Abstract
Sexual dimorphism, the condition in which males and females in a species differ beyond the morphology of sex organs, delineates critical aspects of the biology of higher eukaryotes, including selenium metabolism. While sex differences in selenium biology have been described by several laboratories, delineation of the effects of sex in selenium function and regulation of selenoprotein expression is still in its infancy. This review encompasses the available information on sex-dependent parameters of selenium metabolism, as well as the effects of selenium on sex hormones. Gaps in the current knowledge of selenium and sex are identified and discussed.
Keywords: selenium, selenoproteins, selenocysteine, sex, sexual dimorphism
Graphical abstract
Introduction
It is widely recognized that intrinsic sex differences account for variations observed in eukaryotic biology. Thus, it is not surprising that selenium biology will also display significant sexual dimorphism. The hierarchical nature of selenium physiology [1–6], the unique mechanism for incorporation of this trace element into selenoproteins as the amino acid, selenocysteine, and the different functions attributed to these selenoproteins [7, 8] deem sex-specific effects as an additional regulatory role in our understanding of the sophisticated biology of selenium.
The complexities of sexual dimorphism in selenium biology have been discussed in several detailed review articles [3, 9–11], but recent discoveries warrant an updated examination of the subject. This review will feature novel recent findings and unveil research areas that are still unknown or poorly understood regarding the interaction between sex and selenium. Moreover, it will highlight the need to consider sex differences in any analysis of selenium biology. We will explore the subject from two approaches: the effects of sex on selenium-dependent parameters and the effects of selenium on sex-dependent parameters. These two considerations may be intertwined and difficult to discern, nonetheless are relevant to construct a comprehensive perspective of selenium biology.
Significance
The importance of investigating sex and gender differences in the health sciences has come to the forefront in recent years, after concerns were raised that “a lack of systemic and consistent inclusion of women in NIH-supported clinical research could result in clinical decisions being made about health care for women based solely on findings from studies of men—without any evidence that they were applicable to women.” [12] The Office of Research on Women’s Health Report expands upon this, stating “Over the past 20 years, research has revealed that from single cells to multiple biological systems and mechanisms, sex differences exist —and these differences are not just hormone based. Sex differences research is needed not only in fields such as endocrinology and immunology, but also in rapidly evolving scientific disciplines such as epigenetics, systems biology, and neuroscience; and new technology-enabled fields such as genomics, proteomics, and metabolomics.”
Sexual dimorphism in selenocysteine incorporation, selenium metabolism and selenoproteins
Sex hormones
Androgens, estrogens, and progestogens comprise the three main classes of endogenous sex hormones. Because they are responsible for male sex characteristics, androgens are referred to as the male sex hormones. Perhaps the most well-studied androgen is testosterone. Likewise, estrogens and progestogens are aptly termed the female sex hormones. Sex hormones are primarily synthesized in the gonads. Thus, gonadectomy is a useful tool in determining the influence of endogenous sex steroids by allowing their near-complete removal. GnRH (gonadotropin-releasing hormone) from the hypothalamus stimulates the anterior pituitary to release LH (luteinizing hormone) and FSH (follicle-stimulating hormone) which act on the testes and ovaries to secrete testosterone and estrogen, respectively. Collectively, this regulatory system is known as the HPG (hypothalamic-pituitary-gonadal) axis [13]. In this review, relevant aspects of selenium metabolism and selenoproteins will be discussed in light of their sexual dimorphism and their regulation by sex hormones.
Selenocysteine incorporation
In higher eukaryotes, selenocompounds need to be metabolized and ultimately converted to selenide, which is utilized in the production of selenocysteine, the amino acid present in the active site of selenoenzymes. The selenocysteine incorporation mechanism has been extensively studied [8, 14–16]. Selenide is phosphorylated by the actions of the selenophosphate synthetase 2 (SEPHS2), a selenoenzyme, in order to be attached to a specific serine-charged tRNA, the tRNASer[Sec]. The tRNA is first charged with a serine residue and by the actions of the phosphoseryl-tRNASer[Sec] kinase (PSTK) and selenocysteine synthase (SepSecS), it results in a tRNA charged with selenocysteine. The tRNASer[Sec] is then utilized during selenoprotein translation to insert at in-frame UGA sequences the amino acid selenocysteine. As UGA also specifies termination of translation, other factors are required to ensure the insertion of selenocysteine in the nascent polypeptide chain. These factors include, but are not restricted to, a specific cis structure in the 3’-UTR of the selenoprotein mRNA (SECIS), a selenocysteine-specific elongation factor (EFSec), a SECIS binding protein (SBP2), and a selenocysteine tRNA associated protein (secp43). Once all these factors are properly assembled, selenoproteins can be synthesized.
Among factors involved in selenocysteine incorporation, current evidence does not indicate their expression or activity being affected by sex. For example, expression of the genes for Efsec and Sbp2 were not regulated by ovariectomization in mice, indicating that if sex influences translational efficiency, it probably does so either through post-translational effects or other members of the selenocysteine incorporation complex [17]. Another example is the deletion of the Trsp gene in murine liver, which leads to total ablation of hepatic selenoproteins and equal early mortality rates between females and males [18], confirming the ability to produce selenoproteins during development to be essential for both sexes. It is reasonable to expect that a sex-independent requirement for selenoprotein production, even partially, remains through adulthood. However, this neutral possibility is still untested.
Intracellular selenium metabolism
Selenide can be produced by different pathways. Dietary selenium is consumed mostly as the organic forms selenomethionine, selenocysteine, and selenocystathionine, or the inorganic forms selenite and selenate. Inorganic selenite is metabolized either by the selenoprotein thioredoxin reductase 1 (TXNDR1) into selenide or by reaction with glutathione. Interestingly, in zebrafish TXNDR1 was shown to have a sex-specific pattern of expression, with females having diminished expression after selenite supplementation while males increased their expression of TXNDR1 regardless of the selenium chemical form [19].
Organic form selenomethionine has been suggested to enter the methionine cycle, be converted into selenohomocysteine, then metabolized by the same enzymes that lead to cysteine formation via the transsulfuration pathway [20, 21]. “Trans-selenation” reactions occur sequentially and involve the enzymes cystathionine beta-synthase (CBS) and cystathionine gamma-lyase (CGL), and the end product is selenocysteine. Selenocystathionine can also serve as a substrate for CGL, whose actions lead to the formation of selenocysteine. Evidence in yeast demonstrates that selenomethionine toxicity is mediated by the transsulfuration pathway [22] and that trans-selenation-derived selenocysteine can be misincorporated in place of cysteine [23]. This possibility, if present in mammals, would point towards mechanisms for dealing with selenomethionine cytotoxicity.
Interestingly, both CBS and CGL are regulated by sex hormones. Renal CBS activity is higher in male rodents than in female rodents, but lower in men than in women. Castration of mice led to decreases in CBS expression to similar levels as female mice [24]. Paradoxically, treatment of an androgen-responsive human prostate cell line with testosterone downregulated CBS and negatively impacted the transsulfuration pathway flux [25]. These findings combined indicate that CBS is possibly regulated by testosterone acting through transcriptional and post-transcriptional mechanisms. CGL, on the other hand, is regulated by both androgens and estrogens. Ovariectomized rats treated with 17β-estradiol showed an increase in CGL expression in the myocardium [26]. Conversely, treatment of ovariectomized ewes with 17β-estradiol did not elicit any changes in expression of CGL in arteries [27]. These findings suggest that estrogen regulation of CGL seems to be tissue-specific in females. Interestingly, mice lacking CGL display profound sexual dimorphism, with females, but not males, exhibiting drastically reduced plasma levels of methionine and cysteine [28]. Perplexingly, the androgen receptor physically interacts with CGL [29], also allowing for regulation of this enzyme by testosterone through a post-translational mechanism [30].
The regulation of the trans-selenation pathway by sex hormones strongly implies that selenomethionine metabolism and its consequent selenocysteine formation and availability for selenoprotein synthesis are not the same in both sexes. Consequently, it also raises the interesting possibility that intracellular mechanisms offsetting toxic levels of selenomethionine and affecting the differential selenium distribution that we observe in circulation are sexually dimorphic, and could explain some of the sexual dimorphism found in clinical trials using selenomethionine (see section Sex differences after selenium supplementation).
In addition to the effects discussed above, selenomethionine can also be misincorporated in place of methionine. The methionyl-tRNA synthetase has long been known to not differentiate between methionine and selenomethionine [31]. Hence, when selenomethionine is in excess, it is assumed that a percentage of tRNAMet is charged with selenomethionine. Misincorporation of selenomethionine may alter physiological characteristics of enzymes, affecting either chemical structure or enzymatic activity, and there is not yet evidence of sexual dimorphism in misincorporation.
Selenium recycling
The unique pathway selenocysteine takes to be incorporated must be acknowledged. It is speculated that selenocysteine coming either from dietary intake, selenoprotein degradation or trans-selenation pathways should be first decomposed to selenide. Selenide can then be utilized to synthesize selenocysteine-bound tRNA, the form that can be incorporated into selenoproteins. The decomposition of selenocysteine is catalyzed by the action of selenocysteine lyase (Scly), a pyridoxal-phosphate-dependent enzyme that converts it into selenide and alanine, and this enzyme has been demonstrated to affect the synthesis of selenoproteins in mammals [32]. Although not essential to life, as mice lacking Scly are viable [33], this enzyme sits at an interesting metabolic intersection, between trans-selenation, protein degradation, and selenoprotein synthesis.
Mammalian Scly was first isolated from pig liver and first cloned from the liver of male mice [34, 35]. It is currently unknown if the expression of Scly varies according to sex. Nevertheless, Scly disruption in mice leads to sexually dimorphic phenotypes. While male Scly−/− mice develop obesity, glucose intolerance and hyperinsulinemia [36], females present a milder phenotype, with weight gain but not changes in glucose sensitivity nor hyperinsulinemia [37].
Selenoproteins
The best-studied connection between a selenoprotein and sex is the role of glutathione peroxidase 4 (GPX4) in spermatozoa structure and viability [38], and male fertility [39], a connection that has been reviewed elsewhere in extensive detail [40–43]. GPX4 plays critical roles in spermatogenesis and in detoxifying hydroperoxides produced by the mitochondria-rich sperm midpiece, which generates energy for flagellar motion through oxidative phosphorylation. Additionally, the testes express a novel transcript of GPX4 utilizing an alternative promoter to encode a nuclear localization signal, and this form of the protein is localized to the sperm head, where it protects the germline DNA from oxidative damage.
A single nucleotide polymorphism (SNP) at the corresponding 3’ untranslated region of the Gpx4 gene is found in Hardy-Weinberg equilibrium in the human population [44]. This SNP accounts for sex differences observed in GPX4 expression and activity [45], possibly by differentially interfering on transcriptional mechanisms.
Numerous other examples of sex-specific differences in selenoprotein expression and function have been described. Female C57BL/6 mice have higher mRNA expression of iodothyronine deiodinase 1 (DIO1) in the liver than males. However, DIO1 activity in the liver of females is drastically lower than in male mice. Under conditions of selenium deficiency, Dio1 mRNA levels in females are two-fold greater than in males, while DIO1 activity is unchanged between the sexes [17]. Thus, selenium levels appear to exert a significant impact on the sex dependence of DIO1 activity in the liver, possibly by affecting translational efficiency and/or protein stability.
Dietary selenite affects glutathione peroxidase 1 (GPX1) in the liver of rats in a sex-dependent fashion, with females enhancing its activity twice as the levels of males, despite similar increases in Gpx1 transcripts [46]. In the murine lungs, expression of GPX1 also shows lower Gpx1 mRNA in male than females [47]. In humans, different SNPs in the Gpx1 gene have been demonstrated to be sex-dependent [48, 49], most commonly leading to men having lower enzyme activity than women. Testosterone has been shown to not affect GPX1 expression in the mouse testis [50], while its transcriptional and translational regulation by other sex hormones has not been determined.
The selenium transporter selenoprotein P (SELENOP) is produced mainly in the liver and secreted into the bloodstream, where it supplies selenium for other organs. Because selenium concentration correlates with circulating SELENOP levels, these are used as a biomarker of selenium status. SelenoP mRNA levels are higher in the liver of female rodents than in males, regardless of age [9, 17]. Males seem to prioritize selenium distribution to the testis at the expense of selenium for the brain to maintain neurological function. Male SelenoP−/− mice were infertile, attesting for the importance of this selenoprotein to sexual reproduction either as a selenium-delivery or as local storage of selenium for the selenium-dependent testis [33, 51, 52]. Recently, the development of a calibrated ELISA for human SELENOP detection revealed striking results, with selenium and SELENOP concentrations not differing between men and women. Nevertheless, the correlation between selenium and SELENOP was altered between sexes, with young women presenting a lower correlation between total selenium and SELENOP concentrations than elderly men or women, or young men [53]. This standardized development will allow for an additional understanding of the sex-dependent dynamics of SELENOP in different populations with various health conditions in the future.
The expression of selenoprotein S (SELENOS) in the mouse liver also presents sexual dimorphism. Upon selenite supplementation, male mice increased SELENOS levels while females only reached maximal SELENOS expression after challenge with lipopolysaccharides to induce an acute-phase response, i.e., when their immune system was challenged [54].
A recent comprehensive analysis of the selenotranscriptome revealed compelling insights towards the sexual dimorphism of selenoprotein expression [55]. Using a mouse model with a shortened telomere that mimics the effects of aging on human chromosomes, a differential pattern of downregulation of selenoproteins between males and females emerged, according to levels of dietary selenium and age. While dietary selenium levels affected most selenoproteins in the female kidneys and liver, only the male liver had a comparable extensive downregulation of selenoproteins upon selenium deficiency. Interestingly, older males presented a decrease in Sephs2 mRNA levels, while older females did not, a finding that suggests either an aging fragility of the selenocysteine incorporation machinery according to sex or a consequence of lower demands of the aging testis for selenium.
Sexual dimorphism in selenium absorption and excretion
Selenium absorption
Most selenocompounds are absorbed by the intestinal epithelial mucosa [52]. Organic forms are primarily absorbed in the intestinal epithelial utilizing methionine or dibasic and neutral amino acid transporters [52, 56, 57]. However, the molecular mechanisms of this absorption are still unclear. Inorganic forms most likely utilize passive transport to enter the intestinal cell.
Intestinal absorption of selenomethionine was shown to be ~96% in healthy women [58] and ~76% in healthy men [59], and ~96% in female rats [60] and 88% in male rats [61]. However, the molecular mechanisms of this sexual dimorphism are still not understood. Experimental models used in previously published molecular studies of selenium intestinal transport were either male animals or cell lines derived from men, such as intestinal Caco-2 cells. When female-derived cells were considered, as in studies of selenomethionine and selenocystine cellular absorption using the female opossum-derived kidney cells (OK cells) [56], these mechanisms were compared to studies in male Caco-2 cells, confounding whether the mechanisms of selenium transport into the cell were different because of their tissue origin or their inherent sexual dimorphism. Hence, a full picture of the possible molecular mechanisms of the sexual dimorphism of absorption of selenocompounds in the intestines and other seleno-dependent tissues has yet to emerge.
Afar from the intestines, other cell types in the body also take up selenium in its various forms, utilizing membrane transporters or mechanisms also found in the intestines. Interestingly, cystathionine was recently unveiled to use the cystine/glutamate transporter in brain and immune cells, a transporter involved in the maintenance of intracellular glutathione levels and extracellular redox balance [62]. If selenocystathionine can utilize the same transporter, this possibility as an alternate avenue in some tissues becomes in itself an interesting research point to be tested.
Circulating selenium
Once absorbed by the intestines, selenocompounds are metabolized, entering the bloodstream, where they become part of the pool of selenium molecules. Many of these are considered as biomarkers of selenium status, such as total selenium content, levels of the selenoproteins glutathione peroxidase 3 (GPX3) and SELENOP, and selenium bound to albumin.
The impact of sex on circulating selenium biomarkers in animals has been observed since the late 60’s [63, 64]. In humans, women have been recognized to have higher GPX activity in their circulation than men, but not increased selenium levels [65, 66], with the same sexually dimorphic pattern also reported in rodents [9, 17]. More recently, it was demonstrated that people with the same total selenium levels might differ in their selenium distribution in the plasma as selenium either bound to albumin or as part of the primary structure of SELENOP or GPX3. Nevertheless, using a subcohort from the cross-sectional ATTICA study in Greece [67], researchers determined that, in people with the same intake of selenium and methionine, a competitor for the amino acid transporter, women presented a significant increase in selenium-bound albumin, while men presented an increased ratio of total selenium to GPX3 in relation to total selenium to either SELENOP or selenium-bound albumin [68]. The extent to which these results can also be expanded into other human populations with different genetic backgrounds and dietary habits is uncertain.
Another factor possibly contributing and/or confounding selenium biomarkers in the bloodstream is the chemical form of selenium ingested. In experiments comparing mice fed diets containing either adequate or high levels of selenite, selenate or selenomethionine, these animals developed differential increases in total selenium content and plasma GPX activity, with selenomethionine being the form that most efficiently increased both of these parameters [69]. Nevertheless, these experiments were performed in male mice, remaining to be clarified whether these changes in the allocation of selenium depending on the chemical form would occur in female mice as well.
Sex differences after selenium supplementation
Under a state of selenium deficiency, female rats were shown to take up selenium more efficiently than males [70] and, upon supplementation, to increase plasmatic levels of GPX activity and selenium levels [71]. Furthermore, female rats have a lower selenium requirement than males [64]. Selenium has also been shown to exhibit differential uptake by the reproductive organs. While the retention rate for selenium is highly efficient in the testes [70], the female reproductive system does not appear to take up or retain significant levels of selenium [10, 11].
Selenium supplementation has been demonstrated to enhance the pool of total selenium and alter its distribution in the plasma of selenium-deficient rats, with females being able to maintain circulating selenium in a more efficient manner than males. Interestingly, a supplementation study conducted decades ago with Norwegian women had already demonstrated that both selenite and selenium-rich pea flour (containing selenomethionine) increased serum selenium levels at the same rate without raising GPX values [72].
In humans, the failure of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) to prevent prostate cancer in selenium-replete US men receiving a high dose (200 µg/day) of selenomethionine supplementation still unveiled an interesting side of the sexual dimorphism of selenium physiology that deems further exploration [73]. The SELECT was prematurely terminated due to a statistically non-significant increase in the risk of type 2 diabetes in men [74]. This unexpected outcome raised two different sex perspectives that we will further explore: 1) Could selenium supplementation still help in preventing prostate cancer in selenium-deficient men? 2) Could a high dose of selenomethionine in a selenium-replete population of women also increase the risk of type 2 diabetes?
Effects of selenium supplementation on selenium deficient males
The first question had been previously answered by the Nutritional Prevention of Cancer (NPC) clinical trial. The NPC provided male participants with 200 µg/day of selenium as selenized yeast, which contains approximately 16% selenomethionine [73]. This dose was enough to significantly reduce the incidence of prostate cancer, as the baseline selenium levels of patients were lower than in the SELECT [75]. Nevertheless, it should be noted that none of these trials assessed the men’s serum selenium distribution into selenoproteins, selenium-bound albumin and their ratios in relation to total selenium levels, all information that could enrich the sex-dependent profile of selenium in men.
This information was later carefully gathered in a clinical trial assessing exactly those parameters [76]. In this trial, selenomethionine in four different doses was provided to healthy men and women of a high selenium baseline status. After a year of supplementation, total serum selenium was elevated in all groups receiving treatment, but selenoproteins were at the same levels, suggesting that selenoproteins were already maximized. Supporting that even selenoprotein deiodinases were not affected by selenomethionine supplementation, additional analysis of an ancillary group of a larger trial with this same selenocompound revealed that neither serum thyroid hormones T4, T3 nor thyroid-stimulating hormone (TSH) levels have changed according to sex [76]. Interestingly, the year-long supplementation trial also revealed that women increased their urinary selenium excretion compared to men receiving the same doses [77], pointing towards a sexual dimorphism in selenium metabolism in other tissues of the body. Kidneys have been already demonstrated in mice to differ their expression of SELENOP according to sex [9], and it is known in male mice that competition between brain and testes exists for available selenium under a compromised selenium metabolism circumstance [78].
Sex differences in the relationship between selenium and energy metabolism
The second perspective derived from SELECT was whether selenium supplementation would also raise the incidence of type 2 diabetes in women, a possibility that requires careful examination. Type 2 diabetes is a worldwide epidemic triggered in most cases by carbohydrate-rich dietary habits and sedentary behavior, which leads to excess circulating glucose and disturbances in energy metabolism. Glucose metabolism is coordinated by the actions of insulin, a peptide hormone produced by the endocrine pancreas that triggers a phosphorylation signaling cascade in target tissues that ultimately allows for circulating glucose to enter the cell and provide energy for basic functions. Insulin release is triggered by glycemic state and centrally controlled by hypothalamic neurons that regulate energy metabolism. Insulin action is affected by sex, with females tending to be more insulin sensitive than males [79] while paradoxically also presenting more commonly impaired glucose tolerance [80]. The molecular mechanism of sexual dimorphism in glucose metabolism is starting to be unveiled, and recent results point to androgens as a key effector in the central [81, 82] and peripheral [83] regulation of glucose metabolism.
The effect of selenium on glucose metabolism and type 2 diabetes incidence has been a very controversial issue, with human and animal studies either pointing towards a positive, a negative or even a neutral correlation between serum selenium and glucose [84]. A recent systematic review of the association between serum selenium and type 2 diabetes incidence comparing five studies concluded that there is a positive association between these two factors in populations whose selenium levels are either low (<97.5 µg/l) or high (>132.5 µg/l) [85], once again leaning towards the recognized U-shape nature of selenium effects. Most of these studies did not consider sex effects on glucose metabolism.
Enzymes involved in selenium metabolism have been shown to act on glucose pathways. As mentioned earlier in this review, male Scly−/− mice develop characteristics of a metabolic syndrome phenotype, such as obesity, glucose intolerance, hyperinsulinemia and hypercholesterolemia [36], while females gained weight but did not exhibit other symptoms [37]. Interestingly, male and female Scly−/− mice were equally prone to high-fat diet-induced obesity [86]. Despite these metabolic disturbances, Scly−/− mice had normal selenoprotein levels in their livers except when selenium was limiting.
Selenoproteins have also been shown to act on glucose metabolism. However, a sex difference has yet to be resolved for each case. For example, SELENOP was suggested as a cause of insulin resistance and low adiponectin levels in humans [87, 88]. However, the identification of this selenoprotein as a marker for type 2 diabetes in humans was performed with a mixed sample including patients of both sexes, and results were not reported in light of possible sex differences. Interestingly, when testing for the response of SELENOP to exercise, a common booster of glucose handling and tolerance, female mice were not included in experiments due to an inconsistent phenotype [88]. It is reasonable to speculate that the effects of SELENOP on glucose metabolism and insulin sensitivity are sex-specific, with molecular mechanisms possibly being affected directly or indirectly by sex hormones.
Another selenoprotein closely linked to glucose metabolism is glutathione peroxidase 1 (GPX1). This selenium-dependent enzyme regulates the levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) intracellularly. Mice overexpressing GPX1 have been developed and are obese, hyperinsulinemic and hyperglycaemic [89, 90], but only male mice were reported in these studies. Thus it is currently unclear whether overexpression of GPX1 in females would lead to similar metabolic disturbances.
The endoplasmic reticulum-resident SELENOM appears to affect animal energy metabolism differently according to sexes. Selenom−/− mice of both sexes presented increased adiposity. However, only male mice were hyperinsulinemic and hyperleptinemic [91]. As this enzyme is highly expressed in the brain [92], it is possible that SELENOM is involved in sex-dependent energy regulation at the central level. If the sexual dimorphism presented by the Selenom−/− mice is directly mediated at the molecular level by sex hormones is unknown.
Selenium and sex hormones
The effects of selenium on sex hormones
Selenium has been known to directly influence testosterone production from early on. Selenium deficient rats exhibited defective testosterone secretion in response to GnRH and LH injection, demonstrating that selenium directly affects testosterone production, rather than indirectly through the HPG axis [93]. Despite the reduced testosterone secretion, circulating testosterone demonstrated only a slight, non-significant decrease, which is quite possibly explained by the reported increase in the number of testosterone-producing Leydig cells [93]. A non-significant downward trend of serum testosterone was also described in mice with double knockout of the Scly and SelenoP (Scly−/−/SelenoP−/−) genes when compared to SelenoP−/− only and WT mice, an unsurprising finding, given that testes selenium concentration was also reportedly lower [78]. The importance of Scly in testosterone is further corroborated by the finding that it is highly expressed in Leydig cells, the primary site for male androgen production and secretion [32].
While it is apparent that selenium supports testosterone production, the mechanistic relationship remains to be resolved. SelenoP mRNA expression increased in response to cAMP-mediated testosterone production in cultured Leydig cells, leading to the hypothesis that selenium may protect against free radicals generated during testosterone synthesis [94]. Presumably, excess reactive oxygen species would lead to apoptosis, resulting in an overall loss of testosterone-producing cells. Selenium has indeed been shown to protect the testes against toxic agents [95]. Yet, selenium deficiency in vivo was demonstrated to result in a seemingly compensatory increase in Leydig cells [93]. It is, therefore, more probable that selenium plays a direct role in testosterone synthesis. Strikingly, endoplasmic reticulum-resident selenoproteins, SELENOT [96] and SELENOS [97] were found to be highly expressed in the Leydig cells. Of particular relevance is the finding that SelenoT mediates PACAP (pituitary adenylate cyclase activating polypeptide)-induced neuroendocrine secretion from PC12 cells [98]. PACAP has also been implicated in steroidogenesis in the Leydig cells [99], albeit the contribution of SELENOT in this process remains to be established. Recently, selenium was found to increase testosterone production through the ERK pathway [100]. Nevertheless, identification of the specific selenoproteins expressed within the Leydig cells will elucidate more explicit pathways in which selenium acts on testosterone production.
Selenium undoubtedly plays a direct role in spermatogenesis, demonstrated by the dependence on normal GPX4 expression to maintain the structural integrity of spermatozoa [101]. Interestingly, it appears that selenium also indirectly enhances spermatogenesis through its regulation of testosterone production. Thus, the negative impact of selenium deficiency on male fertility is two-fold.
The effects of selenium on estrogen are less clear. One study in rainbow trout involving long-term selenomethionine exposure showed elevated plasma testosterone and estrogen levels in females as well as increases in expression of key steroidogenic enzymes in the gonads [102]. Conversely, a study in cows demonstrated progesterone levels may be regulated by selenium, but not estrogen [103]. Additional studies are necessary to clarify the relationship between selenium and female sex hormones.
The effects of sex hormones on selenium distribution and selenoprotein expression
None of the genes encoding for selenoproteins or selenocysteine incorporation factors are present in the sex chromosomes of humans or mice. Thus, sex differences in selenoprotein expression or selenocysteine incorporation factors are under the regulation of other factors, such as sex hormones.
For reasons that are yet unknown, sex hormones appear to play a direct role in selenium distribution and metabolism. Sexual dimorphism in selenium distribution and selenoprotein expression has been well-documented [3] and discussed previously in this review. It is, therefore, reasonable to consider that sex hormones may at least partially account for apparent sexual dimorphism in selenoprotein regulation.
Early studies described a positive correlation between plasma selenium levels and GPX activity with estrogen fluctuation in pre-menopausal women, suggesting a direct effect of estrogen on selenium status [104]. This is corroborated by the finding that ovariectomized and estrogen replaced (OVX+E2) rats have higher selenium concentration and GPX activity after 75Se administration compared to OVX rats in the plasma, liver, and brain [105]. In the same study, it was demonstrated that OVX+E2 resulted in an upregulation of hepatic SELENOP mRNA, suggesting that estrogen promotes increased selenium transport through SELENOP. Perplexingly, plasma SELENOP was not significantly different between the two groups [105]. Moreover, OVX did not affect hepatic mRNA expression of selenoprotein synthesis factors SBP2 and EFSec [17]. However, post-translational effects of estrogen should not be overlooked. Currently, the mechanistic relationship governing estrogen-mediated increase in selenium remains elusive.
Considering the significant selenium demands of the testes, it is necessary to highlight that castration results in two possible outcomes which confound the interpretation of gonadectomy with regards to selenium distribution. While castration results in reduced testosterone levels, it also liberates selenium supply which would otherwise be routed to the testes. Thus, while castration of male mice abolished some of the sex differences observed in selenoprotein tissue expression and activity [17], it does not necessarily presuppose an effect of testosterone on selenoprotein expression. One study found that administration of testosterone or anti-androgenic compounds in rats resulted in opposite effects on testes GPX4 expression, which does indeed suggest an effect of testosterone [106], but it is unclear whether these effects are a direct result of testosterone signaling in the testes, or due to negative regulation through the HPG axis. Interestingly, women with polycystic ovarian syndrome, a condition in which plasmatic androgens are elevated, present normal GPX3 as well as plasma selenium levels [107]. A molecular regulatory function for androgens on the maintenance of circulating GPX3 levels is therefore plausible. Additional studies employing testosterone treatment are required to clarify the role of testosterone in the sexual dimorphism observed in plasma and tissue selenoprotein expression.
Concluding Remarks
It is concerning that a significant number of studies have so far neglected to acknowledge the critical physiological differences between males and females in their analysis of results and conclusions. This review highlights the fundamental importance of considering sex differences when analyzing for selenium metabolism, selenoprotein expression and action, and selenium-dependent parameters, and not assuming that a result can be extended to both sexes without proper verification. Furthermore, the impact of sex hormones in the regulation of selenium metabolism and selenoproteins has been explored in this review and is summarized in figure 1. In a moment when women are finally becoming more empowered in many areas of our society, it would be refreshing to broaden scientific outcomes in selenium-related studies towards a more balanced perspective by including more female samples and considering sexual dimorphism as a crucial variable to be taken into consideration during analysis. Such approach of a comprehensive and inclusive understanding of selenium biology can only benefit our scientific knowledge.
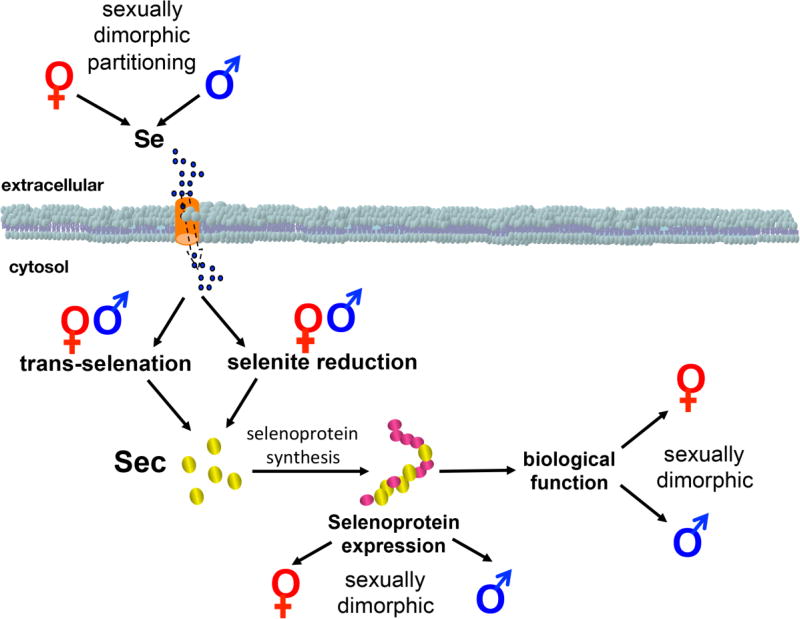
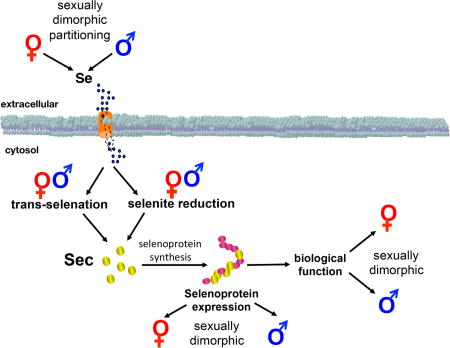
Figure 1.
Schematic illustration representing the several points of selenium biology, including selenium partitioning, selenium metabolism, and selenoprotein function, where sexual dimorphism has been reported. Except for the regulation of members of the selenocysteine incorporation and the absence of selenoprotein genes in sex chromosomes, most current evidence points towards sexual dimorphism as a broad characteristic of selenium biology.
HIGHLIGHTS.
Sexual dimorphism affects selenium biology and its effects on health.
Sex differences have been mostly neglected in past studies of selenium biology.
Sex differences should be considered in the future when analyzing selenium parameters.
Acknowledgments
The authors would like to thank Dr. Regina Brigelius-Flohé and Dr. Elias Arner for the opportunity of writing this manuscript. The authors would like to apologize for the authors of articles on this topic not cited or discussed due to space limits. MJB is supported by the US National Institutes of Health (NIH), grants R01DK47320 and G12MD007601, and LAS is supported by the US NIH grant U54MD007601 (subproject 5544).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Sunde RA, et al. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29(5):329–38. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhbacher M, et al. The brain selenoproteome: priorities in the hierarchy and different levels of selenium homeostasis in the brain of selenium-deficient rats. J Neurochem. 2009;110(1):133–42. doi: 10.1111/j.1471-4159.2009.06109.x. [DOI] [PubMed] [Google Scholar]
- 3.Schomburg L, Schweizer U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim Biophys Acta. 2009;1790(11):1453–62. doi: 10.1016/j.bbagen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Stock T, et al. Disruption and complementation of the selenocysteine biosynthesis pathway reveals a hierarchy of selenoprotein gene expression in the archaeon Methanococcus maripaludis. Mol Microbiol. 2011;82(3):734–47. doi: 10.1111/j.1365-2958.2011.07850.x. [DOI] [PubMed] [Google Scholar]
- 5.Low SC, et al. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19(24):6882–90. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry MJ. Insights into the hierarchy of selenium incorporation. Nat Genet. 2005;37(11):1162–3. doi: 10.1038/ng1105-1162. [DOI] [PubMed] [Google Scholar]
- 7.Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–77. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low SC, Berry MJ. Knowing when not to stop: selenocysteine incorporation in eukaryotes. Trends Biochem Sci. 1996;21(6):203–8. [PubMed] [Google Scholar]
- 9.Schomburg L, et al. Effect of age on sexually dimorphic selenoprotein expression in mice. Biol Chem. 2007;388(10):1035–41. doi: 10.1515/BC.2007.128. [DOI] [PubMed] [Google Scholar]
- 10.Hardy G, Hardy I. Selenium: the Se-XY nutraceutical. Nutrition. 2004;20(6):590–3. doi: 10.1016/j.nut.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Schomburg L. Sex-specific differences in biological effects and metabolism of selenium. In: Hatfield DL, et al., editors. Selenium - Its Molecular Biology and Role in Human Health. Springer; New York, NY: 2016. [Google Scholar]
- 12.Report OoRoWsH. Moving into the Future with New Dimensions and Strategies: A Vision for 2020 for Women’s Health Research. 2010 Available from: https://orwh.od.nih.gov/resources/pdf/ORWH_StrategicPlan2020_Vol1.pdf.
- 13.Melmed S, et al. Williams Textbook of Endocrinology. 13. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 14.Lobanov AV, Hatfield DL, Gladyshev VN. Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta. 2009;1790(11):1424–8. doi: 10.1016/j.bbagen.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty SP, Shah R, Copeland PR. Regulation of selenocysteine incorporation into the selenium transport protein, selenoprotein P. J Biol Chem. 2014;289(36):25317–26. doi: 10.1074/jbc.M114.590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small-Howard A, et al. Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol Cell Biol. 2006;26(6):2337–46. doi: 10.1128/MCB.26.6.2337-2346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riese C, et al. Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology. 2006;147(12):5883–92. doi: 10.1210/en.2006-0689. [DOI] [PubMed] [Google Scholar]
- 18.Carlson BA, et al. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J Biol Chem. 2004;279(9):8011–7. doi: 10.1074/jbc.M310470200. [DOI] [PubMed] [Google Scholar]
- 19.Benner MJ, et al. Zebrafish (Danio rerio) vary by strain and sex in their behavioral and transcriptional responses to selenium supplementation. Comp Biochem Physiol A Mol Integr Physiol. 2010;157(4):310–8. doi: 10.1016/j.cbpa.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajander EO, et al. Metabolism, cellular actions, and cytotoxicity of selenomethionine in cultured cells. Biol Trace Elem Res. 1991;28(1):57–68. doi: 10.1007/BF02990463. [DOI] [PubMed] [Google Scholar]
- 21.Schrauzer GN. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr. 2000;130(7):1653–6. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 22.Lazard M, et al. Trans-sulfuration Pathway Seleno-amino Acids Are Mediators of Selenomethionine Toxicity in Saccharomyces cerevisiae. J Biol Chem. 2015;290(17):10741–50. doi: 10.1074/jbc.M115.640375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plateau P, et al. Exposure to selenomethionine causes selenocysteine misincorporation and protein aggregation in Saccharomyces cerevisiae. Sci Rep. 2017;7:44761. doi: 10.1038/srep44761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitvitsky V, et al. Testosterone regulation of renal cystathionine beta-synthase: implications for sex-dependent differences in plasma homocysteine levels. Am J Physiol Renal Physiol. 2007;293(2):F594–600. doi: 10.1152/ajprenal.00171.2007. [DOI] [PubMed] [Google Scholar]
- 25.Prudova A, et al. Testosterone regulation of homocysteine metabolism modulates redox status in human prostate cancer cells. Antioxid Redox Signal. 2007;9(11):1875–81. doi: 10.1089/ars.2007.1712. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, et al. Estrogens increase cystathionine-gamma-lyase expression and decrease inflammation and oxidative stress in the myocardium of ovariectomized rats. Menopause. 2013;20(10):1084–91. doi: 10.1097/GME.0b013e3182874732. [DOI] [PubMed] [Google Scholar]
- 27.Lechuga TJ, et al. Estrogen Replacement Therapy in Ovariectomized Nonpregnant Ewes Stimulates Uterine Artery Hydrogen Sulfide Biosynthesis by Selectively Up-Regulating Cystathionine beta-Synthase Expression. Endocrinology. 2015;156(6):2288–98. doi: 10.1210/en.2015-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, et al. Sex-specific dysregulation of cysteine oxidation and the methionine and folate cycles in female cystathionine gamma-lyase null mice: a serendipitous model of the methylfolate trap. Biol Open. 2015;4(9):1154–62. doi: 10.1242/bio.013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brancaleone V, et al. Crucial role of androgen receptor in vascular H2S biosynthesis induced by testosterone. Br J Pharmacol. 2015;172(6):1505–15. doi: 10.1111/bph.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao K, et al. Regulation of cystathionine gamma-lyase/H(2)S system and its pathological implication. Front Biosci (Landmark Ed) 2014;19:1355–69. doi: 10.2741/4286. [DOI] [PubMed] [Google Scholar]
- 31.Weller CA, Green M. Methionyl-tRNA synthetase detected by [75Se]selenomethionine in lenses from normal and galactose-fed rats. Exp Eye Res. 1969;8(1):84–90. doi: 10.1016/s0014-4835(69)80084-9. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa S, et al. Mammalian selenocysteine lyase is involved in selenoprotein biosynthesis. J Nutr Sci Vitaminol (Tokyo) 2011;57(4):298–305. doi: 10.3177/jnsv.57.298. [DOI] [PubMed] [Google Scholar]
- 33.Raman AV, et al. Absence of selenoprotein P but not selenocysteine lyase results in severe neurological dysfunction. Genes Brain Behav. 2012;11(5):601–13. doi: 10.1111/j.1601-183X.2012.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihara H, et al. cDNA cloning, purification, and characterization of mouse liver selenocysteine lyase. Candidate for selenium delivery protein in selenoprotein synthesis. J Biol Chem. 2000;275(9):6195–200. doi: 10.1074/jbc.275.9.6195. [DOI] [PubMed] [Google Scholar]
- 35.Esaki N, et al. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J Biol Chem. 1982;257(8):4386–91. [PubMed] [Google Scholar]
- 36.Seale LA, et al. Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice. Mol Cell Biol. 2012;32(20):4141–54. doi: 10.1128/MCB.00293-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa-Wong AN, et al. Sexual Dimorphism in the Selenocysteine Lyase Knockout Mouse. Nutrients. 2018;10(2):E159. doi: 10.3390/nu10020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ursini F, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285(5432):1393–6. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 39.Schneider M, et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23(9):3233–42. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 40.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Boitani C, Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Adv Exp Med Biol. 2008;636:65–73. doi: 10.1007/978-0-387-09597-4_4. [DOI] [PubMed] [Google Scholar]
- 42.Chabory E, et al. Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J Anim Sci. 2010;88(4):1321–31. doi: 10.2527/jas.2009-2583. [DOI] [PubMed] [Google Scholar]
- 43.Flohe L. Selenium in mammalian spermiogenesis. Biol Chem. 2007;388(10):987–95. doi: 10.1515/BC.2007.112. [DOI] [PubMed] [Google Scholar]
- 44.Villette S, et al. A novel single nucleotide polymorphism in the 3' untranslated region of human glutathione peroxidase 4 influences lipoxygenase metabolism. Blood Cells Mol Dis. 2002;29(2):174–8. doi: 10.1006/bcmd.2002.0556. [DOI] [PubMed] [Google Scholar]
- 45.Meplan C, et al. Functional effects of a common single-nucleotide polymorphism (GPX4c718t) in the glutathione peroxidase 4 gene: interaction with sex. Am J Clin Nutr. 2008;87(4):1019–27. doi: 10.1093/ajcn/87.4.1019. [DOI] [PubMed] [Google Scholar]
- 46.Weiss Sachdev S, Sunde RA. Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and -4 in rat liver. Biochem J. 2001;357(Pt 3):851–8. doi: 10.1042/0264-6021:3570851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tondreau MY, et al. Sex-specific perinatal expression of glutathione peroxidases during mouse lung development. Mol Cell Endocrinol. 2012;355(1):87–95. doi: 10.1016/j.mce.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Malling TH, et al. Sex determines the influence of smoking and gene polymorphism on glutathione peroxidase activity in erythrocytes. Scand J Clin Lab Invest. 2009;69(2):295–302. doi: 10.1080/00365510802632155. [DOI] [PubMed] [Google Scholar]
- 49.Donadio JL, et al. Influence of Gender and SNPs in GPX1 Gene on Biomarkers of Selenium Status in Healthy Brazilians. Nutrients. 2016;8(5) doi: 10.3390/nu8050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musicki B, et al. Mechanism of testosterone deficiency in the transgenic sickle cell mouse. PLoS One. 2015;10(5):e0128694. doi: 10.1371/journal.pone.0128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schomburg L, et al. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370(Pt 2):397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burk RF, Hill KE. Regulation of Selenium Metabolism and Transport. Annu Rev Nutr. 2015;35:109–34. doi: 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- 53.Hybsier S, et al. Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated ELISA for selenoprotein P. Redox Biol. 2016;11:403–414. doi: 10.1016/j.redox.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoedter M, et al. Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem J. 429(1):43–51. doi: 10.1042/BJ20091868. [DOI] [PubMed] [Google Scholar]
- 55.Cao L, et al. Analyses of Selenotranscriptomes and Selenium Concentrations in Response to Dietary Selenium Deficiency and Age Reveal Common and Distinct Patterns by Tissue and Sex in Telomere-Dysfunctional Mice. J Nutr. 2017;147(10):1858–1866. doi: 10.3945/jn.117.247775. [DOI] [PubMed] [Google Scholar]
- 56.Nickel A, et al. Characteristics of transport of selenoamino acids by epithelial amino acid transporters. Chem Biol Interact. 2009;177(3):234–41. doi: 10.1016/j.cbi.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Wolffram S, et al. Transport of selenoamino acids and their sulfur analogues across the intestinal brush border membrane of pigs. J Nutr. 1989;119(5):706–12. doi: 10.1093/jn/119.5.706. [DOI] [PubMed] [Google Scholar]
- 58.Griffiths NM, Stewart RD, Robinson MF. The metabolism of [75Se]selenomethionine in four women. Br J Nutr. 1976;35(3):373–82. doi: 10.1079/bjn19760043. [DOI] [PubMed] [Google Scholar]
- 59.Janghorbani M, et al. Selenium metabolism in healthy adults: quantitative aspects using the stable isotope 74SeO3(2-) Am J Clin Nutr. 1982;35(4):647–54. doi: 10.1093/ajcn/35.4.647. [DOI] [PubMed] [Google Scholar]
- 60.Thomson CD, Stewart RD. Metabolic studies of (75Se)selenomethionine and (75Se)selenite in the rat. Br J Nutr. 1973;30(1):139–47. doi: 10.1079/bjn19730015. [DOI] [PubMed] [Google Scholar]
- 61.Mason AC, Weaver CM. Metabolism in rats of selenium from intrinsically and extrinsically labeled isolated soy protein. J Nutr. 1986;116(10):1883–8. doi: 10.1093/jn/116.10.1883. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi S, et al. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J Biol Chem. 2015;290(14):8778–88. doi: 10.1074/jbc.M114.625053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto RE, Bartley W. Changes in glutathione reductase and glutathione peroxidase activities in rat liver related to age and sex. Biochem J. 1968;109(3):34P. doi: 10.1042/bj1090034pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burk RF, Lawrence RA, Correia MA. Sex differences in biochemical manifestations of selenium deficiency in rat liver with special reference to heme metabolism. Biochem Pharmacol. 1980;29(1):39–42. doi: 10.1016/0006-2952(80)90241-5. [DOI] [PubMed] [Google Scholar]
- 65.Clausen J, Nielsen SA. Comparison of whole blood selenium values and erythrocyte glutathione peroxidase activities of normal individuals on supplementation with selenate, selenite, L-selenomethionine, and high selenium yeast. Biol Trace Elem Res. 1988;15:125–38. doi: 10.1007/BF02990131. [DOI] [PubMed] [Google Scholar]
- 66.Rush JW, Sandiford SD. Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin Biochem. 2003;36(5):345–51. doi: 10.1016/s0009-9120(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 67.Pitsavos C, et al. Epidemiology of cardiovascular risk factors in Greece: aims, design and baseline characteristics of the ATTICA study. BMC Public Health. 2003;3:32. doi: 10.1186/1471-2458-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Letsiou S, et al. Gender-specific distribution of selenium to serum selenoproteins: associations with total selenium levels, age, smoking, body mass index, and physical activity. Biofactors. 2014;40(5):524–35. doi: 10.1002/biof.1176. [DOI] [PubMed] [Google Scholar]
- 69.Lennicke C, et al. Individual effects of different selenocompounds on the hepatic proteome and energy metabolism of mice. Biochim Biophys Acta. 2017;1861(1 Pt A):3323–3334. doi: 10.1016/j.bbagen.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 70.Brown DG, Burk RF. Selenium retention in tissues and sperm of rats fed a Torula yeast diet. J Nutr. 1973;103(1):102–8. doi: 10.1093/jn/103.1.102. [DOI] [PubMed] [Google Scholar]
- 71.Debski B, Zarski TP, Milner JA. The influence of age and sex on selenium distribution and glutathione peroxidase activity in plasma and erythrocytes of selenium-adequate and supplemented rats. J Physiol Pharmacol. 1992;43(3):299–306. [PubMed] [Google Scholar]
- 72.Meltzer HM, et al. The form of selenium determines the response to supplementation in a selenium replete population. Eur J Clin Nutr. 1990;44(6):435–46. [PubMed] [Google Scholar]
- 73.Frankel PH, et al. Baseline selenium and prostate cancer risk: comments and open questions. J Natl Cancer Inst. 2014;106(3):dju005. doi: 10.1093/jnci/dju005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark LC, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81(5):730–4. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 76.Combs GF, Jr, et al. Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am J Clin Nutr. 2009;89(6):1808–14. doi: 10.3945/ajcn.2008.27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Combs GF, Jr, et al. Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. Br J Nutr. 2012;107(10):1514–25. doi: 10.1017/S0007114511004715. [DOI] [PubMed] [Google Scholar]
- 78.Pitts MW, et al. Competition between the Brain and Testes under Selenium-Compromised Conditions: Insight into Sex Differences in Selenium Metabolism and Risk of Neurodevelopmental Disease. J Neurosci. 2015;35(46):15326–38. doi: 10.1523/JNEUROSCI.2724-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26(7–8):686–93. doi: 10.1016/j.nut.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basu A, Dube S, Basu R. Men Are from Mars, Women Are from Venus: Sex Differences in Insulin Action and Secretion. Adv Exp Med Biol. 2017;1043:53–64. doi: 10.1007/978-3-319-70178-3_4. [DOI] [PubMed] [Google Scholar]
- 81.Morford J, Mauvais-Jarvis F. Sex differences in the effects of androgens acting in the central nervous system on metabolism. Dialogues Clin Neurosci. 2016;18(4):415–424. doi: 10.31887/DCNS.2016.18.4/fmauvais. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morford JJ, Wu S, Mauvais-Jarvis F. The impact of androgen actions in neurons on metabolic health and disease. Mol Cell Endocrinol. 2017 doi: 10.1016/j.mce.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiffer L, et al. MECHANISMS IN ENDOCRINOLOGY: The sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177(3):R125–R143. doi: 10.1530/EJE-17-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rayman MP, Stranges S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Wang XL, et al. Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr J. 2016;15(1):48. doi: 10.1186/s12937-016-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seale LA, et al. Diet-Induced Obesity in the Selenocysteine Lyase Knockout Mouse. Antioxid Redox Signal. 2015;23(10):761–74. doi: 10.1089/ars.2015.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Misu H, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12(5):483–95. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Misu H, et al. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One. 2012;7(4):e34952. doi: 10.1371/journal.pone.0034952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McClung JP, et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101(24):8852–7. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang XD, et al. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51(8):1515–24. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 91.Pitts MW, et al. Deletion of selenoprotein M leads to obesity without cognitive deficits. J Biol Chem. 2013;288(36):26121–34. doi: 10.1074/jbc.M113.471235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, et al. Comparative analysis of selenocysteine machinery and selenoproteome gene expression in mouse brain identifies neurons as key functional sites of selenium in mammals. J Biol Chem. 2008;283(4):2427–38. doi: 10.1074/jbc.M707951200. [DOI] [PubMed] [Google Scholar]
- 93.Behne D, Weiler H, Kyriakopoulos A. Effects of selenium deficiency on testicular morphology and function in rats. J Reprod Fertil. 1996;106(2):291–7. doi: 10.1530/jrf.0.1060291. [DOI] [PubMed] [Google Scholar]
- 94.Nishimura K, et al. Association of selenoprotein P with testosterone production in cultured Leydig cells. Arch Androl. 2001;47(1):67–76. doi: 10.1080/01485010152104026. [DOI] [PubMed] [Google Scholar]
- 95.Erkekoglu P, et al. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol. 2010;248(1):52–62. doi: 10.1016/j.taap.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Tanguy Y, et al. The PACAP-regulated gene selenoprotein T is highly induced in nervous, endocrine, and metabolic tissues during ontogenetic and regenerative processes. Endocrinology. 2011;152(11):4322–35. doi: 10.1210/en.2011-1246. [DOI] [PubMed] [Google Scholar]
- 97.Windmill K, et al. Localization and expression of selenoprotein S in the testis of Psammomys obesus. J Mol Histol. 2007;38(1):97–101. doi: 10.1007/s10735-006-9073-2. [DOI] [PubMed] [Google Scholar]
- 98.Grumolato L, et al. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. Faseb J. 2008;22(6):1756–68. doi: 10.1096/fj.06-075820. [DOI] [PubMed] [Google Scholar]
- 99.Rossato M, et al. Pituitary adenylate cyclase activating polypeptide stimulates rat Leydig cell steroidogenesis through a novel transduction pathway. Endocrinology. 1997;138(8):3228–35. doi: 10.1210/endo.138.8.5314. [DOI] [PubMed] [Google Scholar]
- 100.Shi L, et al. Effects of selenium on the proliferation, apoptosis and testosterone production of sheep Leydig cells in vitro. Theriogenology. 2017;93:24–32. doi: 10.1016/j.theriogenology.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Ingold I, et al. Expression of a Catalytically Inactive Mutant Form of Glutathione Peroxidase 4 (Gpx4) Confers a Dominant-negative Effect in Male Fertility. J Biol Chem. 2015;290(23):14668–78. doi: 10.1074/jbc.M115.656363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiseman S, et al. Chronic exposure to dietary selenomethionine increases gonadal steroidogenesis in female rainbow trout. Aquat Toxicol. 2011;105(3–4):218–26. doi: 10.1016/j.aquatox.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Cerny KL, et al. Form of supplemental selenium fed to cycling cows affects systemic concentrations of progesterone but not those of estradiol. Theriogenology. 2016;85(5):800–806. doi: 10.1016/j.theriogenology.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 104.Ha EJ, Smith AM. Plasma selenium and plasma and erythrocyte glutathione peroxidase activity increase with estrogen during the menstrual cycle. J Am Coll Nutr. 2003;22(1):43–51. doi: 10.1080/07315724.2003.10719274. [DOI] [PubMed] [Google Scholar]
- 105.Zhou X, et al. Estrogen status alters tissue distribution and metabolism of selenium in female rats. J Nutr Biochem. 2012;23(6):532–8. doi: 10.1016/j.jnutbio.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baek IJ, et al. Effects of endocrine disrupting chemicals on expression of phospholipid hydroperoxide glutathione peroxidase mRNA in rat testes. J Vet Sci. 2007;8(3):213–8. doi: 10.4142/jvs.2007.8.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zagrodzki P, et al. Selenium status parameters in patients with polycystic ovary syndrome. J Trace Elem Med Biol. 2017;44:241–246. doi: 10.1016/j.jtemb.2017.08.012. [DOI] [PubMed] [Google Scholar]