Abstract
Purpose
After chemotherapy for breast cancer, most women will recover some ovarian function, but the timing and extent of this recovery are poorly understood. We studied post-chemotherapy ovarian recovery in women with and without a history of ovarian suppression during chemotherapy.
Methods
Reproductive age breast cancer patients who were seen prior to chemotherapy for fertility preservation consult were consented for follow-up ovarian function assessment (every 3–6 months after chemotherapy) with antral follicle count (AFC) in this prospective cohort study. We restricted our analysis to those with menses present after chemotherapy. Box plots were used to demonstrate the change in follow-up AFC versus time elapsed after chemotherapy. A mixed effects regression model was used to assess differences in AFC.
Results
Eighty-eight patients with a history of newly diagnosed breast cancer were included. Forty-five patients (51%) had ovarian suppression with GnRH agonist (GnRHa) during chemotherapy. AFC recovery appeared to plateau at 1 year after completing chemotherapy at a median of 40% of pre-chemotherapy AFC. After adjustment for age, initial AFC, cyclophosphamide exposure, combined hormonal contraceptive (CHC) use, and tamoxifen use, AFC recovered faster and to a greater degree for those women who underwent GnRHa therapy for ovarian protection during chemotherapy (P = 0.032).
Conclusions
Women with menses after chemotherapy for breast cancer appear to recover their full potential AFC 1 year after their last chemotherapy dose. Treatment with GnRHa during chemotherapy is associated with a higher degree of AFC recovery. The findings of this study can aid in counseling patients prior to chemotherapy about expectations for ovarian recovery and planning post-treatment fertility preservation care to maximize reproductive potential when pre-treatment fertility preservation care is not possible or has limited oocyte yield.
Keywords: Breast cancer, Ovarian reserve, Antral follicle count, Chemotherapy, Ovarian suppression, Fertility preservation
Introduction
Chemotherapy agents have a well-known gonadotoxic effect, significantly compromising reproductive potential in premenopausal cancer patients [1]. With improving long-term survival rates, an important concern for many cancer survivors is how the disease and its treatment will affect their future fertility. Approximately 70–80% of reproductive age women (18–45 years old) who are treated with chemotherapy for breast cancer will resume some ovarian function post-treatment [2, 3]. However, despite resumption of menses, there remains a considerable risk of reproductive compromise and possibly early menopause, and it is currently difficult to predict the extent to which reproductive dysfunction will occur [2].
Typifying the impact of chemotherapy on women that recover ovarian function is challenging. Given that menstrual function is not a reliable predictor of fertility, previous studies looking at ovarian follicular dynamics after chemotherapy have used surrogate markers for ovarian reserve, such as follicle-stimulating hormone (FSH), anti-Mullerian hormone (AMH), and antral follicle count (AFC). These prior studies have shown that FSH levels normalize to pre-treatment levels after chemotherapy, while AMH levels and AFC are severely reduced [4–6]. However, these studies are limited by inclusion of a wide variety of cancer types and chemotherapy regimens, infrequent and short-term follow-up after chemotherapy, and case-control design. Thus, little is known about how and when ovarian reserve recovers after completion of chemotherapy.
In our clinical practice, we prefer to use AFC pre- and post-chemotherapy to describe the extent of ovarian recovery, as it also allows us to estimate oocyte yield after fertility preservation (FP) [7]. Having a better understanding of AFC recovery after chemotherapy could help us provide important references for counseling patients pre-chemotherapy about their expectations for ovarian recovery and post-chemotherapy FP, as well as optimize timing of post-chemotherapy FP. In this study, serial antral follicle counts were used to examine post-chemotherapy ovarian recovery in breast cancer patients with and without a history of GnRHa for ovarian suppression during chemotherapy.
Materials and methods
We performed a prospective cohort study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of California, San Francisco (UCSF) Committee on Human Research and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Study population
Our program has a study to longitudinally survey patients diagnosed with cancer who present to the fertility preservation clinic. A subset of these patients were recruited for longitudinal AFC follow-up. Women were included in the study if they were premenopausal, ages 18–45, and newly diagnosed with breast cancer at their first FP visit. Patients were excluded if they were outside the age ranges above, had received any chemotherapy or radiation treatment prior to enrollment, had a hysterectomy, or had an oophorectomy. Two hundred forty-six breast cancer patients met the inclusion criteria and were enrolled for the study. We contacted all patients after completion of chemotherapy and excluded those that had already started ovarian suppression as part of their cancer treatment. Eighty-eight had return of menses, either with regular or with irregular cycles, at some point after chemotherapy and agreed to follow-up monitoring.
Study visits
At the initial fertility preservation evaluation, patients went through a structured interview including detailed information on demographics, medical history, gynecological history, and obstetrical history. At the first post-treatment visit, additional information regarding the patient’s cancer therapy was obtained and cross-validated with the medical record. GnRH agonists administered during chemotherapy included leuprolide (Lupron Depot 3.75 mg IM every month) and goserelin (Zoladex 3.6 mg SubQ every month). Menstrual history and use of tamoxifen and GnRH agonists were recorded at each post-treatment visit.
Assessment of ovarian reserve
Ovarian reserve was assessed by measuring AFC at the initial fertility preservation consultation. Transvaginal ultrasound was performed on a GE VolusonS8 machine by two experienced clinicians, M.R. and J.M.L. Follicles measuring between 2 and 10 mm in both ovaries were counted in the AFC. Patients were followed with AFC starting 3–6 months after chemotherapy and then every 3–6 months thereafter until a plateau in AFC was observed.
Statistical analysis
Electronic medical record data were extracted and de-identified. Statistical analyses were performed using Stata version 14 (Stata Corp, College Station, TX). Statistical significance was defined by two-sided P values < 0.05. T tests were used, as appropriate, to compare demographic data among the GnRHa versus no GnRHa groups. Follow-up AFC was compared to initial, pre-treatment AFC to create a ratio of the patient’s follow-up AFC versus their original AFC. We used box plots to demonstrate the relationship between time elapsed after chemotherapy and AFC improvement. A subgroup analysis was performed comparing patients with greater than 6 months of combined hormonal contraception (CHC) use immediately preceding the initial visit to patients without CHC use because long-term CHC use is known to suppress the AFC in 50% of patients [8].
A random slopes and intercepts regression model was used to determine whether longitudinal measurements of AFC after completion of chemotherapy were significantly different. This model describes within-person change over time, thus accounting for correlations in a particular repeated measurement within an individual, in addition to averaging data across women. Predictors of interest included age at initial visit, pre-chemotherapy AFC, combined hormonal contraception use at initial visit, cycholphosphamide-based chemotherapy, GnRHa use during chemotherapy, and tamoxifen use after chemotherapy. We assumed that predictors could influence the recovery of ovarian reserve in two distinct ways. First, predictors may influence the level of AFC recovery. In addition, these predictors may affect the rate of recovery over time. Examination of this later influence was performed using statistical tests of interaction between time and certain predictors.
Results
Patient characteristics
Eighty-eight breast cancer patients were seen prior to chemotherapy for fertility preservation consult, had menses return after chemotherapy, and were seen after chemotherapy for a follow-up AFC assessment. Baseline demographics are presented in Table 1. Patients were on average 34 ± 4 years old with mostly clinical stage one or two breast cancer. Median pre-chemotherapy AFC was 12 (1–55). Seventy-six percent of patients received cyclophosphamide as part of their chemotherapy regimen. The median number of AFC assessments to date was 2 (range 1–5) and the median observational time was 2 years (1–5).
Table 1.
Baseline demographics in all patients and GnRHa vs. no GnRHa groups
| All Patients (N = 88) | GnRHa (N = 45) | No GnRHa (N = 43) | |
|---|---|---|---|
| Age (years), median (range) | 35 (24–43) | 35 (26–42) | 34 (24–43) |
| Pre-chemotherapy AFC, median (range) | 12 (1–55) | 11 (1–55)** | 16 (5–54)** |
| Prior parity (≥ 1), % (n) | 15 (13/88) | 13 (6/45) | 16 (7/43) |
| Receptor Status, % (n) | |||
| ER+ | 61 (54/88) | 44 (20/45)** | 79 (34/43)** |
| PR+ | 51 (45/88) | 38 (17/45)** | 65 (28/43)** |
| HER2+ | 41 (36/88) | 42 (19/45) | 40 (17/43) |
| Clinical stage, % (n) | |||
| Positive axillary nodes | 33 (29/88) | 36 (16/45) | 30 (13/43) |
| Positive distal nodes | 1 (1/88) | 0 (0/45) | 2 (1/43) |
| Metastatic | 2 (2/88) | 2 (1/45) | 2 (1/43) |
| Cancer type and regimen | |||
| Breast, % (n) | 100 (88/88) | 100 (45/45) | 100 (43/43) |
| AC-TC | 5 (4/88) | 7 (3/45) | 2 (1/43) |
| ACT | 56 (49/88) | 56 (25/45) | 56 (24/43) |
| T | 2 (2/88) | 2 (1/45) | 2 (1/43) |
| TC (Taxotere, Cytoxan) | 15 (13/88) | 11 (5/45) | 19 (8/43) |
| TC (Taxotere, carboplatin) | 22 (19/88) | 22 (10/45) | 21 (9/43) |
| T-FEC (5FU, epirubicin, Cytoxan) | 1 (1/88) | 2 (1/45) | 0 (0/43) |
| Cyclophosphamide use, % (n) | 76 (67/88) | 76 (34/45) | 77 (33/43) |
| CHC use at initial visit, % (n) | 33 (29/88) | 44 (20/45)** | 21 (9/43)** |
| GnRHa use during chemo, % (n) | 51 (45/88) | 100 (45/45)** | 0 (0/43)** |
| Tamoxifen use after chemo, % (n) | 42 (37/88) | 29 (13/45)** | 46 (24/43)** |
**Denotes a statistically significant difference (P < 0.05) between the GnRHa and no GnRHa groups
Forty-five patients (51%) took a GnRH agonist (GnRHa), such as leuprolide or goserelin, for ovarian suppression during chemotherapy. Table 1 compares the baseline characteristics between the GnRHa and no GnRHa groups. There were no significant differences in age, clinical stage, chemotherapy regimen, and use of cyclophosphamide between the groups. However, initial median AFC was lower in the GnRHa group (GnRHa 11 vs. no GnRHa 16; P = 0.012).
AFC recovery over time
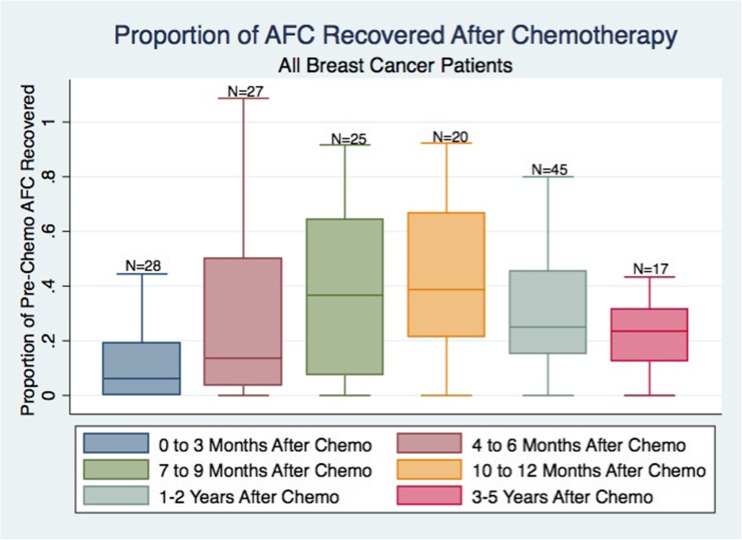
Women who resumed menses after completing chemotherapy generally had a gradual recovery of AFC, reaching maximal recovery approximately 1 year post-treatment (Fig. 1). By 1 year after completing chemotherapy, women appeared to recover 40% of their original AFC. No additional significant AFC recovery was seen between 1 and 2 years post-chemotherapy, and AFC began to decline 1 year after chemotherapy.
Fig. 1.
We report the proportion of the original AFC that have returned at various follow-up time points (AFC at X months/original AFC) in breast cancer patients with return of menses after chemotherapy (N = 88). Boxes represent the 25th and 75th percentiles. Error bars represent range, excluding statistical outliers. The number of patients that have returned at a particular time point are listed over the boxes. AFC appears to improve steadily to about 40% of the original AFC, at 10–12 months after chemotherapy
Combined hormonal contraception subgroup analysis
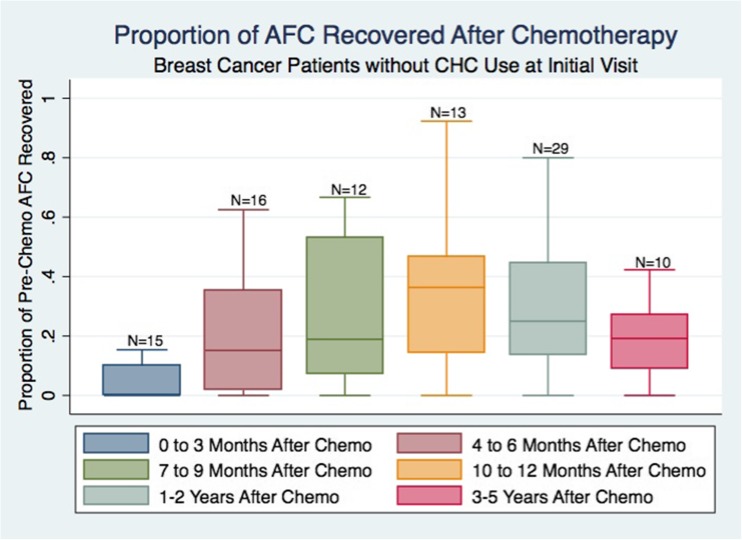
Twenty-nine patients (33%) were using CHC for greater than 6 months immediately preceding their initial visit. The average pre-chemotherapy AFC was higher in women without CHC use at the initial visit, though not statistically significantly different (no CHC use 17 ± 11 vs. CHC use 13 ± 8; P = 0.180). In all patients, CHC use was discontinued after their initial visit. Women without CHC use appeared to recover only 36% of the original AFC, reaching a plateau at 1-year post-treatment (Fig. 2). On the other hand, women with CHC use at the initial visit recovered 67% of the original AFC at 1-year post-chemotherapy (data not shown). A mixed effects regression model demonstrated a statistically significant increase in AFC recovery among women who reported recent CHC use at their initial visit (P = 0.028).
Fig. 2.
Women who were not using combined hormonal contraception (CHC) at the time of their initial visit represented 67% of our study population (N = 59). The boxes represent proportions of the original AFC (AFC at X months/original AFC). Boxes include the 25th and 75th percentiles. Error bars represent range, excluding statistical outliers. The number of patients that have returned at a particular time point are listed over the boxes. Women without CHC use at initial visit appeared to have AFC recovery to only 35% of the original AFC, which plateaus 1 year after chemotherapy
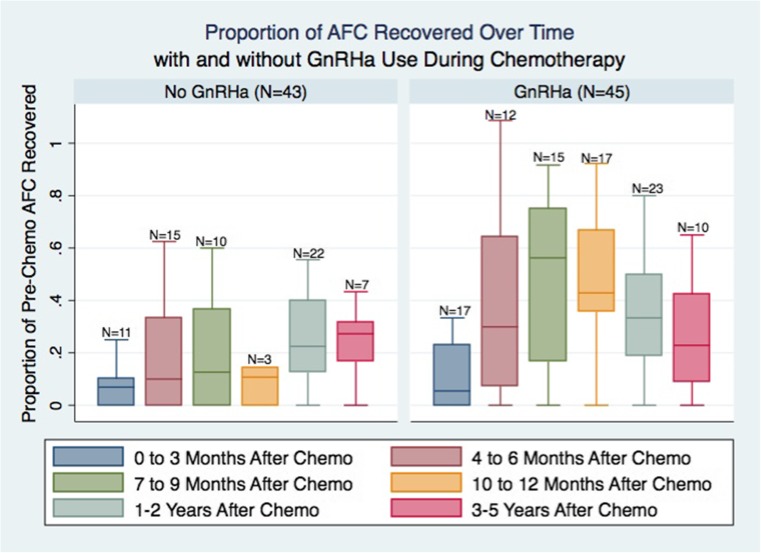
Impact of GnRH agonist co-treatment on AFC recovery
Patients who were treated with GnRHa during chemotherapy had increased and faster AFC recovery after completion of chemotherapy (Fig. 3). Patients treated with GnRH agonist showed maximal ovarian recovery to 56% of their baseline AFC 7–9 months after chemotherapy as compared to only 27% recovery in the untreated group by 3–5 years after chemotherapy. Using a mixed effects regression model (Table 2), GnRHa therapy was associated with increased AFC recovery, after adjusting for age and pre-chemotherapy AFC (P = 0.032). The impact of GnRHa use did not change over time. Older age at the initial visit and lower pre-chemotherapy AFC was associated with lower AFC recovery. Tamoxifen use after chemotherapy did not have a significant impact on AFC recovery. For example, in a patient with initial age of 30 and initial pre-chemotherapy AFC of 15 who was receiving cyclophosphamide, one would expect an AFC of 4 without GnRHa use and 7 with GnRHa use at 1 year after chemotherapy.
Fig. 3.
Women who took GnRHa during chemotherapy represented 51% of our study population. The boxes represent proportions of the original AFC (AFC at X months/original AFC). Boxes include the 25th and 75th percentiles. Error bars represent range, excluding statistical outliers. The number of patients that have returned at a particular time point are listed over the boxes. Women who took GnRHa appeared to have increased and faster AFC recovery, compared to those whose menses resumed after chemotherapy without concurrent GnRHa. For the GnRHa group, AFC appears to improve steadily to about 56% of the original AFC, reaching a maximum by 7–9 months after chemotherapy. For the no GnRHa group, AFC appears to improve steadily to about 27% of the original AFC, only reaching a maximum 3–5 years after chemotherapy
Table 2.
Mixed model results of GnRHa use, covariate, and individual effects on AFC recovery during 5 years of follow-up
| Effect | Description | Coefficient | P value |
|---|---|---|---|
| Time | Time in months; time = 0 at completion of chemotherapy | 0.159 | 0.006 |
| GnRHa use during Chemotherapy | Treatment arm; GnRHa = 1, no GnRHa = 0 | 2.85 | 0.032 |
| Time × GnRHa use | Interaction with time | − 0.064 | 0.118 |
| Age at initial diagnosis | Age in years at initial FP visit | − 0.358 | 0.002 |
| Pre-chemotherapy AFC | AFC at initial FP visit before starting chemotherapy | 0.148 | 0.002 |
| Cyclophosphamide use | Chemotherapy regimen included cyclophosphamide | − 1.88 | 0.087 |
| CHC use at initial visit | CHC use at initial FP visit | 2.15 | 0.028 |
| Tamoxifen use after chemotherapy | Hormonal treatment with tamoxifen after chemotherapy | − 0.496 | 0.593 |
Women who took a GnRHa during chemotherapy represent 51% of our study population. Here, we present the full formal coefficient estimates for the mixed effects model looking at the impact of GnRHa use on post-chemotherapy AFC. GnRHa use was associated with a statistically significant higher ovarian recovery, after adjustment for age, pre-chemotherapy AFC, cyclophosphamide exposure, CHC use at initial FP visit, and tamoxifen use
Discussion
Women with menses after chemotherapy for breast cancer appear to recover 40% of their pre-treatment AFC after chemotherapy. Treatment with a GnRH agonist during chemotherapy may allow for increased AFC recovery. The timing and extent of post-chemotherapy ovarian recovery can be predicted by AFC, age at treatment, time elapsed from chemotherapy, and the use of GnRHa during chemotherapy. The findings of this study may help to personalize fertility care and oncology care after chemotherapy for breast cancer.
Numerous prior studies have reported the impact of chemotherapy using menstruation as a surrogate for ovarian function. However, even as women approach menopause, menstrual cycles are still present, though shorter and more variable, thus questioning the value of relying solely on assessment of resumption of menses after chemotherapy [9]. Among patients with menses post-chemotherapy, we observed a 40% recovery of pre-treatment AFC after chemotherapy. It is known that resumption of menses underestimates the degree of reproductive impairment after chemotherapy and alone is not an indicator of fertility potential [2]. This study provides further evidence of a persistent “partial ovarian injury” after chemotherapy, even among women whose menses return.
Prior studies have evaluated the acute ovarian endocrine response to chemotherapy using known ovarian reserve markers. Most of the initial literature on this topic came from case-control studies, comparing markers between post-chemotherapy cancer patients and healthy age-matched controls [10, 11]. A limitation with these study designs is that even with perfect matching of cases and controls, it is difficult to be sure whether the groups did not differ in ovarian reserve before chemotherapy. Existing evidence using cohort studies has shown a significant reduction in AMH levels following chemotherapy with the rate of AMH recovery after chemotherapy impacted by pre-treatment levels and alkylating agent exposure [6, 12, 13]. Other studies limited by their short-term follow-up have noted a marked drop in AFC to about half of the pre-chemotherapy AFC at 6 to 8 months after chemotherapy, consistent with the results of this study [4, 6, 12]. In a longer, but small study of 51 breast cancer patients, AFC fell from 8.5 pre-chemotherapy to 1.8 2 years after initiation of chemotherapy [14]. The degree of AFC recovery was much lower in this study compared to our study because the majority of patients in this study remained amenorrheic, and likely menopausal after chemotherapy. Similar to the results from our mixed model, Wenners and colleagues observed that AFC levels before chemotherapy were significantly positively correlated with AFC 1 year after chemotherapy and negatively correlated with age. However, we also shed light on how AFC changes up to 5 years after chemotherapy.
Of note, none of these previous studies accounted for CHC use at the initial visit. However, Letourneau et al. found that long-term CHC use suppresses the AFC in 50% of patients [8]. Thus, in patients with long-term CHC use newly diagnosed with cancer, the baseline AFC recorded at the initial FP visit may be suppressed, falsely elevating the proportion of AFC recovery after chemotherapy. In patients with greater than 6 months of CHC use at the intake visit, we observed that women with CHC use appeared to recover almost double their original AFC at 1-year post-chemotherapy. Furthermore, CHC use was significantly associated with an increase in AFC recovery in our mixed effects model. The inclusion of women on hormonal contraceptives is an inherent limitation of any study assessing ovarian reserve in young women with cancer at risk for pregnancy and thus should be accounted for in similar future studies to give an accurate impression of a patient’s baseline ovarian reserve.
We observed that pre-treatment with GnRH agonist may allow for increased AFC recovery. At this time, temporary ovarian suppression with GnRH agonists (GnRHa) during chemotherapy is the only pharmaceutical strategy to preserve ovarian function, but their use in fertility preservation is controversial and still considered experimental by the American Society of Clinical Oncology [15]. The two largest randomized controlled trials in breast cancer patients, POEMS and PROMISE, demonstrated a significantly decreased risk of ovarian failure in the patients treated with GnRHa during chemotherapy [16, 17]. However, other smaller randomized controlled trials in cancer patients have not shown a difference [18, 19]. A challenge with evaluating the efficacy of temporary ovarian suppression by GnRHa is the lack of long-term follow-up and data about fertility outcomes. Because menstrual activity is not the best proxy for ovarian function, it is important to further our understanding of ovarian suppression during chemotherapy using other predictors of future fertility.
Only two studies in the literature have looked at the ability of GnRHa to protect the ovary using quantifiable markers of ovarian reserve and found that the use of GnRHa did not significantly affect AMH and AFC up to 3 years after chemotherapy [20, 21]. To our knowledge, our study is the first to show that GnRHa may improve recovery of post-chemotherapy AFC among menstruating women. Furthermore, GnRH agonist treatment appeared to hasten the AFC recovery post-chemotherapy. Thus, use of a GnRH agonist may allow patients to undergo fertility treatment sooner post-chemotherapy, which is an important consideration given the narrowed reproductive window for these women and the substantial effect of age on fertility potential. Ovarian suppression with a GnRHa may be a more accessible option for patients and can be used in combination with traditional fertility preservation techniques, and thus its impact on ovarian reserve should be investigated further.
The results of this study help to provide a reference for counseling patients prior to chemotherapy about expectations for ovarian recovery, based on pre-chemotherapy antral follicle count, which could assist with making more informed decisions about fertility preservation. Fertility counseling for women with a higher AFC at the time of diagnosis could be different from counseling for women with a lower AFC. For example, a patient with a higher pre-chemotherapy AFC could be counseled about the possibility of harvesting eggs after chemotherapy if the pressure of starting chemotherapy did not permit the opportunity for an ovarian stimulation cycle without significant delays, or if limited oocytes were collected prior to chemotherapy. Post-treatment ovarian stimulation and oocyte/embryo cryopreservation may be a strategy to maximize future reproductive capacity in this population given that that oocyte yield per antral follicle count is similar among post-chemotherapy and chemotherapy-naïve patients [22]. Using a patient’s baseline AFC as a predictor of post-treatment ovarian reserve would facilitate a more informed choice of therapy for physicians and would enable a more patient-centered approach to fertility preservation.
Strengths, limitations, and future research
This study has several strengths and limitations. The principal strength of this study is the longitudinal follow-up at discrete time points out to 5 years from treatment. While it is limited by its incomplete follow-up of the entire cohort at each time point, this is one of the first studies to examine ovarian recovery, using a quantifiable and reliable measure as AFC, past 1 year after exposure to chemotherapy. Our data was collected from a homogeneous population of breast cancer patients seen at a single university medical center, in the largest cohort to date. We have also accounted for potential suppression from long-term combined hormonal contraception, which we have shown in our analyses could give a false impression of a patient’s baseline ovarian reserve. Even though we have a relatively large cohort, our study size does also limit the ability to detect significant differences in ovarian recovery between the GnRHa and no GnRHa treatment groups at various time points. Longer follow-up is necessary to understand the implications of antral follicle count recovery for timing of menopause and pregnancy outcomes. Areas for future research could include determining whether pre-chemotherapy AFC can predict post-cancer treatment reproductive health outcomes in terms of post-chemotherapy ovarian reserve and time to maximum ovarian recovery. This knowledge would allow clinicians to predict a patient’s risk of ovarian follicular depletion after chemotherapy and thus offer a more individualized plan for fertility preservation.
Conclusion
Women with menses after chemotherapy for breast cancer appear to reach the peak of their AFC recovery 1 year after chemotherapy. Among women who resume menses after chemotherapy, 40% of observable pre-treatment antral follicle count is recovered. Thus, patients who could not freeze enough oocytes before cancer treatment might expect to have an oocyte yield of a third of their baseline AFC with ovarian stimulation after chemotherapy, allowing them to maximize their reproductive potential. Finally, treatment with GnRHa during chemotherapy may be strongly considered, given its association with higher recovery of AFC.
Acknowledgements
We would like to make a special thanks to our nurses, Elizabeth Gomes, Cathy Chin-Yu, and Audra Katz, who provided patient education at fertility preservation counseling appointments. We also thank the UCSF Department of OB/GYN for their support of this study.
Author’s roles
N.S.’s and J.M.L.’s roles included study design, data collection, data analysis, and manuscript writing. K.W., P.X., B.L., and E.H.’s roles included data collection and manuscript writing. E.M.-L. and M.I.C.’s roles included data analysis and manuscript writing. M.R.’s roles included study design, data analysis, and manuscript writing.
Funding
This study was supported by departmental research funding within the University of California, San Francisco Department of Obstetrics, Gynecology and Reproductive Sciences and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR001871.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of California, San Francisco (UCSF) Committee on Human Research and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
N. Sinha, Email: nikita.sinha@northwestern.edu
Mitchell P. Rosen, Phone: +1-415-476-5305, Email: Mitchell.Rosen@ucsf.edu
References
- 1.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update Oxford Univ Press. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 2.Letourneau Joseph M., Ebbel Erin E., Katz Patricia P., Oktay Kutluk H., McCulloch Charles E., Ai Wei Z., Chien A. Jo, Melisko Michelle E., Cedars Marcelle I., Rosen Mitchell P. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2011;118(7):1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kil WJ, Ahn SD, Shin SS, Lee S-W, Choi EK, Kim JH, Son BH, Ahn SH, Kim WK, Kim SB. Treatment-induced menstrual changes in very young (<35 years old) breast cancer patients. Breast Cancer Res Treat. 2006;96:245–250. doi: 10.1007/s10549-005-9059-x. [DOI] [PubMed] [Google Scholar]
- 4.D’Avila ÂM, Capp E, Corleta HVE. Antral follicles count and anti-Müllerian hormone levels after gonadotoxic chemotherapy in patients with breast cancer: cohort study. Rev Bras Ginecol Obstet. Thieme-Revinter Publicações Ltda; 2017. [DOI] [PMC free article] [PubMed]
- 5.Anderson RA, Cameron DA. Pretreatment serum anti-Müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 6.Rosendahl M, Andersen CY, la Cour FN, Juul A, Løssl K, Andersen AN. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril. 2010;94:156–166. doi: 10.1016/j.fertnstert.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 7.Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod BioMed Online. 2015;31:486–496. doi: 10.1016/j.rbmo.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Letourneau JM, Cakmak H, Quinn M, Sinha N, I Cedars M, Rosen MP. Long-term hormonal contraceptive use is associated with a reversible suppression of antral follicle count and a break from hormonal contraception may improve oocyte yield. J Assist Reprod Genet. Springer US; 2017;103:1551–8. [DOI] [PMC free article] [PubMed]
- 9.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42:629–636. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 10.Lutchman Singh K, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer Nat Publ Group. 2007;96:1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oktem Ozgur, Oktay Kutluk. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110(10):2222–2229. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RA, Themmen APN, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod Oxford Univ Press. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 13.Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, Gosiengfiao Y, Gracia CR. Pretreatment antimüllerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477–483. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenners A, Grambach J, Koss J, Maass N, Jonat W, Schmutzler A, et al. Reduced ovarian reserve in young early breast cancer patients: preliminary data from a prospective cohort trial. BMC Cancer. 3rd ed. BioMed Central; 2017;17:632. [DOI] [PMC free article] [PubMed]
- 15.Lindsey H. ASCO Updates Fertility-Preservation Guidelines. Oncol Times. 2013;35:16–17. [Google Scholar]
- 16.Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA Am Med Assoc. 2011;306:269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 17.Moore HCF, Unger JM, Phillips K-A, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med Mass Med Soc. 2015;372:923–932. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29:2334–2341. doi: 10.1200/JCO.2010.32.5704. [DOI] [PubMed] [Google Scholar]
- 19.Munster PN, Moore AP, Ismail-Khan R, Cox CE, Lacevic M, Gross-King M, Xu P, Carter WB, Minton SE. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533–538. doi: 10.1200/JCO.2011.34.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elgindy EA, El-Haieg DO, Khorshid OM, Ismail EI, Abdelgawad M, Sallam HN, et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78–86. doi: 10.1097/AOG.0b013e31827374e2. [DOI] [PubMed] [Google Scholar]
- 21.Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD) Hematol Taylor & Francis. 2007;12:141–147. doi: 10.1080/10245330600954072. [DOI] [PubMed] [Google Scholar]
- 22.Chan JL, Johnson LNC, Efymow BL, Sammel MD, Gracia CR. Outcomes of ovarian stimulation after treatment with chemotherapy. J Assist Reprod Genet Springer US. 2015;32:1537–1545. doi: 10.1007/s10815-015-0575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]