Abstract
Purpose
Epidemiologic data suggest that in vitro fertilization (IVF) is associated with an increased risk of disorders of placentation including preeclampsia and fetal growth restriction. Specifically, studies have demonstrated that singleton pregnancies conceived following a fresh embryo transfer are at an increased risk of delivering an infant with low birth weight compared to those conceived following a frozen embryo transfer. The mechanism responsible for this association remains unclear. Procedures utilized in IVF have also been linked with epigenetic changes and gene expression changes in both fetal and maternal tissues. Data suggest that modifications in the maternal endometrium can lead to disordered trophoblast invasion and placentation. This study examines the effect of ovarian stimulation on endometrial gene expression and DNA methylation during the window of implantation to examine potential pathways playing a role in the adverse outcomes associated with IVF.
Methods
Endometrial biopsies were obtained from oocyte donors and age-matched naturally cycling women 11 days following oocyte retrieval in donors or 12 days following luteinizing hormone (LH) surge in naturally cycling women. Global gene expression was analyzed via Affymetrix Human Gene 1.1 ST array and confirmed with RT-qPCR. DNA methylation was assessed with the Infinium DNA methylation 450 K BeadChip.
Results
Analysis of endometrial gene expression from 23 women (11 oocyte donors and 12 controls) demonstrated 165 genes with a greater than twofold change in expression between donors and controls. While there were 785 genes with significant differential methylation in the endometrium of donors when compared with control subjects, none of the genes with altered expression showed significant changes in DNA methylation. Analysis of the differentially expressed genes showed enrichment for genes involved in endometrial remodeling including PLAT, HSPE2, MMP2, and TIMP1. Validation studies using RT-qPCR found a 73% reduction in expression of heparanase 2 (HSPE2) an enzyme associated with both angiogenesis and cell invasion, a greater than twofold increase in tissue-type plasminogen activator (PLAT), a serine protease participating in matrix degradation, and a 70% increase in MMP2, a gelatinase involved in collagen and fibronectin breakdown.
Conclusions
Superovulation alters expression of genes critical to endometrial remodeling during early implantation. Such changes could lead to altered trophoblast migration and impaired endovascular invasion. These findings offer a potential mechanism for the adverse perinatal outcomes observed following embryo transfer during fresh IVF cycles.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1244-z) contains supplementary material, which is available to authorized users.
Keywords: Superovulation, Endometrium, Extracellular matrix, Gene expression, Epigenetics
Introduction
Singleton pregnancies conceived through in vitro fertilization (IVF) have an increased risk of adverse outcomes including growth restriction, preeclampsia, and placental abruption [1–8]. Animal studies have confirmed that at least some of these outcomes are associated with the IVF procedures themselves, and are not solely due to the underlying infertility diagnosis [9–17]. To reduce the incidence of adverse pregnancy outcomes following assisted reproductive technologies (ART), it is critical to identify the modifiable factors contributing to these outcomes.
One intervention that has been consistently associated with adverse outcomes in both human and animal studies is superovulation with gonadotropins [16, 18–22]. In fresh autologous IVF cycles, the oocyte, the developing embryo, and the maternal endometrium are all exposed to a supraphysiologic hormonal environment created through superovulation. Epidemiologic studies have suggested that singleton pregnancies conceived following an autologous fresh IVF cycle with blastocyst transfer may be more likely to be affected by preeclampsia and result in infants small for gestational age [23] when compared to infants born following a frozen embryo transfer cycle, when the hormonal environment during implantation is more physiologic [24–36]. In addition, clinical studies have demonstrated an association between response to gonadotropins and adverse perinatal outcomes in fresh IVF cycles: women with a vigorous response to gonadotropins, reflected by high estradiol levels on the day of hCG administration, have an increased rate of small for gestational age offspring as well a greater risk of preeclampsia [37, 38]. These findings suggest that the peri-implantation hormonal environment is affecting placentation and fetal growth.
These data are not surprising as studies in both humans and in animal models demonstrate that many preexisting maternal conditions, as well as various peri-conception exposures, increase the risk of adverse perinatal outcomes [5, 6, 35, 39–47]. However, the exact mechanisms responsible for the effects of superovulation on placentation are largely unknown. Many adverse pregnancy outcomes, including preeclampsia, intrauterine fetal growth restriction, preterm birth, and placenta accreta, have all been associated with abnormal trophoblast (fetal-derived placental precursors) migration and invasion, a critical step of embryo implantation and placentation [48–51]. While trophoblast invasion is under some autocrine control, there is accumulating evidence that signals originating in maternal decidua regulate early placentation [52]. Therefore, we hypothesized that changes to the maternal decidua, even prior to the arrival of the embryo, were responsible for, at least some of the adverse outcomes associated with IVF. Several prior studies have demonstrated that superovulation leads gene expression changes in the endometrium [53–58]. However, these studies have yet to uncover a link between the reported gene expression changes and the adverse outcomes associated with ART.
We, and others, have demonstrated that IVF leads to epigenetic changes in placental and fetal tissue [16, 59–63]. In addition, previous data has shown that the endometrium undergoes epigenetic changes throughout the menstrual cycle and in response to steroid hormone administration [64, 65]. Specifically, variation in DNA methylation and expression of correlating genes has been observed throughout the menstrual cycle and in response to estrogen and progesterone, demonstrating that the epigenetic profile of the endometrium is dynamic and responsive to steroid hormone exposure [65]. These findings led us to hypothesize that gene expression changes seen in the endometrium following gonadotropin stimulation may be due to epigenetic modifications resulting from superovulation. Therefore, in this study we examine gene expression in the decidualized endometrium following superovulation and during natural cycles to identify novel genes that could affect early placentation and assess whether the changes observed in gene expression are due to changes in DNA methylation following superovulation.
Materials and methods
Human subjects
This is a prospective pilot cohort study of women undergoing donor oocyte IVF cycles and age-matched controls who consented to endometrial biopsy, genetic, and epigenetic studies. Studies were approved by the IRB of the University of Pennsylvania and written informed consent was obtained from all subjects. All women were between the ages of 21 and 40, with no significant medical history and no history of infertility (defined as 1 year of attempted pregnancy without conception) with regular menstrual cycles. Oocyte donors underwent ovarian stimulation with recombinant or purified-urinary follicle stimulating hormone and/or human menopausal in either a luteal phase Lupron protocol or a GnRH antagonist protocol. Gonadotropin dose was chosen based on patient characteristics and was adjusted during stimulation as clinically indicated based on patient response. Oocyte maturation was induced with Novarel® (Ferring) administered intramuscularly followed by transvaginal egg retrieval 36 h later. Fertilization, by intracytoplasmic sperm injection (ICSI) was performed for male factor as clinically indicated. Conventional insemination or ICSI and embryo culture were performed utilizing appropriate media (VitroLife; Gothenburg, Sweden) in microdroplets under oil. Endometrial biopsies were taken from donors 11 days after ultrasound-guided oocyte retrieval. Controls were healthy women with regular menstrual cycles, not attempting pregnancy, with no recent exposure (at least 3 months) to hormonal contraception, no history of infertility (defined as 1 year of attempted pregnancy without conception), and a documented negative pregnancy test. Endometrial biopsy was obtained 12 days after an LH surge, which was detected in the urine by the Clearblue® Ovulation Test. Samples were obtained using a Pipelle® Endometrial Suction Curette (CooperSurgical). Samples were collected in RNA later and stored at − 20 °C until DNA and RNA extraction.
DNA/RNA extraction
Genomic DNA and total RNA were isolated from each sample using Qiagen Allprep DNA/RNA Mini Kit per manufacturer’s instructions. DNA and RNA concentrations and quality were inspected and quantified by a NanoDrop spectrophotometer (Thermo Fischer Scientific).
Gene expression
Synthesis of cDNA, amplification, generation of sense-target cDNA, fragmentation, and labeling were performed using the WT-Ovation Pico RNA Amplification system, WT-Ovation Exon Module, and FL-Ovation cDNA Biotin Module V2 (NuGEN Technologies, Inc.) per the manufacturer’s protocol. All samples were processed together, and 5 μg of RNA from each sample was submitted to the Penn Micro-Array Facility for Gene-Chip Hybridization. Samples of cDNA were hybridized to the Affymetrix GeneChip Array, then washed, and stained on fluidics stations and scanned at a resolution of 3 lm according to the manufacturer’s instructions.
Statistical analysis of the microarray data was performed by the Penn Bioinformatics Core using Partek Genomics Suite to generate a gene list with statistical differences in gene expression. Hierarchical clustering analysis and principal components analysis were performed to assess the variability of the samples. A false-discovery rate of 0.05 was used to generate a list of genes with statistically significant differences in endometrial expression between the endometrium from donors and the controls. Principal component analysis was used to visualize global variation among the samples. The Database for Annotation, Visualization, and Integrated Discovery [66] and Ingenuity Pathway Analysis (IPA) was used to assess whether the resulting gene list represented disruption of specific pathways or molecular interactions.
Validation of gene expression changes
Validation of microarray gene expression by quantitative polymerase chain reaction (qPCR) was performed using the same set of samples used for microarray. Synthesis of cDNA was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) using 2 μg of RNA. Real-time quantitative PCR (qRT-PCR) was performed with the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Each reaction was performed in 10 μl with 50 ng of cDNA template. Each sample was run in triplicate, and the reactions were carried out for 40 cycles. The following taqman probes were used CTSL(Hs00952036_m1), MMP2(Hs01548727_m1), MMP26(Hs00983740_m1), SERPINA5(Hs04333915_m1), TIMP(Hs00171558_m1), HPSE2(Hs00222435_m1), and PLAT (Hs00263492_m1). Gene expression was normalized to reference gene RNAPOL2 (HS00172187_m1), and relative gene expression was calculated using the ΔΔCT method [67]. Fold change is expressed as mean ± standard deviation (SD). Comparison of gene expression between the endometrium of donors and controls was performed using a two-tailed Student’s t test with a p < 0.05 considered significant.
DNA methylation analysis
Genomic DNA (1 μg) was bisulfite converted using the EpiTect bisulfite kit (Qiagen, Valencia, CA USA) according to the manufacturer’s protocol. Samples were submitted to the Center for Applied Genomics (Children’s Hospital of Philadelphia) for methylation analysis on the Illumina Infinium Methylation Assay. Whole-genome DNA methylation of all 24 samples were profiled by the Illumina Infinium HumanMethylation450 BeadChip (Illumina, San Diego, CA), following the Illumina Infinium HD Methylation protocol [68]. The Infinium 450 K methylation array, interrogates over 485,000 CpG sites with an average of 17CpGs per gene, distributed over promoter, 5′UTR, exons, and 3′UTRs. Raw methylation value (β value) of each CpG ranges from 0% (completely unmethylated) to 100% (completely methylated) and was preprocessed by internal control probes specifically designed for the HumanMethylation450 BeadChip. CpG probes were excluded if they had intensities indistinguishable from background noises or were non-CpG probes, SNP-CpG probes, or cross-reactive probes [69]. Smooth quantile normalization approach [70] and beta-mixture quantile normalization method (BMIQ) [71] were applied to adjust for the potential biases due to two color channels and two types of CpG probes. Parametric empirical Bayes frameworks [72] was used to eliminate potential batch effect (3 different chips used for 24 samples), which initially accounted for 5.8% of total variability of the raw DNA methylation data and nearly 0% after data normalization. After above quality control steps, 428,034 eligible CpGs remained for subsequent differential analysis. The majority of CpGs covers 99% of RefSeq genes spanning from promoter region (41%), gene body (31%) to 3′UTR region (3%), and about 25% are located in intergenic regions [68].
Linear regression models were used to compare β levels of 428,034 CpGs between donor and control groups, adjusted for age. Instead of controlling for type I error rates, we controlled the false discovery rate (FDR) at the level of 0.05 [73]. Statistical analysis was carried out using the R package version 3.1.1.
Results
Patient characteristics
Twelve control endometrial biopsies and 12 endometrial biopsies from egg donors were collected and analyzed for DNA methylation and gene expression by microarray. After the biopsies were collected, one donor was excluded from the analysis because she was noted to have received a gonadotropin-releasing hormone agonist (leuprolide acetate) for final oocyte administration, as opposed to hCG. There was no difference in median age between controls and donors (controls 24 years vs donors 25 years, p = 0.83). Of the 11 donor cycles, 4 received a downregulation protocol with luteal phase leuprolide acetate, while 7 received a gonadotropin releasing hormone antagonist protocol. Duration of stimulation ranged from 8 to 12 days (median 11 days); estradiol values in the donors on day of hCG ranged from 1213 to 3270 pg/mL (median 2143 pg/mL), and total gonadotropin dose ranged from 1300 to 7225 IU (median 1550 IU).
Gene expression
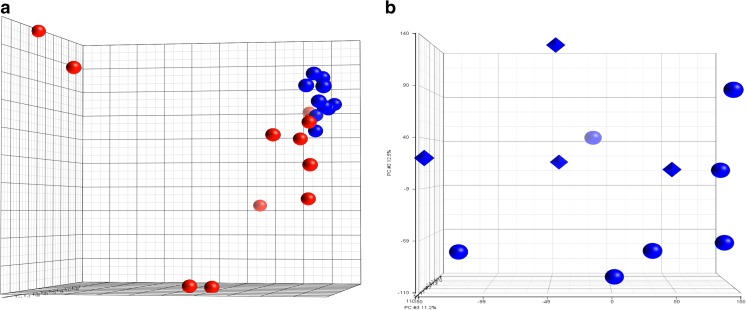
Gene expression changes between the endometrium of donors and controls were compared using the Affymetrix Human Gene 2.0 ST array. This array covers greater than 44,000 genes and contains over six million distinct probes. Using a false discovery rate of 0.05, we found that 641 genes had a greater than 1.5-fold change in expression, and 165 genes had a greater than twofold change in gene expression between donors and controls (Supplemental Table 1). Principle component analysis showed tight clustering of the donor samples while endometrial expression in the controls showed marked variability between samples (Fig. 1a). As our donors had received two different stimulation protocols, we performed principle component analysis on the endometrium of oocyte donors based on protocol and found no clustering of gene-expression by stimulation protocol (Fig. 1b).
Fig. 1.
Pricipal Component Analysis oof Endometrial gene expression. a Principal Component Analysis performed on naturally cycling controls compared to oocyte donors based on the results of microarray gene analysis. (Red: Controls, Blue: Oocyte donors) b Principal Component Analysis performed on oocyte donors only base on stimulation protocol (Circles: Antagonist, Diamonds: Luteal-phase lupron)
Ingenuity pathway analysis identified a number of networks and pathways playing a role in early trophoblast invasion and placentation. Specifically, the top network identified in our differentially expressed gene list was connective tissue disorders, while the top three canonical pathways identified were natural killer cell signaling, coagulation system, and extrinsic prothrombin activation pathway. As expected, among the top upstream regulators was progesterone, in addition to dexamethasone and IL1β. Progesterone mediated expression of 20 of the differentially expressed genes including MMP26.
Further analysis of our networks and pathways showed enrichment for multiple genes involved in extracellular matrix degradation, specifically, proteases, glycosidases, and their inhibitors. Real-time qPCR was utilized to validate a number of these genes on the microarray. We found a 73% reduction in expression of heparanase 2 (HSPSE2), an enzyme associated with both angiogenesis and cell invasion (p = 0.0007), as well as a 81% reduction in cathepsin L, a cysteine protease that cleaves the N-terminal of collagen (p = 0.01). (Fig. 2a, b). There was also a statistically significant reduction in SERPINA5 and MMP26 (p < 0.05), though there was great variation among individuals for these transcripts. (Fig. 2c, d) Conversely, superovulated endometrium demonstrated a greater than twofold increase in tissue-type plasminogen activator (PLAT), a serine protease participating in matrix degradation (p = 0.01) (Fig. 2e). There was also a 70% increase in MMP2, a gelatinase involved in collagen and fibronectin breakdown (p = 0.01) (Fig. 2f).
Fig. 2.
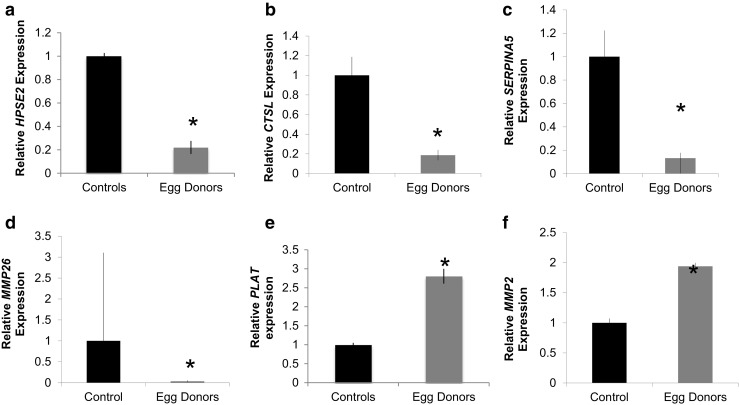
RT-qPCR validation of microarray analysis. Validation of microarray results by Q-PCR shows significant gene expression changes in a HPSE2, b CTSL, c SERPINA5, d MMP26, e PLAT, and f MMP2. Data are expressed as mean ± standard deviation. * p < 0.05
Differential methylation
In order to identify epigenetic changes resulting from superovulation, DNA methylation in samples from the endometrium of oocyte donors was compared to the endometrium from control subjects. In order to limit our analysis to clinically relevant changes, we considered genes differentially methylated if the difference in β value was > 0.1, corresponding to a difference in methylation of at least 10% between the endometrium of oocyte donors and controls. Using this cutoff as well as a false discovery rate of 0.05, we found 3322 genes with differential methylation in at least one CpG site. As the biological significance of hypo- or hyper-methylation in a single CpG sites remains unclear, we further limited our list to only include genes demonstrating methylation changes in the same direction in at least three CpG sites and at least 20% of the represented CpGs [74]. Using these stringent criteria we found 785 genes with significantly different methylation in the endometrium of oocyte donors when compared with control subjects. Of these genes, 89 were hypomethylated, while 696 were hypermethylated in the superovulated endometrium. Supplementary Table 2 lists the 20 genes with the greatest proportion of abnormally methylated CpGs in our data set. Supplementary Table 3 lists the 20 genes with the greatest mean difference in methylation among differentially methylated CpG sites. We compared gene lists of differential expression between the endometrium of oocyte donors and controls with all genes with significant differences in DNA methylation and found no overlap in the gene lists.
Discussion
Fresh IVF cycles have been associated with an increased risk of abnormal placentation leading to lower birth weight when compared to pregnancies occurring after either natural conceptions or frozen embryo transfer [25, 28, 35, 36]. This phenomenon has been suggested to be due the altered peri-implantation environment created through superovulation that occurs during fresh IVF cycles [20, 46]. Here, we demonstrate that superovulation alters endometrial expression genes critical to endometrial remodeling during early placentation. This data provides a potential mechanism for the increased incidence of disorders of placentation observed following fresh IVF cycles. Though this study compares the endometrium of superovulated oocyte donors to the endometrium of naturally cycling women, the hormonal milieu during frozen embryo transfer cycles more closely resembles the latter group. Nevertheless, future studies examining endometrial gene expression following hormonal preparation for a frozen embryo transfer cycle are necessary to confirm these gene expression changes persist.
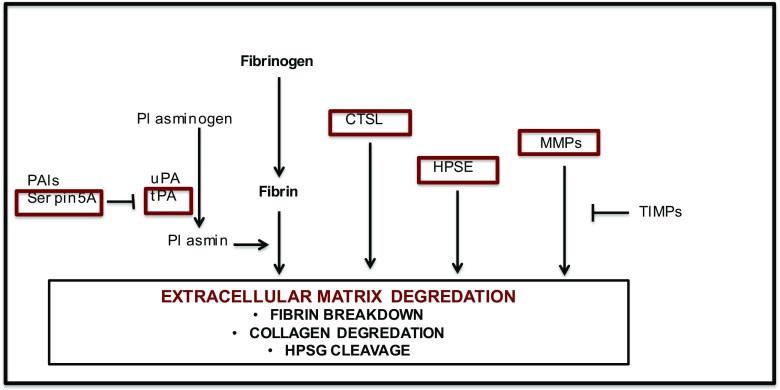
Previous studies have shown difference in endometrial gene expression between superovulated and natural cycles, with alterations seen in numerous pathways including the Wnt-signaling pathway and immunomodulation [42, 75, 76]. However, many of these studies were performed prior to the published epidemiologic data demonstrating differences in perinatal outcomes between fresh and frozen embryo transfer cycles. Animal studies have also demonstrated changes in endometrial gene expression following superovulation, though these results differed from our gene lists [77]. However, the phenotype following IVF and frozen embryo transfers differs in animal models compared to what is observed in humans, and these differences may be attributed to inherent differences in endometrial receptivity and placentation in an epitheliochorial versus hemochorial model. In this study, we identify a potential mechanism by which superovulation may affect early trophoblast invasions and placentation in humans and lead to the observed increase risk of adverse fetal outcomes observed following fresh IVF cycles. Placentation is controlled by both fetal and maternal factors, allowing trophoblasts to migrate into the maternal decidua and penetrate the maternal spiral arteries and replace the maternal endothelium. This process involves tissue remodeling and the degradation of extracellular matrix (ECM) by proteases and glycosidases and their inhibitors. A correct balance of these factors is necessary to prevent abnormal placentation and adverse fetal outcomes [78]. In this study, we identify and confirm altered expression of multiple members of the proteolytic pathway involved in ECM breakdown in the endometrium following superovulation during the period of early implantation (Fig. 3), suggesting that the hormonal milieu following a fresh IVF cycle may result in an imbalance of these factors resulting in altered growth and placentation.
Fig. 3.
Schematic of endometrial remodeling in embryo invasion and early placentation. We found altered expression of multiple members of the proteolytic pathway involved in ECM breakdown in the endometrium following superovulation during the period of early implantation
The extracellular matrix in the maternal decidua is composed of multiple components including laminin, collagen, heparin sulfate proteoglycan, and fibronectin. Trophoblast invasion and ECM degradation are critical to implantation, tissue remodeling, and uteroplacental vascular development. One family of proteases known to be critical to ECM degradation during trophoblast invasion is the matrix metalloproteinases (MMPs) [79]. MMPs target many ECM proteins including proteoglycans, fibronectin, and laminin. In this study, we found a significant increase in MMP2 gene expression in the endometrium of oocyte donors. MMP2 is a gelatinase that has been localized to the placental bed in early pregnancy and can be found in the maternal glandular epithelium and vascular endothelial cells as well as the extravillous trophoblasts in the first trimester [80] [81, 82]. Elevated levels of MMP2 have been detected in serum of patients with early pregnancy failure [83]. We also identified a significant reduction in MMP26, known to have spatiotemporal changes in expression in the rhesus monkey during the menstrual cycle [84]. MMP26 cleaves fibrinogen, fibronectin, and vitronectin and denatures collagen, and in tumor cells be critical for tumor progression [85]. Interestingly, the imbalance between matrix metalloproteinases and their tissue inhibitors has been hypothesized to be involved in the pathogenesis of preeclampsia and gestational trophoblastic diseases [86]. In these data, these pathways were under epigenetic regulation by histone trimethylation as opposed to altered DNA methylation.
We also detected other changes in the fibrin degradation pathway. Following superovulation, there was increased expression of PLAT, the gene encoding tissue-type plasminiogen activator (tPA), a serine protease responsible for plasminogen conversion to plasmin, a fibrinolytic enzyme. The balance between plasminogen activator and its inhibitor, PAI-1, is believed to regulate trophoblast invasion. In addition, there was a reduced expression of SERPINA5, also known as protein C inhibitor, a serine peptidase inhibitor known to modulate PLAT activity [87].
Similarly, we found changes in proteases critical to collagen and HSPG breakdown. Both cathepsin L and heparanase were downregulated in our superovulated endometrium. Cathepsin L is a cysteine protease that cleaves the n-terminal peptide of collagen. In the rodent model, partial inhibition of cathepsin was found to lead to stunted embryonic growth, while increased inhibition led to implantation failure [88]. Heparanase is a glucoronidase that cleaves heparin sulfate. Animal models have shown that heparanase expression increases during pregnancy [89, 90], and lower levels have been associated with spontaneous abortions in humans [91, 92].
Gene expression can be modulated through multiple mechanisms including direct binding of transcription factors as well as epigenetic mechanisms including DNA methylation and histone modifications. We and others have identified changes in methylation in cord blood and the placenta in children conceived through IVF when compared to naturally conceived children. DNA methylation has been recognized as a strong contributor to fetal growth and placentation [93]. In addition, recent studies have demonstrated changes in DNA methylation during the menstrual cycle and in response to steroid hormone exposure [65]. These findings led us to hypothesize that superovulation could affect early placentation through epigenetic changes in DNA methylation in the endometrium. However, in this study, we failed to correlate the changes in gene expression to the changes in DNA methylation. This suggest that alternate methods may be controlling the aberrant expression of differentially expressed genes found in our data. Further studies examining histone modifications and miRNA are currently being performed to investigate alternate epigenetic mechanisms that may be regulating our endometrial gene expression.
While other epigenetic changes not examined in this study may be responsible for the gene expression changes observed, the changes seen in gene expression may not be related to epigenetics, but rather, may be secondary to alternations in other factors affected by superovulation. In vitro studies suggest that the plasminogen activator pathway and MMP9 may be regulated by VEGF [94]. VEGF is expressed by the endometrium, the embryo, and the corpus luteum and has been shown to be elevated following gonadotropin stimulation. Therefore, the alterations in placentation seen following fresh IVF cycles may be due to the elevated serum VEGF levels altering ECM breakdown during early placentation. Clearly, further studies in animal models are necessary to further investigate this pathway.
While this is a pilot study and thus limited in sample size, the data are strengthened by the fact that the samples were similar in age, and neither the control, nor the experimental group had an infertility diagnosis. We understand there are limitations of this study, including acknowledgement that endometrial biopsy recovers multiple cell types and more subtle differences in DNA methylation or gene expression in a single cell type may be lost in these samples. Additionally, the donors underwent different stimulation protocols and did not receive luteal support, though all received an hCG trigger. We believe the significant changes in gene expression that we found, despite the variation in treatment between the donors, represent clinically relevant changes that may be influencing outcome. Since the adverse effects observed clinically following fresh IVF cycles do not appear to be affected by stimulation protocol, this further strengthens our hypothesis that altered ECM invasion may be responsible for the adverse outcome. As early implantation is nearly impossible to examine in vivo, it is critical to further investigate this pathway in animal models. The information gained by these data not only can aid us in modifying our current treatment decisions, but can also inform our understanding of the mechanism(s) responsible for disorders of placentation and perinatal growth.
Funding/support
NIH K12HD001265 (SS), Penn Presbyterian George L and Emily McMichael Harrison Fund for Research in Obstetrics and Gynecology (MM), NIH 2K12HD000849-26 (MM), P50-HD068157 (CC, MM).
Electronic supplementary material
(DOCX 28 kb)
References
- 1.Chung Karine, Coutifaris Christos, Chalian Raffi, Lin Kathleen, Ratcliffe Sarah J., Castelbaum Arthur J., Freedman Martin F., Barnhart Kurt T. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertility and Sterility. 2006;86(6):1634–1641. doi: 10.1016/j.fertnstert.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103(6):1144–1153. doi: 10.1097/01.AOG.0000127037.12652.76. [DOI] [PubMed] [Google Scholar]
- 3.Pinborg A., Wennerholm U. B., Romundstad L. B., Loft A., Aittomaki K., Soderstrom-Anttila V., Nygren K. G., Hazekamp J., Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Human Reproduction Update. 2012;19(2):87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 4.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103(3):551–563. doi: 10.1097/01.AOG.0000114989.84822.51103/3/551. [DOI] [PubMed] [Google Scholar]
- 5.Shevell Tracy, Malone Fergal D., Vidaver John, Porter T Flint, Luthy David A., Comstock Christine H., Hankins Gary D., Eddleman Keith, Dolan Siobhan, Dugoff Lorraine, Craigo Sabrina, Timor Ilan E., Carr Stephen R., Wolfe Honor M., Bianchi Diana W., D’Alton Mary E. Assisted Reproductive Technology and Pregnancy Outcome. Obstetrics & Gynecology. 2005;106(5, Part 1):1039–1045. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- 6.Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21(9):2353–2358. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- 7.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Grady R, Alavi N, Vale R, Khandwala M, McDonald SD. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril. 2012;97(2):324–331. doi: 10.1016/j.fertnstert.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Li Tao, Vu Thanh H., Ulaner Gary A., Littman Eva, Ling Jian-Qun, Chen Hui-Ling, Hu Ji-Fan, Behr Barry, Giudice Linda, Hoffman Andrew R. IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. MHR: Basic science of reproductive medicine. 2005;11(9):631–640. doi: 10.1093/molehr/gah230. [DOI] [PubMed] [Google Scholar]
- 10.Wright K, Brown L, Brown G, Casson P, Brown S. Microarray assessment of methylation in individual mouse blastocyst stage embryos shows that in vitro culture may have widespread genomic effects. Hum Reprod. 2011;26(9):2576–2585. doi: 10.1093/humrep/der201. [DOI] [PubMed] [Google Scholar]
- 11.Mann M. R. W. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131(15):3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- 12.Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27(2):153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]
- 13.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62(6):1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 14.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17(1):1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- 15.de Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, et al. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod. 2014;90(2):22. 10.1095/biolreprod.113.114785. [DOI] [PMC free article] [PubMed]
- 16.Fortier AL, Lopes FL, Darricarrere N, Martel J, Trasler JM. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet. 2008;17(11):1653–1665. doi: 10.1093/hmg/ddn055. [DOI] [PubMed] [Google Scholar]
- 17.Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014;90(4):80. doi: 10.1095/biolreprod.113.113134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. 2001;16(2):221–225. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, Otsu E, Negishi H, Utsunomiya T, Arima T. Aberrant DNA methylation of imprinted loci in superovulated oocytes. Hum Reprod. 2006;22(1):26–35. doi: 10.1093/humrep/del316. [DOI] [PubMed] [Google Scholar]
- 20.Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90(2):26. doi: 10.1095/biolreprod.113.110411.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LY, Wang N, Le F, Li L, Lou HY, Liu XZ, et al. Superovulation induced changes of lipid metabolism in ovaries and embryos and its probable mechanism. PLoS One. 2015;10(7):e0132638. doi: 10.1371/journal.pone.0132638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortier AL, McGraw S, Lopes FL, Niles KM, Landry M, Trasler JM. Modulation of imprinted gene expression following superovulation. Mol Cell Endocrinol. 2014;388(1–2):51–57. doi: 10.1016/j.mce.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Bangsgaard N, Lund CO, Ottesen B, Nilas L. Improved fertility following conservative surgical treatment of ectopic pregnancy. BJOG : Int J Obstet Gynaecol. 2003;110(8):765–770. doi: 10.1111/j.1471-0528.2003.02253.x. [DOI] [PubMed] [Google Scholar]
- 24.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95(3):959–963. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 25.Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95(2):548–553. doi: 10.1016/j.fertnstert.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato Osamu, Kawasaki Nami, Bodri Daniel, Kuroda Tomoko, Kawachiya Satoshi, Kato Keiichi, Takehara Yuji. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2012;161(1):46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu SY, Teng B, Fu J, Li X, Zheng Y, Sun XX. Obstetric and neonatal outcomes after transfer of vitrified early cleavage embryos. Hum Reprod. 2013;28(8):2093–2100. doi: 10.1093/humrep/det104. [DOI] [PubMed] [Google Scholar]
- 28.Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98(2):368–377. doi: 10.1016/j.fertnstert.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod. 2010;25(4):914–923. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 30.Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 31.Wikland M., Hardarson T., Hillensjo T., Westin C., Westlander G., Wood M., Wennerholm U. B. Obstetric outcomes after transfer of vitrified blastocysts. Human Reproduction. 2010;25(7):1699–1707. doi: 10.1093/humrep/deq117. [DOI] [PubMed] [Google Scholar]
- 32.Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28(9):2545–2553. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 33.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101(1):128–133. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Kalra SK. Adverse perinatal outcome and in vitro fertilization singleton pregnancies: what lies beneath? Further evidence to support an underlying role of the modifiable hormonal milieu in in vitro fertilization stimulation. Fertil Steril. 2012;97(6):1295–1296. doi: 10.1016/j.fertnstert.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118(4):863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imudia AN, Awonuga AO, Kaimal AJ, Wright DL, Styer AK, Toth TL. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertil Steril. 2013;99(1):168–173. doi: 10.1016/j.fertnstert.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 37.Imudia Anthony N., Awonuga Awoniyi O., Doyle Joseph O., Kaimal Anjali J., Wright Diane L., Toth Thomas L., Styer Aaron K. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertility and Sterility. 2012;97(6):1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Farhi J, Ben Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod BioMed Online. 2010;21(3):331–337. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Radulescu L, Munteanu O, Popa F, Cirstoiu M. The implications and consequences of maternal obesity on fetal intrauterine growth restriction. J Med Life. 2013;6(3):292–298. [PMC free article] [PubMed] [Google Scholar]
- 40.Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol. 2007;36(2):412–419. doi: 10.1093/ije/dyl271. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds LP, Borowicz PP, Palmieri C, Grazul-Bilska AT. Placental vascular defects in compromised pregnancies: effects of assisted reproductive technologies and other maternal stressors. Adv Exp Med Biol. 2014;814:193–204. doi: 10.1007/978-1-4939-1031-1_17. [DOI] [PubMed] [Google Scholar]
- 42.Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102(1):10–18. doi: 10.1016/j.fertnstert.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poston Lucilla, Caleyachetty Rishi, Cnattingius Sven, Corvalán Camila, Uauy Ricardo, Herring Sharron, Gillman Matthew W. Preconceptional and maternal obesity: epidemiology and health consequences. The Lancet Diabetes & Endocrinology. 2016;4(12):1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 44.Sasson IE, Vitins AP, Mainigi MA, Moley KH, Simmons RA. Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia. 2015;58(3):615–624. doi: 10.1007/s00125-014-3466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mainigi MA, Weinerman R, Ord T, Coutifaris C, editors. Low birthweight during fresh IVF cycles is due to altered placental vasculogenesis: evidence from a mouse model. American Society of Reproductive Medicine 2016; 2016.
- 46.Weinerman R, Ord T, Bartolomei MS, Coutifaris C, Mainigi M. The superovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model. Biol Reprod. 2017;97(1):133–142. doi: 10.1093/biolre/iox067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Waal E, Vrooman LA, Fischer E, Ord T, Mainigi MA, Coutifaris C, et al. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet. 2015;24(24):6975–85. 10.1093/hmg/ddv400. [DOI] [PMC free article] [PubMed]
- 48.Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639–645. doi: 10.1016/j.placenta.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 50.Kim Yeon Mee, Bujold Emmanuel, Chaiworapongsa Tinnakorn, Gomez Ricardo, Yoon Bo Hyun, Thaler Howard T, Rotmensch Siegfried, Romero Roberto. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology. 2003;189(4):1063–1069. doi: 10.1067/S0002-9378(03)00838-X. [DOI] [PubMed] [Google Scholar]
- 51.Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol. 2002;187(1–2):233–238. doi: 10.1016/S0303-7207(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 52.Knofler M, Pollheimer J. IFPA award in placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33(Suppl):S55–S62. doi: 10.1016/j.placenta.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab. 2004;89(11):5742–5752. doi: 10.1210/jc.2004-0605. [DOI] [PubMed] [Google Scholar]
- 54.Horcajadas José Antonio, Riesewijk Anne, Polman Jan, van Os Roselinde, Pellicer Antonio, Mosselman Sietse, Simón Carlos. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. MHR: Basic science of reproductive medicine. 2004;11(3):195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 55.Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20(12):3318–3327. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- 56.Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93(11):4500–4510. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril. 2008;90(6):2152–2164. doi: 10.1016/j.fertnstert.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24(6):1436–1445. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katari Sunita, Turan Nahid, Bibikova Marina, Erinle Oluwatoyin, Chalian Raffi, Foster Michael, Gaughan John P., Coutifaris Christos, Sapienza Carmen. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Human Molecular Genetics. 2009;18(20):3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19(1):36–51. doi: 10.1093/hmg/ddp465. [DOI] [PubMed] [Google Scholar]
- 61.Song S, Ghosh J, Mainigi M, Turan N, Weinerman R, Truongcao M, et al. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin Epigenetics. 2015;7(1):41. 10.1186/s13148-015-0071-7. [DOI] [PMC free article] [PubMed]
- 62.Ghosh J, Mainigi M, Coutifaris C, Sapienza C. Outlier DNA methylation levels as an indicator of environmental exposure and risk of undesirable birth outcome. Hum Mol Genet. 2016;25(1):123–129. doi: 10.1093/hmg/ddv458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh J, Coutifaris C, Sapienza C, Mainigi M. Global DNA methylation levels are altered by modifiable clinical manipulations in assisted reproductive technologies. Clin Epigenetics. 2017;9:14. doi: 10.1186/s13148-017-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16(5):297–310. doi: 10.1093/molehr/gaq010. [DOI] [PubMed] [Google Scholar]
- 65.Houshdaran S, Zelenko Z, Irwin JC, Giudice LC. Human endometrial DNA methylome is cycle-dependent and is associated with gene expression regulation. Mol Endocrinol. 2014;28(7):1118–1135. doi: 10.1210/me.2013-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Cuilin, Qiu Chunfang, Hu Frank B., David Robert M., van Dam Rob M., Bralley Alexander, Williams Michelle A. Maternal Plasma 25-Hydroxyvitamin D Concentrations and the Risk for Gestational Diabetes Mellitus. PLoS ONE. 2008;3(11):e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 68.Sandoval Juan, Heyn Holger, Moran Sebastian, Serra-Musach Jordi, Pujana Miguel A., Bibikova Marina, Esteller Manel. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics. 2011;6(6):692–702. doi: 10.4161/epi.6.6.16196. [DOI] [PubMed] [Google Scholar]
- 69.Chen Yi-an, Lemire Mathieu, Choufani Sanaa, Butcher Darci T., Grafodatskaya Daria, Zanke Brent W., Gallinger Steven, Hudson Thomas J., Weksberg Rosanna. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics. 2013;8(2):203–209. doi: 10.4161/epi.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24(13):1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 71.Teschendorff Andrew E., Marabita Francesco, Lechner Matthias, Bartlett Thomas, Tegner Jesper, Gomez-Cabrero David, Beck Stephan. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2012;29(2):189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 73.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gemma Carolina, Ramagopalan Sreeram V, Down Thomas A, Beyan Huriya, Hawa Mohammed I, Holland Michelle L, Hurd Paul J, Giovannoni Gavin, David Leslie R, Ebers George C, Rakyan Vardhman K. Inactive or moderately active human promoters are enriched for inter-individual epialleles. Genome Biology. 2013;14(5):R43. doi: 10.1186/gb-2013-14-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devroey P, Bourgain C, Macklon NS, Fauser BCJM. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15(2):84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Macklon NS, van der Gaast MH, Hamilton A, Fauser BC, Giudice LC. The impact of ovarian stimulation with recombinant FSH in combination with GnRH antagonist on the endometrial transcriptome in the window of implantation. Reprod Sci. 2008;15(4):357–365. doi: 10.1177/1933719107311781. [DOI] [PubMed] [Google Scholar]
- 77.Forde N., Carter F., di Francesco S., Mehta J. P., Garcia-Herreros M., Gad A., Tesfaye D., Hoelker M., Schellander K., Lonergan P. Endometrial response of beef heifers on day 7 following insemination to supraphysiological concentrations of progesterone associated with superovulation. Physiological Genomics. 2012;44(22):1107–1115. doi: 10.1152/physiolgenomics.00092.2012. [DOI] [PubMed] [Google Scholar]
- 78.Salamonsen LA. Role of proteases in implantation. Rev Reprod. 1999;4(1):11–22. doi: 10.1530/ror.0.0040011. [DOI] [PubMed] [Google Scholar]
- 79.Zhu JY, Pang ZJ, Yu YH. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Reviews in Obstetrics & Gynecology. 2012;5(3–4):e137–e143. [PMC free article] [PubMed] [Google Scholar]
- 80.Seval Y, Akkoyunlu G, Demir R, Asar M. Distribution patterns of matrix metalloproteinase (MMP)-2 and -9 and their inhibitors (TIMP-1 and TIMP-2) in the human decidua during early pregnancy. Acta Histochem. 2004;106(5):353–362. doi: 10.1016/j.acthis.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Naruse K., Lash G. E., Innes B. A., Otun H. A., Searle R. F., Robson S. C., Bulmer J. N. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Human Reproduction. 2008;24(3):553–561. doi: 10.1093/humrep/den408. [DOI] [PubMed] [Google Scholar]
- 82.Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS, Zhuang LZ. Expression of matrix metalloproteinase-2, -9, and -14, tissue inhibitors of metalloproteinase-1, and matrix proteins in human placenta during the first trimester. Biol Reprod. 2000;62(4):988–994. doi: 10.1095/biolreprod62.4.988. [DOI] [PubMed] [Google Scholar]
- 83.Nissi R, Talvensaari-Mattila A, Kotila V, Niinimaki M, Jarvela I, Turpeenniemi-Hujanen T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reproductive Biology and Endocrinology: RB&E. 2013;11(2):2. doi: 10.1186/1477-7827-11-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, Wang H, Zhao Y, Lin H, Sang QA, Zhu C. Identification and specific expression of matrix metalloproteinase-26 in rhesus monkey endometrium during early pregnancy. Mol Hum Reprod. 2002;8(10):934–940. doi: 10.1093/molehr/8.10.934. [DOI] [PubMed] [Google Scholar]
- 85.Marchenko GN, Ratnikov BI, Rozanov DV, Godzik A, Deryugina EI, Strongin AY. Characterization of matrix metalloproteinase-26, a novel metalloproteinase widely expressed in cancer cells of epithelial origin. Biochem J. 2001;356(Pt 3):705–718. doi: 10.1042/bj3560705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahat B, Sharma R, Bagga R, Hamid A, Kaur J. Imbalance between matrix metalloproteinases and their tissue inhibitors in preeclampsia and gestational trophoblastic diseases. Reproduction. 2016;152(1):11–22. doi: 10.1530/REP-16-0060. [DOI] [PubMed] [Google Scholar]
- 87.Espana F, Estelles A, Fernandez PJ, Gilabert J, Sanchez-Cuenca J, Griffin JH. Evidence for the regulation of urokinase and tissue type plasminogen activators by the serpin, protein C inhibitor, in semen and blood plasma. Thrombosis and Haemostasis. 1993;70(6):989–994. doi: 10.1055/s-0038-1649712. [DOI] [PubMed] [Google Scholar]
- 88.Afonso S, Romagnano L, Babiarz B. The expression and function of cystatin C and cathepsin B and cathepsin L during mouse embryo implantation and placentation. Development. 1997;124(17):3415–3425. doi: 10.1242/dev.124.17.3415. [DOI] [PubMed] [Google Scholar]
- 89.D'Souza SS, Daikoku T, Farach-Carson MC, Carson DD. Heparanase expression and function during early pregnancy in mice. Biol Reprod. 2007;77(3):433–441. doi: 10.1095/biolreprod.107.061317.. [DOI] [PubMed] [Google Scholar]
- 90.D'Souza SS, Fazleabas AT, Banerjee P, Sherwin JR, Sharkey AM, Farach-Carson MC, et al. Decidual heparanase activity is increased during pregnancy in the baboon (Papio anubis) and in in vitro decidualization of human stromal cells. Biol Reprod. 2008;78(2):316–323. doi: 10.1095/biolreprod.107.063891.. [DOI] [PubMed] [Google Scholar]
- 91.Nadir Y, Henig I, Naroditzky I, Paz B, Vlodavsky I, Brenner B. Involvement of heparanase in early pregnancy losses. Thromb Res. 2010;125(5):e251–e257. doi: 10.1016/j.thromres.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 92.Wirstlein PK, Mikolajczyk M, Skrzypczak J. Correlation of the expression of heparanase and heparin-binding EGF-like growth factor in the implantation window of nonconceptual cycle endometrium. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2013;51(2):127–134. doi: 10.5603/fhc.2013.0020.. [DOI] [PubMed] [Google Scholar]
- 93.Lim AL, Ferguson-Smith AC. Genomic imprinting effects in a compromised in utero environment: implications for a healthy pregnancy. Semin Cell Dev Biol. 2010;21(2):201–208. doi: 10.1016/j.semcdb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Anteby E.Y. Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Molecular Human Reproduction. 2004;10(4):229–235. doi: 10.1093/molehr/gah031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 28 kb)