Abstract
Purpose
To provide a commentary on our understanding of the role that the Hippo signaling pathway may play in patients with polycystic ovarian syndrome (PCOS) and how this understanding may impact the diagnosis of PCOS.
Methods
We assessed publications discussing the role of the Hippo signaling pathway in the ovary. In particular, we discuss how Hippo signaling disruption after ovarian fragmentation, combined with treating ovarian fragments with phosphatase and tensin homolog (PTEN) inhibitors and phosphoinositide-3-kinase stimulators to augment AKT signaling, has been used in treatment of patients with primary ovarian insufficiency. Furthermore, we discuss our own data on variations in Hippo signaling pathway gene expression in cumulus cells isolated from women undergoing IVF with a previous diagnosis of PCOS.
Results and conclusions
Aberrant Hippo signaling in PCOS patients is likely a contributing mechanism to the multifactorial etiology of the disease. Given the challenge of discerning the underlying etiology of oligo-ovulation in some patients, especially those with normal body mass indices, and the need for customized stimulation protocols for PCOS patients who have an increased risk of over-response and higher percentage of immature oocyte yield, it is important to identify these patients prior to treatment. Hippo gene expression fingerprints could potentially be used to more accurately define patients with PCOS. Additionally, targeting this pathway with pharmacologic agents could lead to non-surgical therapeutic options for PCOS.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1235-0) contains supplementary material, which is available to authorized users.
Keywords: PCOS, HIPPO signaling, Cumulus cells, IVF
Introduction
The regulation of organ growth during development is essential for optimal function in later life. In particular, organs that are composed of different cell types must have appropriate proportions, or relative numbers, of the different cell types that make up the organ as well as the correct number of total cells. The Hippo pathway [1, 2] has been identified as one key modality organs use to regulate growth. It is a kinase pathway with WWTR1 and YAP as downstream effectors. The Hippo pathway is a highly conserved pathway that controls organ size by strictly regulating cell growth and apoptosis [1, 2]. It is known that control of cell proliferation, apoptosis, and differentiation are required for homeostasis and reproduction. Dysregulation within the Hippo pathway has been shown to lead to cellular overgrowth, dysfunction, and tumorigenesis [3]. It has been implicated in disease states ranging from juvenile polycystic kidney disease [4] to arrhythmogenic right ventricular cardiomyopathy to malignancies of the lung, brain, skin, breast, colon, kidney, pancreas, and liver to name a few [2]. As such, this pathway is an area of interest for diseases involving tissue and/or organ overgrowth and dysfunction.
The Hippo pathway and the ovary
The ovary is composed of stromal, surface epithelial, and germ cells whose differential growth is under tight control [5], not only during development but also in the mature organ throughout reproductive life. The continuous turnover and development of follicles from the primordial to mature state is of particular interest. During this development, the ovarian stroma is invariably subjected to differing mechanical forces due to follicular growth and rupture. These forces have not been characterized but are likely to include tension, spring, and frictional forces [6–8].
In Drosophila, the Hippo pathway is involved intimately in germline differentiation. Huang et al. [9] recently showed that Hedgehog (Hh) signaling induces the Hippo pathway effector Yorkie (Yki) to promote proliferation and maintenance of somatic follicle stem cells, but Hh also signals to escort cells, which are quiescent. They also showed that in escort cells, both Hh and Yki limit production of BMP ligands to allow germline differentiation. Escort cells promote differentiation of the germline stem cells in Drosophila and provide signals for the follicle stem cells and derive directly from follicle stem cells in adult Drosophila. In addition, Polesello and Tapon [10] have also demonstrated that Salvador-Warts-Hippo signaling is important in follicular maturation and oogenesis in Drosophila.
In humans, the role of the Hippo pathway is less understood, however, it has been shown that the Hippo signaling pathway may be implicated in the process of follicular maturation [reviewed by [11]]. By manipulating expression of key genes in the Hippo pathway in patients with primary ovarian insufficiency, Kawamura et al. [12] successfully promoted follicle growth, retrieved mature oocytes, and performed in vitro fertilization on the resultant oocytes. The translation of our understanding of the Hippo pathway to a clinical application has led us to explore other roles that the Hippo pathway may play in ovarian function and disease.
PCOS and the Hippo pathway
Polycystic ovarian syndrome (PCOS) affects 4 to 12% of women of reproductive age [13, 14]. Despite the high prevalence of PCOS, various professional societies have set forth different criteria for the diagnosis of PCOS. Two of the agreed upon findings for PCOS are hyperandrogenism/hyperandrogenemia and ovulatory dysfunction [15, 16], both of which can be expressed in a spectrum. The importance of polycystic appearing ovaries by ultrasound for the diagnosis of PCOS remains a subject of debate [17].
Why have the professional societies found it difficult to agree upon an official set of diagnostic criteria for PCOS? Interestingly, little is understood of the underlying molecular and cellular mechanisms involved in PCOS and there is still conjecture on whether it is a syndrome or disease. Schmidt et al. [18] have shown that a number of inflammation related genes are differentially expressed in PCOS ovaries, while Fan et al. [19] showed that HSP10, which is involved in protein folding, had increased expression. The majority of our knowledge is based upon a characteristic hormonal profile seen in PCOS and some even postulate that the elevated androgenic periovarian environment is the root cause of the condition [20]. Several years ago, Kawamura et al. [12, 21] highlighted a possible role for the Hippo pathway in the evolution of PCOS and suggested that mechanical tension resulting from the thickened ovarian capsule may be the underlying etiology of arrested folliculogenesis. Given that one of the characteristics of PCOS is that the ovary appears to lose its ability to regulate cellular growth and apoptosis, in particular when examining ovarian volume [22] and cortical thickness, we propose that this may be linked to aberrant expression of Hippo pathway genes.
A major characteristic of ovaries in PCOS patients is that they are generally larger than in patients without PCOS. This is analogous to granulosa cell tumors that are hormonally active (estrogen producing) tumors and lead to an enlarged ovary. Fu et al. [23] have shown that compared to age-matched normal ovaries, those with granulosa cell tumors exhibited increased YAP expression. Non-tumor ovarian stromal cells expressed very low levels of YAP while YAP knockdown reduced follicle stimulating hormone (FSH)-induced aromatase (CYP19A1) and was postulated as a possible mechanism for hyperandrogenemia/hyperandrogenism. The Hippo/YAP pathway is involved in regulation of steroidogenesis in human granulosa cell tumor tissues. Although there is no direct correlation between granulosa cell tumors and PCOS, this does demonstrate a possible mechanism for hyperandrogenemia/hyperandrogenism resulting from YAP knockdown causing decrease in aromatase and therefore an increase in androgens.
A further relationship between the Hippo pathway and the PCOS is that patients with PCOS are known to be at an increased risk of insulin resistance and Metformin has been utilized in isolation or in conjunction with selective estrogen receptor modifiers (SERMs) or aromatase inhibitors (AIs) for ovulation induction. Wang et al. [24] have shown that YAP/TAZ regulate PI3K/Akt signaling, which is the main pathway cascade of insulin/IGF1 signaling. Studies of siYAP/TAZ have suggested that YAP/TAZ is a downstream target of Metformin and might be more effective for patients with low YAP/TAZ levels versus those with higher levels.
Hippo gene expression in cumulus cells of PCOS patients
In our own studies, we characterized the expression of genes in the Hippo signaling pathway in cumulus cells from patients with PCOS and compared them to a control group composed of non-PCOS, non-infertile patients (see supplementary material for Methodology). We hypothesized that patients with PCOS may exhibit Hippo cumulus cell gene expression profiles that are different from those of control patients. Our specific objectives were to (1) characterize the expression of genes in the Hippo signaling pathway in cumulus cells obtained at the time of oocyte retrieval in patients with PCOS undergoing in vitro fertilization (IVF) and compare these to a control group, (2) determine if the Hippo pathway gene expression fingerprint changes differentially in cumulus cells cultured under mechanical stress, and (3) evaluate if an objective diagnostic test could be developed for PCOS.
Baseline characteristics of subjects and controls are depicted in Table 1. Twenty-six subjects with PCOS and 28 controls were included in the study. Cumulus cells were recovered from the eggs of patients undergoing IVF by trimming the cumulus-oocyte complexes prior to insemination. No differences were found between the study groups with respect to baseline serum anti-mullerian hormone (AMH), antral follicle count, baseline FSH, baseline estradiol, pretrigger luteinizing hormone (LH), posttrigger LH, posttrigger progesterone, nor number and maturity of oocytes retrieved. Although a statistically significant difference was noted in baseline FSH, this difference is not clinically relevant. Factors that were both clinically relevant and statistically significantly different include patient age, body mass index (BMI), pretrigger progesterone, and total ovarian volume (Table 1).
Table 1.
Baseline patient demographics for control and PCOS patients who had cumulus cells analyzed for Hippo gene expression
| Parameter | Number of Controls | Control Mean ± SD |
Number of PCOS | PCOS Mean ± SD |
p value |
|---|---|---|---|---|---|
| Age (years) | 28 | 28.36 ± 4.53 | 26 | 33.15 ± 5.12 | 0.00 |
| BMI | 28 | 23.66 ± 3.74 | 26 | 27.49 ± 6.75 | 0.01 |
| AMH (ng/ml) | 27 | 3.6 ± 2.04 | 24 | 5.33 ± 4.59 | 0.08 |
| Baseline FSH (mIU/ml) | 11 | 7.94 ± 2.02 | 24 | 6.12 ± 2.32 | 0.03 |
| Baseline estradiol (pg/ml) | 10 | 34.07 ± 32.73 | 24 | 40.2 ± 20.20 | 0.51 |
| Peak estradiol (pg/ml) | 27 | 4239.15 ± 1945.86 | 26 | 3262.92 ± 2393.96 | 0.11 |
| Pretrigger progesterone (ng/ml) | 18 | 1.63 ± 0.74 | 21 | 1.10 + 0.44 | 0.01 |
| Posttrigger progesterone (ng/ml) | 24 | 11.89 ± 8.89 | 14 | 8.45 ± 5.73 | 0.20 |
| Pretrigger LH (mIU/ml) | 11 | 1.04 ± 1.24 | 8 | 1.11 ± 0.76 | 0.88 |
| Posttrigger LH (mIU/ml) | 24 | 59.74 ± 38.65 | 14 | 55.79 ± 41.26 | 0.77 |
| Number of oocytes retrieved | 28 | 23.46 ± 12.93 | 26 | 18.31 ± 9.85 | 0.11 |
| Number of mature cumulus oocyte complexes | 28 | 20.71 +/1 12.77 | 26 | 16.35 ± 8.67 | 0.15 |
| Percent of mature cumulus oocyte complexes | 28 | 87.98 ± 12.83 | 26 | 89.66 ± 10.21 | 0.63 |
| Total antral follicle count | 23 | 21.26 ± 9.81 | 17 | 25.41 ± 21.96 | 0.43 |
| Total ovarian volume (cm3) | 20 | 13.33 ± 6.19 | 15 | 22.10 ± 10.76 | 0.01 |
Subjects with PCOS were found to have higher levels of expression for genes in the Hippo signaling pathway. Statistically significant differences were noted in MOB1A (p < 0.001), MOB1B (p < 0.001), WWTR1 (p < 0.001), and YAP1 (p < 0.01) (Fig. 1). The greatest change in expression levels was noted in MOB1A and WWTR1. The relative fold change in Hippo pathway gene expression levels between the individual control and PCOS patients is shown in Fig. 1. The major difference was that PCOS patients showed a greater heterogeneity in gene expression when compared to the control population (Fig. 1b). Controls represent a much tighter group with fairly homogeneous expression levels per gene. Conversely, PCOS patients have much more heterogenous expression levels, some patients aligning well with the control population and others with clear differentiation. This correlates well with the heterogeneous clinical presentation of patients with PCOS. Interestingly, it did not correlate with the degree of dysfunction based upon our current criteria for PCOS, indicating that one or a combination of these markers could have diagnostic potential.
Fig. 1.
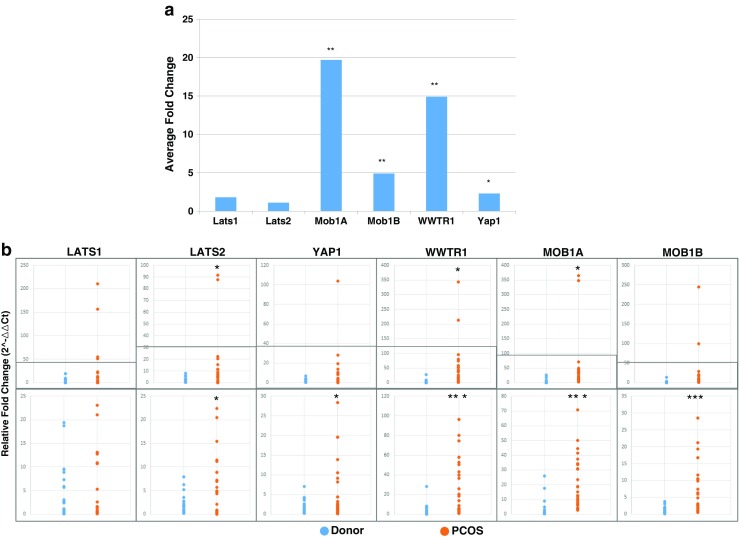
Relative expression levels of Hippo signaling genes when comparing a the average fold change between control and PCOS patients and b the relative fold change between control and PCOS patients in individual samples. The top panel contains all tested individuals, while the bottom panel shows sample distribution without outliers. Statistically significant differences between control and PCOS in fold change sample distributions are denoted by * = p < 0.05 > 0.001, *** = p < 0.001
When looking at expression levels of all tested genes in the Hippo pathway and averaging them, there was a significant difference between PCOS patients and controls (p < 0.001). When averaging the expression levels from the genes with statistically significant differences in expression levels [MOB1A, MOB1B, WWTR1, and YAP1], a better discrimination could be obtained between the two groups.
In vitro induction of PCOS Hippo gene expression
Induction of PCOS-like Hippo gene expression in cumulus cells from eight control patients was attempted under two-dimensional, three-dimensional, and dynamic three-dimensional culture conditions. These conditions were introduced to mimic the mechanical forces that might be experienced by cells in a PCOS ovary.
Table 2 shows a comparison of gene expression levels from static control cells to those from standard plastic culture, biologic scaffold culture, and dynamic biologic scaffold culture. YAP1 demonstrated a significant increase in gene expression on the plastic compared to the static sample. YAP1 also demonstrated a significant increase in gene expression on the dynamic scaffold compared to the static sample. Both plastic and dynamic scaffold increase the mechanical forces experienced by the cumulus cells compared to the static state. In the standard tissue culture dish and dynamic device, the cells experience different mechanical forces, whereas, in the 3D biological scaffold alone, cells have been previously shown to maintain a more in vivo like profile [25].
Table 2.
The Hippo gene expression profiles of cumulus cells without culture (static) compared to those cultured on platforms that produce variable mechanical forces. Only those values with three arrows show a significant change. Changes depicted with one or two arrows depict a general change in direction of expression. The mean difference compared to an ActinB housekeeping gene (ACTB) is provided along with the p value in parentheses. Control static cells from eight patients were compared to those plated on standard polystyrene tissue culture dishes (donor plastic), an inert 3D biologic scaffold (donor scaffold), and the biological scaffold on a dynamic piston device which created a convex displacement of the scaffold (donor scaffold stretch)
| Donor genes static | Donor plastic | Donor scaffold | Donor scaffold stretch | |||
|---|---|---|---|---|---|---|
| LATS1 | 0.833 (0.25) | ↑ | 0.334 (0.64) | – | 0.624 (0.38) | ↑ |
| LATS2 | 0.916 (0.26) | ↑ | 0.793 (0.33) | ↑ | 0.626 (0.44) | ↑ |
| YAP1 | 1.584 (0.05) | ↑↑↑ | 0.416 (0.60) | – | 1.543 (0.05) | ↑↑↑ |
| WWTR1 | 1.459 (0.06) | ↑↑ | 0.084 (0.91) | – | 0.291 (0.71) | – |
| MOB1A | − 0.959 (0.29) | ↓ | 0.375 (0.68) | – | − 0.918 (0.31) | ↓ |
| MOB1B | 0.291 (0.65) | – | − 0.207 (0.75) | – | 0.168 (0.80) | – |
|
ACTB
Control |
− 0.209 (0.80) | 0.166 (0.84) | 0.041 (0.96) | |||
↑↑ Non-significant (0.05 < P < 0.1) and ↑ (0.1 < P < 0.5) upregulation compared to static non-cultured donor controls
↓ Non-significant (01 < P < 0.5) downregulation compared to static non-cultured donor controls
↑↑↑ Statistically significant (p < 0.05) upregulation compared to static non-cultured donor controls
Is the Hippo signaling pathway relevant to ovarian physiology?
Kawamura et al. [12] demonstrated that the Hippo signaling pathway is involved in controlling intraovarian follicular growth and oocyte maturation. This control involves a delicate balance of permitting follicular growth at an appropriate time of the menstrual cycle, allowing follicular and, therefore, oocyte maturation, and inhibiting excessive growth. The Hippo pathway responds to upstream regulation as well as cell to cell contact/density, mechanical forces on the cell, stress signals, cellular polarity and architecture, and stage of the cell cycle [26]. In PCOS, the ovarian cellular density is greater than in a normal ovary [27]. Additionally, the ovarian cortex is thicker in a PCOS ovary and, therefore, the cortical cells are likely exposed to different mechanical forces in a PCOS ovary compared to a non-PCOS ovary [27–30]. Our own data reveals that genes within the Hippo pathway are upregulated in patients with PCOS when compared to controls. This upregulation likely leads to the loss of controlled orderly proliferation as well as apoptotic dysfunction. Consequently, dysregulation of the Hippo pathway in patients with PCOS is a potential contributing factor for cellular overgrowth. The genes found to have statistically significant higher expression levels (MOB1A, MOB1B, WWTR1, YAP1) code for proteins and transcription factors involved in control of cell growth and proliferation (Fig. 1). In the traditional Hippo pathway, under higher cell density scenarios, there is tight regulation of the balance between growth and apoptotic factors trending towards restriction of growth. Our results indicate that this delicate balance has been perturbed in patients with PCOS.
It is unclear if this is the underlying etiology of PCOS or if it is a result of the PCOS ovarian anatomy. Interestingly, cumulus cells from study controls that were exposed to mechanical forces in vitro expressed higher levels of the Hippo signaling genes compared to static samples from the same patient. This in vitro model may be able to induce PCOS Hippo gene expression levels; however, additional research must be conducted to determine if this can serve as a model for in vitro PCOS studies.
Clues from cumulus and granulosa cells
Cumulus and granulosa cells are directly involved in oocyte growth and maturation as well as sex hormone production. As discussed previously, granulosa cell tumors have been shown to be implicated in the Hippo pathway [23]. This is of particular interest in patients with PCOS who have biochemical and/or clinical hyperandrogenism as well as failure to develop mature follicles and ovulate mature oocytes. Cumulus cells provide a modality to study, not only oocyte development, but also underlying ovarian function and follicular development [5]. Additionally, they are readily acquired from patients undergoing in vitro fertilization or oocyte donation and provide an excellent surrogate for studying ovarian physiology [31]. They are also well accepted as a surrogate marker of oocyte viability [32–34]. Cumulus cells have also been previously used in studies to characterize PCOS. Adams et al. [35] showed that intra-follicular androgens and cytokines likely comprise a local regulatory loop that impacts granulosa cell expression of cytokines and chemokines.
One limitation of using cumulus cells is the extrapolation of a collection of cells to gross ovarian PCOS physiology. Cumulus cells in a mature follicle may not be exposed to the same forces as oocytes nor the stroma of a PCOS ovarian cortex. Additionally, analysis of the postovulatory cumulus oocyte complexes (COCs) may not appropriately reflect what oocyte Hippo gene expression was in the embryologic gonadal and oogonial development nor in early folliculogenesis. Furthermore, Hippo gene expression in cumulus cells, obtained after exogenous high dose gonadotropin stimulation of the ovaries, does not necessarily directly correlate with gene expression in cumulus cells from ovaries stimulated via physiologic endogenous gonadotropins. However, the differences in PCOS and control cumulus cell Hippo gene expression demonstrated in this snapshot of time, when cellular exposures of both PCOS and control cells to mechanical forces are likely most similar, suggest that PCOS patients have underlying Hippo pathway dysfunction. Although our own data examined gene expression, it does not necessarily mean that protein expression follows the same pattern. Due to the paucity of material, it was not feasible to examine protein levels in concurrent material.
The limitations and benefits of using cumulus cells have been highlighted above. In trying to use them as a surrogate marker for ovarian tension possibly experienced in PCOS, we are hypothesizing that the cumulus cells are representative. The tension experienced by these cells may however differ, firstly because they are within a follicle and secondly they may be somewhat protected from the overall ovarian architecture. It is however clear that the expression of Hippo genes dramatically differs in control versus PCOS patients. Interestingly, our in vitro stretching model did show that Hippo gene expression was altered in control cumulus cells and that the Hippo gene expression fingerprint can change differentially in cumulus cells cultured under mechanical stress. This failed to reach similar levels seen in the PCOS cumulus cells but did indicate that the cells are susceptible to mechanical stretching and that this could be a pathway that is influenced by the increased ovarian volumes seen in PCOS patients. Although our model was not an ideal system to test this theory, in vitro models are now being developed that could allow us to better understand the interplay between gene expression and tissue mechanics, in particular that of the ovary [34–36]. The largest limitation is the isolated study of selective Hippo pathway gene expression without inclusion of the end effector, phosphorylated YAP, nor a larger panel of genes within the Hippo pathway. It is difficult to extrapolate isolated gene expression levels to the cellular localization of YAP and resultant transcription effects.
Who is a PCOS patient?
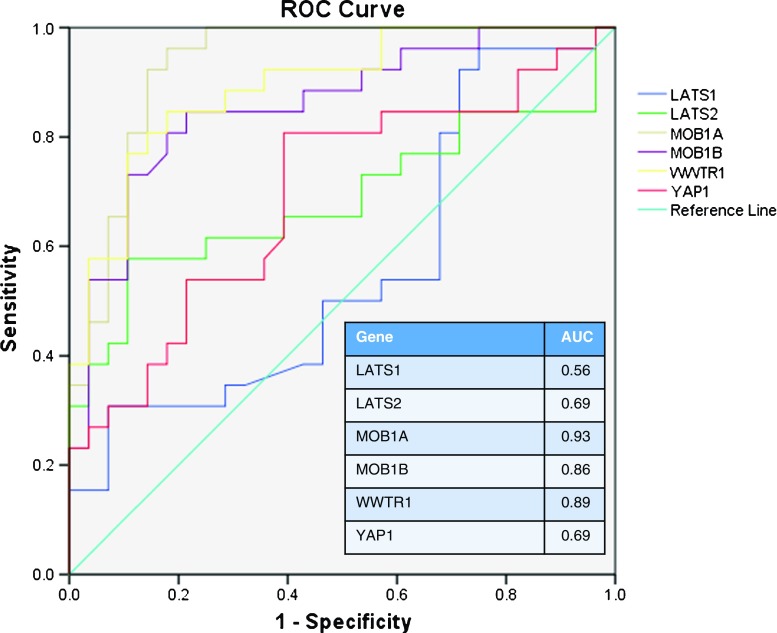
Phenotypic presentation of PCOS is quite variable; even among providers caring for patients with PCOS there is inconsistency in proposed diagnostic criteria [15, 16, 36]. As a result, it can be difficult to determine which patients should be categorized as having PCOS. We demonstrated that, not only do patients with PCOS have a heterogeneous phenotypic presentation, but the differential Hippo gene expression is also heterogeneous. When compared to controls, whose gene expression levels in the tested Hippo pathway genes are relatively homogenous (Fig. 1), the Hippo pathway gene expression varies from PCOS patient to PCOS patient. Some patients with a clinical diagnosis of PCOS demonstrated Hippo gene expression profiles more consistent with control profiles. Additionally, there does not appear to be a strict correlation between PCOS phenotypic clinical characteristics and gene expression levels. Therefore, single PCOS patients did show some variability in Hippo gene expression levels with some falling either outside or inside the normal range. That said, when we plotted the receiver operating curve (ROC) curve and calculated the corresponding area under the curve (AUC), we found that the MOB1A, MOB1B, and WWTR1 gene expression levels had a high sensitivity for predicting PCOS (Fig. 2). This indicates that any future validation using Hippo markers for PCOS may involve only a subset of related markers.
Fig. 2.
Receiver operating curves (ROC) demonstrating the predictive value of individual Hippo gene expression levels for PCOS. Area under the curve (AUC) signifies better predictive value when it is closer to 1
Using a threshold of average gene expression could represent a highly specific, but less sensitive test for PCOS. The need for objective criteria was highlighted in a recent review by Jones and Goodarzi [37] who stated that the potential for gene discovery to improve diagnosis and treatment of PCOS is promising, though there is much to be done in the field before the current findings can be translated to the clinic.
Clinical relevance and future directions
Testing for Hippo pathway gene expression and phosphorylation levels could be useful for both diagnostic and therapeutic purposes. Theoretically, a test could be designed to measure expression levels of genes within the Hippo pathway along with phosphorylation levels to determine normal reference ranges. Ranges might be identified that are consistent with what is currently a clinical diagnosis of PCOS [15, 16, 36]. Alternatively, gene expression and phosphorylation levels may be combined with specific phenotypic features of PCOS to better delineate the spectrum of this condition. Phosphorylation studies could clarify the ultimate effects of mechanical forces on transcription. Additional studies should be conducted to correlate cumulus cell Hippo gene expression with that from cells readily available in a less invasive manner such as serum, buccal or vaginal swabs, or other sources. This is also dependent on understanding whether the relationship between PCOS and the Hippo pathway is due to a systemic upregulation or a phenomenon that would be limited to the ovaries. If it is the latter, it may be more difficult to draw an association to non-ovarian tissue. Development of this test may be particularly helpful for discerning PCOS patients with a normal BMI, often labeled lean PCOS, from those with anovulation of another etiology. This becomes important when considering the long-term health implications of PCOS [38]. Patients with PCOS must be counseled regarding their increased risk of metabolic syndrome as well as undergo appropriate screening for its components [39].
Additionally, appropriate diagnosis of patients with PCOS is important when treating infertility. Recent studies have demonstrated that patients with PCOS have improved outcomes when undergoing ovulation induction with letrozole versus clomiphene citrate [40]. Furthermore, patients with PCOS are at increased risk of over-response when undergoing controlled ovarian hyperstimulation with gonadotropins [41]. This risk must be considered when designing the stimulation protocol, deciding to include estrogen priming, selecting a trigger medication, and deciding to transfer in a fresh cycle to balance the risk of over-response while maintaining the best chance at live birth.
Therapeutic treatment of PCOS could potentially be targeted to the gene level in the setting of abnormal expression profiles. Hippo gene-targeted therapeutics would likely not only have significant reproductive implications but may also impact the systemic symptoms of PCOS such as increased risk of hyperlipidemia, hypertension, diabetes, and hyperandrogenism [15, 16]. Treatments for the components of metabolic disorder seen in PCOS patients that are targeted to the underlying etiology may be more beneficial than those that provide general treatment which may be due to a different underlying pathology. Additionally, targeted pharmacologics could reduce the occurrence of surgical management via ovarian wedge resection and/or ovarian drilling which have been used to induce ovulation and reset the ovulatory cycle in patients with PCOS [42]. By eliminating surgical management, as well as capturing patients that are poor candidates for surgical treatments based upon surgical risk factors and/or poor prognosis, pharmacologic approaches may improve patient access to therapy and reduce its associated risks. Research into directed therapies is already being conducted, and possible pharmacologic agents have been proposed [11].
Conclusion
Our own results have demonstrated that patients with PCOS have higher expression levels of genes found in the Hippo signaling pathway in cumulus cells obtained after exogenous gonadotropin stimulation. Dysregulation of the Hippo pathway is likely one of the underlying etiologies for the clinical findings of PCOS including: enlarged ovaries with multiple small follicles, theca hyperplasia, and thickened ovarian cortices. Li et al. [43] also concluded that a key effector in the Hippo signaling pathway, YAP1, is differentially expressed in PCOS patients compared to controls in the Han Chinese when using RTqPCR and SNP analysis for GWA significance. Their study also indicated that the alleles are associated with insulin sensitivity and resistance and LH levels, further validating the association with clinical PCOS. Additionally, aberrancy in YAP1 methylation patterns among patients with PCOS has been demonstrated by Jiang et al. [44]. Although our pilot study did not demonstrate differential expression of LATS1 among PCOS patients and controls, Sun et al. [45] found that a deletion in Lats 1 resulted in ovarian germ cell apoptosis and follicular cysts. They also demonstrated that LATS1, LATS2, and MOB1B are localized to germ and somatic cells of primordial to antral follicles. Shah et al. [46] have also recently reviewed the complexities of the integral role that the Hippo pathway plays in mechanical and signaling pathways in the ovary and concluded that a tight relationship exists between both. They also proposed that disruption of ovarian biomechanics may be the mechanism by which ovarian disease phenotypes, such as PCOS, evolve.
Despite the marked differences in study design, the abundance of studies now indicate that aberrant signaling within the Hippo pathway is correlated with PCOS or clinical findings attributed to patients with PCOS. Further research is therefore needed to clarify the genomics, proteomics, and metabolomics underlying PCOS. Once elucidated, these can serve as targets for diagnostic testing and therapeutic interventions for patients with PCOS.
Electronic supplementary material
(DOCX 19 kb)
Reference
- 1.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 2.Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13:324–337. doi: 10.1038/nrgastro.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Happé Hester, de Heer Emile, Peters Dorien J.M. Polycystic kidney disease: The complexity of planar cell polarity and signaling during tissue regeneration and cyst formation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(10):1249–1255. doi: 10.1016/j.bbadis.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Bonfiglio R, Banerji S, Jackson DG, Salustri A, Richter RP. Micromechanical analysis of the hyaluronan-rich matrix surrounding the oocyte reveals a uniquely soft and elastic composition. Biophys J. 2016;110:2779–2789. doi: 10.1016/j.bpj.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, Leppert PC. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol Reprod. 2015;92:25. doi: 10.1095/biolreprod.114.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn PF, Picologlou BF. Viscoelastic properties of cumulus oophorus. Biorheology. 1976;13:379–384. doi: 10.3233/BIR-1976-13605. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Reilein A, Kalderon D. Yorkie and Hedgehog independently restrict BMP production in escort cells to permit germline differentiation in the Drosophila ovary. Development. 2017;144:2584–2594. doi: 10.1242/dev.147702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol. 2007;17:1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farah L, Lazenby AJ, Boots LR, Azziz R. Prevalence of polycystic ovary syndrome in women seeking treatment from community electrologists. Alabama Professional Electrology Association Study Group. J Reprod Med. 1999;44:870–874. [PubMed] [Google Scholar]
- 14.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 15.Goodman Neil F., Cobin Rhoda H., Futterweit Walter, Glueck Jennifer S., Legro Richard S., Carmina Enrico. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME -PART 2. Endocrine Practice. 2015;21(12):1415–1426. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- 16.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary. Endocr Pract. 2015;21:1291–300. [DOI] [PubMed]
- 17.Dewailly D, Alebic MS, Duhamel A, Stojanovic N. Using cluster analysis to identify a homogeneous subpopulation of women with polycystic ovarian morphology in a population of non-hyperandrogenic women with regular menstrual cycles. Hum Reprod. 2014;29:2536–2543. doi: 10.1093/humrep/deu242. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20:49–58. doi: 10.1093/molehr/gat051. [DOI] [PubMed] [Google Scholar]
- 19.Fan L, Fan L, Ling J, Ma X, Cui YG, Liu JY. Involvement of HSP10 during the ovarian follicular development of polycystic ovary syndrome: study in both human ovaries and cultured mouse follicles. Gynecol Endocrinol. 2009;25:392–397. doi: 10.1080/09513590902730796. [DOI] [PubMed] [Google Scholar]
- 20.Hewlett M, Chow E, Aschengrau A, Mahalingaiah S. Prenatal exposure to endocrine disruptors: a developmental etiology for polycystic ovary syndrome. Reprod Sci. 2016; [DOI] [PMC free article] [PubMed]
- 21.Kawamura K, Cheng Y, Sun YP, Zhai J, Diaz-Garcia C, Simon C, Pellicer A, Hsueh AJ. Ovary transplantation: to activate or not to activate. Hum Reprod. 2015;30:2457–2460. doi: 10.1093/humrep/dev211. [DOI] [PubMed] [Google Scholar]
- 22.Hahn S., Bering van Halteren W., Roesler S., Schmidt M., Kimmig R., Tan S., Mann K., Janssen O. The Combination of Increased Ovarian Volume and Follicle Number is Associated with More Severe Hyperandrogenism in German Women with Polycystic Ovary Syndrome. Experimental and Clinical Endocrinology & Diabetes. 2006;114(04):175–181. doi: 10.1055/s-2006-924063. [DOI] [PubMed] [Google Scholar]
- 23.Fu D, Lv X, Hua G, He C, Dong J, Lele SM, Li DWC, Zhai Q, Davis JS, Wang C. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer. 2014;21:297–310. doi: 10.1530/ERC-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Jeong K, Jiang H, Guo W, Gu C, Lu Y, Liang J. YAP/TAZ regulates the insulin signaling via IRS1/2 in endometrial cancer. Am J Cancer Res. 2016;6:996–1010. [PMC free article] [PubMed] [Google Scholar]
- 25.Derda R, Laromaine A, Mammoto A, Tang SK, Mammoto T, Ingber DE, et al. Paper-supported 3D cell culture for tissue-based bioassays. Proc Natl Acad Sci U S A. 2009;106:18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng Zhipeng, Moroishi Toshiro, Guan Kun-Liang. Mechanisms of Hippo pathway regulation. Genes & Development. 2016;30(1):1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Ozaki T, Okada M, Uchida A, Kitao M. Relationship between ultrasonography and histopathological changes in polycystic ovarian syndrome. Hum Reprod. 1994;9:2255–2258. doi: 10.1093/oxfordjournals.humrep.a138432. [DOI] [PubMed] [Google Scholar]
- 28.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–1379. doi: 10.1016/S0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 29.Atiomo WU, Pearson S, Shaw S, Prentice A, Dubbins P. Ultrasound criteria in the diagnosis of polycystic ovary syndrome (PCOS) Ultrasound Med Biol. 2000;26:977–980. doi: 10.1016/S0301-5629(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 30.Swanson M, Sauerbrei EE, Cooperberg PL. Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound. 1981;9:219–222. doi: 10.1002/jcu.1870090504. [DOI] [PubMed] [Google Scholar]
- 31.Fragouli E, Lalioti MD, Wells D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. Hum Reprod Update. 2014;20:1–11. doi: 10.1093/humupd/dmt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, Sirard MA. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod. 2008;23:1118–1127. doi: 10.1093/humrep/den048. [DOI] [PubMed] [Google Scholar]
- 33.Haouzi D, Assou S, Monzo C, Vincens C, Dechaud H, Hamamah S. Altered gene expression profile in cumulus cells of mature MII oocytes from patients with polycystic ovary syndrome. Hum Reprod. 2012;27:3523–3530. doi: 10.1093/humrep/des325. [DOI] [PubMed] [Google Scholar]
- 34.Hamamah S, Matha V, Berthenet C, Anahory T, Loup V, Dechaud H, Hedon B, Fernandez A, Lamb N. Comparative protein expression profiling in human cumulus cells in relation to oocyte fertilization and ovarian stimulation protocol. Reprod BioMed Online. 2006;13:807–814. doi: 10.1016/S1472-6483(10)61028-0. [DOI] [PubMed] [Google Scholar]
- 35.Adams Jaye, Liu Zhilin, Ren Yi Athena, Wun Wan-Song, Zhou Wei, Kenigsberg Shlomit, Librach Clifford, Valdes Cecilia, Gibbons William, Richards JoAnne. Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women With Polycystic Ovarian Syndrome. The Journal of Clinical Endocrinology & Metabolism. 2016;101(9):3459–3468. doi: 10.1210/jc.2015-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotterdam ESHRE/ ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Jones MR, Goodarzi MO. Genetic determinants of polycystic ovary syndrome: progress and future directions. Fertil Steril. 2016;106(1):25–23. doi: 10.1016/j.fertnstert.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 38.El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124. doi: 10.3389/fphys.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardestani Amin, Lupse Blaz, Maedler Kathrin. Hippo Signaling: Key Emerging Pathway in Cellular and Whole-Body Metabolism. Trends in Endocrinology & Metabolism. 2018;29(7):492–509. doi: 10.1016/j.tem.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin H, Li Y, Li L, Wang W, Yang D, Zhang Q. Is a GnRH antagonist protocol better in PCOS patients? A meta-analysis of RCTs. PLoS One. 2014;9:e91796. doi: 10.1371/journal.pone.0091796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu HH. Predictors of success of laparoscopic ovarian drilling in women with polycystic ovary syndrome: an evidence-based approach. Arch Gynecol Obstet. 2015;291:11–18. doi: 10.1007/s00404-014-3447-6. [DOI] [PubMed] [Google Scholar]
- 43.Li T, Zhao H, Zhao X, Zhang B, Cui L, Shi Y, Li G, Wang P, Chen ZJ. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49:254–257. doi: 10.1136/jmedgenet-2011-100727. [DOI] [PubMed] [Google Scholar]
- 44.Jiang LL, Xie JK, Cui JQ, Wei D, Yin BL, Zhang YN, Chen YH, Han X, Wang Q, Zhang CL. Promoter methylation of yes-associated protein (YAP1) gene in polycystic ovary syndrome. Medicine (Baltimore) 2017;96:e5768. doi: 10.1097/MD.0000000000005768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun T, Pepling ME, Diaz FJ. Lats1 deletion causes increased germ cell apoptosis and follicular cysts in mouse ovaries. Biol Reprod. 2015;93:22. doi: 10.1095/biolreprod.114.118604. [DOI] [PubMed] [Google Scholar]
- 46.Shah Jaimin S., Sabouni Reem, Cayton Vaught Kamaria C., Owen Carter M., Albertini David F., Segars James H. Biomechanics and mechanical signaling in the ovary: a systematic review. Journal of Assisted Reproduction and Genetics. 2018;35(7):1135–1148. doi: 10.1007/s10815-018-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb)