Abstract
Understanding the physiology underlying the complex dialog between the oocyte and its surrounding somatic cells within the ovarian follicle has been crucial in defining optimal procedures for the development of clinical approaches in ART for women suffering from infertility and ovarian dysfunction. Recent studies have implicated oocyte-secreted factors like growth differentiation factor 9 (GDF-9) and bone morphogenetic protein 15 (BMP-15), members of the transforming growth factor-beta (TGFβ) superfamily, as potent regulators of folliculogenesis and ovulation. These two factors act as biologically active heterodimers or as homodimers in a synergistic cooperation. Through autocrine and paracrine mechanisms, the GDF-9 and BMP-15 system has been shown to regulate growth, differentiation, and function of granulosa and thecal cells during follicular development playing a vital role in oocyte development, ovulation, fertilization, and embryonic competence. The present mini-review provides an overview of recent findings relating GDF-9 and BMP-15 as fundamental factors implicated in the regulation of ovarian function and discusses their potential role as markers of oocyte quality in women.
Keywords: TGFβ, GDF-9, BMP-15, Ovarian function, Oocyte quality, Folliculogenesis
The oocyte—a key player in ovarian function
The production of developmentally competent mammalian oocytes engages a series of intercellular signaling events within the ovarian follicle. Neural, endocrine, neuroendocrine, and paracrine/autocrine act in harmony to synchronize communication between the oocyte and somatic cells at sequential stages of folliculogenesis. This two-way communication is essential for the nuclear and cytoplasmic maturation processes that drive the meiotic, fertilization, and early embryonic competencies sustaining embryogenesis. [1–9]
As long ago as 1959, the experiments of Falck and colleagues implicated the oocyte as a source of a luteinization inhibitor in transplantation studies using rabbit follicles placed in ectopic sites [10]. This study was the first to establish the oocyte in controlling follicle luteinization. Although further studies in the 1970s have shown that ablation of the oocyte results in impaired folliculogenesis with spontaneous transformation of Graafian follicles into the corresponding corpus luteum [11, 12], the regulatory capacity of the oocyte in directing its own fate as well as growth and differentiation of the follicle through secretion of oocyte-specific factors has only been discussed for the last two decades [13–20]. The oocyte is in fact an important regulator of GCs and cumulus cell (CC) function. The paracrine factors synthetized and secreted by the oocyte act locally to direct the differentiation and function of GCs. Besides having a mitogenic action, these factors play a critical role in regulating hormones like follicle-stimulating hormone (FSH), growth factor similar to insulin-I and androgens, and overall improve oocyte developmental competence [16, 17, 20–29]. Oocyte factors also have a protective role towards GCs promoting expression of anti-apoptotic proteins Bcl-2 and suppression of pro-apoptotic protein Bax in GCs and CCs by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins [30]. Nowadays, it has been established that ovary-derived transforming growth factor-β (TGF-β) superfamily members play an integral role in inhibiting GC progesterone production with subsequent luteinization and are important modulators of ovarian function [31].
TGF-β superfamily protein members are important regulators of folliculogenesis
The TGF-β superfamily is the largest family of protein secretors in mammals and includes TGF-βs, anti-Mullerian hormone (AMH), activins, inhibins, bone morphogenetic proteins (BMPs), and growth differentiation factors (GDFs). These proteins are synthesized as prepropeptide precursors and then processed and secreted as homodimers or heterodimers. Genes belonging to the TGF-β superfamily regulate many aspects of development by activating transmembrane serine/threonine kinase receptors [32].
Several members of the TGF-β superfamily which include bone morphogenetic proteins (BMPs) and growth differentiation factors (GDFs) have been identified to play essential roles in the regulation of folliculogenesis by paracrine/autocrine mechanisms [31, 33–37]. Initial work showed that two theca cell-derived morphogenetic proteins, BMP-4 and BMP-7, inhibit FSH-dependent progesterone production while stimulating FSH-dependent estradiol production in rat GCs in vitro [38]. Subsequently, it was demonstrated that BMP-15 and BMP-6 also inhibit FSH-stimulated progesterone production without changing estradiol production in rat GCs [37]. In theca cells, BMP-7 has been implicated in promoting vascular endothelial growth factor (VEGF) responsible for the vascular network between theca cell layers and granulosa cells that turn the resource of nutrients to the follicle [39, 40]. Among the oocyte-derived BMP family members, the biological and physiological activities of growth and differentiation factor-9 (GDF-9) and BMP-15 (also called GDF-9B) have been extensively researched, and essential roles for both factors in female fertility have been identified in several mammalian species [34, 37, 41–44].
GDF-9 and BMP-15 signaling pathways
BMP-15 gene is an X-linked gene and encodes a protein that is secreted from oocytes beyond the primordial/primary stages [44]. The GDF-9 gene is located in the long (q) arm of chromosome 5. GDF-9 mRNA is synthesized only in the oocyte from the primary 1-layer follicle stage until after ovulation [45]. Both genes have rapidly evolved when compared to other TGFß family members and have been subjected to positive selection in the mammalian clade evidencing the importance and particular functions of the GDF-9 and BMP-15 proteins produced and consequently their importance in female fertility [46, 47]. The mRNAs of both proteins are expressed in female mice oocytes throughout folliculogenesis with a very low or no expression in oocytes of primordial follicles and a strong expression in oocytes at all stages of developing follicles. GDF-9 mRNA is also detectable in ovulated oocytes [42]. Like other members of the TGF-β superfamily, GDF-9 is synthesized as a prepropeptide which needs processing by furin-like proteases to result in an active mature protein [48].
GDF-9 and BMP-15 have a high degree of amino acid homology and similar protein structure. Both factors are closely associated by expression pattern and function in the ovary and interact with each other [49–51]. Increasing evidence has shown a synergistic relationship between GDF-9 and BMP-15 as GDF-9/BMP-15 heterodimers, recently named as cumulin, act as potent regulators of GCs and CC function and improve oocyte quality [52, 53].
GDF-9 and BMP-15 bind to their corresponding receptors in GCs, leading to a cascade of reactions of downstream genes [49]. Besides significantly improving oocyte developmental competence, these factors act directly on the GCs having critical effects on ovarian function, namely GC proliferation, differentiation, steroidogenesis, apoptosis, and cumulus expansion [6, 13, 15, 17, 30, 34, 35, 37, 68]. Both BMP-15 and GDF-9 bind to specific transmembrane serine-threonine kinase receptors that comprise the TGFβ superfamily and are divided into two subclasses [69]: type I receptors which include seven receptors (ALK 1 to 7) and type II receptors that comprises five receptors (Act RII, Act RIIB, AMHRII, TGF-βRII, and BMPRII). GDF-9 binds to ALK5 [70, 71] and to BMPRII [72, 73]. BMP-15 binds to receptor ALK6 and to BMPRII [34, 52]. When attached to their ligands, type I and type II receptors form a heterodimeric complex where the type II receptor (BMPRII) activates the type I receptor (ALK5 and 6). Once activated, the type I receptor phosphorylates specific receptor-regulatory SMAD proteins (R-SMAD) that propagate the signal to the nucleus via interaction with the common SMAD (Co-SMAD or SMAD4). This SMAD complex then translocates to the nucleus to interact with specific transcription factors to regulate the expression of target genes [40, 52, 69, 74–76]. While BMP-15 joins BMPRII and ALK6 and subsequently activates SMADS 1, 5, and 8, GDF-9 binds to BMPRII and ALK5 receptors and activates SMAD 2 and 3 [40, 70–73, 77]. The BMP-15/GDF-9 heterodimer cumulin needs to bind to the receptor BMPRII-ALK4/5/7-ALK6 complex to activate SMAD 2 and 3 signaling [52, 78–80]. BMP-15 and GDF-9 have synergistic effects and can act through miR-375 to affect the expression levels of type I receptor ALK4 and type II receptor BMPRII and the activation of SMAD signaling pathway, which subsequently affected the proliferation, spread, and apoptosis of cumulus cells [81]. The activation of SMAD signaling by the oocytes and the requirement of SMAD2/3 signaling for cumulus cell expansion can also vary between species. For instance, it is notably contrasted in pigs and mice and involves essential MAPK signals [82]. Furthermore, it has been shown that human GDF-9 and BMP-15 act synergistically to stimulate granulosa cell proliferation, a response that also involves species-specific non-SMAD signaling pathways [79].
Lessons from knock-out mice
The importance of GDF-9 and BMP-15 factors in rats, mice, sheep, porcine, and humans has been shown in individuals that have mutations or deletions of these genes or through in vitro studies through recombinant forms that mimic the oocyte paracrine actions on GCs and CCs [6, 48, 67–72]. The production of a GDF-9-null mouse model by the Matzuk lab was fundamental to unraveling the role of GDF-9 in ovarian function [68]. GDF-9 knockout female mice showed no Graafian follicles, exhibiting a complete absence of normal follicles beyond the primary stage and no differentiation of the follicle compartments, no ovulation, and no pregnancies. While GDF-9 null oocytes displayed normal meiotic competence for early stage follicles, the most striking change that occurs in these oocytes is its increase in size (a 70% increase in volume) in addition to an absence of cortical granules and clustering of organelles around the germinal vesicle suggesting that late secretory events are affected. At the granulosa cell level, changes reflected a decreased mitosis, lack of FSH receptor, overexpression of the kit ligand, and no apoptosis. These mice exhibited large numbers of primordial follicles form and many advances to the point at which the oocyte is fully grown and there is a single layer of cuboidal GCs, but no theca. In the absence of GDF-9, folliculogenesis stops at the primary stage. At about this stage, the oocyte degenerates and an aberrant form of spontaneous luteinization of the follicle occurs [68, 73, 74].
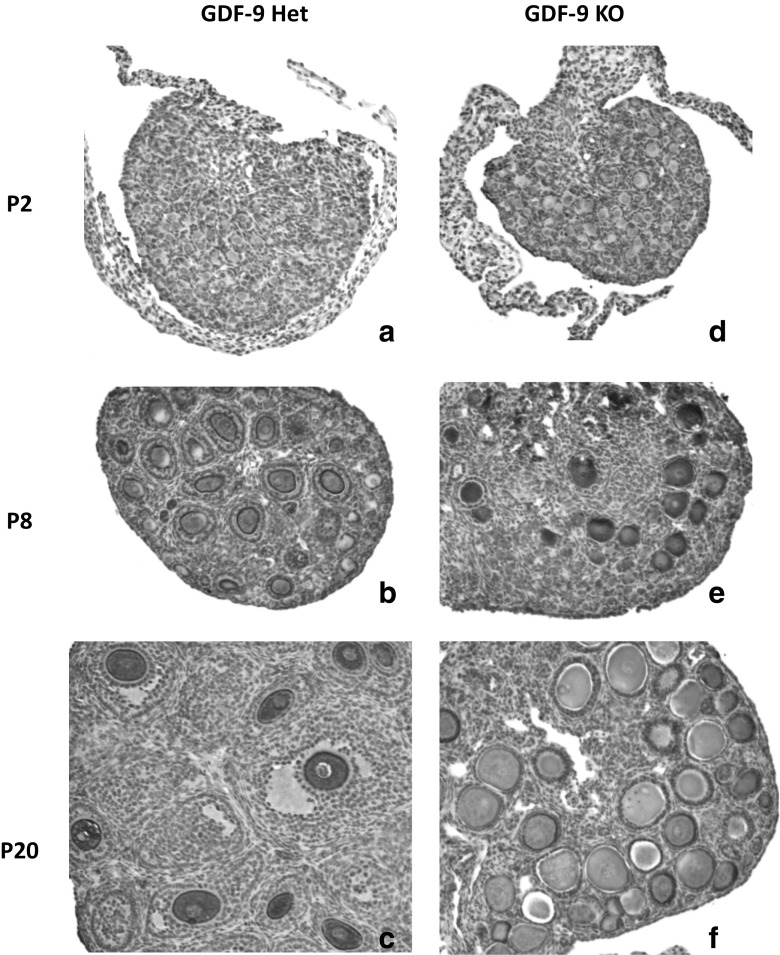
We have established a GDF-9 mice colony with two founder pairs of two null males and two heterozygous females (generous gift from Dr. Martin Matzuk’s lab). Ovaries from null and heterozygous females at different postnatal ages (days 2, 8, and 20) were collected, fixed in 2%PFA, embedded in paraffin, and sectioned. Some of these sections were stained with mouse vasa homolog (MVH, a cytoplasmic germ cell specific protein, gift from Dr. Noce), diluted 1:1200 in 1% BSA, and counterstained with Harris hematoxylin containing 4% acetic acid, developed with horseradish peroxidase avidin D (HRP; Vector; 1:500); diaminobenzidine (DAB) was used as a substrate for HRP. MVH staining allowed us to confirm previous observations regarding follicle and oocyte growth more accurately. Fig. 1c, f clearly illustrate no follicle growth beyond primary stage with fully-grown oocytes (Fig. 1f). We have also observed that at postnatal day 2 (P2), null-GDF-9 ovaries already seem to have more primary follicles than heterozygous ovaries, which may be a sign of premature primordial follicle activation (Fig. 1a, d) [93]. At P8, almost all follicles had fully-grown oocytes in GDF-9 null ovaries (Fig. 1e), whereas the GDF-9 heterozygous ovaries exhibited regular follicle development with several follicle stages, from primordial to secondary (Fig. 1b). Our MVH staining of the oocytes cytoplasm also illustrated well oocyte degeneration in some of the oocytes within the P20 GDF-9 null ovaries (Fig. 1f).
Fig. 1.
Paraffin sections of P2, P8, and P20 ovaries in GDF-9 heterozygous (a, b, c) and GDF-9 KO mice (d, e, f). Stereoscopic images showing the phenotype of the GDF-9 KO mice: absence of secondary follicles (f), activation of the follicular reserve (d), and oocyte continuing growth (f)
These findings are consistent with the fact that in humans, mutations in GDF-9 and BMP-15 have been associated with subfertility, high incidence of dizygotic twins, ovulation defects, and with premature ovarian failure [76–80]. Altered expression of these factors may cause damage to ovarian function and fertility in several species [35, 37, 81, 82].
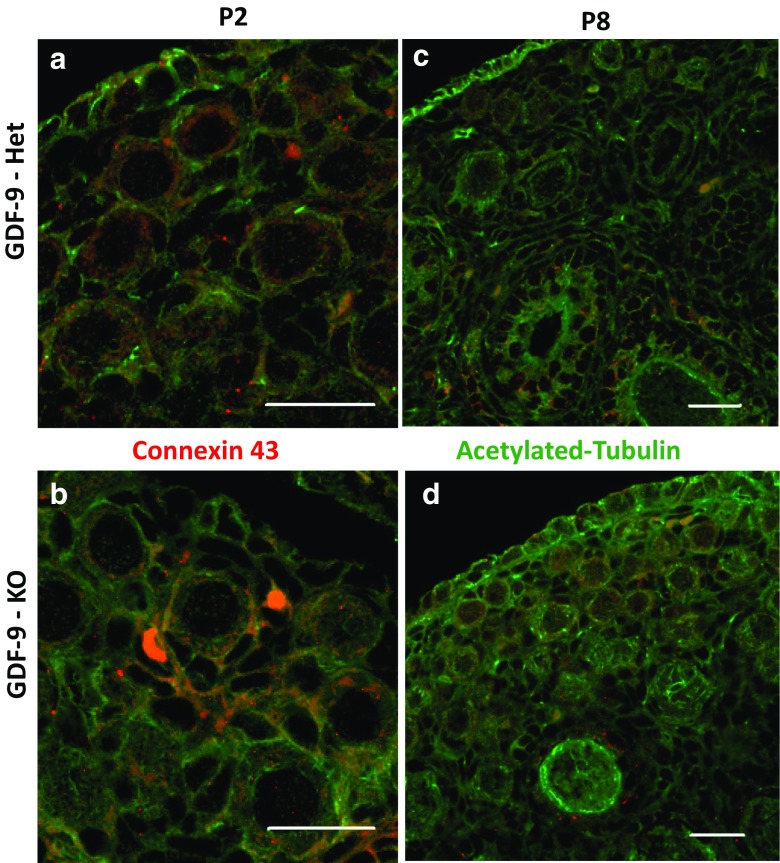
In order to better illustrate the effects of the absence of GDF-9 within the ovary, paraffin sections of P2 and P8 GDF-9-null and heterozygous ovaries were evaluated. After antigen retrieval, and immunolocalization for stable microtubules (acetylated-tubulin, green) and gap junctions (connexin 43 red), these sections were counterstained with Ethidiumhomodimer-2 (Eth-D2) for nucleus staining (white) (Fig. 2a–d). Results have shown that the structural differences between GDF-9 heterozygous and null ovaries were accompanied by the cytoskeleton with acetylated tubulin shaping the follicle structure (Fig. 2a–d). Interestingly, as previously observed, the oocytes in GDF-9-null ovaries at P8 have shown different tubulin distribution and single granulosa cells layer (Fig. 2d), while similar sized oocytes in GDF-9 heterozygous ovaries exhibited at least two layers of granulosa cells (Fig. 2c). Regarding gap-junction distribution, greater differences were observed between GDF-9-null and heterozygous ovaries. The null ovaries have shown irregular gap junctions and in some cases an overexpression of connexin 43 protein in the extracellular matrix of the primordial follicles, if compared with GDF-9 heterozygous follicles (Fig. 2a–b). Unpaired cell-cell communication in GDF-null mice further confirms the importance of GDF-9 in follicle and oocyte development and ultimately in female reproduction in the mouse model.
Fig. 2.
Paraffin sections of P2 and P8 mouse ovaries stained for a microtubule marker, acetylated-tubulin (green), and a gap-junction marker, connexin-43 (red), in GDF-9 Heterozygous (a, c) and GDF-9 KO mice (b, d). It is possible to observe irregular gap junction distribution among the GDF-9 KO ovaries (b–d) within preganulosa and granulosa cells and the absence of secondary or graffian follicles
GDF-9 and BMP impact folliculogenesis and oocyte quality
Both GDF-9 and BMP-15 have been shown to impact folliculogenesis and oocyte quality. Overall GDF-9 has been implicated in follicular development at the transition of the primary follicle to the secondary follicle stage. GDF-9 is also implicated in GC differentiation, specifically in the transition of preantral GC to CC in mice [56]. BMP-15 promotes follicle maturation since the primordial gonadotropin independent phases, regulates GC sensitivity to FSH action, prevents GC apoptosis, increases oocyte developmental competence, and regulates the ovulation quota [34, 36, 40, 57].
Apart from the gonadotrophins secreted by the pituitary gland, oocyte-derived BMP-15 and GDF-9 participate in the modulation of certain target genes related to ovulation and luteinization [34, 58, 59]. Recently, gene expression studies have shown how complex signaling systems are at follicle activation and suggest ways, direct and indirect, through which GDF-9 function may be manifest in normal or pathological ovarian conditions [60].
Both GDF-9 and BMP-15 have been show to interact in modulating the expression of certain proteins in GCs. For example, Kit Ligand (KITL) is critical for the growth of oocytes, and its level of expression is differentially controlled by paracrine and hormonal factors. It has been shown that GDF-9 inhibits the expression of ligand kit by GCs, whereas BMP-15 stimulates its expression [16]. Oocyte-derived GDF-9 and BMP-15 have also been shown to be involved in regulating glycolysis and cholesterol synthesis by granulosa cells, essential processes for nourishing the oocyte with pyruvate, lactate, and products resulting from the cholesterol biosynthetic pathway via gap junctions [54, 55].
In human GCs, intra-ovarian BMPs and TGF-βI modulate expression of connexin 43 (Cx43) and intercellular communication. Specifically, BMP-4, BMP-7, and BMP-15 suppress Cx43 expression, whereas TGF-βI increase Cx43 expression indicating regulatory roles for these factors [61, 62].
BMP-15 inhibits follicular luteinization by decreasing progesterone production through downregulating the expression of the steroidogenic acute regulatory protein (StAR) [83]. Additionally, GDF-9 interacts with pituitary gonadotrophins to further reduce progesterone production. After ovulation, the rapid decrease of these oocyte factors in the corpus luteum leads to elevation of StAR expression and consequent progesterone production [34].
The antral follicle stage is characterized by differentiation of the GCs that surround the oocyte into two functional and anatomical distinct sub lineages, the CCs and the mural GCs [84]. CCs, in contrast to mural GCs, exhibit higher proliferation rate, higher anti-Mullerian hormone expression, lower steroidogenic capacity, and lower LH-receptor expression and have the ability to secrete hyaluronic acid for cumulus oocyte complex (COC) expansion [23, 27, 85].
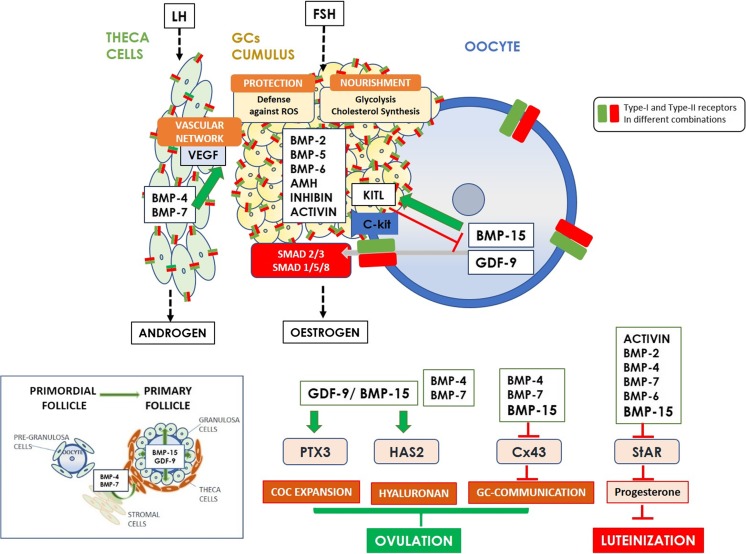
The process of cumulus expansion represents the secretion and apposition of hyaluronan-rich extracellular matrix in the COC and is controlled by endocrine, paracrine, and oocyte-derived factors [86–89]. Hyaluronan, the backbone of the COC extracellular matrix, is the product of the hyaluronan synthase 2 (Has2) and its expression is upregulated by LH. These structure is further stabilized by a complex network of binding proteins, including versican, tumor necrosis factor-stimulated gene 6 protein (TSG-6), inter-α trypsin inhibitor, and pentraxin 3 (PTX3) [90, 91]. BMP-15 and GDF-9 have been implicated in upregulation of the cumulus expansion-related genes Has2 and PTX3 [34, 52] and therefore might be involved in maintaining the CC phenotype by promoting cumulus expansion. Epidermal growth factor (EGF)-like peptides and GDF-9 are also involved in cumulus expansion as GDF-9 induces HAS2 expression and hyaluronan synthesis as well as prostaglandin E2 production which is essential for normal ovulation [92]. Other BMPs have been implicated in remodeling extracellular matrix formation, namely the theca/stroma-derived BMPs (BMP4 and BMP7), decomposing the structure of the extracellular matrix in the neighboring mural GCs to facilitate separation of COC and mural GCs in the human ovary and thus regulating ovulation [34]. A schematic diagram exhibiting the functional roles of the main TGF-β superfamily member proteins in regulating ovarian function is shown in Fig. 3.
Fig. 3.
Schematic diagram exhibiting the functional roles of the main TGF-β superfamily member proteins in regulating ovarian function. GDF-9 and BMP-15 are implicated in the transition of the primordial follicle to the primary follicle stage. In theca cells BMP-7 regulates VEGF and thus the flow of nutrients within the follicle. GCs contribute to oocyte antioxidant defense against reactive oxygen species (ROS) [118] and to oocyte nutrients through glycolysis and cholesterol biosynthesis which is regulated by oocyte-derived GDF-9 and BMP-15. The paracrine interactions between BMP-15 and KITL are an example of a negative feedback system: while oocyte-derived BMP-15 induces KITL expression in granulosa cells, KITL from granulosa cells inhibits BMP-15 secretion by the oocyte. Overall, the roles of GDF-9 and BMP-15 are evidenced in regulating the function of GCs, GC communication, COC expansion and hyaluronan production leading to ovulation, and in preventing luteinization through inhibition of progesterone production
Although GDF-9 and BMP-15 regulate ovarian function, it is important to reinforce the specific species differences between these oocyte factors. These differences may reflect inherent variations between mono- and poly-ovulatory mammals [51, 63, 64]. In fact, BMP-15 action is more important in sheep (mono-ovulatory) rather than mice (poly-ovulatory) in the first stages of follicle development [17]. Knocking-out the BMP-15 gene in mice unexpectedly revealed minimal alterations in follicle development, but defects are confined to the ovulation process, cumulus expansion, and subsequent fertilization and fertility [51, 65]. In contrast, GDF-9 has been shown to be a critical player of follicle growth in mice [66]; it is latent in some species (notably human) and requires activation [67].
Importance of BMP-15 and GDF-9 in human fertility
The importance of BMP-15 and GDF-9 as fertility markers in the human has received wide attention once these factors play a critical role in granulosa cell differentiation and ovarian architecture interfering considerably in all stages of oocyte development and embryonic quality [34, 36, 94]. Abnormal expression of these factors may be related to female infertility. [16]
Higher mature GDF-9 levels in the follicular fluid were significantly correlated with oocyte nuclear maturation and embryo quality [95].
Several BMPs have been implicated in female reproductive pathologies namely failure in embryo implantation and female sterility [96, 97], polycystic ovary syndrome (PCOS), primary ovarian insufficiency (POI) [98], and endometriosis [99]. Also, given that reduced levels of BMP-15 and/or GDF-9 in ewes are associated with increased ovulation rate and litter size, women with GDF-9 (and possibly BMP-15) mutations may have an increased number of dominant follicles, resulting in an increased likelihood of bearing dizygotic twins [100, 101].
Although expression levels of BMP-15 and GDF-9 in the oocytes have shown to be higher in PCOS women, the expression levels of both BMP-15 and GDF-9 in CCs is lower in PCOS women which may result in premature luteinization, poor oocyte competence, and luteal dysfunction, leading to higher miscarriage rates in these patients [102]. In vitro maturation (IVM) protocols that take into account these two factors might have potential therapeutic clinical applications and may help women with PCOS to overcome infertility treatments. Indeed, treatment of COCs with recombinant GDF-9, BMP-15, or denuded oocytes during IVM led to higher rates of blastocyst formation and fetal yield after IVM and in vitro fertilization (IVF) of COCs in cattle [19, 103, 104], in mice [105, 106], in goats [107], and in pigs [108].
Several BMP-15 gene mutations have been reported in POI patients [109–113]. GDF-9 variants do not seem to be involved in POI [114] but the BMP-15 mutations reported in POI patients reduce the synergy between BMP-15 and GDF-9 [115] evidencing the importance of the BMP-15/GFD-9 heterodimers in regulating ovarian function.
Modulation of TGF-β pathways might also influence reproductive disorders. Recently, it was shown that dysregulation of ALK6 in human GCs is associated with reduced ovarian reserve and age-related decline in fertility [116]. Recent studies have demonstrated a favorable effect of BMP-15 in combination with FSH on the in vitro development of small size mouse follicles to antral stage [117].
The BMP/GDF system has been show to control GC development and function, cell-cell communication, steroidogenesis, COC formation and expansion, oocyte maturation, ovulation, and luteolysis. Thus, the physiological roles of BMP-15 and GDF-9 in directing the follicular symphony will provide a significant understanding on ovarian architecture and function and the development of new methodologies in ART. The use of drugs that modulate TGF-β pathways (Kushnir et al. in press) or the inclusion of BMPs and GDFs in in vitro protocols will provide new approaches in fertility treatments.
References
- 1.Richards JS, Fitzpatrick SL, Clemens JW, Morris JK, Alliston T, Sirois J. Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res. 1995;50:223–254. doi: 10.1016/b978-0-12-571150-0.50014-7. [DOI] [PubMed] [Google Scholar]
- 2.McGinnis LK, Limback SD, Albertini DF. Signaling modalities during oogenesis in mammals. Curr Top Dev Biol. 2013;102:227–242. doi: 10.1016/B978-0-12-416024-8.00008-8. [DOI] [PubMed] [Google Scholar]
- 3.Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. [PubMed] [Google Scholar]
- 4.Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Oogenesis: prospects and challenges for the future. J Cell Physiol. 2008;216(2):355–365. doi: 10.1002/jcp.21473. [DOI] [PubMed] [Google Scholar]
- 5.Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226(2):167–179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- 6.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82-83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 8.Albertini DF. The Mammalian Oocyte, in Knobil and Neill's Physiology of Reproduction (Fourth Edition) Editor: A.Z. Tony Plant; 2015. The Mammalian Oocyte; pp. 59–97. [Google Scholar]
- 9.Monniaux D. Driving folliculogenesis by the oocyte-somatic cell dialog: lessons from genetic models. Theriogenology. 2016;86(1):41–53. doi: 10.1016/j.theriogenology.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Falck B. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol Scand Suppl. 1959;47(163):1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 11.el-Fouly MA, et al. Role of the ovum in follicular luteinization. Endocrinology. 1970;87(2):286–293. [PubMed] [Google Scholar]
- 12.Nekola MV, Nalbandov AV. Morphological changes of rat follicular cells as influenced by oocytes. Biol Reprod. 1971;4(2):154–160. doi: 10.1093/biolreprod/4.2.154. [DOI] [PubMed] [Google Scholar]
- 13.Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod BioMed Online. 2007;14(6):758–764. doi: 10.1016/s1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- 14.Erickson GF, Shimasaki S. The role of the oocyte in folliculogenesis. Trends Endocrinol Metab. 2000;11(5):193–198. doi: 10.1016/s1043-2760(00)00249-6. [DOI] [PubMed] [Google Scholar]
- 15.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 16.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 17.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122(6):829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 18.Eppig JJ, Chesnel F, Hirao Y, O'Brien MJ, Pendola FL, Watanabe S, Wigglesworth K. Oocyte control of granulosa cell development: how and why. Hum Reprod. 1997;12(11 Suppl):127–132. [PubMed] [Google Scholar]
- 19.Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296(2):514–521. doi: 10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderhyden BC, et al. Evaluation of members of the TGFbeta superfamily as candidates for the oocyte factors that control mouse cumulus expansion and steroidogenesis. Reprod Suppl. 2003;61:55–70. [PubMed] [Google Scholar]
- 22.Vanderhyden BC, Tonary AM. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol Reprod. 1995;53(6):1243–1250. doi: 10.1095/biolreprod53.6.1243. [DOI] [PubMed] [Google Scholar]
- 23.Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56(4):976–984. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- 24.Gilchrist RB, et al. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119(Pt 18):3811–3821. doi: 10.1242/jcs.03105. [DOI] [PubMed] [Google Scholar]
- 25.Eppig JJ, Pendola FL, Wigglesworth K. Mouse oocytes suppress cAMP-induced expression of LH receptor mRNA by granulosa cells in vitro. Mol Reprod Dev. 1998;49(3):327–332. doi: 10.1002/(SICI)1098-2795(199803)49:3<327::AID-MRD13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 26.Gilchrist RB, Ritter LJ, Armstrong DT. Mouse oocyte mitogenic activity is developmentally coordinated throughout folliculogenesis and meiotic maturation. Dev Biol. 2001;240(1):289–298. doi: 10.1006/dbio.2001.0451. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong DT, Xia P, de Gannes G, Tekpetey FR, Khamsi F. Differential effects of insulin-like growth factor-I and follicle-stimulating hormone on proliferation and differentiation of bovine cumulus cells and granulosa cells. Biol Reprod. 1996;54(2):331–338. doi: 10.1095/biolreprod54.2.331. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. 2000;63(3):839–845. doi: 10.1095/biolreprod63.3.839. [DOI] [PubMed] [Google Scholar]
- 29.Coskun S, Uzumcu M, Lin YC, Friedman CI, Alak BM. Regulation of cumulus cell steroidogenesis by the porcine oocyte and preliminary characterization of oocyte-produced factor(s) Biol Reprod. 1995;53(3):670–675. doi: 10.1095/biolreprod53.3.670. [DOI] [PubMed] [Google Scholar]
- 30.Hussein TS, et al. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118(Pt 22):5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 31.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 32.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23(6):787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 33.Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11(2):143–160. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- 34.Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. 2016;23(1):1–18. doi: 10.1093/humupd/dmw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Castro FC, Cruz MH, Leal CL. Role of growth differentiation factor 9 and bone morphogenetic protein 15 in ovarian function and their importance in mammalian female fertility - a review. Asian-Australas J Anim Sci. 2016;29(8):1065–1074. doi: 10.5713/ajas.15.0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persani L, Rossetti R, di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update. 2014;20(6):869–883. doi: 10.1093/humupd/dmu036. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78(1):9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimasaki S, Zachow RJ, Li D, Kim H, Iemura SI, Ueno N, Sampath K, Chang RJ, Erickson GF. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A. 1999;96(13):7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and localisation of vascular endothelial growth factor and basic fibroblast growth factor during the final growth of bovine ovarian follicles. J Endocrinol. 2000;167(3):371–382. doi: 10.1677/joe.0.1670371. [DOI] [PubMed] [Google Scholar]
- 40.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25(1):72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 41.McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268(5):3444–3449. [PubMed] [Google Scholar]
- 42.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9(1):131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 43.Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O. A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech Dev. 1998;78(1–2):135–140. doi: 10.1016/s0925-4773(98)00161-0. [DOI] [PubMed] [Google Scholar]
- 44.Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12(12):1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 45.Incerti B, Dong J, Borsani G, Matzuk MM. Structure of the mouse growth/differentiation factor 9 gene. Biochim Biophys Acta. 1994;1222(1):125–128. doi: 10.1016/0167-4889(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad Hafiz Ishfaq, Liu Guiqiong, Jiang Xunping, Edallew Shishay Girmay, Wassie Teketay, Tesema Birhanu, Yun Yu, Pan Liu, Liu Chenhui, Chong Yuqing, Yu Zhao Jia, Jilong Han. Maximum-likelihood approaches reveal signatures of positive selection in BMP15 and GDF9 genes modulating ovarian function in mammalian female fertility. Ecology and Evolution. 2017;7(21):8895–8902. doi: 10.1002/ece3.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad HI, Ahmad MJ, Adeel MM, Asif AR, du X. Positive selection drives the evolution of endocrine regulatory bone morphogenetic protein system in mammals. Oncotarget. 2018;9(26):18435–18445. doi: 10.18632/oncotarget.24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mottershead DG, Pulkki MM, Muggalla P, Pasternack A, Tolonen M, Myllymaa S, Korchynskyi O, Nishi Y, Yanase T, Lun S, Juengel JL, Laitinen M, Ritvos O. Characterization of recombinant human growth differentiation factor-9 signaling in ovarian granulosa cells. Mol Cell Endocrinol. 2008;283(1–2):58–67. doi: 10.1016/j.mce.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Paulini F, Melo EO. The role of oocyte-secreted factors GDF-9 and BMP-15 in follicular development and oogenesis. Reprod Domest Anim. 2011;46(2):354–361. doi: 10.1111/j.1439-0531.2010.01739.x. [DOI] [PubMed] [Google Scholar]
- 50.McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL. The proregion of mouse BMP-15 regulates the cooperative interactions of BMP-15 and GDF-9. Biol Reprod. 2008;79(5):889–896. doi: 10.1095/biolreprod.108.068163. [DOI] [PubMed] [Google Scholar]
- 51.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 52.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110(8):E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mottershead DG, Sugimura S, al-Musawi SL, Li JJ, Richani D, White MA, Martin GA, Trotta AP, Ritter LJ, Shi J, Mueller TD, Harrison CA, Gilchrist RB. Cumulin, an oocyte-secreted heterodimer of the transforming growth factor-beta family, is a potent activator of granulosa cells and improves oocyte quality. J Biol Chem. 2015;290(39):24007–24020. doi: 10.1074/jbc.M115.671487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP-15 and GDF-9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 55.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP-15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134(14):2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 56.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 2007;305(1):300–311. doi: 10.1016/j.ydbio.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashimoto O, Moore RK, Shimasaki S. Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci U S A. 2005;102(15):5426–5431. doi: 10.1073/pnas.0409533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pangas SA, Matzuk MM. The art and artifact of GDF-9 activity: cumulus expansion and the cumulus expansion-enabling factor. Biol Reprod. 2005;73(4):582–585. doi: 10.1095/biolreprod.105.042127. [DOI] [PubMed] [Google Scholar]
- 59.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120(Pt 8):1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 60.Ernst EH, Franks S, Hardy K, Villesen P, Lykke-Hartmann K. Granulosa cells from human primordial and primary follicles show differential global gene expression profiles. Hum Reprod. 2018;33(4):666–679. doi: 10.1093/humrep/dey011. [DOI] [PubMed] [Google Scholar]
- 61.Chang HM, Cheng JC, Leung PC. Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J Clin Endocrinol Metab. 2013;98(3):E437–E445. doi: 10.1210/jc.2012-3851. [DOI] [PubMed] [Google Scholar]
- 62.Chang HM, Cheng JC, Taylor E, Leung PCK. Oocyte-derived BMP-15 but not GDF-9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells. Mol Hum Reprod. 2014;20(5):373–383. doi: 10.1093/molehr/gau001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 64.Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP-15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25(3):279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 65.Gueripel X, Brun V, Gougeon A. Oocyte bone morphogenetic protein 15, but not growth differentiation factor 9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion. Biol Reprod. 2006;75(6):836–843. doi: 10.1095/biolreprod.106.055574. [DOI] [PubMed] [Google Scholar]
- 66.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13(6):1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 67.Li Jing-Jie, Sugimura Satoshi, Mueller Thomas D., White Melissa A., Martin Georgia A., Ritter Lesley J., Liang Xiao-Yan, Gilchrist Robert B., Mottershead David G. Modifications of Human Growth Differentiation Factor 9 to Improve the Generation of Embryos From Low Competence Oocytes. Molecular Endocrinology. 2015;29(1):40–52. doi: 10.1210/me.2014-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hennet ML, Combelles CM. The antral follicle: a microenvironment for oocyte differentiation. Int J Dev Biol. 2012;56(10–12):819–831. doi: 10.1387/ijdb.120133cc. [DOI] [PubMed] [Google Scholar]
- 69.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15(1):1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem. 2003;278(1):304–310. doi: 10.1074/jbc.M207362200. [DOI] [PubMed] [Google Scholar]
- 71.Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJW. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol. 2004;18(3):653–665. doi: 10.1210/me.2003-0393. [DOI] [PubMed] [Google Scholar]
- 72.Vitt UA, Mazerbourg S, Klein C, Hsueh AJW. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod. 2002;67(2):473–480. doi: 10.1095/biolreprod67.2.473. [DOI] [PubMed] [Google Scholar]
- 73.Mazerbourg S, Hsueh AJ. Genomic analyses facilitate identification of receptors and signalling pathways for growth differentiation factor 9 and related orphan bone morphogenetic protein/growth differentiation factor ligands. Hum Reprod Update. 2006;12(4):373–383. doi: 10.1093/humupd/dml014. [DOI] [PubMed] [Google Scholar]
- 74.Franzen P, et al. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993;75(4):681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 75.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 76.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7(12):1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 77.Reader KL, Heath DA, Lun S, McIntosh CJ, Western AH, Littlejohn RP, McNatty KP, Juengel JL. Signalling pathways involved in the cooperative effects of ovine and murine GDF-9+BMP-15-stimulated thymidine uptake by rat granulosa cells. Reproduction. 2011;142(1):123–131. doi: 10.1530/REP-10-0490. [DOI] [PubMed] [Google Scholar]
- 78.Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF-9 and BMP-15. Mol Hum Reprod. 2012;18(3):121–128. doi: 10.1093/molehr/gar056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reader KL, Mottershead DG, Martin GA, Gilchrist RB, Heath DA, McNatty KP, Juengel JL. Signalling pathways involved in the synergistic effects of human growth differentiation factor 9 and bone morphogenetic protein 15. Reprod Fertil Dev. 2016;28(4):491–498. doi: 10.1071/RD14099. [DOI] [PubMed] [Google Scholar]
- 80.Chen H, Liu C, Jiang H, Gao Y, Xu M, Wang J, Liu S, Fu Y, Sun X, Xu J, Zhang J, Dai L. Regulatory role of miRNA-375 in expression of BMP-15/GDF-9 receptors and its effect on proliferation and apoptosis of bovine cumulus cells. Cell Physiol Biochem. 2017;41(2):439–450. doi: 10.1159/000456597. [DOI] [PubMed] [Google Scholar]
- 81.Liu C, Yuan B, Chen H, Xu M, Sun X, Xu JJ, Gao Y, Chen C, Jiang H, Zhang J. Effects of MiR-375-BMPR2 as a key factor downstream of BMP-15/GDF-9 on the Smad1/5/8 and Smad2/3 signaling pathways. Cell Physiol Biochem. 2018;46(1):213–225. doi: 10.1159/000488424. [DOI] [PubMed] [Google Scholar]
- 82.Gilchrist RB, Ritter LJ. Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion. Reproduction. 2011;142(5):647–657. doi: 10.1530/REP-11-0196. [DOI] [PubMed] [Google Scholar]
- 83.Chang HM, Cheng JC, Klausen C, Leung PCK. BMP-15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol. 2013;27(12):2093–2104. doi: 10.1210/me.2013-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albertini DF, et al. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 85.Grondahl ML, et al. Anti-Mullerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod BioMed Online. 2011;22(4):389–398. doi: 10.1016/j.rbmo.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Dekel N, Kraicer PF. Induction in vitro of mucification of rat cumulus oophorus by gonadotrophins and adenosine 3′,5′-monophosphate. Endocrinology. 1978;102(6):1797–1802. doi: 10.1210/endo-102-6-1797. [DOI] [PubMed] [Google Scholar]
- 87.Eppig JJ. Regulation of cumulus oophorus expansion by gonadotropins in vivo and in vitro. Biol Reprod. 1980;23(3):545–552. doi: 10.1095/biolreprod23.3.545. [DOI] [PubMed] [Google Scholar]
- 88.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 89.Fang L, Cheng JC, Chang HM, Sun YP, Leung PCK. EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J Clin Endocrinol Metab. 2013;98(12):4932–4941. doi: 10.1210/jc.2013-2662. [DOI] [PubMed] [Google Scholar]
- 90.Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24(4):217–227. doi: 10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- 91.Baranova NS, Inforzato A, Briggs DC, Tilakaratna V, Enghild JJ, Thakar D, Milner CM, Day AJ, Richter RP. Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. J Biol Chem. 2014;289(44):30481–30498. doi: 10.1074/jbc.M114.568154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, Tian S, Klausen C, Zhu H, Liu R, Leung PCK. Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells. Mol Cell Endocrinol. 2016;428:17–27. doi: 10.1016/j.mce.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 93.Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF-9 and BMP-15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol Reprod. 2004;70(4):900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 94.Wu YT, et al. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod. 2007;22(6):1526–1531. doi: 10.1093/humrep/dem029. [DOI] [PubMed] [Google Scholar]
- 95.Gode F, et al. Influence of follicular fluid GDF-9 and BMP-15 on embryo quality. Fertil Steril. 2011;95(7):2274–2278. doi: 10.1016/j.fertnstert.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 96.Clementi C, et al. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 2013;9(11):e1003863. doi: 10.1371/journal.pgen.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagashima T, et al. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest. 2013;123(6):2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takebayashi K, et al. Mutation analysis of the growth differentiation factor-9 and -9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril. 2000;74(5):976–979. doi: 10.1016/s0015-0282(00)01539-9. [DOI] [PubMed] [Google Scholar]
- 99.Crispi S, et al. Transcriptional profiling of endometriosis tissues identifies genes related to organogenesis defects. J Cell Physiol. 2013;228(9):1927–1934. doi: 10.1002/jcp.24358. [DOI] [PubMed] [Google Scholar]
- 100.Belli M, Shimasaki S. Molecular aspects and clinical relevance of GDF-9 and BMP-15 in ovarian function. Vitam Horm. 2018;107:317–348. doi: 10.1016/bs.vh.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tong S, Short RV. Dizygotic twinning as a measure of human fertility. Hum Reprod. 1998;13(1):95–98. doi: 10.1093/humrep/13.1.95. [DOI] [PubMed] [Google Scholar]
- 102.Zhao SY, et al. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94(1):261–267. doi: 10.1016/j.fertnstert.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 103.Dey SR, et al. Coculturing denuded oocytes during the in vitro maturation of bovine cumulus oocyte complexes exerts a synergistic effect on embryo development. Theriogenology. 2012;77(6):1064–1077. doi: 10.1016/j.theriogenology.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 104.Oocyte-secreted factors in oocyte maturation media enhance subsequent development of bovine cloned embryos - Su - 2014 - Mol Reprod Dev - Wiley Online Library. 2018. [DOI] [PubMed]
- 105.Sudiman J, et al. Effects of differing oocyte-secreted factors during mouse in vitro maturation on subsequent embryo and fetal development. J Assist Reprod Genet. 2014;31(3):295–306. doi: 10.1007/s10815-013-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeo CX, et al. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod. 2008;23(1):67–73. doi: 10.1093/humrep/dem140. [DOI] [PubMed] [Google Scholar]
- 107.Romaguera R, et al. Oocyte secreted factors improve embryo developmental competence of COCs from small follicles in prepubertal goats. Theriogenology. 2010;74(6):1050–1059. doi: 10.1016/j.theriogenology.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 108.Gomez MN, et al. Effect of oocyte-secreted factors on porcine in vitro maturation, cumulus expansion and developmental competence of parthenotes. Zygote. 2012;20(2):135–145. doi: 10.1017/S0967199411000256. [DOI] [PubMed] [Google Scholar]
- 109.Ferrarini E, et al. Clinical characteristics and genetic analysis in women with premature ovarian insufficiency. Maturitas. 2013;74(1):61–67. doi: 10.1016/j.maturitas.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 110.Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol. 2010;45(5):257–279. doi: 10.1677/JME-10-0070. [DOI] [PubMed] [Google Scholar]
- 111.Tiotiu D, et al. Variants of the BMP-15 gene in a cohort of patients with premature ovarian failure. Hum Reprod. 2010;25(6):1581–1587. doi: 10.1093/humrep/deq073. [DOI] [PubMed] [Google Scholar]
- 112.Auclair S, et al. Positive selection in bone morphogenetic protein 15 targets a natural mutation associated with primary ovarian insufficiency in human. PLoS One. 2013;8(10):e78199. doi: 10.1371/journal.pone.0078199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Di Pasquale E, et al. Identification of new variants of human BMP-15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 2006;91(5):1976–1979. doi: 10.1210/jc.2005-2650. [DOI] [PubMed] [Google Scholar]
- 114.Kumar R, et al. BMP-15 and GDF-9 gene mutations in premature ovarian failure. J Reprod Infertil. 2017;18(1):185–189. [PMC free article] [PubMed] [Google Scholar]
- 115.Patino LC, et al. BMP-15 mutations associated with primary ovarian insufficiency reduce expression, activity, or synergy with GDF-9. J Clin Endocrinol Metab. 2017;102(3):1009–1019. doi: 10.1210/jc.2016-3503. [DOI] [PubMed] [Google Scholar]
- 116.Regan SL, et al. Dysregulation of granulosal bone morphogenetic protein receptor 1B density is associated with reduced ovarian reserve and the age-related decline in human fertility. Mol Cell Endocrinol. 2016;425:84–93. doi: 10.1016/j.mce.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 117.Salehnia M, Pajokh M, Ghorbanmehr N. Short term organ culture of mouse ovary in the medium supplemented with bone morphogenetic protein 15 and follicle stimulating hormone: a morphological, hormonal and molecular study. J Reprod Infertil. 2016;17(4):199–207. [PMC free article] [PubMed] [Google Scholar]
- 118.Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86(2):27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]