Abstract
Purpose
The study aims to determine differences in micro-RNA (miRNA) expression in granulosa (GC) and cumulus cells (CC) between young women with diminished ovarian reserve (DOR) or normal ovarian reserve (NOR). Secondary objective was to identify downstream signaling pathways that could ultimately indicate causes of lower developmental competence of oocytes from young women with DOR.
Methods
The method of the study is prospective cohort study.
Results
Of the miRNA, 125 are differentially expressed in GC between DOR and NOR. Only nine miRNA were different in CC; therefore, we focused analysis on GC. In DOR GC, miR-100-5p, miR-16-5p, miR-30a-3p, and miR-193a-3p were significantly downregulated, while miR-155-5p, miR-192-5p, miR-128-3p, miR-486-5p, miR130a-3p, miR-92a-3p, miR-17-3p, miR-221-3p, and miR-175p were increased. This pattern predicted higher cell proliferation in the DOR GC. The primary pathways include MAPK, Wnt, and TGFbeta.
Conclusions
The miRNA pattern identified critical functions in cell proliferation and survival associated with DOR. GC in women with DOR seems to respond differently to the LH surge.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1239-9) contains supplementary material, which is available to authorized users.
Keywords: Micro-RNA, Granulosa cells, Cumulus cells, Diminished ovarian reserve
Introduction
Folliculogenesis, the process through which primordial follicles develop into mature antral follicles, hinges on a complex communication between the oocyte with its neighboring granulosa cells. The cumulus granulosa cells (CC) envelop the oocyte and have intimate communication across the zona pellucida with the oocyte, while the mural granulosa cells (GC) are separated from the oocyte by the CC and the fluid-filled antral cavity [1, 2]. Therefore, even though cumulus and mural granulosa begin in early development as a single-cell type, during folliculogenesis, they are separated physically and differentiate into two distinct cell types, each with their own gene expression profiles [3].
Micro-RNA (miRNA) have been recently identified as potent regulators of gene expression that act through posttranscriptional modification of messenger RNA (mRNA) to fine-tune the production of proteins involved in cell signaling pathways [4]. Micro-RNAs are a class of endogenous, non-coding, small single-stranded RNA of approximately 18 to 25 nucleotides that are highly conserved across species. It is predicted that humans express thousands of miRNA (~2588 known human miRNA in miRBase [5]). Micro-RNA are transcribed in the nucleus as long single-stranded transcripts referred to as primary miRNA which fold back upon themselves to form complex 2-D structures. These pre-miRNAs are then processed by an endonuclease complex to yield short 70–100 base pair, pre-miRNA that are exported into the cytoplasm where they are further processed by another endonuclease complex to yield mature miRNA. The mature miRNA is associated with a complex of proteins called the RNA-induced silencing complex (RISC) [6]. The RISC binds to target transcripts, which are homologous with the miRNA sequence, to block protein production either by repressing translation or by causing transcript degradation [7]. A single miRNA can regulate multiple mRNA transcripts that have related functions or that are expressed in the same cell. Therefore, modulating a single miRNA could change a whole phenotype [8].
Micro-RNA have critical roles in many cellular processes and diseases including cell proliferation, apoptosis, cardiovascular and autoimmune diseases, and in a variety of cancers [9, 10]. Within the field of reproduction, there is increasing literature demonstrating that miRNA regulates biologically important pathways of follicle growth and oocyte health [11, 12]. As the ovarian follicle grows, the antral cavity is formed by the secretion of fluid from the surrounding cells. This follicular fluid is rich in nutrients as well as molecular signaling molecules, which includes miRNA, enabling communication and nutritional support between the mural granulosa cells, cumulus cells, and the growing oocyte [12]. Numerous studies have examined the roles of miRNA in ovarian biology, and the main focus of most of these studies has been the granulosa cells [11, 13–19].
Research comparing miRNA expression profiles in human granulosa cells, cumulus cells, follicle fluid, and oocytes under varying conditions such as age or disease have found that miRNA expression profiles vary in association with these changes in state. For instance, 37 miRNA have been found upregulated in human antral follicle fluid (FF) compared to serum [20]. Another study showed four miRNAs, which were differentially expressed in the FF in young (< 31 years) compared to older (> 38 years) patients undergoing assisted reproduction [21]. Micro-RNA expression has been profiled in follicular fluid of women with polycystic ovarian syndrome (PCOS), and the miRNA expression pattern was found to be associated with the regulation of steroidogenesis [22, 23]. Oocytes from women with PCOS may have reduced developmental competence, and their miRNA expression profiles distinguish them from oocytes of non-PCOS patients [24–26]. Once identified, the differences in miRNA expression can be analyzed using biological functional databases to determine the biological pathways known or predicted to be associated with these specific miRNA expression profiles (expression increased or decreased). Micro-RNA expression profiles thus have the potential to enable the determination of both the underlying causes of ovarian failure as well as guiding future potential treatment options to improve reproduction for women suffering from infertility.
One such group of women are young women with diminished ovarian reserve (DOR) from unknown causes [27]. Oocytes of young women with DOR have reduced developmental competence compared to young women with normal ovarian reserve (NOR), but the cause of this lower oocyte quality is unknown [28]. Since communication between the CC and GC with the developing oocyte is critical, and CC/GC creates the follicular environment in which the oocyte matures [29], differences in gene expression in the CC and GC may be indicative of the causes of reduced oocyte quality.
We hypothesized that CC and GC would have distinct miRNA profiles between DOR and NOR. We further hypothesized that because the CC are in direct contact with and have access to the interior environment of the oocyte, that there might be a greater difference in miRNA expression in CC than in the mural granulosa cells. Our primary objective is to determine differences in miRNA expression in CC and GC between young women with DOR or NOR by using next-generation sequencing. Our secondary objective was to determine the potential downstream mRNA targets of the identified miRNA as well as the signaling pathways that may be associated with these targets. These downstream mRNA targets may ultimately be implicated with the lower developmental competence of oocytes from women with DOR.
Materials and methods
Patient population
For this study, we recruited Caucasian women ≤ 37 years of age with NOR (n = 7) and idiopathic DOR (n = 8) undergoing ovarian stimulation for in vitro fertilization at an academic fertility practice between 2015 and 2016. Six patient samples were used for the miRNAseq, and a separate set of nine samples were used for the quantitative-polymerase chain reaction (qPCR) to validate the miRNAseq study. DOR was defined using a modification of the Bologna criteria [30]. Patients with two or more of the following were classified as DOR: (1) anti-Mullerian hormone (AMH) < 2 ng/mL, follicle-stimulating hormone (FSH) > 10 IU/L, antral follicle county (AFC) < 7 ng/mL, and previous cycle with less < 6 oocytes collected. The AMH was assayed as a standard part of each patient’s infertility work-up using the Ansh Labs AMH ELISA. All patients received 10,000 IU hCG to induce ovulation (trigger). Patients underwent oocyte retrieval at 34–36 h after trigger. The patient population was limited to Caucasians to reduce population-based genetic differences that could confound NOR/DOR effects in this small number of patient samples. To reduce the likelihood of health-related confounding factors that might affect miRNA gene expression, we excluded all patients with diabetes, obesity, history of iatrogenic causes of DOR (i.e., radiation, chemotherapy), endometriosis, and/or PCOS. Statistical analyses were conducted using Stata 14.2 (Stata Corp., College Station, TX). Patient and cycle characteristics were evaluated using independent t test.
This study was approved by the Institutional Review Board (HS-15-00756 and HS-15-00859).
Granulosa cells, cumulus cells, and follicular fluid collection
At the time of oocyte retrieval, aspirate from the first punctured follicle was collected for use in this study. Surgeons were not blinded to patient group; however, they were instructed to aspirate the largest follicle first on each side. In our clinic, the dominant follicle would be approximately 16–20 mm. Any sample that contained visible blood contamination was discarded. The embryologist searched the FF and removed the CC-enclosed oocyte, then manually trimmed a piece of the CC mass from oocyte, and transferred the CC into a cryotube. The oocyte was transferred to the fertilization dish and continued through standard embryology lab procedures for fertilization and culture. GC were isolated from fresh FF by centrifugation at 1000g for 30 min. All GC, CC, and FF samples were immediately frozen on dry ice and stored at – 80 °C.
Total RNA collection
To disrupt the cells and preserve RNA, 500 μL Qiazol was added to the frozen GC and CC samples prior to thaw. Micro-RNAs were extracted from CC and GC using microRNeasy Kit (Qiagen Inc., Germantown, MD) according to manufacturer’s instructions. The integrity of the RNA was assessed with a picochip on the Agilent Bioanalyzer.
Library preparation and miRNA deep sequencing
The global miRNA profiles of DOR-granulosa, DOR-cumulus, NOR-granulosa, and NOR-cumulus were interrogated using Illumina’s small RNA sequencing technology (TruSeq Small RNA Sample Prep Kit). The experiments consisted of 12 samples collected from six patients, three with DOR and three in the NOR group. Both granulosa and cumulus cells were isolated from each patient. The small RNA-seq samples were sequenced at a 50-cycle single end read resolution in a HiSeq2500 sequencing system (Illumina). This generated around 12.3 and 16.8 million reads per sample. The samples were first checked for read quality using the FastQC software [31]. Adaptors were then removed and the resulting reads were mapped to the human genome (GRCh38.rel77) using the bwa software [32]. Between 8.7 and 13.8 million reads per sample mapped to the genome.
The mapped reads were further processed as follows. The reads from all 12 samples were merged and scanned for high-density regions defined as a contiguous region whose read count at each base is not less than 20% of the highest base read count for the locus (a continuous stretch of mapped reads). These high-density regions formed the effective region of the locus and its length was its effective length. Loci with an effective length greater than or equal to 17 (shortest length of a miRNA) were retained. The number of reads mapped to the effective region in each sample formed the effective read counts. Loci were further filtered on their normalized effective read counts (normalized to the number of counts per million reads (CPM)), retaining only those loci with a CPM greater than or equal to 10 in all three replicate samples in at least one of the groups being compared. These loci were used for downstream analysis. The effective regions were annotated for genomic features with the Ensemble gene annotation file for human (GRCh38.rel77) and miRBase (release 21; http://www.mirbase.org).
A total of 264 known and novel miRNAs were identified from these loci through a systematic filtering process beginning with a length filter that filtered loci to those with an effective length between 17 and 30 bases. Effective regions that mapped to annotated human mature miRNA were first identified. The remaining effective regions were compared to known miRNA from both human and other species found in miRBase (release 21). A region was labeled as a miRNA by homology if it passed the following criteria; a gapless alignment of the effective region to the mature reference miRNA with at most two mismatches in the core and at most one gap/mismatch at the five and three prime ends and less than 10% mismatches in the alignment of the reference hairpin sequence to the locus region in the genome. This resulted in further two miRNAs. The remaining 45 miRNAs were identified as novel computationally validated miRNA. The validation is based on the criteria that the extended effective region should have a predicted pre-miRNA-like hairpin structure [33] with the effective region falling in the stem region with at least 80% pairing. In other words, to be classified as a novel miRNA, the predicted structure must match the definition of a miRNA.
Generalized linear models (GLM) developed for multi-group experiments available from the edgeR software package [34] were used to determine significantly differently expressed miRNA between the different conditions. The edgeR package employs advance empirical Bayes methods to estimate miRNA-specific biological variation under minimal levels of biological replication. The RNA composition in each sample was normalized in edgeR using the trimmed mean of M value (TMM) method. The associated p values were corrected for multiple-hypothesis testing by the Benjamini and Hochberg method [35]. All results from miRNAseq will be deposited into the National Center for Biotechnology Information-Gene Expression Omnibus (NCBI-GEO).
miRNA target gene prediction and functional annotation
Interaction between differentially expressed miRNA and their target mRNA was predicted using Ingenuity Pathway Analysis software (Qiagen Inc.; https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis). Only the highly stringent experimentally observed evidence in IPA were used for identifying mRNA targets. miRNA associated with cellular proliferation was specifically evaluated between NOR and DOR groups and further analysis of target mRNA function.
Validation of candidate miRNA by qPCR
Four differentially expressed candidate miRNA identified by NGS (miR-17-3p, miR-17-5p, miR-92a-3p, and miR-486-5p) along with U6 control were selected for qPCR validation. The relative abundance of these candidate miRNA was assessed in the granulosa cells of nine patients (four in the NOR group and five in the DOR group—meeting all previous eligibility criteria). PCR was completed on three replicates of each patient. First-strand cDNA was synthesized from equal amounts of total RNA input using miRCURY LNA Universal Reverse Transcriptase microRNA PCR Kit (Exiqon Inc., Woburn, MA, USA) according to the manufacturer’s instruction. PCR primers were also purchased from Exiqon: hsa-miR-17-3p (#206008), hsa-17-5p (#204771), hsa-92a-3p (#204258), and hsa-486-3p (#204001).
Quantitative PCR was conducted by the Institutional Genomics Core Laboratory using a QuantStudio 7 Flex Real-Time PCR system (Applied Biosystems Inc., Forest City, CA, USA). Triplicate Ct readings were averaged and delta Ct values were calculated for each sample as the difference between Ct values for the miRNA and the U6 spike-in control. Mean delta Ct values were compared between DOR and NOR samples using Student’s t test. Relative miRNA expression (DOR versus NOR) was calculated as difference in delta Ct values. Statistical analyses were carried out using Stata 14.2 (Stata Corp., College Station, TX).
Results
Patient demographics are presented in Table 1. In comparing DOR with NOR patients, DOR patients had significantly lower AMH (0.9 versus 6.0 ng/mL, p = 0.02), higher FSH (10 versus 5 IU/L, p = 0.05, lower AFC (11 versus 22, p = 0.01), fewer oocytes collected (9 versus 16, p = 0.02), and fewer mature oocytes (8 versus 14, p = 0.02). There was no significant difference in the percentage of oocytes that had matured to metaphase-II by the time of oocyte retrieval (87 versus 90%, p = 0.42). Estradiol levels were numerically lower in DOR patients but this was not statistically significant (1948 versus 3037 pg/mL, p = 0.11).
Table 1.
Patient demographics
| Group | Age | BMI | AMH | FSH | AFC | Max E2 | Number of oocytes collected | Number of mature oocytes | Percent of mature |
|---|---|---|---|---|---|---|---|---|---|
| NOR | 32 (20–37) | 24.0 (19–30) | 6.0 (3.7–14) | 5 (3–8) | 22 (9–31) | 3037 (1199–5848) | 16 (7–24) | 14 (7–19) | 90 (69–100) |
| DOR | 35 (33–37) | 22.7 (19–26) | 0.9 (0.3–1.8) | 10 (4–19) | 11 (7–18) | 1948 (777–2708) | 9 (2–16) | 8 (2–16) | 87 (50–100) |
| p | 0.48 | 0.43 | 0.02* | 0.05* | 0.01* | 0.11 | 0.02* | 0.02* | 0.42 |
Range of values = ()
age years, BMI body mass index, AMH anti-Mullerian hormone, FSH follicle-stimulating hormone, AFC antral follicle count, max E2 maximum levels of estradiol detected prior to trigger
*p ≤ 0.05 is significant
The miRNA detected accounted for approximately 10% of the total small RNAs which included long non-coding RNA (lincRNA), small nucleolar RNA (snoRNA), small nuclear RNA (snRNA), ribosomal RNA (rRNA), and ribosomal and transfer RNA from mitochondria (mt_rRNA and mt_tRNA, respectively) (Table 2). We identified 234 loci that map to an annotated pre-miRNA (stem-loop) region of which 217 map to the annotated mature miRNA region. Within this group, two miRNAs were novel having not been detected in humans, but had homology with miRNA previously reported in canines and rodents, cfa-miR-1843 and rno-miR-1839-5p, respectively.
Table 2.
Distribution of small RNA detected by next-generation sequencing
| Feature | Count |
|---|---|
| lincRNA | 262 |
| snoRNA | 226 |
| miRNA | 234 |
| snRNA | 56 |
| rRNA | 86 |
| Mt_rRNA | 19 |
| Mt_tRNA | 12 |
Detection of small RNA with normalized read counts per million mapped reads (CPM) ≥ 10 in all three replicate samples in any one of the treatment-cell groups
The majority of the detected miRNA were equally expressed in both GC and CC. In the GC, 125 miRNAs are differentially expressed between DOR and NOR groups (with a false discovery rate (FDR) q < 0.05 and at least a 2-fold change). Of these, 20 mature miRNAs were significantly enriched in NOR granulosa cells (Table S1), while 85 miRNAs were higher in DOR granulosa cells (Table S2). Micro-RNA expressions in CC of NOR and DOR patients were very similar to each other, with only nine miRNAs that were significantly increased in DOR, while none were decreased in CC (Table S3).
Since the greatest distinction between DOR and NOR cells was in the GC, we focused on this group for further analysis. Ingenuity Pathway Analysis on predicted target genes of the 85 differentially expressed miRNAs was once known or predicted to regulate cellular development, growth, and proliferation. A group of 13 miRNAs that were upregulated or downregulated in DOR granulosa cells are associated specifically with cellular proliferation (Table S4). Of these 13, nine genes were higher and four were lower in DOR GC (Fig. 1, green and red, respectively). The pattern of expression of these genes would predict higher cell proliferation in the GC of DOR patients. Table 3 lists the known genes associated with cell proliferation that are regulated by these 13 miRNAs. These genes are primarily associated with MAPK, Wnt, and TGFbeta signaling pathways, which are critical for proliferation and cell survival.
Fig. 1.
Ingenuity Pathway Analysis of differentially expressed miRNA. The analysis identified 13 miRNAs that regulate cell proliferation and cell survival and were significantly different between NOR and DOR granulosa cells. Micro-RNA were either significantly lower (green) or higher (red) in NOR granulosa cells as compared to DOR (corresponding to Table 3). Lines with arrows indicate miRNA that lead to increased cell proliferation. Lines with blunt ends indicate miRNA that inhibit cell proliferation
Table 3.
miRNA and their target genes associated with cell proliferation
| miR-16-5p | miR-17-5p | miR-17-3p | miR-30a-3p | miR-92a-3p | miR-100-5p | miR-128-3p |
|---|---|---|---|---|---|---|
| ABCF2 | APP | CASP3 | ANKFY1 | AGO2 | AFP | ADORA2B |
| ABHD10 | ARID4B | CASP9 | AQP4 | BCL2L11 | AGO2 | AFF1 |
| ACP2 | BAMBI | ARMC8 | BMPR2 | CCND1 | AGO1 | |
| ACTR1A | BCL2 | CC2D1B | CCND1 | CCND3 | BMI1 | |
| ADSS | BCL2L11 | CDK6 | CCND2 | CD209 | DBI | |
| AGO2 | BIRC5 | CYR61 | CCNE1 | Cyclin E | DCX | |
| ANAPC16 | BMP2 | FMR1 | CCNE2 | EGR2 | E2F3 | |
| ANLN | BMPR2 | HDAC2 | CDK7 | EIF4EBP1 | KMT2A | |
| ARHGDIA | BNIP2 | HELZ | CDKN1A | FGF16 | LDLR | |
| ARL2 | CAMTA1 | KIF1B | CDKN1C | FGFR3 | NTRK3 | |
| ASXL2 | CCND1 | LIMA1 | ENPP6 | ID1 | RELN | |
| ATF6 | CCND2 | MCCC2 | FBXW7 | IGF1R | SNAP25 | |
| ATG9A | CCNE1 | PIK3C2A | HIPK3 | IGF2 | TGFBR1 | |
| BCL2 | CDK7 | IKZF1 | MMP13 | TXNIP | ||
| BCL2L2 | CDKN1A | IL6 | MTOR | |||
| miR-130a-3p | miR-155-5p | miR-192-5p | miR-193a-3p | miR-221-3p | miR-486-5p | |
| AGO2 | ABHD16A | BIRC5 | CCND1 | AGO2 | ADARB1 | |
| AQP4 | AGTR1 | DHFR | E2F6 | APAF1 | AFF3 | |
| ATG2B | AICDA | DTL | ESR1 | BBC3 | ANKRD12 | |
| CSF1 | AMIGO2 | IGF1 | ETS1 | BCL2L11 | ARID4B | |
| DICER1 | ANKFY1 | IGF1R | MCL1 | BMF | CREBL2 | |
| HOXA5 | ARFIP1 | TYMS | MIR31HG | BNIP3L | DCBLD2 | |
| MAFB | ARFIP2 | ZEB1 | PLAU | CDKN1B | EMP1 | |
| MEOX2 | ARID2 | ZEB2 | PTK2 | CDKN1C | FOXO1 | |
| SMAD4 | ARL10 | SRSF2 | DDIT4 | FOXP1 | ||
| TAC1 | ARL5B | DIRAS3 | GABRE | |||
| ZEB1 | ATG3 | ESR1 | PIK3AP1 | |||
| ZFPM2 | ATP6V1C1 | FOS | PTEN | |||
| BACH1 | FOXO3 | RFFL | ||||
| BET1 | GAS5 | SLC10A7 | ||||
| BRPF3 | ICAM1 | |||||
Target genes predicted by Ingenuity Pathway Analysis
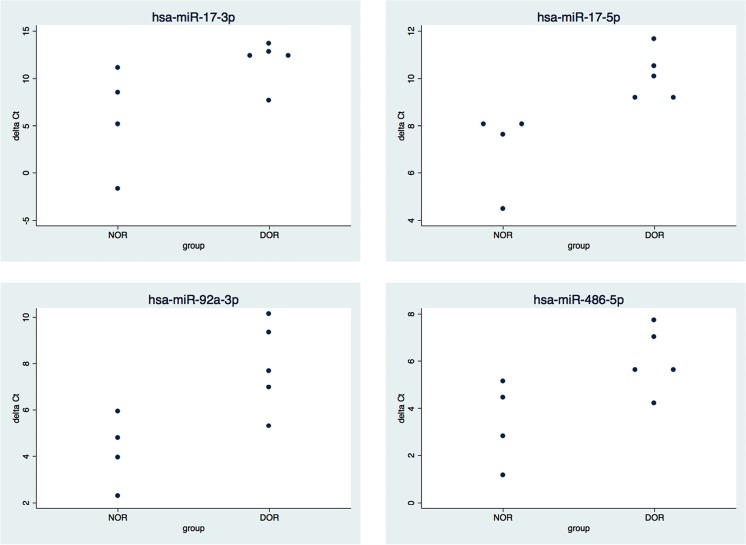
To validate the results of the miRNAseq, we selected four of the miRNAs that were upregulated in DOR GC and associated with cell proliferation (miR-17-5p, miR-17-3p, miR-92a-3p, and miR-486-5p) and measured miRNA expression levels by qPCR, using nine patient samples that were independent from those used for the miRNAseq. The results demonstrate the sensitivity of these assays. Table 4 shows the results of the qPCR (delta Ct), and Fig. 2 shows the distribution of average delta Ct results for each patient. These results agree with the miRNAseq and confirmed a significant increase of three (miR-17-5p, miR-92a-3p, miR-486-5p) of the four miRNAs in granulosa cells of DOR patients. Micro-RNA-17-3p also measured 2-fold higher in DOR samples; however, due to a high standard deviation for this miRNA, this value did not reach statistical significance (p = 0.06).
Table 4.
qPCR confirms the gene expression differences between DOR and NOR granulosa cells
| miRNA | NOR | DOR | p value |
|---|---|---|---|
| Delta Ct | Delta Ct | ||
| miR-17-5p | 7.1 ± 1.7 | 10.1 ± 1.0 | 0.01* |
| miR-17-3p | 5.8 ± 5.5 | 11.8 ± 2.3 | 0.06 |
| miR-92a-3p | 4.2 ± 1.5 | 7.9 ± 1.9 | 0.02* |
| miR-486-5p | 3.4 ± 1.8 | 6.1 ± 1.4 | 0.04* |
Values are means ± SD
*p ≤ 0.05 is significant
Fig. 2.
qPCR results confirmed the differential expression of miRNA in granulosa cells. To validate the miRNAseq results, four miRNAs were selected for further testing by qPCR. Each dot represents the average delta Ct value for each patient sample (n = 3 replicates). Three miRNA (miR-17-5p, miR-92a-3p, and miR-486-5p) were significantly higher in DOR versus NOR GC. Micro-RNA-17-3p was 2-fold higher in DOR GC, although this value did not reach significance (p = 0.06)
Discussion
In this study, we have quantitated miRNA expression in GC and CC of follicles from young women (≤ 37 years) undergoing ovarian stimulation and egg retrieval for assisted reproduction. We compared the expression profiles between women with normal (NOR) versus diminished (DOR) ovarian reserve to determine if there are differences in gene expression patterns in these cells. Granulosa and cumulus cells are in close communication with the oocyte, and the health of each is dependent on the others. Previous studies have shown a significantly lower developmental potential for oocytes from women with DOR but the cause is unknown. We theorized that gene expression in GC and/or CC would correlate with physiological differences within the antral follicle environment and thus provide the first insights into the causes of lower developmental potential in women with DOR. By understanding the physiology of the DOR follicle, treatments may be developed that would improve egg quality and increase pregnancy rates for these patients.
We chose to use miRNA profiling because a small number of miRNA can regulate the expression of multiple genes at once; thus, a small change in the miRNA pattern can have a large effect on cell function. Using next-generation sequencing, we identified 105 miRNAs that were differentially expressed in the granulosa with 85 increased (Table S2) and 20 decreased (Table S1) in DOR versus NOR, respectively. Interestingly, only nine miRNAs were differentially expressed in cumulus cells with all of them higher in young women with DOR (Table S3) compared to NOR and none were lower in DOR cumulus. This was surprising since it is the cumulus cells that have direct contact and closest communication with the developing oocyte and we had theorized that they would have the higher chance of influencing oocyte quality.
Since there were few changes in the miRNA expressed in cumulus cells, we focused on the granulosa cells for more in-depth analysis. We used Ingenuity Pathway Analysis software to determine genes that were targets for the miRNA that were differentially expressed in granulosa cells. The overall combined pattern of expression was consistent with gene regulation relating to cellular development, proliferation, and survival. This pattern indicated a higher rate of cell proliferation in granulosa cells of DOR patients.
The GCs obtained from ovulatory follicles during oocyte collection for assisted reproductive technology (ART) procedures have been exposed in vivo to the HCG trigger. Therefore, it is expected that the granulosa cells would have begun differentiation, luteinization, and reduced cell proliferation [36]. In vitro data have suggested that the pre-ovulatory rise in progesterone directly inhibits GC mitosis, limiting GC proliferation as these cells gain LH receptors. Therefore, the pattern of miRNA expression that we found in NOR GC is consistent with known biological function in normal follicle development. However, in the GC from the DOR group, the pattern of miRNA expression does not indicate a similar reduction in cell proliferation, suggesting that they do not respond to the LH surge as would be expected.
To validate the findings of our miRNAseq, we selected miR-17-3p, miR-17-5p, miR-92a-3p, and miR-486-5p, all of which were more than 7-fold higher in DOR versus NOR granulosa cells. Micro-RNAs 17, 18a, 19a, 19b, 20a, and 92a are members of a miRNA gene cluster, genes located closely together on the same chromosome and often transcribed together as a single 800-bp-long transcript that is later processed into six individual miRNAs. Four of this cluster were significantly increased in DOR granulosa (miR-17-3p, miR-17-5p, miR-18a-3p, and miR-92a-3p) [37]. Interestingly, this same cluster was recently reported as increased in DOR cumulus cells [38]. In our study, we also found them increased in cumulus, but the increase was not significant (data not shown). This difference between studies may be due to differences in the specifics of the bioinformatics analysis. The miRNAs in this cluster are expressed in almost all tissues but levels of expression vary depending on cell type or stage of development. High levels of this cluster are associated with high rates of cell proliferation and low cell death [27], which fits the profile of granulosa cells in the rapidly growing antral follicle [38]. After the LH surge, we would predict a decrease in the levels of these miRNAs, which agrees with the lower levels that we detected in the GC of NOR patients. Interestingly, miR-92a has been reported to target smad7, causing its degradation and protecting granulosa cells from apoptosis and cell death [39]. Micro-RNA-486-5p is also of special interest for our study because miR-486-5p has been shown to target and degrade PTEN. Loss or reduction of PTEN is associated with increased granulosa cell survival [40]. A decrease in miR-486-5p in cumulus cells of PCOS patients is associated with subfertility and abnormal follicle development [41].
Additional correlations with known miRNA functions
Overall, the miRNA patterns detected in our study agree with known functions in cell proliferation and survival. In NOR granulosa cells, miR-100-5p, miR-16-5p, miR-30a-3p, and miR-193a-3p were upregulated. These four miRNAs target 265 downstream mRNAs including FGFR3 and IGF1R. One of the target genes of miR-100-5p, upregulated in the GCs in the NOR group, is FGFR3, fibroblast growth factor receptor 3. FGFR3 is one of the four FGF receptors identified; its signals are transmitted via cell-surface tyrosine kinase receptors. FGF ligand has been shown to promote development of rat primordial follicles in vitro [42, 43]. Basic FGF and its receptors are localized in both oocytes and granulosa cells in humans [44]. Therefore, GCs of NOR patients are more likely than GCs of DOR patients to check the development of primordial follicles. This has an interesting implication for the low number of growing follicles in DOR patients and implies a negative feedback loop preventing the recruitment of follicles into the growing pool. Further studies are needed to determine if this is indeed the case, and if true, then could this be disrupted to allow for more follicle growth for these patients.
Another target of miR-100-5p and miR-16-5p is IGF1R, insulin-like growth factor 1—this receptor expression in ovarian granulosa cell is also essential for granulosa cell proliferation [45]. Granulosa cells of women with NOR are significantly enriched with miRNA that targets and inhibits IGF1R expression and therefore limits steroidogenesis in the developing oocyte. Another study reported that IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells from pre-antral to pre-ovulatory granulosa cell [46]. Therefore, NOR GCs may have the ability to maintain tight control of this progression.
Micro-RNA-16-5p targets WNT3a. Studies have shown concomitant activation of FSH and WNT pathways results in marked reduction of ovarian differentiation factors, LH receptor (Lhcgr), and inhibin-alpha (Inha). Therefore, WNT inhibits FSH target genes and steroid production associated with maturation and differentiation of the ovarian follicle [47]. Enriched miR-16-5p therefore reduces WNT function resulting in elevated steroid production in NOR GCs.
Micro-RNA-16-5p also targets MAP2K1, MAPK3 (mitogen-activated protein kinase kinase 1, mitogen-activated protein kinase kinase kinase). Previous studies have noted that MAPK signaling pathways are associated with regulation of steroidogenic acute regulatory protein expression and steroidogenesis. MAPK signaling pathway regulates cell proliferation, differentiation, and apoptosis [48].
Cyclin E and CDK (cyclin-dependent kinase) are targeted by miR-100-5p and miR-30a-3p, miR-16-5p. Cyclin E and CDK have been shown to continue granulosa cell proliferation for up to 10 h after an ovulatory stimulus. In NOR granulosa cells, overexpression of these miRNAs target to inhibit cyclin E and CDK action, resulting in limiting granulosa proliferation [49]. Overall, the miRNA expression profile and known mRNA targets show that NOR GCs are able to limit cellular proliferation, control steroidogenesis, and restrain differentiation of granulosa cells.
In DOR granulosa cells, the following miRNAs were upregulated: miR-155-5p, miR-192-5p, miR-128-3p, miR-486-5p, miR130a-3p, miR-92a-3p, miR-17-3p, miR-221-3p, and miR-175p. Micro-RNA-128-3p targets TGFBR1, transforming growth factor, beta receptor I. There is a difference in age-related expression of TGF beta family receptors in human cumulus ophorus cells [50]. Genes implicated in TGF-b signaling pathway were underexpressed in older women [51]. Overexpression of miR-128-3p in DOR GCs reveals that women with DOR have suppressed expression of TGF-b pathway, similar to older women.
Study limitations
Our classification of women with DOR was mainly based on AMH of < 2 ng/mL. This is our clinic’s cutoff for diminishing ovarian reserve. Though AMH is a continuum and different laboratories may use different cutoffs, we used a level for which we have the most consistent results. It is difficult to compare AMH cutoff values between clinics due to the variability and non-standardized testing of AMH conducted between differing testing labs [26]. For AMH-level run in our clinical IVF laboratory, we used the Ansh labs AMH ELISA immnunoassay. Despite using an AMH cutoff that may be considered less stringent, we identified significant differences in the miRNA expression profiles between NOR and DOR, and these were confirmed independently by qPCR testing of an additional nine NOR/DOR patient samples. Another limitation of our study was the small sample size. Due to high cost of next-generation sequencing, we were limited in the number of samples that could be processed. Nevertheless, our results are consistent with other published literature and the pathways implicated here match to known physiology of granulosa and cumulus cells. This is the first exploratory study to evaluate for next-generation sequencing of miRNA expression profile in granulosa cells and cumulus cells in young women with NOR or idiopathic DOR.
Conclusions
Based on the combined miRNA results in our study, we predicted key molecular pathways important in ovarian follicle development in NOR versus DOR that are potentially targeted by the miRNA described in this paper. These pathways included WNT, TGF-b, P13K-Akt, and MAPK signaling pathways in the granulosa cells [48]. These results have discovered a biological difference between DOR and NOR follicles for which future research may lead to new treatment options and potentially improve oocyte quality for young women with idiopathic DOR.
Electronic supplementary material
(DOCX 25 kb)
Acknowledgements
The research was supported by NIH grant HD082484 awarded to LKM and LKC.
References
- 1.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 2.Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88:399–413. doi: 10.1139/Y10-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118:5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 4.Gilabert-Estelles J, Braza-Boils A, Ramon LA, Zorio E, Medina P, Espana F, Estelles A. Role of microRNAs in gynecological pathology. Curr Med Chem. 2012;19:2406–2413. doi: 10.2174/092986712800269362. [DOI] [PubMed] [Google Scholar]
- 5.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack GS. MicroRNA gets down to business. Nat Biotechnol. 2007;25:631–638. doi: 10.1038/nbt0607-631. [DOI] [PubMed] [Google Scholar]
- 7.Oliveto S, Mancino M, Manfrini N, Biffo S. Role of microRNAs in translation regulation and cancer. World J Biol Chem. 2017;8:45–56. doi: 10.4331/wjbc.v8.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passetti F, Ferreira CG, Costa FF. The impact of microRNAs and alternative splicing in pharmacogenomics. Pharmacogenomics J. 2009;9:1–13. doi: 10.1038/tpj.2008.14. [DOI] [PubMed] [Google Scholar]
- 9.Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, Singha RS, Malakar AK, Chakraborty S. Interplay between miRNAs and human diseases. J Cell Physiol 2017. [DOI] [PubMed]
- 10.Ha TY. MicroRNAs in human diseases: from Cancer to cardiovascular disease. Immune Netw. 2011;11:135–154. doi: 10.4110/in.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiedler SD, Carletti MZ, Hong X, Christenson LK. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod. 2008;79:1030–1037. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGinnis LK, Luense LJ, Christenson LK. MicroRNA in ovarian biology and disease. Cold Spring Harb Perspect Med. 2015;5:a022962. doi: 10.1101/cshperspect.a022962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W. Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA. 2007;13:2366–2380. doi: 10.1261/rna.754207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain MM, Ghanem N, Hoelker M, Rings F, Phatsara C, Tholen E, Schellander K, Tesfaye D. Identification and characterization of miRNAs expressed in the bovine ovary. BMC Genomics. 2009;10:443. doi: 10.1186/1471-2164-10-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silveira JC, Winger QA, Bouma GJ, Carnevale EM. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-? signalling during follicle development in the mare. Reprod Fertil Dev 2015. [DOI] [PubMed]
- 17.McBride D, Carre W, Sontakke SD, Hogg CO, Law A, Donadeu FX, Clinton M. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction. 2012;144:221–233. doi: 10.1530/REP-12-0025. [DOI] [PubMed] [Google Scholar]
- 18.Yin M, Lu M, Yao G, Tian H, Lian J, Liu L, Liang M, Wang Y, Sun F. Transactivation of microRNA-383 by steroidogenic factor-1 promotes estradiol release from mouse ovarian granulosa cells by targeting RBMS1. Mol Endocrinol. 2012;26:1129–1143. doi: 10.1210/me.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navakanitworakul R, Hung WT, Gunewardena S, Davis JS, Chotigeat W, Christenson LK. Characterization and Small RNA content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci Rep. 2016;6:25486. doi: 10.1038/srep25486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzi P, Rizzari S, Maugeri M, Scollo P, Tatone C, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102:1751–1761. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, Benoit Y, Espeel M, D'Herde K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil (Camb) 2014;17:90–98. doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- 22.Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:355–362. doi: 10.1007/s10815-013-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Xing Q, Jin L, He L, Wu L, Wang L. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98:3068–3079. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 24.Wood JR, Dumesic DA, Abbott DH, Strauss JF., 3rd Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 25.La Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, Fiorentino F, Greco E. Female age, serum antimullerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2017;108:777–783. doi: 10.1016/j.fertnstert.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Iwase A, Nakamura T, Osuka S, Takikawa S, Goto M, Kikkawa F. Anti-Mullerian hormone as a marker of ovarian reserve: what have we learned, and what should we know? Reprod Med Biol. 2016;15:127–136. doi: 10.1007/s12522-015-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jevnaker AM, Khuu C, Kjole E, Bryne M, Osmundsen H. Expression of members of the miRNA17-92 cluster during development and in carcinogenesis. J Cell Physiol. 2011;226:2257–2266. doi: 10.1002/jcp.22562. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J, Mounsambote L, Prier P, Mathieu D’Argent E, Selleret L, Chabbert-Buffet N, et al. Outcomes of first IVF/ICSI in young women with diminished ovarian reserve. Minerva Ginecol. 2016; [DOI] [PubMed]
- 29.Pacella L, Zander-Fox DL, Armstrong DT, Lane M. Women with reduced ovarian reserve or advanced maternal age have an altered follicular environment. Fertil Steril. 2012;98:986–994. doi: 10.1016/j.fertnstert.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. Definition EwgoPOR. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 31.Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 32.Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang P, Wu H, Wang W, Ma W, Sun X, MiPred LZ. Classification of real and pseudo microRNA precursors using random forest prediction model with combined features. Nucleic Acids Res. 2007;35:W339–W344. doi: 10.1093/nar/gkm368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995:289–300.
- 36.Chaffin CL, Schwinof KM, Stouffer RL. Gonadotropin and steroid control of granulosa cell proliferation during the periovulatory interval in rhesus monkeys. Biol Reprod. 2001;65:755–762. doi: 10.1095/biolreprod65.3.755. [DOI] [PubMed] [Google Scholar]
- 37.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, Zhang ZG. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem. 2013;288:12478–12488. doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Yao W, Yao Y, Du X, Zhou J, Ma B, Liu H, Li Q, Pan Z. MiR-92a inhibits porcine ovarian granulosa cell apoptosis by targeting Smad7 gene. FEBS Lett. 2014;588:4497–4503. doi: 10.1016/j.febslet.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi L, Liu S, Zhao W, Shi J. miR-483-5p and miR-486-5p are down-regulated in cumulus cells of metaphase II oocytes from women with polycystic ovary syndrome. Reprod BioMed Online. 2015;31:565–572. doi: 10.1016/j.rbmo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol. 2004;214:19–25. doi: 10.1016/j.mce.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131:1007–1015. doi: 10.1530/rep.1.00978. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Haroush A, Abir R, Ao A, Jin S, Kessler-Icekson G, Feldberg D, Fisch B. Expression of basic fibroblast growth factor and its receptors in human ovarian follicles from adults and fetuses. Fertil Steril. 2005;84(Suppl 2):1257–1268. doi: 10.1016/j.fertnstert.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R Expression in Ovarian Granulosa Cells is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology 2017. [DOI] [PMC free article] [PubMed]
- 46.Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:2995–3004. doi: 10.1210/jc.2014-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stapp AD, Gomez BI, Gifford CA, Hallford DM, Hernandez Gifford JA. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS One. 2014;9:e86432. doi: 10.1371/journal.pone.0086432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zielak-Steciwko AE, Browne JA, McGettigan PA, Gajewska M, Dzieciol M, Szulc T, Evans AC. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol Genomics. 2014;46:735–745. doi: 10.1152/physiolgenomics.00036.2014. [DOI] [PubMed] [Google Scholar]
- 49.Cannon JD, Cherian-Shaw M, Chaffin CL. Proliferation of rat granulosa cells during the periovulatory interval. Endocrinology. 2005;146:414–422. doi: 10.1210/en.2004-0581. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro A, Freitas C, Matos L, Gouveia A, Gomes F, Silva Carvalho JL, Almeida H. Age-related expression of TGF beta family receptors in human cumulus oophorus cells. J Assist Reprod Genet. 2017;34:1121–1129. doi: 10.1007/s10815-017-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Edani T, Assou S, Ferrieres A, Bringer Deutsch S, Gala A, Lecellier CH, Ait-Ahmed O, Hamamah S. Female aging alters expression of human cumulus cells genes that are essential for oocyte quality. Biomed Res Int. 2014;2014:964614. doi: 10.1155/2014/964614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 25 kb)