Abstract
Purpose
To study the presence and distribution of genes encoding free radical scavengers in human granulosa cells from primordial and primary ovarian follicles.
Methods
A class comparison study on existing granulosa cell transcriptome from primordial (n = 539 follicles) and primary (n = 261) follicles donated by three women having ovarian tissue cryopreserved before chemotherapy was performed and interrogated.
Results
In granulosa cells from primordial follicles, 30 genes were annotated ‘mitochondrial dysfunction’ including transcripts (PRDX5, TXN2) encoding enzymatic free radical scavengers peroxiredoxin 5 and thioredoxin 2. Several apoptosis regulation genes were noted (BCL2, CAS8, CAS9, AIFM1). In granulosa cells from primary follicles, mitochondrial dysfunction signalling pathway was annotated. High expression of transcripts encoding the free radical scavenger peroxiredoxin 3, as well as anti-apoptotic enzyme BCL2, was found. Interestingly, PARK7 encoding the deglycase (DJ-1) protein was expressed in granulosa cells from primary follicles. DJ-1 is implicated in oxidative defence and functions as a positive regulator of the androgen receptor and as a negative regulator of the phosphatidylinositol 3-kinase (PI3K)/phosphatase and tensin homolog (PTEN)/serine-threonine protein kinase (AKT) signalling pathway suppressor PTEN.
Conclusions
The results indicate extensive energy production and free radical scavenging in the granulosa cells of primordial follicles with potential implications for ovarian ageing, cigarette smoking, premature ovarian failure and polycystic ovarian syndrome. Furthermore, DJ-1 may be involved in androgen responsiveness and the regulation of follicle growth via PI3K/PTEN/AKT signalling pathway regulation in the granulosa cells of primary follicles. The involvement of mitochondrial free radical production in the age-related decline of competent oocytes is becoming apparent.
Keywords: Human primordial follicles, Ovarian ageing, Granulosa cells, Mitochondria, Integrity, Reactive oxygen species (ROS), Reactive nitrogen species (RNS), Antioxidant, ROS scavenger, Free radical, Fertility, DJ-1 (PARK7), Thioredoxin, Peroxiredoxin, BCL2
Introduction
The female reproductive capacity is dependent on the quantity and quality of the poll of primordial follicles [1, 2]. Laid down during foetal life, oocytes halt meiosis in the dictyotene stage of prophase I and remain enclosed by one layer of granulosa cells during alleged dormancy [3]. Here they remain arrested until they are activated into growth or atresia [4]. Increasing lines of evidence suggest that bi-directional signalling between the oocyte and the granulosa cells is crucial in regulating dormancy and contributes towards activation and integrity of the primordial follicle-complex across decades [5, 6]. The female reproductive potential expires upon the exhaustion of the primordial follicle pool at 51 years of age on average [7, 8]. During the prolonged arrested period, the metabolically active oocyte and surrounding granulosa cells must maintain integrity in order to ensure oocyte competence [9–11]. The chance of a pregnancy occurring per cycle decreases dramatically with age from 25% at the age of 25 years to 6% at the age of 42 years. This is indicative of detrimental age-associated effects on the ovarian function [12, 13]. Consistent with this, chromosomal aneuploidies are known to increase with age [9]. Recently, the importance of mitochondrial functions in ovarian ageing was recognized [14, 15]. Mitochondria, being the ‘powerhouse’ of the cell, produce energy (ATP) for all cellular functions through oxidative phosphorylation [14]. The production of ATP via the electron transport chain in the inner mitochondrial membrane produces reactive oxygen species (ROS) and reactive nitrogen species (RNS) (collectively ‘free radicals’) as side products [16, 17]. Free radicals include hydroxyls, superoxides, nitric oxides, nitrogen dioxides, peroxyls and lipid peroxyls. Additionally, non-free radicals such as hydrogen peroxide and lipid peroxide rapidly transform to free radicals. Most free radicals are highly unstable molecules reacting in a detrimental way with DNA, lipids and protein [18]. The detrimental effects of free radicals occur upon mismatch between ROS/RNS production and free radical scavenging mechanisms (antioxidants) [11]. Ovarian antioxidants are numerous and include vitamins (A, C and E), tripeptide glutathione (GSH) and free radical scavenging enzymes such as glutathione peroxidase (GPX), superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), thioredoxins (TXN) and peroxiredoxins (PRDX) [19]. ROS are prominent and are commonly involved in oxidative stress. Mitochondria are central for ROS production, a process that has been shown to increase with age [20]. It was reported that levels of 8-oxodeoxyguanosine (8-OHdG) are a marker of oxidative stress, and 8-OHdG was found higher in ageing oocytes [21]. However, free radical scavenging enzymes have been shown to decrease with age in mammalian granulosa cells [22, 23]. Furthermore, oral antioxidants (vitamin C and E) have been shown to counteract the negative effects of ageing on oocyte quantity and quality, probably through increased free radical scavenging mechanisms in the follicle [24]. The involvement of free radicals in ageing, the ‘free radical theory of ageing’, was proposed more than 60 years ago by Harman [25]. However, the free radical theory of ageing is not the only theory that could explain the mechanism involved in ageing at the molecular level, including genomic stability and mitochondria functions [26]. Thus, mitochondrial dysfunction with decreased ATP production and increased free radical production has been associated with having deleterious consequences on chromosome segregation, meiotic spindle abnormalities and decreased preimplantation potential [11, 14, 27]. In pre-ovulatory follicles, ROS has been proposed to affect several processes. Only the dominant oocyte enters meiosis I, a process targeted by increased ROS and decreased antioxidants. Interestingly, as increased antioxidants are required for the progression of meiosis II, this suggests a cross-talk between ROS and antioxidants in the ovary [28].
Recent studies have also highlighted the protective role for correct mitochondrial function of human granulosa cells in protecting the ovarian reserve [29]. During follicle development, most follicles undergo follicular atresia. Abnormal follicular atresia could accelerate follicular depletion causing ovarian dysfunction. Recent studies indicated that apoptosis in granulosa cells is linked to follicular atresia [30, 31]. Oxidative stress is believed to be the cause of granulosa cell apoptosis in an age-dependent manner [8, 11]. Free radicals in granulosa cells that cause subsequent oxidative stress have been linked to several important deleterious ovarian processes such as follicular apoptosis [32], follicular ageing [19, 22], cyclophosphamide treatment-induced premature ovarian failure (POF) [33] and polycystic ovarian syndrome (PCOS) [34]. In fact, PCOS has been found associated with decreased antioxidant concentrations [35, 36], and the decrease in mitochondrial O2 consumption and GSH levels alongside increased ROS production has been suggested to be the cause of mitochondria dysfunction in PCOS patients [37]. Recently, it was found that PCOS women have lowered nitric oxide due to reduced iNOS/eNOS expression, low H2O2, high asymmetric dimethyl arginine (ADMA) levels and decreased arginine bioavailability, suggesting that redox biology represents a potential treatment option for PCOS patients [38]. In addition, lifestyle factors such as obesity and smoking have been associated with mitochondrial dysfunction and with the increased production of free radicals and compromised oocyte development [28, 39].
The association between mitochondrial dysfunction and compromised fertility seen in a variety of conditions (high maternal age, POF, PCOS, obesity) calls for greater understanding of the mechanisms that ensure structural integrity in primordial follicles across decades of dormancy.
Several lines of evidence thus confirm the key role of oxidative stress in aged granulosa cells [40]. Knowing the detailed expression of genes encoding free radical scavenger proteins in human granulosa cells is a first step towards the search for good candidate as a predictive marker and therapeutic target in new strategies for improving reproductive counselling in ageing women.
A previous study explored the presence of canonical signalling pathways in granulosa cells from human primordial and primary follicles [41]. This study interrogates the canonical signalling pathway ‘mitochondrial dysfunction’ that was found highly enriched in granulosa cells from primordial follicles as compared to granulosa cells from primary follicles. The canonical signalling pathway mitochondrial dysfunction contains several free radical scavengers, including the PRDX3, PRDX5 and TXN2 genes. Their presence suggests their potential involvement in maintaining the integrity of dormant human primordial follicles and the regulated process of follicle development.
Materials and methods
Human cortical ovarian tissue and follicle isolation
Normal human ovarian tissue was donated by three women undergoing cryopreservation prior to gonadotoxic treatment (Danish Scientific Ethical Committee Approval Number: KF 299017 and J7KF/01/170/99) [42]). The patients were aged 26, 34 and 34 years old, respectively. The primary diagnoses were unrelated to ovarian malignancies. Patients were normo-ovulatory, with normal reproductive hormones and not received ovarian stimulation with exogenous gonadotropins. All methods were carried out in accordance with relevant guidelines and regulations, and The Central Denmark Region Committees on Biomedical Research Ethics and the Danish Data Protection Agency approved the study. Written informed consent was obtained from all participants before inclusion. Patients consented to the research conducted. Oocytes and follicles (oocytes with surrounding granulosa cells) from the primordial and primary stages respectively were isolated based on morphological appearance via laser capture microdissection (Veritas™ Microdissection Instrument Model 704, ArcturusXT™, Molecular Devices, Applied Biosystems, Life Technologies, Foster City, CA, USA) [41, 43] from cryopreserved biopsies. Until use, the cortical piece was stored in liquid nitrogen (− 196 °C), as previously described [44, 45]. Although healthy children have been born as a result of the ovarian cryopreservation procedure [44], oxidative stress on the tissue cannot be excluded. Primordial follicles were defined as oocytes surrounded by three to five flattened granulosa cells, and primary follicles were defined as oocytes surrounded by one layer of cuboidal granulosa cells.
Laser capture microdissection
Ovarian cortical fragments with a size of 2 × 2 × 1 mm were thawed and fixed by direct immersion into 4% paraformaldehyde (PFA) at 4 °C for 4 h followed by dehydration and embedding in paraffin, and LCM was performed using the Veritas™ Microdissection instrument Model 704 (ArcturusXT™, Molecular Devices, Applied Biosystems®, Life Technologies, Foster City, CA, USA), as described [46]. Isolates of oocytes and oocytes with surrounding granulosa cells (follicles) from the primordial and primary stage, respectively (four main groups in total), from each of the three patients, with a total number of 12 samples, were isolated using laser capture microdissection [41].
Library preparation, sequencing and bio-informatically management
Total RNA was extracted from isolates (KIT0312-l, Arcturus® Picopure® RNA Isolation Kit, Applied Biosystems, Life Technologies™, CA, USA), converted to cDNA (#3312 NuGen Inc., CA, USA) and sequenced on the Illumina HiSeq2000 platform (Illumina Inc., CA, USA) at an external facility (AROS Biotechnology, Aarhus, Denmark).
Bio-informatically management included BAM file generation (Tophat 2.0.4., Cufflink 2.0.2.), mapping to the human reference genome (hg19) (BWA 0.6.2.) and normalization (FPKM) (Software R, R Development Core Team (2015), R: language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria; URL http://www.R-project.org) (Team, 2012)).
Mean FPKM values for all transcripts were calculated based on triplicate FPKM values using a one-sample t test. All transcripts were ranked based on consistency across triplicates as indicated by one-sample t test p value (the cut-off value for inclusion in downstream analysis: p < 0.2 across triplicates). Based on global transcription lists for (a) oocytes from primordial follicles, (b) oocytes from primary follicles, (c) primordial follicles (oocytes with surrounding granulosa cells) and (d) primary follicles (oocytes with surrounding granulosa cells), gene contribution in granulosa cells from primordial and primary follicles was analysed [41].
In silico extraction of granulosa cell transcriptomes was performed by applying three filters on global transcriptome data from patient triplicates of oocytes [46] and oocytes with surrounding granulosa cells (follicle) for both the primordial and primary stage [41]. First, consistency in mean gene expression level (FPKM) for all detected transcripts was quantified by performing a t test on patient triplicate samples of same type ((a) primordial oocyte, patient 1 + 2 + 3; (b) primordial follicle, patient 1 + 2 + 3; (c) primary oocyte, patient 1 + 2 + 3; and (d) primary follicle, patient 1 + 2 + 3). The level of consistency was sorted based on p value, with a low p value indicating a high degree of consistency in FPKM mean across patient triplicates. The cut-off in the level of consistency for all transcripts was set at p < 0.2 across triplicates for being included in all downstream analyses.
Significantly, differentially expressed genes between the granulosa cells from primordial follicles and the granulosa cell from primary follicles were defined as FPKM mean fold change > 2 and/or paired t test significance (p < 0.05) [41].
Enrichment analysis
We extracted two main lists: stage-specific consistently expressed genes (SSCEGs) for oocytes and granulosa cells from primordial and primary follicle transcriptomes, respectively, and differentially expressed genes (DEG) comparing the transcriptomes of the two morphological stages. SSCEGs were defined as genes significantly expressed (p < 0.05) in either oocytes or granulosa cells from primordial or primary follicles in the three different patients. SSCEGs were identified using a one-sample t test on FPKM values for each identified transcript from patient triplicates within each follicle stage in relation to a ‘0’ value. This in silico merging of transcriptomes from three patients was performed to account for biological variance. DEGs were analysed in order to investigate genes consistently down- or upregulated during the primordial-to-primary transition. We identified DEG on the basis of genes consistently expressed in primordial and primary follicles by comparing FPKM values. Towards this, we used the Ingenuity® Pathway Analysis (IPA®) software (Qiagen) according to their instructions (Qiagen). The canonical pathway enrichment analysis of (I) consistently expressed genes in granulosa cells from primordial follicles, (II) consistently expressed genes in granulosa cells from primary follicles, (III) downregulated genes in the granulosa cells during the primordial-to-primary transition and (IV) upregulated genes in the granulosa cells during the primordial-to-primary transition and was performed using the IPA®software. The molecular and Cellular annotation foundation was likewise defined using the IPA® software (Qiagen).
Real-time quantitative PCR
The validation of the sequencing results was performed for a selected gene (TXN2) using a predesigned and validated gene expression assay (TXN2, Hs00429399_g1, TaqMan®, Applied Biosystems, Life Technologies™, CA, USA). GAPDH (Hs02758991_g1, TaqMan®, Applied Biosystems, Life Technologies™, CA, USA) was selected as the reference gene. Triplicate expression values of the gene (TXN2) were set relative to GAPDH via the ΔΔCt method [47, 48]. Experiments were repeated at least three times. As a negative control, cDNA from no template RT PCR reactions was used. All qPCR data were analysed using Prism 6, version 6.0 (GraphPad Software Inc., CA, USA). Statistical analysis was carried out using Student’s unpaired t test. Data are represented as mean ± SEM. RT-qPCR validation of the dataset used was previously performed for several other genes [41].
Results
The mitochondrial dysfunction canonical signalling pathway
A recent study previously explored the most enriched and significant canonical pathways in granulosa cells from primordial and primary follicles were defined using a transcriptome-based approach [41]. Interestingly, this revealed that although the number of significant canonical pathways were comparable in the granulosa cells during the primordial to primary transition (n = 46 and n = 37 in granulosa cells from primordial and primary follicles, respectively), the biological contents of the canonical pathways differed remarkably, suggesting a dynamic nature in human granulosa cells during the primordial to primary transition.
In granulosa cells from primordial follicles, the mitochondrial dysfunction canonical signalling pathway was the foremost enriched signalling pathway (p = 4.11E−05) with 30 molecules assigned (Table 1; Fig. 1). In granulosa cells from primary follicles, mitochondrial dysfunction was the 29th most enriched canonical signalling pathway (p = 3.6E−02) with 12 molecules assigned (Table 1). Of the assigned genes from the mitochondrial dysfunction pathway, only four genes (CYB5R3, NDUFB2, SDHB and BCL2) were present in both granulosa cells from primordial and primary follicles. This clearly suggests that the genes assigned to the mitochondrial dysfunction pathway are dynamic during the primordial to primary transition, with different genes assigned to the mitochondrial dysfunction.
Table 1.
‘Mitochondrial dysfunction’ signalling pathway annotation of transcripts identified in granulosa cells from primordial and primary follicles, respectively
| ‘Mitochondrial dysfunction’ pathway annotations | |||
|---|---|---|---|
| Gene name | Gene symbol | FPKM mean value | p value |
| GCs from primordial follicles | |||
| ATP synthase, H+ transporting, mitochondrial Fo complex subunit D | ATP5H | 7.003722754 | 0.01670744 |
| NADH/ubiquinone oxidoreductase subunit A2 | NDUFA2 | 2.963325614 | 0.01874323 |
| Presenilin 2 | PSEN2 | 3.263341286 | 0.024146621 |
| NADH/ubiquinone oxidoreductase subunit B9 | NDUFB9 | 1.830962863 | 0.031371209 |
| ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1 | ATP5C1 | 3.989803598 | 0.039197685 |
| Apoptosis inducing factor, mitochondria associated 1 | AlFM1 | 3.613715001 | 0.044079763 |
| ATP synthase, H+ transporting, mitochondrial Fo complex subunit Cl (subunit 9) | ATP5G 1 | 1.874935038 | 0.050183137 |
| Cytochrome C oxidase subunit 8C | COX8C | 1.810614952 | 0.0566006 |
| Cytochrome B5 reductase 3 | CYB5R3 | 2.094835367 | 0.056641305 |
| NADH/ubiquinone oxidoreductase subunit B10 | NDUFB10 | 3.008997935 | 0.060705184 |
| Aph-1 homolog A, gamma-secretase subunit | AP H1A | 3.478406037 | 0.072182448 |
| Ubiquinol-cytochrome C reductase, Rieske iron-sulphur polypeptide 1 | UQCRFS1 | 2.540184794 | 0.084315517 |
| Caspase 8 | CASP8 | 3.658460561 | 0.085398141 |
| NADH/ubiquinone oxidoreductase subunit AB1 | NDUFAB1 | 1.88869655 | 0.086915174 |
| NADH/ubiquinone oxidoreductase subunit B2 | NDUFB2 | 4.091466578 | 0.091428443 |
| Thioredoxin 2 | TXN2 | 2.058542691 | 0.093586399 |
| ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit | ATP5O | 2.956570579 | 0.114821485 |
| NADH/ubiquinone oxidoreductase subunit B3 | NDUF53 | 2.984935364 | 0.115612421 |
| Peroxiredoxin 5 | PRDX5 | 3.728725125 | 0.129264328 |
| Xanthine dehydrogenase | XDH | 2..709244397 | 0.132521709 |
| COX10, heme A:farnesyltransferase cytochrome C oxidase assembly factor | COX10 | 3.018504989 | 0.133332747 |
| Succinate dehydrogenase complex iron-sulphur subunit B | SDHB | 2.676785352 | 0.146443724 |
| COX17, cytochrome C oxidase copper chaperone | COX17 | 3.038353629 | 0.171886696 |
| SURF1, cytochrome C oxidase assembly factor | SURF1 | 2.314066164 | 0.183536671 |
| Mitogen-activated protein kinase 9 | MAPK9 | 3.079312273 | 0.1845136 |
| Cytochrome C oxidase subunit 7A2 | COX7A2 | 1.820117208 | 0.187572763 |
| Leucine-rich repeat kinase 2 | LRRK2 | 1.742929277 | 0.18382465 |
| Caspase 9 | CASP9 | 0.357300941 | 0.191902227 |
| Ubiquinol-cytochrome C reductase complex III subunit VII | UQCRQ | 2.795960319 | 0.197783654 |
| BCL2, apoptosis regulator | BCL2 | 2.977907347 | 0.199595519 |
| GCs from primary follicles | |||
| Peroxiredoxin 3 | PRDX3 | 4.122496649 | 0.031217155 |
| NADH/ubiquinone oxidoreductase core subunit S8 | NDUFS8 | 3.123248644 | 0.051406125 |
| Cytochrome B5 reductase 3 | CYB5R3 | 2.431150003 | 0.084522964 |
| NADH/ubiquinone oxidoreductase subunit 52 | NDUFB2 | 2.937883812 | 0.137007167 |
| Cytochrome B5 type A | CYB5A | 3.106697839 | 0.141002458 |
| Succinate dehydrogenase complex iron-sulphur subunit B | SDHB | 2.541296738 | 0.150507621 |
| NADH/ubiquinone oxidoreductase subunit A8 | NDUFA8 | 3.023299272 | 0.155284585 |
| Parkinsonism associated deglycase | PARK7 | 3.22257735 | 0.172431902 |
| NADH/ubiquinone oxidoreductase subunit A6 | NDUFA6 | 3.119066403 | 0.179699014 |
| Aph-1 homolog a, gamma-secretase subunit | APH1B | 3.08893234 | 0.192116622 |
| BCL2, apoptosis regulator | BCL2 | 2.05073274 | 0.196819022 |
| Ras homolog family member T2 | RHOT2 | 1.338057874 | 0.199400508 |
FPKM mean values were calculated based on triplicate expression values of the same transcript using a one-sample t test. The noted p value is indicative of the consistency in expression pattern across triplicates
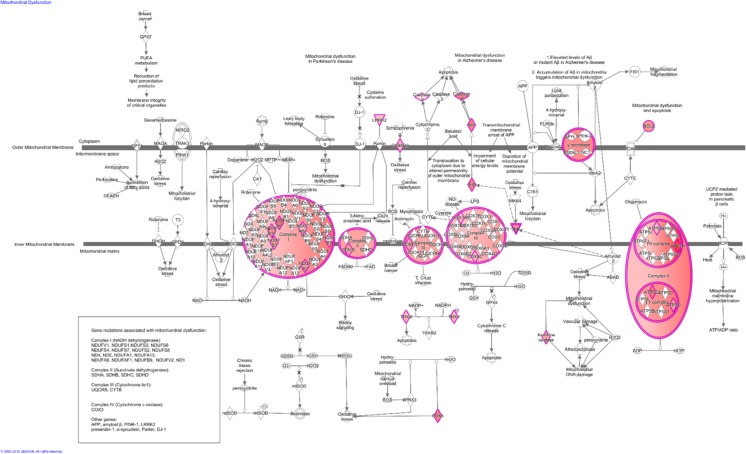
Fig. 1.
Pathway of mitochondrial dysfunction in granulosa cells from primordial follicles. Illustrative presentation of the ‘mitochondrial dysfunction’ signalling pathway (n = 30) in granulosa cells from primordial follicles. Annotated genes are heavily involved in mitochondrial energy production and free radical scavenging mechanisms (see text for details). The figure was generated based on transcripts annotated the mitochondrial dysfunction signalling pathway using Ingenuity® Pathway Analysis software. Colour intensities are based on FPKM mean values where high significance is most intensive in colour (red)
The signalling pathway in mitochondrial dysfunction
The mitochondrial dysfunction pathway from granulosa cells from primordial follicles was subjected to molecular pathway analysis in the IPA® software (Fig. 1) in order to reveal the presence of the gene candidates from this study against the broader context of mitochondria function. This clearly indicates that the vast majority of the genes from this study are associated with complexes within the inner mitochondria membrane (Fig. 1). A few genes associated with the outer mitochondria membrane was noted, such as the genes encoding components of the gamma secretase complex [49]. The gamma secretase complex consists of four individual proteins presenilin-1 (PSEN1) nicastrin (NCT), anterior pharynx-defective 1 (APH-1) and presenilin enhancer 2 (PEN-2) that were noted in the granulosa cells (Fig. 1).
The inner mitochondria membrane contained genes encoding NADH/ubiquinone oxidoreductase supernumerary subunits (NDUP making up the complex I, and some components of the complex II, III and IV such as succinate dehydrogenase (SDHD) and cytochrome c oxidase (COX) 7A) (Fig. 1). In the inner mitochondria membrane complex V, genes encoding adenotriphosphate (ATP)5G, 5H, SC and S0 were noted (Fig. 1).
The inner membrane of the mitochondrion is involved in the final step in aerobic respiration, suggesting that energy production and cellular activity associated with this is prominent in granulosa cells. It was observed that the caspase 8 and caspase 9 apoptosis components are present in the granulosa cells of the primordial follicle, supporting the well-known fact that apoptosis contributes towards atresia during follicle development.
Differentially expressed genes in mitochondrial dysfunction
We further analysed differentially expressed genes (DEG) between granulosa cells from primordial and primary follicles. We found that 11 genes assigned with mitochondrial dysfunction (p = 3.72E−02) were significantly downregulated in the granulosa cells during the primordial-to-primary transition, whilst three genes were significantly upregulated (ns enrichment) (Table 2). Interestingly, we note that the gene encoding the apoptosis-inducing factor, mitochondria-associated 1 (AIFM1) is highly expressed in granulosa cells from primordial follicles and downregulated by a 2.5-fold factor.
Table 2.
Differentially expressed genes when comparing the transcriptomes of granulosa cells from primordial follicles with that of granulosa cells from primary follicles
| DEG annotated ‘mitochondrial dysfunction’ pathway | |||||||
|---|---|---|---|---|---|---|---|
| Gene name | Gene symbol | GCs from PDF mean FP KM value | p value | GCs from PMF mean FPKM value | p value | Significance (paired t test) | Fold change down |
| Significantly downregulated in GCs during primordial-to-primary transition | |||||||
| Caspase 9 | CASP9 | 0.35730C941 | 0.191902227 | ND | ND | . | – |
| Leucine-rich repeat kinase 2 | LRRK2 | 1.742929277 | 0.18882465 | 0.152619841 | 0.422649731 | 0.202859976 | 11.42007003 |
| Xanthine dehydrogenase | XDH | 2.709244357 | 0.132521709 | 0.694350157 | 0.422649731 | 0.280037462 | 3.901841698 |
| Caspase 8 | CASP8 | 3.658460561 | 0.085398141 | 1.013842009 | 0.330037104 | 0.150990301 | 3.608511511 |
| NADH/ubiquinone oxidoreductase subunit B10 | NDUFB10 | 3.008997935 | 0.060705184 | 0.877799646 | 0.25403594 | 0.052881546 | 3.427886932 |
| SURF1, cytochrome C oxidase assembly factor | SU RF1 | 2.314066164 | 0.183536671 | 0.751575033 | 0.388497206 | 0.270072995 | 3.078955608 |
| Apoptosis inducing factor, mitochondria associated 1 | AIFM1 | 3.613715001 | 0.044079763 | 1.417073168 | 0.213958626 | 0.240541281 | 2.550125909 |
| Presenilin 2 | PSEN2 | 3.263341286 | 0.024146621 | 1.400426297 | 0.40453294-9 | 0.203847324 | 2.330248505 |
| Mitogen-activated protein kinase 9 | MAPK9 | 3.075312273 | 0.1845136 | 1.441810509 | 0.422649731 | 0.358332488 | 2.135726056 |
| ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1 | ATP5C1 | 3.989803o98 | 0.039197685 | 1.904638856 | 0.312564394 | 0.291986732 | 2.094782266 |
| Thioredoxin 2 | TXN2 | 2.058542691 | 0.093586399 | 1.00385669 | 0.309227225 | 0.409842284 | 2.0S0634032 |
| Significantly upregulated in GCs during primordial-to-primary transition | |||||||
| NADH/ubiquinone oxidoreductase core subunit S8 | NDUFS8 | ND | ND | 3.123248644 | 0.051406125 | – | – |
| Ras homolog family member T2 | RHOT2 | 0.347494344 | 0.422649731 | 1.838057874 | 0.199400508 | 0.263934146 | 5.289461278 |
| Cytochrome B5 type A | CYB5A | 1.166231252 | 0.163820909 | 3.106697839 | 0.141002458 | 0.206470227 | 2.663878055 |
Eleven transcripts were significantly (fold change > 2) downregulated, whilst three were significantly upregulated (fold change > 2) in the granulosa cells during the primordial-to-primary follicle transition
ND not determined
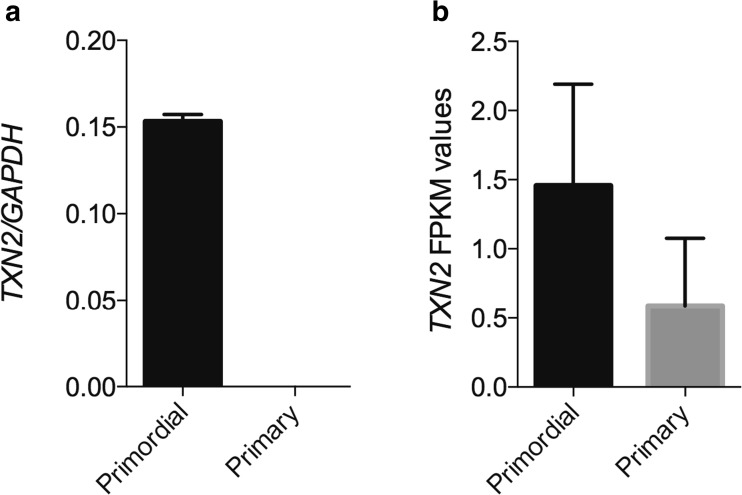
Thioredoxin 2 (TXN2) was likewise downregulated by a 2-fold change during the primordial-to-primary transition and was selected for RT-qPCR confirmation. This showed that the TNX2 gene is significantly (p < 0.002) downregulated granulosa cells during the primordial and primary follicle transition (Fig. 2a) and, moreover, confirmed the RNA seq. data (p = 0.38) for TXN2 (Fig. 2b). In this regard, it should be noted that FPKM values below 1 regards the gene expression not to be present.
Fig. 2.
TXN2 transcript analysis by RT-qPCR compared to RNA seq. data. Real-time quantitative PCR of a TXN2 RNA in primordial and primary follicles, respectively, relative to the reference gene GAPDH (p < 0.200, unpaired t test with Welch correlation) compared to the b TXN2 expression from RNA seq. data (FPKM values).
Molecular and cellular functions
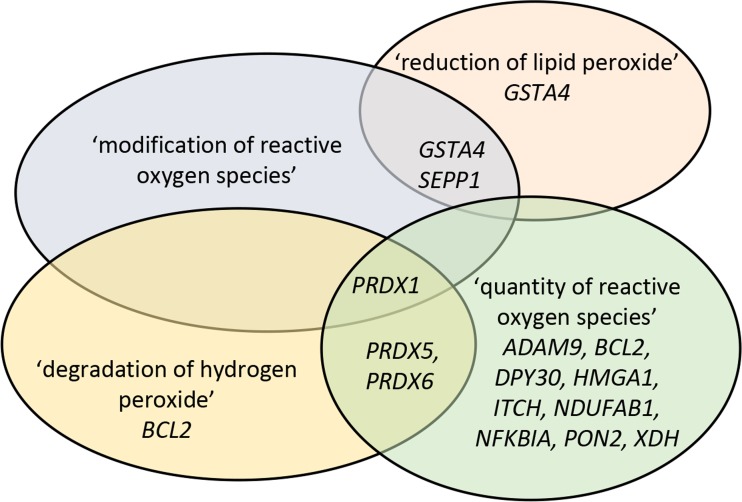
As a next step, the ‘molecular and cellular function’ from the IPA analysis was investigated and, in line with the canonical pathway analysis, the ‘free radical scavenger’ category was restricted to granulosa cells from primordial follicles (Table 3), including four functional annotations ‘modification of reactive oxygen species’ (containing; GSTA4, PRDX1, SEPP1), ‘quantity of reactive oxygen species’ (containing; ADAM9, BCL2, DPY30, HMGA1, ITCH, NDUFAB1, NFKBIA, PON2, PRDX1, PRDX5, PRDX6, XDH), ‘reduction of lipid peroxide’ (containing; GSTA4, SEPP1) and ‘degradation of hydrogen peroxide’ (containing; BCL2, PRDX1, PRDX5, PRDX6), with the quantity of reactive oxygen species being the most abundant in terms of number of genes. Several genes in the functional annotations overlap restrictively (Fig. 3).
Table 3.
The molecular and cellular function ‘free radical scavenging’ and the function annotations with genes assigned, found in granulosa cells from primordial follicles
| Category | Function annotation | p values | Molecules |
|---|---|---|---|
| Free radical scavenging | ‘Modification of reactive oxygen species’ | 9.65E−03 | GSTA4, PRDX1, SEPP1 |
| ‘Quantity of reactive oxygen species’ | 1.02E−02 | ADAM9, BCL2, DPY30, HMGA1, ITCH, NDUFAB1, NFKBIA, PON2, PRDX1, PRDX5, PRDX6, XDH | |
| ‘Reduction of lipid peroxide’ | 1.98E−02 | GSTA4, SEPP1 | |
| ‘Degradation of hydrogen peroxide’ | 3.16E−02 | BCL2, PRDX1, PRDX5, PRDX6 |
Fig. 3.
Schematic presentation of ‘free radical scavenger’ functional annotations and genes assigned. Presentation of the genes assigned into each of the four functional annotations in the free radical scavenger category in granulosa cells from primordial follicles, revealing that whilst some genes overlap between the annotations, some are unique
Discussion
The present study showed that genes associated with mitochondrial functions are highly expressed in granulosa cells from primordial follicles and less in granulosa cells from primary follicles. We observed that the genes annotated the mitochondrial dysfunction pathway are highly dynamic, with several transcripts encoding important proteins that exhibit differential expression when comparing granulosa cells from the two key stages of human follicle development.
In granulosa cells from primordial follicles, mitochondrial dysfunction was the foremost enriched pathway, with 30 molecules assigned. Large portions of the proteins that these transcripts encode reside at the inner mitochondrial membrane, where they are involved in ATP generation. These findings indicated that the alleged dormant primordial follicles are indeed highly active in energy production. Interestingly, we noted expression of transcripts encoding the ROS scavengers PRDX5, as well as TXN2 in granulosa cells from primordial follicles. In granulosa cells from primary follicles, we detected high expression of PRDX3. In mammals, six different isoforms of the free radical scavenging enzymes, peroxiredoxins (PRDX1–6), have been identified [50]. In an acellular assay, PRDX5 was previously reported to protect mitochondrial DNA from oxidative damage [51]. Furthermore, in hamster ovary cells, the overexpression of Prdx5 reduced the damage caused by hydrogen peroxide on mitochondrial DNA [51]. In mice, Prdx3 and Txn2 have been found to decrease significantly with age coinciding with increased oxidative damage to lipids, proteins and DNA [19]. The PRDX4 protein, and mRNA, mainly expressed in the granulosa cells of mature human follicles, was reported lower in PCOS patients as compared to controls, possibly due to increased oxidative stress in the follicles of PCOS patients [52]. A deficiency of mitochondrial redox protein TXN2 was recently associated with early-onset neuro-degeneration [53]. Here, patient-derived samples exhibited increased ROS levels, impaired free radical defence and showed oxidative phosphorylation dysfunction. Furthermore, upon cell culture antioxidant treatment (MitoQ, Idebenone, Trolox), these detrimental effects were counteracted [53]. The transcript level of TXN2 was previously reported significantly higher in granulosa cells of normally responding women undergoing IVF treatment as compared to poor responders [54]. Interestingly, we noted significant downregulation of TXN2 in granulosa cells during the primordial-to-primary follicle transition. These findings suggested the importance for TXN2 in maintaining the integrity of the granulosa cells during prolonged primordial follicle dormancy. Additionally, peroxiredoxin 5 may be another important protector of free radicals in the granulosa cells from primordial follicles. We found indications that peroxiredoxin 3 may be the dominant peroxiredoxin in granulosa cells from primary follicles. As Prdx3 and Txn2 were previously found to decrease with increasing age in mice [19], it would be interesting to investigate whether age has a similar effect on human granulosa cells TXN2, PRDX3 and PRDX5 expression. The potential involvement of TXN2, PRDX3 and PRDX5 in the patho-ethiology of human POF remains to be determined.
We found transcripts encoding the known anti-apoptotic factor BCL2 [16] expressed in granulosa cells of both primordial and primary follicles. The BCL2 apoptosis regulator is under the regulatory control of AKT of the phosphatidylinositol 3-kinase (PI3K)/phosphatase and tensin homolog (PTEN)/AKT signalling pathway, which is known to be crucial in controlling primordial follicle dormancy, activation and integrity [55]. Bcl2 was shown to be pivotal in maintaining the pool of primordial follicles in rats [56]. In addition, we found transcripts encoding the pro-apoptotic enzymes caspase 8 and 9 expressed in granulosa cells of primordial follicles with a significantly lower expression in granulosa cells of primary follicles. AKT has previously been proposed to directly inhibit caspase 9 [57]. Thus, the present data indicated a complex interplay between pro-apoptotic and anti-apoptotic enzymes governing the survival of primordial follicles in dormancy and that BCL2 may have an important role in supporting the survival of human granulosa cells from primordial and primary follicles.
We found high expression of the transcript Parkinson associated deglycase (PARK7) encoding the protein deglycase (DJ-1) in the granulosa cells of primary follicles. A mutation in the DJ-1 protein was recently found to decrease the anti-oxidative capacity of cells [58]. Interestingly, DJ-1 acts as a positive regulator of androgen receptor-dependent transcription [59]. Androgen receptor mRNA has been found expressed from transitional human follicles (mixed flattened and cuboidal granulosa cells) onwards [60], indicative of androgen responsiveness. An in vitro culture of mouse ovaries with testosterone has previously been shown to increase the primordial-to-primary transition [61]. This is theoretically consistent with an increased number of small growing pre-antral follicles seen in hyper-androgenetic PCOS patients [62]. Additionally, in IVF treatments, low responder patients had increased ovarian response when pre-treated with low doses of dehydroepiandrosterone (DHEA) [63]. Finally, DJ-1 is a negative regulator of PTEN, a known suppressor of the PI3K/PTEN/AKT signalling pathway [64]. The actions of the PI3K/AKT signalling pathway have been associated with primordial follicle activation [55]. One may speculate whether PARK7 expression in the granulosa cells of primary follicles augments the transcriptional effects of androgens and in parallel relieves the PI3K/PTEN/AKT signalling pathway from the PTEN suppressive effect, thus increasing cell proliferation and the growth of early pre-antral follicles.
In conclusion, these findings indicate that granulosa cells of primordial and primary follicles are highly active in energy production. Furthermore, in order to cope with the free radical load generated in connection with this, our findings suggest that granulosa cells may have developed defence mechanisms involving several free radical scavengers such as peroxiredoxins (PRDX3, PRDX5) and thioredoxin (TXN2), with the free radical scavenger category from the molecular and cellular function being specific to granulosa cells from primordial follicles. Moreover, the anti-apoptotic factor BCL2 may prove to be important for sustaining human granulosa cell integrity in both the dormant primordial follicle and the activated primary follicle. As ROS-induced damage progresses with increasing age [19], a strong free radical defence in dormant primordial follicles, as well as anti-apoptotic factor BCL2, may prove important in ensuring granulosa cell integrity in human primordial follicle senescence from birth to menopause. We were surprised to identify expression of the DJ-1 encoding transcript PARK7 in granulosa cells of primary follicles. Recent literature on the function of DJ-1 as a positive regulator of androgen receptor transcriptional effects, and a regulator of the PI3K/PTEN/AKT signalling pathway, as well as free radical scavenging, makes this a highly interesting finding.
As free radicals have been linked to follicular ageing [19], PCOS [34], and cyclophosphamide treatment-induced premature ovarian failure (POF) [33], it is conceivable that the dysregulated transcription of PRDX3-, PRDX5-, TXN2, and PARK7 may be involved in compromised dormancy and early pre-antral human follicle development. Despite the fact that mitochondria and their membrane proteins have been intensively studies for many years, they still represent a fascinating aspect with relevance to human health [65]. Our results raise many questions. It is important to keep in mind that there might also be detectable differences in what is considered dormant follicles. Primordial follicles do not belong in only one category, and it is unknown whether the primordial follicles analysed represent those still in the resting pool or those from the cohort that have entered the growing pool. How are the mitochondria of dormant primordial follicles involved in ovarian ageing? And how do the granulosa cells help to protect against oxidative damage to the oocyte, if at all? Or would this represent a building up of factors that can combat oxidative stress, in case of need? Are there any specific functional roles for the respiratory complex proteins? How does this affect the ‘cross-talk’ between the oocyte and the granulosa cells? It is likely that the combat against oxidative stress could play a role in maintenance of the oocyte integrity to protect the follicular reserve. Interestingly, a study showed that pre-pubertal ovaries contained a high proportion of morphologically abnormal non-growing follicles, and these follicles showed reduced capacity for in vitro growth [66], which may suggest that age-related lower levels of ROS may contribute towards this. Functional studies may be able to address these questions in the future.
Acknowledgements
The authors wish to thank the clinical, paramedical and laboratory team of the Fertility Clinic, Aarhus University Hospital. We thank Lykke-Hartmann laboratory (AU) for scientific discussions. Anders Heuck is also thanked for excellent laboratory work.
Funding
E.H. Ernst was supported by a PhD scholarship from the Health Faculty, Aarhus University. The work was further supported by grants from Kong Christian Den Tiendes Fond (to EHE and KLH), Th. Maigaards Eft. Fru Lily Benthine Lunds Fond and Fonden til Lægevidenskabens Fremme (to KLH).
Compliance with ethical standards
All methods were carried out in accordance with relevant guidelines and regulations, and the Central Denmark Region Committees on Biomedical Research Ethics and the Danish Data Protection Agency approved the study. Written informed consent was obtained from all participants before inclusion. Patients consented to the research conducted.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 2.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23(3):699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari A, Fowler PA. Primordial follicular assembly in humans—revisited. Zygote. 2008;16(4):285–296. doi: 10.1017/S0967199408004802. [DOI] [PubMed] [Google Scholar]
- 4.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh AJ, et al. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monniaux D. Driving folliculogenesis by the oocyte-somatic cell dialog: lessons from genetic models. Theriogenology. 2016;86(1):41–53. doi: 10.1016/j.theriogenology.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30(5):438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 8.Broekmans FJ, Knauff EAH, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Hassold T, Hunt PA, Sherman S. Trisomy in humans: incidence, origin and etiology. Curr Opin Genet Dev. 1993;3(3):398–403. doi: 10.1016/0959-437X(93)90111-2. [DOI] [PubMed] [Google Scholar]
- 10.Tarin JJ. Aetiology of age-associated aneuploidy: a mechanism based on the ‘free radical theory of ageing’. Hum Reprod. 1995;10(6):1563–1565. doi: 10.1093/HUMREP/10.6.1563. [DOI] [PubMed] [Google Scholar]
- 11.Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14(2):131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- 12.van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302(6789):1361–1365. doi: 10.1136/bmj.302.6789.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N Engl J Med. 1982;306(7):404–406. doi: 10.1056/NEJM198202183060708. [DOI] [PubMed] [Google Scholar]
- 14.May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L'Hotellier V, Morinière C, Descamps P, Procaccio V, Reynier P. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22(6):725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 15.Kristensen SG, Pors SE, Andersen CY. Diving into the oocyte pool. Curr Opin Obstet Gynecol. 2017;29:112–118. doi: 10.1097/GCO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 16.Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131(7–8):536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 19.Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011;84(4):775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33(1):37–44. doi: 10.1016/S0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 21.Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48(5):642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12(11):655–660. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- 23.Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9(11):639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- 24.Tarin JJ, Perez-Albala S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002;61(3):385–397. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- 25.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 26.Muller FL, Lustgarten MS, Jang Y, Richardson A, van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16(10):841–850. doi: 10.1038/sj.cr.7310095. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Z, Huang M, Liu Z, Xie P, Dong Y, Wu X, Qu Z, Shen B, Huang X, Zhang T, Li J, Liu J, Yanase T, Zhou C, Xu Y. Focused screening of mitochondrial metabolism reveals a crucial role for a tumor suppressor Hbp1 in ovarian reserve. Cell Death Differ. 2016;23(10):1602–1614. doi: 10.1038/cdd.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asselin E, Xiao CW, Wang YF, Tsang BK. Mammalian follicular development and atresia: role of apoptosis. Biol Signals Recept. 2000;9(2):87–95. doi: 10.1159/000014627. [DOI] [PubMed] [Google Scholar]
- 31.Tilly JL, Kowalski KI, Johnson AL, Hsueh AJW. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology. 1991;129(5):2799–2801. doi: 10.1210/endo-129-5-2799. [DOI] [PubMed] [Google Scholar]
- 32.Tsai-Turton M, Luderer U. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology. 2006;147(3):1224–1236. doi: 10.1210/en.2005-1281. [DOI] [PubMed] [Google Scholar]
- 33.Luderer U. Ovarian toxicity from reactive oxygen species. Vitam Horm. 2014;94:99–127. doi: 10.1016/B978-0-12-800095-3.00004-3. [DOI] [PubMed] [Google Scholar]
- 34.Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med. 2013;59(2):91–98. doi: 10.3109/19396368.2012.743197. [DOI] [PubMed] [Google Scholar]
- 35.Palacio JR, Iborra A, Ulcova-Gallova Z, Badia R, Martinez P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin Exp Immunol. 2006;144(2):217–222. doi: 10.1111/j.1365-2249.2006.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avila J, et al. Oxidative stress in granulosa-lutein cells from in vitro fertilization patients. Reprod Sci. 2016;23(12):1656–1661. doi: 10.1177/1933719116674077. [DOI] [PubMed] [Google Scholar]
- 37.Victor VM, Rocha M, Bañuls C, Alvarez A, de Pablo C, Sanchez-Serrano M, Gomez M, Hernandez-Mijares A. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab. 2011;96(10):3115–3122. doi: 10.1210/jc.2011-0651. [DOI] [PubMed] [Google Scholar]
- 38.Krishna MB, Joseph A, Thomas PL, Dsilva B, Pillai SM, Laloraya M. Impaired arginine metabolism coupled to a defective redox conduit contributes to low plasma nitric oxide in polycystic ovary syndrome. Cell Physiol Biochem. 2017;43(5):1880–1892. doi: 10.1159/000484107. [DOI] [PubMed] [Google Scholar]
- 39.Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod. 2016;31(9):2090–2097. doi: 10.1093/humrep/dew181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatone C, Amicarelli F. The aging ovary—the poor granulosa cells. Fertil Steril. 2013;99(1):12–17. doi: 10.1016/j.fertnstert.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 41.Ernst E H, Franks S, Hardy K, Villesen P, Lykke-Hartmann K. Granulosa cells from human primordial and primary follicles show differential global gene expression profiles. Human Reproduction. 2018;33(4):666–679. doi: 10.1093/humrep/dey011. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt KL, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18(6):1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 43.Ernst EH, Grøndahl ML, Grund S, Hardy K, Heuck A, Sunde L, Franks S, Andersen CY, Villesen P, Lykke-Hartmann K. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation. Hum Reprod. 2017;32(8):1684–1700. doi: 10.1093/humrep/dex238. [DOI] [PubMed] [Google Scholar]
- 44.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KLT, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 45.Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, Andersen AN, Andersen CY. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod BioMed Online. 2011;22(2):162–171. doi: 10.1016/j.rbmo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Ernst EH et al. Dormancy and activation of human oocytes from primordial and primary follicles: Molecular clues to oocyte regulation. Human Reproduction, 2017. Accepted. [DOI] [PubMed]
- 47.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 48.Kaether C, Haass C, Steiner H. Assembly, trafficking and function of gamma-secretase. Neurodegener Dis. 2006;3(4–5):275–283. doi: 10.1159/000095267. [DOI] [PubMed] [Google Scholar]
- 49.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Banmeyer I, Marchand C, Clippe A, Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 2005;579(11):2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Meng Y, Qian Y, Gao L, Cai LB, Cui YG, Liu JY. Downregulated expression of peroxiredoxin 4 in granulosa cells from polycystic ovary syndrome. PLoS One. 2013;8(10):e76460. doi: 10.1371/journal.pone.0076460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzerova E, Danhauser K, Haack TB, Kremer LS, Melcher M, Ingold I, Kobayashi S, Terrile C, Wolf P, Schaper J, Mayatepek E, Baertling F, Friedmann Angeli JP, Conrad M, Strom TM, Meitinger T, Prokisch H, Distelmaier F. Human thioredoxin 2 deficiency impairs mitochondrial redox homeostasis and causes early-onset neurodegeneration. Brain. 2016;139(Pt 2):346–354. doi: 10.1093/brain/awv350. [DOI] [PubMed] [Google Scholar]
- 53.Kishi I, Ohishi M, Akiba Y, Asada H, Konishi Y, Nakano M, Kamei K, Yoshimura Y, Maruyama T. Thioredoxin, an antioxidant redox protein, in ovarian follicles of women undergoing in vitro fertilization. Endocr J. 2016;63(1):9–20. doi: 10.1507/endocrj.EJ15-0210. [DOI] [PubMed] [Google Scholar]
- 54.Makker A, Goel MM, Mahdi AA. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: an update. J Mol Endocrinol. 2014;53(3):R103–R118. doi: 10.1530/JME-14-0220. [DOI] [PubMed] [Google Scholar]
- 55.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136(8):3665–8. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 56.Friedrich A, Pechstein J, Berens C, Lührmann A. Modulation of host cell apoptotic pathways by intracellular pathogens. Curr Opin Microbiol. 2017;35:88–99. doi: 10.1016/j.mib.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Guo J, He D, Wang L, Kang J, Li N, Yan X, Tang B. L10P mutation in DJ-1 gene induced oxidative stress and mitochondrial disfunction. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(12):1285–1291. doi: 10.11817/j.issn.1672-7347.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Tillman JE, Yuan J, Gu G, Fazli L, Ghosh R, Flynt AS, Gleave M, Rennie PS, Kasper S. DJ-1 binds androgen receptor directly and mediates its activity in hormonally treated prostate cancer cells. Cancer Res. 2007;67(10):4630–4637. doi: 10.1158/0008-5472.CAN-06-4556. [DOI] [PubMed] [Google Scholar]
- 59.Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Mullerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92(3):1034–1040. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- 60.Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, Fan CH, Cai H, Ren Y, Hu ZY, Gao F, Liu YX. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 2010;151(2):774–782. doi: 10.1210/en.2009-0751. [DOI] [PubMed] [Google Scholar]
- 61.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. doi: 10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Niu W, Wang Y, Xu J, Bao X, Wang L, du L, Sun Y. Dehydroepiandrosterone treatment in women with poor ovarian response undergoing IVF or ICSI: a systematic review and meta-analysis. J Assist Reprod Genet. 2016;33(8):981–991. doi: 10.1007/s10815-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim RH, Peters M, Jang YJ, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7(3):263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Kuhlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson RA, McLaughlin M, Wallace WHB, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29(1):97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]