Abstract
Purpose
A microwell culture system that facilitates group culture, such as well-of-the-well (WOW), improves embryonic development in an individual culture. We examined the effect of WOW on embryonic development in vitro with commercially available human single culture media.
Methods
Using four different commercial human single culture media, in vitro development and imprinted gene expression of bovine embryos cultured in WOW were compared to droplet culture (one zygote per drop). To determine the effects of microwell and group culture on embryonic development, different numbers of embryos were cultured in droplet or WOW. Diffusion simulation of accumulating metabolites was conducted using the finite volume method.
Results
WOW had a positive effect on bovine embryonic development, regardless of the type of single culture media. Imprinted gene expression was not different between droplet- and WOW-derived blastocysts. The microwell and group cultures in WOW showed a significant positive effect on the rate of total blastocysts and the rate of development to the expanded and hatching blastocyst stages. The assumed cumulative metabolite concentration of WOW with one embryo was 1.47 times higher than that of droplet culture with one embryo. Furthermore, the concentration of WOW with three embryos was 1.54 times higher than that of WOW with one embryo.
Conclusions
In using human single culture media, a microwell culture system that allows group culture could be a powerful clinical tool for improving the success of assisted reproductive technologies.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1252-z) contains supplementary material, which is available to authorized users.
Keywords: Bovine embryo assay, Group culture, Human single medium, Metabolites, Microwell
Introduction
Assisted reproductive technology (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) is associated with several complications including low pre-implantation embryonic development, abnormal fetal development, and adverse neonatal and long-term health outcomes attributed to genomic imprinting disorders [1, 2]. ART success is affected by several factors such as culture condition, particularly composition of the culture medium [3]. There are two types of culture media that take on very different approaches. These are referred to as “back to nature” and “let the embryo choose.”
Sequential culture medium based on the “back to nature” approach mimics the in vivo conditions when the zygote moves from the fallopian tube to the uterus during early embryonic development [4]. To mimic the in vivo environment, the sequential medium contains different compositions during the different days of culture [4]. The compositions are based on animal models reporting a change of energy requirements during in vitro culture [5, 6]. Moreover, the exchange of medium has the effect of removing detrimental factors such as ammonium from the developmental environment of the embryonic development [7].
The single culture medium is based on the “let the embryo choose” approach. It is constant and contains all components required for development. Importantly, the composition of this single medium does not change during the in vitro embryo culture [5]. Proponents of this paradigm argue that there is no direct experimental evidence that sequential media are required for optimal embryonic development [5]. Although it is not clear which type of culture media (sequential or single) is the best for human ART, in recent years, the use of single culture media has increased due to the worldwide spread of time-lapse cinematography (TLC), blastocyst transfer, and preimplantation genetic diagnosis (PGD)/preimplantation genetic screening (PGS).
Significant improvements in ART have been achieved in recent years; however, two major problems still exist. Firstly, clinical pregnancy rates in Europe are still only around 34.5% per transfer. This is because it is difficult to objectively determine which embryo has the highest competence for implantation [8]. Secondly, the high rate of multiple deliveries is still approximately 18.0%. This is because of the multiple embryos that are transferred into the uterus per cycle [8]. In order to address the high multiple pregnancy rate, it would be necessary to reduce the number of transferred embryos and move to single embryo transfer. Time-lapse imaging that allows selection based on morphokinetic markers, such as the number of pronuclei or nuclei, timing of cleavage, and number of blastomeres, is helping to address these problems [9, 10].
Despite the compatibility between TLC and single medium, the culturing system suitable for single culture media is unclear. In human ART, a single embryo is placed in a 10 to 50 μl droplet for in vitro embryo culture [11]. The low embryo density can result in defects such as embryonic development impaired blastocyst formation, low cell numbers, and decreased production of pregnancy recognition factors [12–14]. Conversely, culturing embryos in droplets of small volumes could lead to the accumulation of detrimental factors such as ammonium [15] and oxygen-derived free radicals [16]. Media change can remove these detrimental factors but is harmful to embryonic development because positive-acting metabolites such as growth factors may also be removed [17].
For overcoming the problems associated with the above methods, the well-of-the-well (WOW) culture system with microwells at the bottom of conventional culture dishes has been developed [18]. The WOW culture system provides both a suitable macroenvironment, which may be responsible for nutritional support and dilution of metabolized detrimental factors, and microenvironment, which may be accumulation of metabolites such as growth factors from the embryos themselves and adjacent embryos, resulting in improved embryonic development and altered gene expression profile [18–20].
Previously, we developed a TLC-compatible culture system with 25 microwells allowing group culture [21, 22], based on the WOW approach [18]. Embryos cultured in the system can be fixed in alternative microwells, which provide good visibility during time-lapse imaging. Studies of the bovine embryo model, using medium for bovine embryo culture but not for human embryo culture, demonstrated no negative developmental effects from the culture dish in vitro. Furthermore, blastocysts from the WOW culture have a low apoptosis rate and high pregnancy rate compared with those cultured in a conventional droplet culture system [21]. These beneficial effects may be due to the accumulation of metabolites such as growth factors that are secreted from the embryos themselves (microwell effect) as well as from adjacent embryos in each microwell (group culture effect) [21]. However, the developmental roles of these microwell and group culture effects on embryonic development and metabolites secretion from embryos in a microwell are poorly understood. The objective of the present study is to evaluate the efficacy of WOW with human single culture media. We examined in vitro development and expression profiles of imprinted genes of bovine IVF embryos, which are a well-established animal model for human IVF [23], cultured in conventional droplet or WOW with different types of human single culture media (experiment 1). We also evaluated the participation of microwell and group culture effects of WOW on embryonic development (experiment 2). Finally, the concentration of metabolites from embryos was simulated by the finite volume method based on Fick’s second law of diffusion (experiment 3).
Materials and methods
Ethical
This study has been approved by the local ethics committee (MMYC ethic committee agenda: agreements for in vitro determination of the best medium for human embryos (MMYC_2017–2) and analysis of imprinted genes of blastocysts generated in single medium (MMYC_2017-3)).
Design and fabrication of WOW
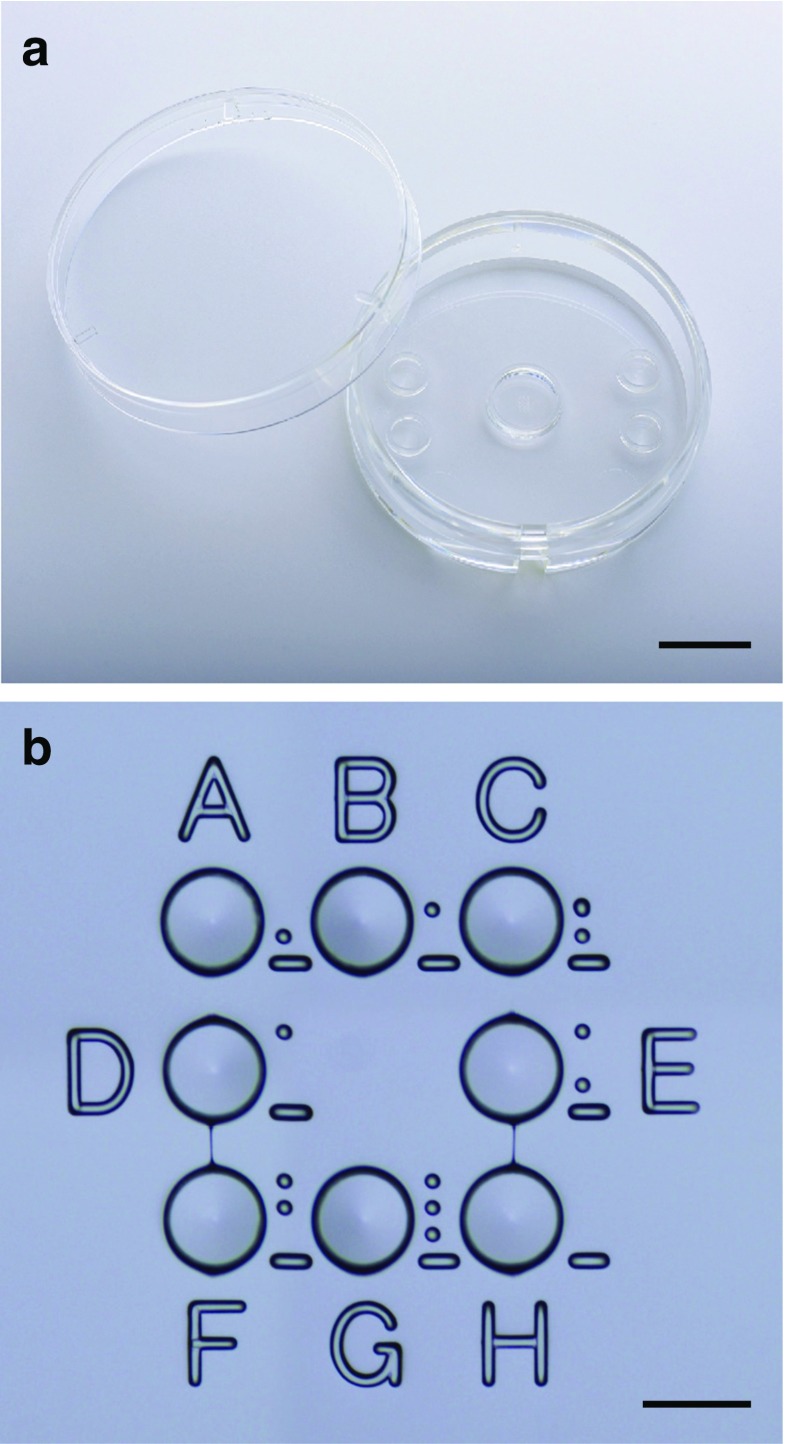
The WOW dish (LinKID® micro8; Dai Nippon Printing Co., Ltd., Chiba, Japan) has eight microwells with a circular wall in the center of a 35-mm culture dish (Fig. 1). Each well is 280 μm in diameter and 160 μm in depth. The eight wells are arranged in three columns and three rows. There is no microwell in the center since there are two adjacent embryos when filling all eight wells. The wells were 170 μm apart, and the bottom of each well slopes down toward the center of the well with a slope angle of 7°. The circular wall is 7 mm in diameter and 2 mm in height and is used to form a microdroplet of culture medium. ID codes A to H are tagged on the left/right and top/bottom of the microwells. Furthermore, recognition dots are tagged on the right of the microwells for recognizing the position of microwells even at high magnification. The WOW system was fabricated based on the conventional injection molding method. All WOW received hydrophilic treatment and were sterilized.
Fig. 1.
Photographs of the fabricated well-of-the-well (WOW) system by injection molding with polystyrene (LinKIDⓇ micro8; Dai Nippon Printing Co., Ltd., Chiba, Japan). WOW has a circular wall (7 mm in diameter and 2 mm in height) (a) surrounding eight microwells (160 μm in depth, 280 μm in diameter, 170 μm in distance with a slope angle of 7°) in the center of the 35-mm culture dish (b). ID codes A to H are tagged on the left/right and top/bottom of the microwells. Recognition dots are tagged on the right of the microwells for recognizing their position even at high magnification. Bar = 10 mm (a) and 300 μm (b)
Embryo culture and quality assessment
Oocyte collection
A total of 214 ovaries from adult cattle at any estrus stage were collected at a local slaughterhouse. Cumulus oocyte complexes (COCs) were aspirated from 2 to 6 mm follicles using a 10-ml syringe with a 19-gauge needle.
In vitro maturation (IVM)
HEPES-buffered TCM 199 supplemented with 5% new born calf serum (NBCS; Biowest, USA) and 0.1 IU/ml follicle-stimulating hormone (Follistim; MSD, Tokyo, Japan) was used as IVM medium. A total of 1280 compacted COCs with three or more layers of cumulus cells and a homogeneous ooplasm were cultured in 500 μl IVM medium covered with paraffin oil (Paraffin Liquid; Nakalai Tesuque, Inc., Kyoto, Japan) in four-well dishes at 38.5 °C in humidified atmosphere of 5% CO2 in air for 20–22 h (40–50 COCs/well).
In vitro fertilization (IVF)
IVF was performed as previously described [24]. At the end of IVM for 20–22 h, sperm samples were thawed and then centrifuged in 3 ml of 90% Percoll solution (GE Healthcare, Uppsala, Sweden) at 750×g for 10 min. The pellet was re-suspended and centrifuged in 6 ml of sperm washing solution (Brackett and Oliphant solution, BO) [25], supplemented with 10 mM hypotaurine (Sigma) and 4 U/ml heparin (Novo-Heparin Injection 1000; Aventis Pharma Ltd., Tokyo, Japan) at 550×g. After centrifugation, the pellet was re-suspended in sperm washing and BO solutions supplemented with 20 mg/ml bovine serum albumin (BSA) (Sigma) to achieve a final concentration of 3 × 106 sperm/ml. The suspensions of 100 μl were aliquoted in 35-mm dishes under paraffin oil as fertilization droplets. COCs were washed in BO supplemented with 10 mg/ml BSA and cultured in fertilization droplets for 6 h at 38.5 °C in humidified atmosphere of 5% CO2 in air (20–30 COCs/drop).
Preparation of WOW
The top four used in Japan was selected as commercially available human single culture media. G-TL™ (Vitrolife, Goteborg, Sweden), SAGE 1-Step™ (ORIGIO, Malov, Denmark), globalⓇ (LifeGlobal, Brussels, Belgium), or ONESTEP medium (NAKA medical, Tokyo, Japan) of 60 μl was placed within the circular wall and covered with paraffin oil, which gave the maximum blastocyst yield and did not disturb embryo manipulation in the preliminary study (data not shown). Air bubbles inside the microwells were removed by tapping the dish wall from outside with an awl. WOW was pre-incubated for at least 3 h before use.
In vitro culture (IVC)
IVC was performed at 38.5 °C in 5% CO2, 5% O2, and 90% N2 with saturated humidity for 8 days [24]. Zygotes were completely denuded from cumulus cells and spermatozoa in pre-incubated IVC medium. Eight zygotes were placed in 60 μl droplet in microwells of WOW or individually in eight conventional 30 μl droplets in a 35-mm dish.
Assessment of embryonic development and morphological quality
We calculated the rates of cleavage and > 5-cell per zygote at day 2, as well as the rates of total, expanding/expanded, hatching/hatched, and Code 1 blastocyst development per cleaved embryo at day 8 derived from data set of 10 replicates. Code 1 (excellent/good) was based on criteria of International Embryo Technology Society (IETS) Manual [26].
Collection of in vivo-derived embryos
In vivo-derived embryos were flushed from superovulated cattle as described previously [27]. Superovulation was performed by inserting an intravaginal progesterone releasing device (controlled internal drug release (CIDR); Pfizer, Tokyo, Japan) on day − 9, injecting 0.8 mg estradiol benzoate (OVAHORMON® INJECTION; ASUKA Animal Health Co. Ltd., Tokyo, Japan) on day − 8, a total of 20 armor unit (A.U.) follicle-stimulating hormone (ANTORIN®-R•10; Kyoritsu Seiyaku Co., Tokyo, Japan) twice daily in decreasing doses (5, 5, 3, 3, 2, 2 A.U., respectively) on days − 4 to − 2, 0.75 mg prostaglandin F2α (cloprostenol, RESIPRON®-C; ASUKA Animal Health Co. Ltd.) on the morning, and CIDR removal on the evening of day − 2. On the morning of day 0, 100 μg gonadotropin-releasing hormone analog (fertirelin acetate, Supolnen; Kyoritsu Seiyaku Co.) was administered and artificial insemination (AI) was performed on the evening of the same day and the morning of day 1. On day 6 or 7 after first AI, embryos were recovered by uterine flushing. Collected code1/2 blastocysts were stored in 20 mg/ml BSA in H2O in − 80 °C until gene expression was analyzed.
Quantitative real-time RT-PCR analysis
Five genes including histone H2A.Z (H2AFZ), insulin-like growth factor 2 receptor (IGF2R), small nuclear ribonucleoprotein-associated protein N (SNRPN), GNAS complex locus (GNAS), and maternally expressed 3 (MEG3) were analyzed using real-time PCR [24]. Four imprinting genes were selected from which the expression was confirmed in blastocyst stage from the results of RNA-seq in a preliminary study.
Total RNA from individual code1/2 blastocysts lysed in 50 μl extraction buffer (Arcturus, Carlsbad, CA, USA) was extracted using a PicoPure RNA Isolation Kit (Arcturus). Each RNA sample was reverse transcribed into cDNA using a ReverTra Ace qPCR RT Kit (Toyobo Bio, Osaka, Japan). Reaction product of 20 μl was diluted with nuclease-free water to a final volume of 40 μl. Real-time RT-PCR was performed using StepOnePlus™ Systems (Applied Biosystems, Foster City, CA, USA) in a 20 μl reaction volume containing 2 μl cDNA, 2.5 μl each of the forward and reverse primers (Table S1), 3 μl nuclease-free water, and 10 μl Fast SYBR Green PCR Master Mix (Applied Biosystems). In each set of PCR reactions, duplicate cDNA samples were run to control for the reproducibility of the real-time RT-PCR results. Universal thermal cycling parameters such as initial step of 20 s at 95 °C, followed by 45 cycles of 3 s at 95 °C, 10 s at 60 °C, and 20 s at 72 °C, were used. Melting curve analysis was performed for checking the specificity of the reaction. A standard curve was generated in every PCR run using serial fivefold dilutions of cDNA derived from blastocysts. The results were normalized to H2FAZ and expressed relative to a mean value of in vivo derived blastocysts set at 1.
Numerical calculations of diffused metabolites
The assumed concentration of embryo-derived metabolites in droplet or WOW was numerically calculated by the finite volume method based on Fick’s second law of diffusion [28]. Numerical calculations were performed using Gambit, ANSYS Fluent, and ANSYS CFD-Post software (ANSYS Japan K.K., Tokyo, Japan).
First, two-dimensional (2D) mesh models of four culture systems (three or one embryo in 60 μl of medium in WOW (models A and B), and one embryo in 60 or 30 μl of medium in droplet (models C and D)) were created using the Gambit software. The medium shapes were set to square in models A and B, or semicircle in models C and D. The embryos were set to be 110 μm in diameter and located center-bottom in all (three) microwells (model A), in the central microwell (model B), or the center-bottom of the droplet (models C, D). For lowering the distortion of the mesh, the embryos were set to be located 3 μm floated from the bottom in all models.
Subsequently, the time valuation of the concentration of the metabolites at all meshed cells was calculated using the ANSYS Fluent software. For calculation, Fick’s second law of diffusion (Eq. (1)) was used.
| 1 |
In Eq. (1), x and y are the position and t is the time. C represents the concentration of the metabolites. Dx and Dy represent the diffusion coefficients (D) of the metabolites in water in the x and y directions, respectively. In this calculation, the diffusion coefficients (D) of the metabolites, such as proteins, is set to 1 × 10−11 m2/s based on the diameter of macromolecules (2–10 nm diameter; 5–200 kDa) [28]. The density of the mixture of the metabolites and water is selected as volume-weighted-mixing law. The viscosity of the mixture is set to 1.72 × 10−5 kg/m, which is the value of the viscosity of water.
The secreted value of the metabolites from one embryo A(t) is described as a single exponential in Eq. (2).
| 2 |
In Eq. (2), K is a kinetic constant and Amax is the maximum concentration of the metabolites. The time constant 1/K of protein secretion (metabolites) was set to approximately 27.7 h, which is based on the previous study [29]. The total amount of metabolites, Amax, was considered to be 100 pmol for 16.7 h based on previous publications [28, 30].
The time step size for all calculations is set to 100 s. Calculations were performed from 100 s to 60,000 s (= 1000 min = 16.7 h). The results are presented as the time valuations of the sum of the concentration of the metabolites at all neighboring cells of the embryo.
Statistical analysis
All statistical analyses were performed using JMP software version 4.0.5 (SAS Institute Inc., Cary, NC, USA). Data were analyzed using two-way analysis of variance (ANOVA) followed by Student’s t test or Tukey–Kramer test. All percentage data were arcsine transformed prior to ANOVA. Cluster analysis using the Ward’s method was performed to identify embryo groups similar to the imprinted gene expression pattern of in vivo embryos. For all data, a P value < 0.05 was considered significant.
Results
Effect of WOW with different types of human single step culture media on embryonic development and imprinting genes (experiment 1)
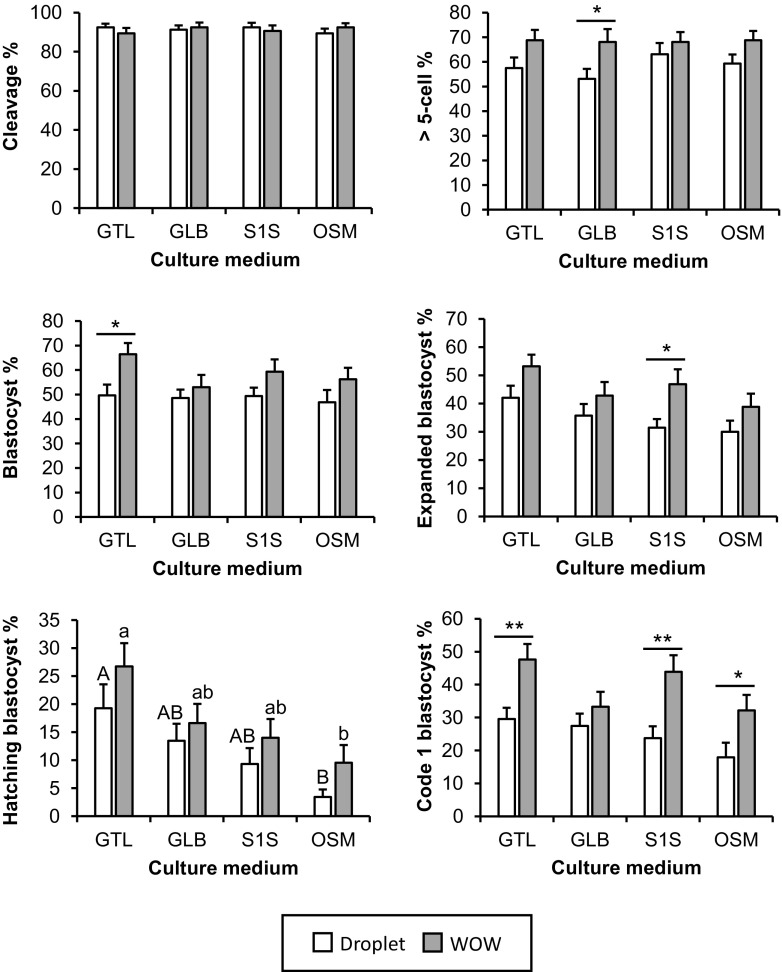
The effect of a culture system (droplet or WOW) with four different human single media (GTL, GLB, S1S, or OSM) on embryonic development (Table 1 and Fig. 2) and imprinted gene expression (IGF2R, SNRPN, GNAS, and MEG4) (Table 2 and Fig. 3) was analyzed. There was no notable effect of the culture system or medium on the cleavage rate at day 2. However, the rate of > 5 cells on day 2 was higher in WOW system. In the GLB group, the rate of embryonic development with WOW was significantly higher than that in the droplet system (P < 0.05). The rate of blastocyst development on day 8 was also affected by the culture system. The blastocyst development rate was higher with WOW in all media, and significant differences between droplet and WOW were observed in the GTL group (P < 0.05). The rates of development to the expanded, hatching, and code 1 blastocyst stages were all affected by both the culture system and medium, and those rates were higher with WOW in all culture media. In the S1S group, the rate of development to the expanded blastocyst stage was significantly higher with the WOW system (P < 0.05). The rate of development to the hatching blastocyst stage was higher in the GTL group, regardless of whether droplet or WOW was used. Furthermore, in the GTL, S1S, and OSM groups, the code 1 blastocyst development rate was significantly higher in WOW compared to the droplet system (P < 0.05). Even by using the medium designed for bovine embryos as a control, the effectiveness of the WOW system including improved rates of total, expanded, and hatching blastocyst and code 1 was observed compared with droplet, which was in a similar manner to human culture media (Supplementary Table 2).
Table 1.
Effect of WOW and types of human single media on in vitro embryonic development at day 2 and day 8
| Factorsa | P valueb | |||||
|---|---|---|---|---|---|---|
| Cleavage % | > 5-cell % | Total blastocyst % | Expanded blastocyst % | Hatching blastocysts % | Code 1 blastocyst % | |
| System | 0.6747 | 0.0158 | 0.0069 | 0.0046 | 0.0275 | < 0.00001 |
| Medium | 0.9719 | 0.7311 | 0.3339 | 0.027 | 0.0001 | 0.0163 |
| System × Mediumc | 0.4009 | 0.7163 | 0.516 | 0.9576 | 0.8601 | 0.3609 |
aFactors are defined as the culture system (System; Droplet and WOW) and culture medium (Medium; GTL, GLB, S1S, and OSM)
bP values are derived from the two-way ANOVA
cSystem × Medium represents the synergistic effect of the system and medium
Fig. 2.
Effect of WOW and human single step media on embryonic development. Zygotes were cultured in a droplet with 30 μl (one zygote per droplet) or WOW with 60 μl (eight zygotes per WOW) of G-TL™ (GTL), global (GLB), SAGE 1-Step (S1S), or ONESTEP medium (OSM). The effect of culture systems and culture media on embryonic development was examined by measuring the following points of embryonic development: cleavage, > 5-cell embryo at day 2, embryos developed to blastocysts at day 8, those that reached the expanded and hatching/hatched blastocyst stages, and embryos developed to code 1 blastocyst. Code 1 indicates excellent/good morphological quality based on IETS criteria [26]. Rates of development to the total blastocyst, expanded blastocyst, hatching blastocyst, and code 1 blastocyst stages were expressed as percentage of cleavage embryos. All values are represented as the mean ± SEM of 20 replicate experiments. Number of total cultured zygotes was 160 in each group. For each trial, 64 zygotes were divided into eight groups. Asterisks indicate significant differences between droplet and WOW at **P < 0.01 or *P < 0.05. Different letters indicate significant differences among culture media at P < 0.05 (A–B: droplet and a–b: WOW)
Table 2.
Effect of WOW and types of human single media on imprinted gene expression at day 8 blastocyst stage
| Factorsa | P valueb | |||
|---|---|---|---|---|
| IGF2R | SNRPN | GNAS | MEG3 | |
| System | 0.5165 | 0.6384 | 0.5426 | 0.5746 |
| Medium | 0.0734 | 0.8601 | 0.5828 | 0.4803 |
| System × Mediumc | 0.4486 | 0.7291 | 0.9483 | 0.6238 |
aFactors are defined as the culture system (System; Droplet and WOW) and types of culture media (Medium; GTL, GLB, S1S, and OSM)
bP values are derived from two-way ANOVA
cSystem × Medium represents the synergistic effects of the system and medium
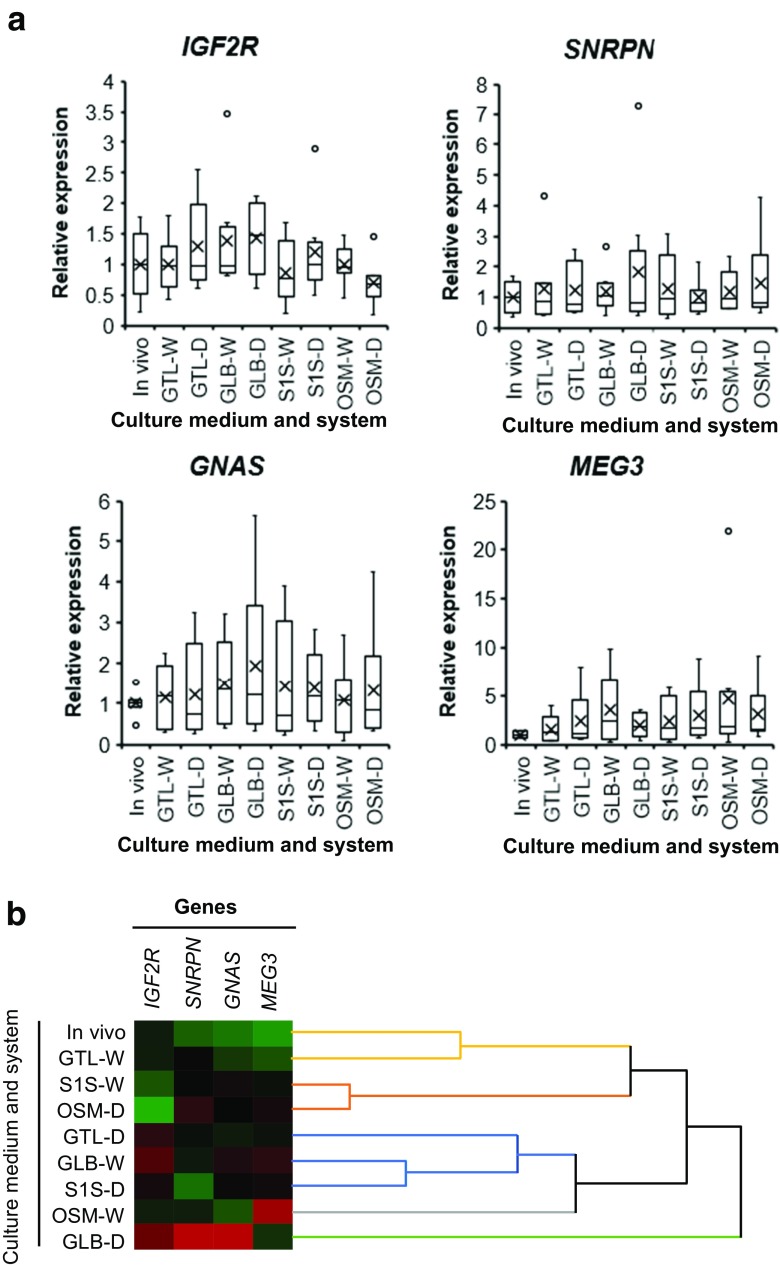
Fig. 3.
Effect of WOW and human single step media on the expression of imprinted genes. Zygotes were cultured in droplet with 30 μl (D) or WOW with 60 μl (W) of G-TL™ (GTL), global (GLB), SAGE 1-Step (S1S), or ONESTEP medium (OSM). Embryos reached the blastocyst stage at day 8 and in vivo-derived blastocysts were examined following expression of IGF2R, SNRPN, GNAS, and MEG3. Gene expression was represented as box-and-whisker plots. Boxes reflect two quartiles, the 25th and 75th percentiles, and the interior horizontal line indicates the median. Whiskers indicate the maximum and minimum values within the acceptable range defined by the two quartiles. Open circles denote outliers. Crosses indicate the mean value (a). Embryos displaying similar patterns of expression for these four genes were grouped together on closely connected branches of the dendrogram with the same colors. Red, black, and green colors of the color map represent high, medium, and low expression, respectively (b)
There was no main effect of the culture system or medium on the expression of any of the four imprinted genes (Table 2 and Fig. 3a). Furthermore, there were no significant differences in the expression of imprinted genes in any of the groups compared with in vivo-derived blastocysts (Fig. 3a). However, cluster analysis revealed that the expression pattern of the imprinted genes of blastocysts derived from GTL-W was most similar to that of in vivo-derived blastocysts (Fig. 3b). Therefore, these results demonstrate that WOW could improve embryonic development without negative effects on the transcription of imprinted genes, regardless of the type of human single step culture medium used.
Effect of microwell and group cultures on embryonic development (experiment 2)
The effects of microwell and group culture were examined by culturing in two different culture systems, droplet (without microwell) or WOW (with microwell), with a different number of zygotes per dish (1, 2, 4, or 8). The culture medium was 60 μl of GTL in both droplet and WOW (Table 3 and Fig. 4). There was no significant effect of the culture system and number of zygotes on the cleavage rate at day 2. The positive effect of the microwell system was observed at day 2 with rates of > 5 cells and at day 8 with total blastocyst development. In experiments with two zygotes per dish, the total blastocyst development rate was significantly higher with WOW (P < 0.05). However, the rates of development to the expanded, hatching, and code 1 blastocyst stages were affected by the number of embryos per dish. These rates were improved by increasing this number of embryos. The hatching rate in the WOW system with eight zygotes was significantly higher than that in WOW with only one zygote (P < 0.05).
Table 3.
Effect of microwell and group culture on in vitro embryonic development at day 2 and day 8
| Factorsa | P valueb | |||||
|---|---|---|---|---|---|---|
| Cleavage % | > 5-cell % | Total blastocyst % | Expanded blastocyst % | Hatching blastocysts % | Code 1 blastocyst % | |
| System | 0.8974 | 0.0100 | 0.0325 | 0.3012 | 0.2721 | 0.1979 |
| Number of embryos | 0.9562 | 0.7340 | 0.1473 | 0.0432 | 0.0001 | 0.0066 |
| System × Number of embryosc | 0.8991 | 0.8032 | 0.9025 | 0.9574 | 0.8000 | 0.747 |
aFactors are defined as the culture system (System; Droplet and WOW) and number of embryos per dish (number of embryos = 1, 2, 4, and 8)
bP values are derived from two-way ANOVA
cSystem × Number of embryos represent synergistic effects of the system and the number of embryos
Fig. 4.
Effect of microwell and group culture on embryonic development. Different numbers of zygotes (1, 2, 4, and 8) per dish were cultured in a droplet with 60 μl or WOW with 60 μl of G-TL™. The effect of microwell and group culture on embryonic development was examined by measuring the following points of embryonic development: cleavage, > 5-cell embryo at day 2, embryos developed to blastocysts at day 8, those that reached the expanded and hatching/hatched blastocyst stages, and embryos developed to code 1 blastocyst. Code 1 indicates excellent/good morphological quality based on IETS criteria [26]. The rates of total blastocyst, expanded blastocyst, hatching blastocyst, and code 1 blastocyst development were expressed as the percentage of cleavage embryos. All values are represented as the mean ± SEM of 10 replicate experiments. Number of total cultured zygotes was 80 in each group. For each trial, 64 zygotes were divided into eight groups. Asterisks indicate significant differences between droplet and WOW at P < 0.05. Different letters indicate significant differences among the number of zygotes per dish at P < 0.05 (A–B: droplet and a–b: WOW)
Taken together, development to the blastocyst stage can be supported by the microwell effect, but the group effect may be necessary for cell proliferation and quality improvement in blastocysts.
Diffusion simulation of embryo-derived metabolites in different culture systems (experiment 3)
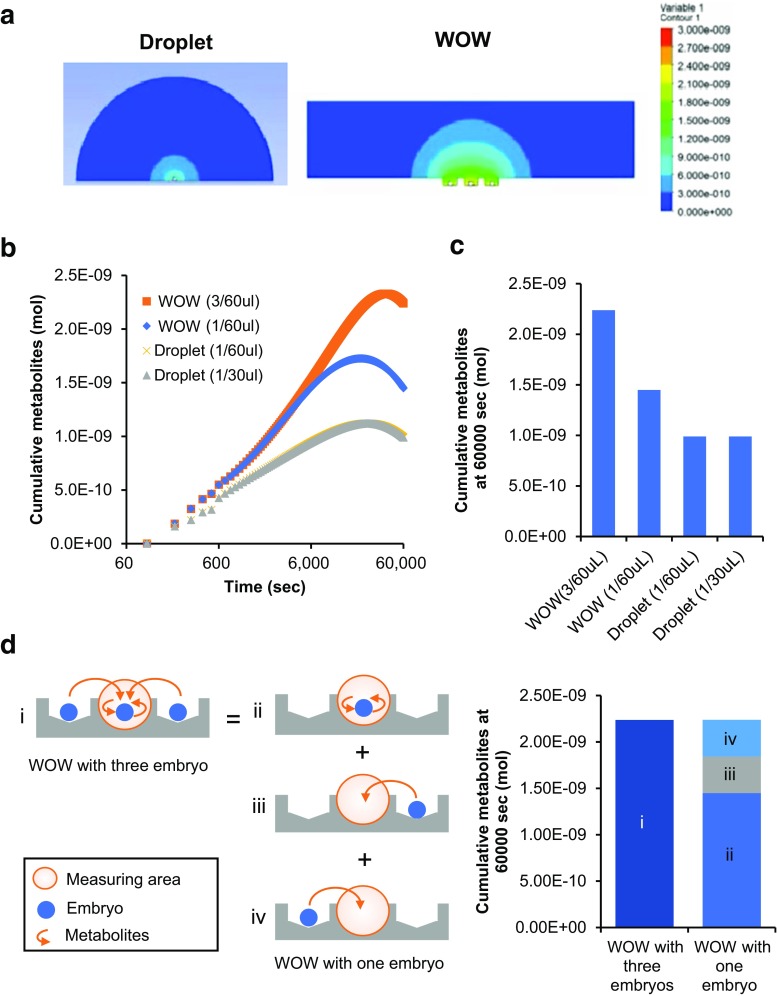
The assumed concentration of metabolites was compared between droplet and WOW using the mathematical simulation model (Fig. 5). The concentration of cumulative metabolites around an embryo adjacent to the two embryos in WOW was 226% of the final concentration around an embryo in a droplet at 60,000 s (Supplementary movie S1 and Fig. 5a). In order to analyze the mechanism of metabolite accumulation in WOW, we calculated the concentrations of cumulative metabolites in WOW with three embryos, in WOW with one embryo, in a 30 μl droplet with one embryo, and in a 60 μl droplet with one embryo. The mean concentration was the highest in the WOW system with three embryos (Fig. 5a), and its final concentration was 154% of WOW with one embryo (Fig. 5c). However, the final concentration of WOW with one embryo was still 147% of the droplet with one embryo (Fig. 5c). The volume of medium did not affect the accumulation of metabolites because the diffusion kinetics and final concentration did not differ between the 60 μl and 30 μl droplets (Fig. 5b, c). The metabolite concentration of WOW with three embryos was equal to the sum of the metabolites from the embryo itself and metabolites from the two adjacent embryos (Fig. 5d). The percentage of metabolites from the embryo itself and those from two adjacent embryos in the sum was 64.7 and 35.3%, respectively (Fig. 5d).
Fig. 5.
Diffusion simulation of embryo-derived metabolites in droplet and WOW. Cumulative metabolites in droplets with one embryo and WOW with three embryos. The colors indicate the metabolite concentration. Minimum concentration, 0 mol, is blue and the maximum value, 3.0E−09 mol, is red on the right color scale (a). Diffusion kinetics of metabolites from embryos cultured in droplet and WOW. The diffusion kinetics (b) and final metabolite concentration at 6000 s (c) in a droplet of 30 μl with one embryo (Droplet (1/30 μl)), droplet of 60 μl with one embryo (Droplet (1/60 μl)), WOW of 60 μl with one embryo (WOW (1/60 μl)), and WOW of 60 μl with three embryos (WOW (3/60 μl)) were simulated. The metabolite concentration of WOW (1/60 μl) and WOW (1/30 μl) were simulated in a center microwell. The mechanism of metabolite accumulation in WOW (d). Cumulative metabolites at 60,000 s in WOW with three embryos (i) consist of the embryo metabolites (ii) and metabolites from the right (iii) and left (iv) adjacent embryos
Therefore, these results indicate that metabolites in WOW are theoretically accumulated around embryos following two effects: (1) the microwell structure suppresses the diffusion of metabolites (microwell effect), and (2) metabolites derived from adjacent embryos further accumulate (group effect).
Discussion
In culture using human single media, the WOW system allowing group culture improved bovine embryonic development compared to the individual droplet culture system. Furthermore, no negative effects of WOW were observed based on the expression of imprinted genes. In contrast, the expression pattern was similar to that derived from in vivo analyses. Embryo culture assays (in vitro) and diffusion simulation of embryo secreted metabolites (in silico) demonstrated that WOW has both microwell and group-culture effects, and these two effects may participate to improve in vitro embryonic development.
In the present study, microwell culture system that facilitates group culture based on the WOW system had a positive effect on embryonic development and no adverse effects on the expression of imprinted genes in all single media evaluated. Preliminary human clinical outcome using the WOW system with human single media showed a delivery rate per transfer of 50.6% (39/77) under the age of 39 and no neonatal abnormalities. Improvement of embryonic development by the WOW culture system compared to the conventional droplet culture system with a flat bottom dish in various animal species has been reported [29]. However, to our knowledge, this is the first report showing the effectiveness of the WOW culture system using various human single culture media of different medium composition.
The increase in detrimental factors including ammonium in group culture is a known disadvantage. Ammonium from the breakdown of amino acids such as unstable l-glutamine and secretion from the embryos themselves gradually accumulates in the culture medium during embryo culture [15]. This affects pre-implantation embryonic development, fetal development, embryonic metabolism, and the expression of imprinted H19 [31, 32]. Therefore, particularly in the case of group culture, sequential medium requiring medium exchange is considered to be effective for reducing the ammonia concentration [7, 33]. However, to reduce the ammonium accumulation in embryo culture media, unstable glutamine has been replaced with a dipeptide form (e.g., alanyl-glutamine or glycyl-glutamine) in commercially available human single media [34]. Ammonium concentration and medium composition in all single media were not calculated, but the excellent compatibility of GT-L with WOW in terms of embryonic development and expression of imprinted genes may be attributed to reduced ammonium concentrations.
The WOW culture system has been shown to improve embryonic development compared with the conventional droplet culture system (one zygote per droplet); however, the microwell or group culture effect involved in embryonic development is unclear [35]. Here, we demonstrate that although the microwell effect can support blastocyst formation, the group effect is needed for improving subsequent cell proliferation and developmental kinetics such as expansion and hatching as well as morphological embryo quality. A previous mouse model revealed that group culture in WOW improved the expansion and hatching of blastocysts, which corresponds to the results obtained in our study [35]. Conversely, in our previous study, the small number of embryos per WOW dish did not affect blastocyst expansion, blastocyst cell numbers, or developmental kinetics [36, 37]. In these studies, the cultures contained five zygotes as a minimum number per group. It may be that a sufficient group culture effect was established in these conditions that improved blastocyst quality. Indeed, in this study, no differences were observed between four and eight zygotes per dish with regard to the developmental rates to the expanded hatching and code 1 blastocyst stages.
It is speculated that a suitable concentration of metabolites such as basic fibroblast growth factor (bFGF), transforming growth factor beta (TGF), insulin growth factor 1 (IGF), platelet activation factor (PAF), and ubiquitin provided by the microwell and group culture effect in the WOW concept culture system may improve embryonic development [18, 21, 38]. However, differences in these metabolite concentrations between WOW and conventional droplet culture have not been determined. This is because it is difficult to accurately measure local metabolites around the embryo with high sensitivity. To investigate the mechanism underlying the improved embryonic development by WOW, the assumed concentration of metabolites secreted from embryos were simulated by the finite volume method based on Fick’s second law of diffusion. In the present study, we demonstrated that the diffusion of metabolites was suppressed by the microwell structure and metabolites from adjacent embryos were supplied in the microwells. Therefore, improvement of in vitro embryonic development by WOW may be associated with the increase in local metabolites such as bFGF, TGFB, IGF1, PAF, and ubiquitin around the embryo in the microenvironment by both microwell and group-culture effects. Using a mathematical model, Matsuura et al. (2014) showed that higher concentrations of macromolecules such as growth factors were to be expected compared with the conventional droplet culture system [28]. Furthermore, we demonstrated using in vitro embryo culture assays (experiment 2) and diffusion simulation (experiment 3) that development to the blastocyst stage can be supported by the concentration of embryo metabolites. However, higher concentrations of those metabolites may be required for improving blastocyst quality with the help of adjacent embryos. The bovine embryo model has revealed that PAF improves the quality of blastocysts, such as cell number, in a dose-dependent manner [39]. Furthermore, high concentration of PAF in conditioned culture media may be considered as an indicator of embryo viability and for predicting pregnancy outcome [40].
Since the metabolites may include detrimental factors in addition to beneficial factors such as PAF, accumulation of these factors from adjacent low quality/incompetent embryo is feared. Wydooghe et al. showed that cleaved or arrested embryos do not hamper embryonic development in the embryo group culture with Primo Vision dish, which is similar to the WOW culture system [38]. Instead, it is necessary to determine how effectively the group effect is obtained when the number of viable zygotes is limited such as with natural cycle/minimal stimulation IVF.
In conclusion, further study with human zygotes will be required, but the combination of microwell culture systems that allows group culture such as the WOW concept system and human single culture media could be a powerful tool for improving human ART success.
Electronic supplementary material
Diffusion kinetics of metabolites from embryos cultured in droplet and LinKIDⓇ micro8 (LinKID). Cumulative metabolites in droplet with one embryo (left) and LinKID with three embryos (right) which color indicates the metabolites concentration. Minimum concentration, zero mol is colored with blue and maximum value, 3.0E−09 mol is colored with red on the right color scale (AVI 554 kb)
(DOCX 17 kb)
Acknowledgements
We thank Editage (https://www.editage.jp/) for editing the draft of this manuscript.
Compliance with ethical standards
This study has been approved by Minotomirai Yume Clinic ethics committee.
Contributor Information
Satoshi Sugimura, Phone: +81 (42) 367 5819, Email: satoshis@cc.tuat.ac.jp.
Hirotsune Kaijima, Phone: +81 (45) 228 3131, Email: h-kaijima@mm-yumeclinic.com.
References
- 1.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rebar RW. What are the risks of the assisted reproductive technologies (ART) and how can they be minimized? Reprod Med Biol. 2013;12:151–158. doi: 10.1007/s12522-013-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9:557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- 4.Gardner DK, Lane M. Culture of viable human blastocysts in defined sequential serum-free media. Hum Reprod. 1998;13(Suppl 3):148–159. doi: 10.1093/humrep/13.suppl_3.148. [DOI] [PubMed] [Google Scholar]
- 5.Biggers JD, Summers MC. Choosing a culture medium: making informed choices. Fertil Steril. 2008;90:473–483. doi: 10.1016/j.fertnstert.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 7.Lane M, Gardner DK. Increase in postimplantation development of cultured mouse embryos by amino acids and induction of fetal retardation and exencephaly by ammonium ions. J Reprod Fertil. 1994;102:305–312. doi: 10.1530/jrf.0.1020305. [DOI] [PubMed] [Google Scholar]
- 8.Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod. 2017;32:1957–1973. doi: 10.1093/humrep/dex264. [DOI] [PubMed] [Google Scholar]
- 9.Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on day 3. Hum Reprod. 2016;31:2231–2244. doi: 10.1093/humrep/dew188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update. 2015;21:727–747. doi: 10.1093/humupd/dmu064. [DOI] [PubMed] [Google Scholar]
- 11.Smith GD, Takayama S, Swain JE. Rethinking in vitro embryo culture: new developments in culture platforms and potential to improve assisted reproductive technologies. Biol Reprod. 2012;86:62. doi: 10.1095/biolreprod.111.095778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Doherty EM, Wade MG, Hill JL, Boland MP. Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology. 1997;48:161–169. doi: 10.1016/S0093-691X(97)00199-4. [DOI] [PubMed] [Google Scholar]
- 13.Larson MA, Kubisch HM. The effects of group size on development and interferon-tau secretion by in-vitro fertilized and cultured bovine blastocysts. Hum Reprod. 1999;14:2075–2079. doi: 10.1093/humrep/14.8.2075. [DOI] [PubMed] [Google Scholar]
- 14.Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000;54:741–756. doi: 10.1016/S0093-691X(00)00387-3. [DOI] [PubMed] [Google Scholar]
- 15.Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 1993;48:377–385. doi: 10.1095/biolreprod48.2.377. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- 17.Fukui Y, Lee ES, Araki N. Effect of medium renewal during culture in two different culture systems on development to blastocysts from in vitro produced early bovine embryos. J Anim Sci. 1996;74:2752–2758. doi: 10.2527/1996.74112752x. [DOI] [PubMed] [Google Scholar]
- 18.Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, Callesen H. New method for culture of zona-included or zona-free embryos: the well of the well (WOW) system. Mol Reprod Dev. 2000;55:256–264. doi: 10.1002/(SICI)1098-2795(200003)55:3<256::AID-MRD3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoelker M, Rings F, Lund Q, Phatsara C, Schellander K, Tesfaye D. Effect of embryo density on in vitro developmental characteristics of bovine preimplantative embryos with respect to micro and macroenvironments. Reprod Domest Anim. 2010;45:e138–e145. doi: 10.1111/j.1439-0531.2009.01535.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoelker M, Rings F, Lund Q, Ghanem N, Phatsara C, Griese J, Schellander K, Tesfaye D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction. 2009;137:415–425. doi: 10.1530/REP-08-0370. [DOI] [PubMed] [Google Scholar]
- 21.Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M, Hattori H, Kobayashi S, Hashiyada Y, Konishi K, Imai K. Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod. 2010;83:970–978. doi: 10.1095/biolreprod.110.085522. [DOI] [PubMed] [Google Scholar]
- 22.Sugimura S, Akai T, Imai K. Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes. J Reprod Dev. 2017;63:353–357. doi: 10.1262/jrd.2017-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menezo YJ, Herubel F. Mouse and bovine models for human IVF. Reprod BioMed Online. 2002;4:170–175. doi: 10.1016/S1472-6483(10)61936-0. [DOI] [PubMed] [Google Scholar]
- 24.Sugimura S, Akai T, Hashiyada Y, Somfai T, Inaba Y, Hirayama M, Yamanouchi T, Matsuda H, Kobayashi S, Aikawa Y, Ohtake M, Kobayashi E, Konishi K, Imai K. Promising system for selecting healthy in vitro-fertilized embryos in cattle. PLoS One. 2012;7:e36627. doi: 10.1371/journal.pone.0036627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod. 1975;12:260–274. doi: 10.1095/biolreprod12.2.260. [DOI] [PubMed] [Google Scholar]
- 26.Stringfellow DA, Givens SM. Manual of the International Embryo Transfer Society, 4th ed. International Embryo Transfer 2010.
- 27.Hashiyada Y, Okada M, Imai K. Transition of the pregnancy rate of bisected bovine embryos after co-transfer with trophoblastic vesicles prepared from in vivo-cultured in vitro-fertilized embryos. J Reprod Dev. 2005;51:749–756. doi: 10.1262/jrd.17032. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura K. Numerical calculations for diffusion effects in the well-of-the-well culture system for mammalian embryos. Reprod Fertil Dev. 2014;26:742–751. doi: 10.1071/RD13025. [DOI] [PubMed] [Google Scholar]
- 29.Swain JE, Smith GD. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update. 2011;17:541–557. doi: 10.1093/humupd/dmr006. [DOI] [PubMed] [Google Scholar]
- 30.Dominguez F, Gadea B, Esteban FJ, Horcajadas JA, Pellicer A, Simon C. Comparative protein-profile analysis of implanted versus non-implanted human blastocysts. Hum Reprod. 2008;23:1993–2000. doi: 10.1093/humrep/den205. [DOI] [PubMed] [Google Scholar]
- 31.Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod. 2003;69:1109–1117. doi: 10.1095/biolreprod.103.018093. [DOI] [PubMed] [Google Scholar]
- 32.Gardner DK, Hamilton R, McCallie B, Schoolcraft WB, Katz-Jaffe MG. Human and mouse embryonic development, metabolism and gene expression are altered by an ammonium gradient in vitro. Reproduction. 2013;146:49–61. doi: 10.1530/REP-12-0348. [DOI] [PubMed] [Google Scholar]
- 33.Virant-Klun I, Tomazevic T, Vrtacnik-Bokal E, Vogler A, Krsnik M, Meden-Vrtovec H. Increased ammonium in culture medium reduces the development of human embryos to the blastocyst stage. Fertil Steril. 2006;85:526–528. doi: 10.1016/j.fertnstert.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Kleijkers SH, van Montfoort AP, Bekers O, Coonen E, Derhaag JG, Evers JL, Dumoulin JC. Ammonium accumulation in commercially available embryo culture media and protein supplements during storage at 2–8 degrees C and during incubation at 37 degrees C. Hum Reprod. 2016;31:1192–1199. doi: 10.1093/humrep/dew059. [DOI] [PubMed] [Google Scholar]
- 35.Vajta G, Korosi T, Du Y, Nakata K, Ieda S, Kuwayama M, Nagy ZP. The well-of-the-well system: an efficient approach to improve embryo development. Reprod BioMed Online. 2008;17:73–81. doi: 10.1016/S1472-6483(10)60296-9. [DOI] [PubMed] [Google Scholar]
- 36.Sugimura S, Akai T, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Kobayashi E, Konishi K, Imai K. Effect of embryo density on in vitro development and gene expression in bovine in vitro-fertilized embryos cultured in a microwell system. J Reprod Dev. 2013;59:115–122. doi: 10.1262/jrd.2012-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SS, Ofuji S, Imai K, Huang W, Koyama K, Yanagawa Y, Takahashi Y, Nagano M. The efficacy of the well of the well (WOW) culture system on development of bovine embryos in a small group and the effect of number of adjacent embryos on their development. Zygote. 2015;23:412–415. doi: 10.1017/S096719941400001X. [DOI] [PubMed] [Google Scholar]
- 38.Wydooghe E, Vandaele L, Piepers S, Dewulf J, Van den Abbeel E, De Sutter P, Van Soom A. Individual commitment to a group effect: strengths and weaknesses of bovine embryo group culture. Reproduction. 2014;148:519–529. doi: 10.1530/REP-14-0213. [DOI] [PubMed] [Google Scholar]
- 39.Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction. 2006;131:269–277. doi: 10.1530/rep.1.00677. [DOI] [PubMed] [Google Scholar]
- 40.Roudebush WE, Wininger JD, Jones AE, Wright G, Toledo AA, Kort HI, Massey JB, Shapiro DB. Embryonic platelet-activating factor: an indicator of embryo viability. Hum Reprod. 2002;17:1306–1310. doi: 10.1093/humrep/17.5.1306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diffusion kinetics of metabolites from embryos cultured in droplet and LinKIDⓇ micro8 (LinKID). Cumulative metabolites in droplet with one embryo (left) and LinKID with three embryos (right) which color indicates the metabolites concentration. Minimum concentration, zero mol is colored with blue and maximum value, 3.0E−09 mol is colored with red on the right color scale (AVI 554 kb)
(DOCX 17 kb)