serum-Derived Hyaluronan-Associated Protein Is a Novel Biomarker for Inflammatory Bowel Diseases. Digestion. 2017;95(2):146–155. doi: 10.1159/000456071. [DOI] [PubMed] [Google Scholar]
- 59.Chang X, Yamada R, Yamamoto K. Inhibition of antithrombin by hyaluronic acid may be involved in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2005;7(2):R268–73. doi: 10.1186/ar1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell JD, Lee R, Hodakowski GT, Neya K, Harringer W, Valeri CR, Vlahakes GJ. Prevention of postoperative pericardial adhesions with a hyaluronic acid coating solution. Experimental safety and efficacy studies. J Thorac Cardiovasc Surg. 1994;107(6):1481–8. [PubMed] [Google Scholar]
- 61.Verheye S, Markou CP, Salame MY, Wan B, King SB, 3rd, Robinson KA, Chronos NA, Hanson SR. Reduced thrombus formation by hyaluronic acid coating of endovascular devices. Arterioscler Thromb Vasc Biol. 2000;20(4):1168–72. doi: 10.1161/01.atv.20.4.1168. [DOI] [PubMed] [Google Scholar]
- 62.Selroos O. Thrombocytosis in rheumatoid arthritis. Scand J Rheumatol. 1972;1(3):136–40. doi: 10.3109/03009747209103013. [DOI] [PubMed] [Google Scholar]
- 63.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113(19):2278–84. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 64.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot CV, Coward J, Deavers MT, Vasquez HG, Urbauer D, Landen CN, Hu W, Gershenson H, Matsuo K, Shahzad MM, King ER, Tekedereli I, Ozpolat B, Ahn EH, Bond VK, Wang R, Drew AF, Gushiken F, Lamkin D, Collins K, DeGeest K, Lutgendorf SK, Chiu W, Lopez-Berestein G, Afshar-Kharghan V, Sood AK. Paraneoplastic thrombocytosis in ovarian cancer. The New England journal of medicine. 2012;366(7):610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rondina MT, Weyrich AS. Regulation of the genetic code in megakaryocytes and platelets. J Thromb Haemost. 2015;13(Suppl 1):S26–32. doi: 10.1111/jth.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lood C, Amisten S, Gullstrand B, Jonsen A, Allhorn M, Truedsson L, Sturfelt G, Erlinge D, Bengtsson AA. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116(11):1951–7. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 67.Deutsch VR, Tomer A. Megakaryocyte development and platelet production. British journal of haematology. 2006;134(5):453–66. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- 68.Goncharova V, Serobyan N, Iizuka S, Schraufstatter I, de Ridder A, Povaliy T, Wacker V, Itano N, Kimata K, Orlovskaja IA, Yamaguchi Y, Khaldoyanidi S. Hyaluronan expressed by the hematopoietic microenvironment is required for bone marrow hematopoiesis. The Journal of biological chemistry. 2012;287(30):25419–33. doi: 10.1074/jbc.M112.376699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosel M, Khaldoyanidi S, Zawadzki V, Zoller M. Involvement of CD44 variant isoform v10 in progenitor cell adhesion and maturation. Exp Hematol. 1999;27(4):698–711. doi: 10.1016/s0301-472x(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 70.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 71.Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, Ehrlicher A, Larson MK, Hoffmeister K, Hartwig JH, Italiano JE., Jr Cytoskeletal mechanics of proplatelet maturation and platelet release. The Journal of cell biology. 2010;191(4):861–74. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khaldoyanidi S, Moll J, Karakhanova S, Herrlich P, Ponta H. Hyaluronate-enhanced hematopoiesis: two different receptors trigger the release of interleukin-1beta and interleukin-6 from bone marrow macrophages. Blood. 1999;94(3):940–9. [PubMed] [Google Scholar]
- 73.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 74.Kaushansky K. The molecular mechanisms that control thrombopoiesis. The Journal of clinical investigation. 2005;115(12):3339–47. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Currao M, Malara A, Di Buduo CA, Abbonante V, Tozzi L, Balduini A. Hyaluronan based hydrogels provide an improved model to study megakaryocyte-matrix interactions. Exp Cell Res. 2015 doi: 10.1016/j.yexcr.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbonante V, Di Buduo CA, Gruppi C, Malara A, Gianelli U, Celesti G, Anselmo A, Laghi L, Vercellino M, Visai L, Iurlo A, Moratti R, Barosi G, Rosti V, Balduini A. Thrombopoietin/TGF-beta1 Loop Regulates Megakaryocyte Extracellular Matrix Component Synthesis. Stem Cells. 2016;34(4):1123–33. doi: 10.1002/stem.2285. [DOI] [PubMed] [Google Scholar]
- 77.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–5. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 78.Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C, Laghi L, Kaplan DL, Balduini A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32(4):926–37. doi: 10.1002/stem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaushansky K. Thrombopoietin and the hematopoietic stem cell. Ann N Y Acad Sci. 2005;1044:139–41. doi: 10.1196/annals.1349.018. [DOI] [PubMed] [Google Scholar]
- 80.Kapsoritakis AN, Potamianos SP, Sfiridaki AI, Koukourakis MI, Koutroubakis IE, Roussomoustakaki MI, Manousos ON, Kouroumalis EA. Elevated thrombopoietin serum levels in patients with inflammatory bowel disease. Am J Gastroenterol. 2000;95(12):3478–81. doi: 10.1111/j.1572-0241.2000.03364.x. [DOI] [PubMed] [Google Scholar]
- 81.Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19(7):757–60. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 82.Wolber EM, Fandrey J, Frackowski U, Jelkmann W. Hepatic thrombopoietin mRNA is increased in acute inflammation. Thromb Haemost. 2001;86(6):1421–4. [PubMed] [Google Scholar]
- 83.Eicher JD, Chen MH, Pitsillides AN, Lin H, Veeraraghavan N, Brody JA, Metcalf GA, Muzny DM, Gibbs RA, Becker DM, Becker LC, Faraday N, Mathias RA, Yanek LR, Boerwinkle E, Cupples LA, Johnson AD. Whole exome sequencing in the Framingham Heart Study identifies rare variation in HYAL2 that influences platelet aggregation. Thromb Haemost. 2017;117(6):1083–1092. doi: 10.1160/TH16-09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrey AC, Obery DR, Kessler SP, Flamion B, de la Motte CA. Hyaluronan Depolymerization by Megakaryocyte Hyaluronidase-2 Is Required for Thrombopoiesis. The American journal of pathology. 2016;186(9):2390–403. doi: 10.1016/j.ajpath.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jadin L, Wu X, Ding H, Frost GI, Onclinx C, Triggs-Raine B, Flamion B. Skeletal and hematological anomalies in HYAL2-deficient mice: a second type of mucopolysaccharidosis IX? FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22(12):4316–26. doi: 10.1096/fj.08-111997. [DOI] [PubMed] [Google Scholar]
- 86.Onclinx C, Dogne S, Jadin L, Andris F, Grandfils C, Jouret F, Mullier F, Flamion B. Deficiency in mouse hyaluronidase 2: a new mechanism of chronic thrombotic microangiopathy. Haematologica. 2015;100(8):1023–30. doi: 10.3324/haematol.2015.123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beaulieu LM, Lin E, Morin KM, Tanriverdi K, Freedman JE. Regulatory effects of TLR2 on megakaryocytic cell function. Blood. 2011;117(22):5963–74. doi: 10.1182/blood-2010-09-304949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward JR, Bingle L, Judge HM, Brown SB, Storey RF, Whyte MK, Dower SK, Buttle DJ, Sabroe I. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94(4):831–8. [PubMed] [Google Scholar]