Abstract
Objectives:
To evaluate the association between PSA nadir level and time to nadir (TTN) with biochemical recurrence (BCR) risk after radical prostatectomy (RP) in the SEARCH database.
Methods:
Retrospective analysis of 1,939 men from the SEARCH database treated with RP between 1998–2015 with available ultrasensitive PSA nadir within 1–6 months after RP. Uni- and multivariable analyses of PSA nadir and TTN with time from nadir to BCR were done with Cox models (adjusted for demographics, tumor features and preoperative PSA).
Results:
Among men with an undetectable PSA nadir, the TTN was unrelated to BCR (1–2.9 vs. 3–6 months: HR 0.86, p=0.46). Regardless of TTN, men with detectable nadir had increased risk of BCR (TTN 3–6 months: HR 1.81, p=0.024; TTN 1–2.99 months: HR 3.75, p<0.001 vs. undetectable nadir and TTN 3–6 months). Among men with a detectable PSA at 1–3 months, 53% had a lower follow-up PSA 3–6 months after RP which was undetectable in 32% and lower but still detectable in 21%.
Conclusions:
In the post-RP setting, men with both a detectable nadir and a shorter TTN had an increased risk of BCR. Intriguingly, about half of the men with a detectable PSA in the first 3 months after RP had a lower follow-up PSA between 3 and 6 months after RP. If confirmed in future studies, this has important implications for patients considering adjuvant therapy based upon post-operative PSA values in the first 3 months after RP.
Keywords: Prostate Cancer, Radical Prostatectomy, Biochemical Recurrence, Time to PSA Nadir
Introduction
Prostate cancer (PCa) is the second leading cause of cancer death. Radical prostatectomy (RP) is one of the most widely used treatment modalities for early stage, localized disease. However, 33% of patients will experience a biochemical recurrence (BCR) within 10 years after surgery.1–4 While some patients who experience BCR may have indolent slow growing disease, others will have rapid clinical progression to metastasis.5 Patients who experience BCR are at increased risk of metastases and PCa death.6, 7
PSA testing after RP is routinely used as a surveillance strategy for early detection of recurrence. Ideally following RP, PSA should decrease to an undetectable level after 4 weeks.8 As such, it is common practice to check the first PSA sometime between 1 and 3 months after surgery and every approximately 3 months thereafter, at least for the first year or two.5
Previous research indicates that the lowest PSA level after RP (PSA nadir) using ultrasensitive PSA assay is highly correlated with time to BCR and overall mortality.9 However, patients achieve PSA nadir at different time points after surgery, which may have an impact on their prognosis. For example, after radiotherapy, short time to nadir (TTN) is associated with increased risk of BCR and distant metastasis.10 Conversely, after androgen deprivation therapy, longer TTN is associated with higher overall mortality.11 However, to date only one small study of 319 men has examined the prognostic utility of TTN after RP.12 Moreover, given the most widely accepted definition for BCR is a PSA >0.2 ng/ml, it is not uncommon for the first PSA level in the early postoperative setting to be detectable but <0.2 ng/ml. Whether some of these men later on achieve an undetectable PSA is unknown and whether for those men, their outcomes are comparable to men whose first PSA is undetectable is unknown.
We analyzed the association between PSA nadir level measured with ultrasensitive PSA and TTN with BCR risk after RP among patients from the Shared Equal-Access Research Cancer Hospital (SEARCH) Database. We hypothesized that higher nadir levels, regardless of when achieved, would be associated with worse outcomes and that TTN would not be related to outcomes.
Material and Methods:
Study population
After obtaining Institutional Review Board approval, data from patients undergoing RP between 1998 and 2015 at six Veteran Affairs Medical Center (West Los Angeles, San Diego, and Palo Alto, CA; Augusta, GA; Durham and Asheville, NC) were combined into the SEARCH database.13 Patients operated before 1998 were not included in the study because ultrasensitive PSA assays were not available at all of our centers before 1998. An undetectable PSA was defined as a value <0.01 ng/mL. Patients treated with preoperative hormonal therapy or radiotherapy were excluded. Of 4,098 patients in SEARCH treated during this time frame, we excluded patients who did not have PSA values within 3 months of surgery (n=1343), received secondary treatment within 6 months (n=361), recurred within 6 months (n=182), were missing PSA nadir data within 6 months of surgery (n=143), had a nadir ≥0.2ng/mL (i.e. they recurred based upon nadir PSA value and thus are not at risk for future BCR) (n=5), and had an absence of follow-up after PSA nadir (n=15). Additionally, we excluded patients missing data on race (n=13), BMI (n=30), pre-operative PSA (n=2), pathological grade group (n=6), or pathological features (n=59). This resulted in a study population of 1,939 subjects.
All patients were followed with serial PSA determinations and clinical visits at intervals according to the attending physician discretion. Additional treatment after surgery was at the judgment of the patient and treating physician. All patients were required to have a PSA within 1 and 2.99 months after surgery. Among those who had a detectable PSA (PSA≥0.01) within 1 and 2.99 months, they had to have at least one PSA within 3 to 6 months in order to discern whether their PSA was still declining after the first 3 months. Among patients who had an undetectable PSA between 1 and 2.99 months or a detectable PSA between 1 and 2.99 months with a follow-up PSA between 3 and 6 months, we defined PSA nadir as the lowest PSA level between 1 and 6 months after surgery and before BCR.14 TTN was defined as the time interval from surgery to PSA nadir. When multiple nadirs were present (i.e. repeated identical lowest PSA values), the first PSA nadir was used to determine TTN. BCR was defined as a single PSA above 0.2ng/mL, 2 concentrations at 0.2ng/mL or secondary treatment for an elevated PSA.15
Statistical analysis
TTN was dichotomized into 2 groups (<3 months and 3–6 months) and patient characteristics between these groups were compared using chi-squared tests for categorical data and rank-sum tests for continuous variables. The cut-point of 3 months was chosen as the midpoint of the surgery to 6 month interval. For analysis, we also stratified patients by undetectable PSA nadir (PSA below the limit of detection) vs. detectable PSA nadir (any PSA that was above the limit of detection).
Univariable analysis of time to BCR was performed using Kaplan-Meier plots and log-rank tests. Time zero was considered as 6 months after surgery. Multivariable analysis of TTN and time to BCR was conducted using a Cox proportional hazards model. The analysis was adjusted for race (white, black, non-white-non-black), body-mass index (BMI, continuous, log-transformed), age at surgery (continuous), year of surgery (continuous), Veteran Affairs (VA) medical center (1–6), preoperative PSA (continuous, log-transformed) and pathological grade group (1, 2, ≥3) as covariates. We also included positive surgical margins, seminal vesicle invasion and extracapsular extension (all categorized as yes or no). Given the number of PSA measurements after surgery may correlate with TTN and time to BCR (e.g. the more PSA levels checked the shorter TTN and time to BCR), we performed two separate sensitivity analyses first adjusting our multivariable models for the number of PSA measurements obtained between surgery and PSA nadir and second accounting for the number of all PSA measurements within 1 to 6 months after RP. Finally, given very high preoperative PSA levels may take a longer time reach undetectable levels, we performed a sensitivity analysis excluding patients with preoperative PSA >20ng/mL. All statistical analyses were performed using Stata 14.1 (StataCorp, College Station, TX). A P <0.05 was considered to indicate statistical significance.
Results:
Compared to those with TTN <3 months, patients with TTN 3–6 months were more likely to be black (49% vs. 36%, p=0.001), had a higher rate of positive surgical margins (45% vs. 35%, p=0.003), higher biopsy (p=0.005) and pathological (p=0.042) Gleason score, higher preoperative PSA (6.6 vs. 6.0 ng/mL, p=0.017), more extracapsular extension (21% vs. 14%, p=0.011), and had slightly higher PSA nadir levels (p<0.001, Table 1). There were no differences in age, BMI, clinical stage, or seminal vesicle invasion (all p>0.05).
Table 1:
Baseline characteristics
| Variables | TTN 1–3months No. (%) |
TTN 3–6months No. (%) |
P |
|---|---|---|---|
| Total number of patients | 1723(89) | 216(11) | - |
| Age at surgery (years) | 0.668a | ||
| Median (IQR) | 62 (58–66) | 62 (58–65) | |
| Ethnic group | 0.001b | ||
| White | 1031 (60) | 105(49) | |
| Black | 618(36) | 106(49) | |
| Others | 74(4) | 5(2) | |
| Body mass index (kg/m2) | 0.210a | ||
| Median (IQR) | 28.2(25.7–31.2) | 27.7(25.1–31.1) | |
| Clinical stage | 0.059b | ||
| T1 | 1118(66) | 128(59) | |
| T2–3 | 582 (35) | 88(41) | |
| Biopsy Gleason score | 0.005b | ||
| 2–6 | 774 (45) | 75 (35) | |
| 3+4 | 495 (29) | 67(31) | |
| 4+3–10 | 436 (26) | 74 (34) | |
| Preoperative PSA (ng/mL) | 0.017a | ||
| Median (IQR) | 6.0 (4.6–8.4) | 6.6 (4.8–9.8) | |
| Pathological Gleason score | 0.042b | ||
| 2–6 | 532(31) | 49 (23) | |
| 3+4 | 692 (40) | 94 (44) | |
| 4+3–10 | 499 (29) | 73 (34) | |
| Extracapsular extension | 246 (14) | 45(21) | 0.011b |
| Positive surgical margins | 596 (35) | 97 (45) | 0.003b |
| Seminal vesicle invasion | 100(6) | 17(8) | 0.229b |
| Prostate weight (g) | 0.996a | ||
| Median (IQR) | 41 (33–52) | 41 (33–52) | |
| PSA nadir level (ng/mL) | <0.001a | ||
| Median (IQR) | 0 (0–0) | 0 (0–0.02) |
IQR: interquartile range
TTN: time to nadir
p-value calculated using
Wilcoxon rank sum test or
chi-squared test
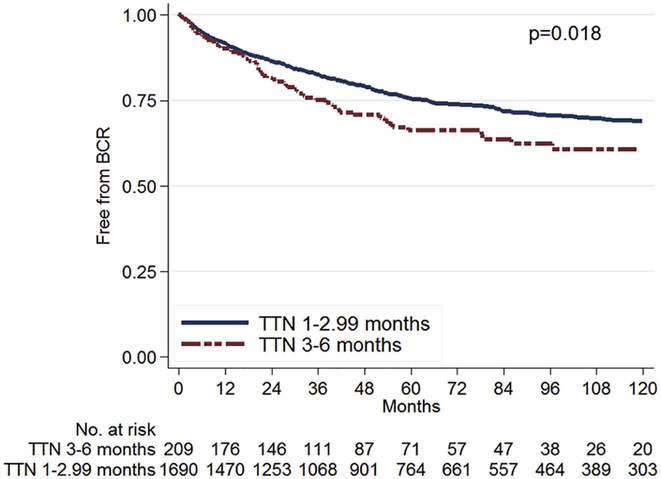
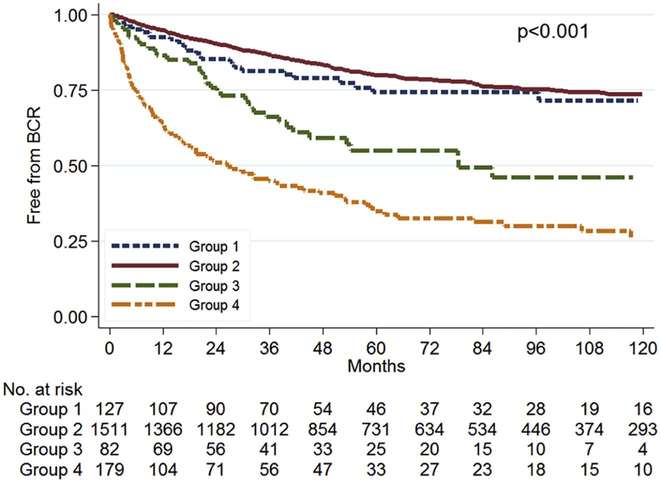
After stratification by TTN (<3 months vs. 3–6 months), men with TTN <3 months had decreased risk for BCR (log-rank, p=0.018; Figure 1). After further stratification by PSA nadir level (detectable vs. undetectable), men with an undetectable nadir, regardless of the TTN, had the best BCR-free survival while those with detectable nadir and a shorter TTN had the worst survival (log-rank; p<0.001; Figure 2). In multivariable analysis, compared to men with undetectable nadir and TTN of 3–6 months, those with undetectable nadir and shorter TTN of <3 months had similar risk of BCR (HR=0.86, 95% CI 0.58–1.28, p=0.462, Table 2). However, those with detectable nadir and TTN 3–6 months (HR=1.81, 95% CI 1.08–3.03, p=0.024) and those with detectable nadir and shorter TTN (HR=3.75, 95% CI 2.44–5.74, p<0.001) had progressively higher BCR risk compared to men with undetectable nadir and TTN of 3–6 months (Table 2). Specifically, patients with a detectable PSA nadir and TTN <3 months had significantly higher risk of BCR compared to patients with a detectable nadir and longer TTN (HR=2.07, 95% CI 1.40–3.05, p<0.001).
Figure 1:
Disease-free survival after radical prostatectomy by TTN
Figure 2:
Disease-free survival after radical prostatectomy by PSA nadir level and TTN
Group 1: Undetectable nadir, TTN 3–6 months
Group 2: Undetectable nadir, TTN 1–2.99 months
Group 3: Detectable nadir, TTN 3–6 months
Group 4: Detectable nadir, TTN 1–2.99 months
Table 2:
Risk of biochemical recurrence after radical prostatectomy by PSA nadir level and time to PSA nadir
| Variables* | BCR/Total N | HR | 95% Cl | P |
|---|---|---|---|---|
| Undetectable PSA nadir | ||||
| TTN 3–6 months | 28/130 | ref | - | - |
| TTN 1–2.99 months | 317/1537 | 0.86 | 0.58–1.28 | 0.462 |
| Detectable PSA nadir | ||||
| TTN 3–6 months | 36/86 | 1.81 | 1.08–3.03 | 0.024 |
| TTN 1–2.99 months | 110/186 | 3.75 | 2.44–5.74 | <0.001^ |
Analysis adjusted for race, body-mass index, age, year, VA medical center, preoperative PSA, pathological Gleason score, surgical margins, seminal vesicle invasion and extracapsular extension
Among those with a detectable PSA nadir, those with TTN 1–2.99 months have significantly higher risk of recurrence compared to those with TTN 3–6 months (p<0.001)
HR: Hazard ratio
CI: Confidence interval
TTN: time to nadir
In a sensitivity analysis excluding patients with PSA >20ng/mL, the results were virtually unchanged (data not shown). After adjusting our multivariable models for either the number of PSA measurements before PSA nadir or in separate analyses for the number of PSA measurements within 1 to 6 months after surgery, results were similar (data not shown) suggesting shorter TTN among patients with detectable PSA nadir is associated with increased risk of BCR regardless of the intensity of post-operative PSA follow-up.
Comments:
The goal of RP is complete removal of the prostate and all tumor. As such, after a successful RP, the PSA should be undetectable. Indeed, multiple studies have shown that higher PSA nadir values portend a worse prognosis.9, 16 However, whether the time it takes to reach PSA nadir also plays a role in predicting BCR is unknown. To address this, we examined the nadir value and TTN nadir among nearly 2,000 men undergoing RP. Consistent with prior studies, we found that having a detectable PSA nadir was associated with increased risk of BCR.17 When patients were stratified based on both TTN and the PSA nadir level, for men who reached an undetectable nadir, the TTN did not affect BCR risk. However, for men with a detectable nadir level, those with a shorter TTN had the greatest risk of BCR. Most intriguingly, 130 men reached an undetectable PSA, but it took 3–6 months implying that not all elevated PSAs in the first 3 months after RP will remain elevated. Moreover, another 86 had a detectable PSA nadir but a TTN of 3–6 months further implying that some early post-operative elevated PSA values may decline with continued observation. This fact has important implications for patients considering adjuvant therapy based upon post-operative PSA values in the first 3 months after RP.
Multiple studies have shown that higher PSA nadir values portend a worse prognosis. Specifically, Moreira et al. found that PSA persistence (defined as PSA nadir ≥0.03 ng/ml) was an independent predictor of recurrence and mortality.9 Likewise, for men treated with other modalities including radiation or androgen deprivation therapy, PSA nadir values are extremely prognostic.10, 18 Given this, it is not surprising that we too found men with a detectable PSA after RP were at higher risk of BCR.
While higher nadirs are clearly associated with worse outcomes, what is less clear is whether the time to nadir matters. For example, among men undergoing hormonal therapy the risk of prostate cancer specific mortality increased significantly with a longer time to undetectable PSA.19 In contrast, for men undergoing radiation, a longer time to nadir predicted improved freedom from distant metastatic disease.20 A single-institution study of 319 men found that PSA nadir >0.1 ng/mL and TTN <3 months are independent predictors of BCR.12 However, the authors did not stratify by both PSA nadir and TTN. When analyzed in our data, we found men with a detectable nadir and a shorter TTN had the greatest risk of BCR. If confirmed in future larger studies using more definitive long-term end-points, men who reach a detectable nadir in a shorter amount of time should be considered for earlier adjuvant therapy.
The exact rationale for a shorter TTN to be associated with higher BCR risk is not clear, though as noted, similar results were seen after radiation.10 In regards to radiotherapy, for those who ultimately recur, there are competing effects on PSA kinetics. There is the effect of the initial treatment to kill both benign and malignant tumor, which is reflected in a decline in PSA levels. However, there is the effect of the residual tumor to grow and produce PSA at an ever increasing rate, ultimately and prematurely halting the PSA decline from the initial treatment resulting in an early PSA nadir. The more aggressive the residual disease, the faster the rate of PSA rise, resulting in a shorter time to reach the nadir PSA. As such, it is intuitive that an early nadir would correlate with more aggressive tumors and higher risk for disease progression. Though the prostate gland is thought to be removed in its entirety in RP, for men who develop a PSA recurrence, obviously some source of residual PSA production was left behind. Similar to that of radiotherapy, these lingering residual cells produce PSA which would in turn limit the decline of PSA levels and thus lead to the observation of a shortened TTN. Ultimately, regardless of the exact reasons for the shorter TTN, if confirmed in future studies this information can be used clinically to help risk stratify patients.
There were a total of 402 men who had a detectable PSA nadir within 1–2.99 months. Of these men, 86/402 (21%) had a lower, but detectable, PSA value in the 3–6 month time frame. There were another 130/402 (32%) who had a PSA drop to undetectable levels in the 3–6 month time frame. As such, 53% of all men who had a detectable PSA at 1–3 months, had a lower follow-up PSA 3–6 months after RP. It is common practice to check the first PSA within 1 to 3 months following RP. Moreover, given the potential benefits of adjuvant radiation,21 these early PSA values are often used in decision making regarding adjuvant therapies. Our data would caution that for nearly half of these men, the PSA will be lower when re-checked just a few months later and half of these men will actually achieve an undetectable PSA by 6 months after RP. This is contrary to common thinking that the first PSA after surgery is the nadir. Indeed, previous studies have reported TTN as 2.0–3.3 months after RP.4, 12, 22 If confirmed in future studies, for patients where the early post-operative PSA value is crucial to deciding on adjuvant radiation or not and the PSA is detectable, but below the threshold of recurrence (i.e. ≤0.2 ng/ml), it may be reasonable to give more time for the PSA to decline before documenting a nadir, which may spare some patients from needless radiation.
The present study is limited by the retrospective nature of our cohort. PSA follow-up scheme was not standardized. However, when we performed sensitivity analysis adjusting for the number of PSA measurements, results were unchanged. In addition, the effect of different PSA assays among the VA centers and throughout the study interval, which is a potential source of bias, was minimized by controlling for both calendar year and center in our multivariable analyses. Lastly, although BCR is a clinically relevant end-point, not all patients who develop recurrence will necessarily develop systemic disease progression and eventually die of prostate cancer. Therefore, in future studies with more patients and events it will be important to correlate TTN with more distal clinical end-points (e.g. all-cause and disease-specific mortality) to assess its usefulness as an independent risk factor for overall adverse prognosis in patient with prostate cancer.
Conclusions:
With the widespread availability of early adjuvant therapies, it is essential to determine the risk of disease recurrence and progression early after surgery. Using both the PSA nadir value and the time it takes to reach nadir can be valuable tools in predicting a patient’s BCR risk and potential need for early secondary therapy. Patients who had a detectable nadir and reached nadir within 1 to 3 months after surgery had the greatest BCR risk. Importantly, about half of men with a detectable PSA within 1 to 3 months after surgery had a lower value when checked 3 to 6 months after surgery. If confirmed in future studies, using these data can better risk stratify men early in their disease course after RP.
Acknowledgments
Research support: Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131–01A1 (WJA), the Georgia Cancer Coalition (MKT), the Department of Defense, Prostate Cancer Research Program, (DMM and SJF), and the American Urological Association Foundation/Astellas Rising Star in Urology Award (SJF).
Footnotes
Publisher's Disclaimer: Disclaimer: Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interest.
References:
- 1.Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. The Journal of urology. 2003;170:1872–1876. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. [DOI] [PubMed] [Google Scholar]
- 3.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. The Journal of urology. 2004;172:910–914. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Nishimura K, Yamaguchi S, Nonomura N, Hara T. Prostate-specific antigen measured 3 months after radical prostatectomy as a new predictor of biochemical recurrence. International journal of clinical oncology. 2015;20:171–175. [DOI] [PubMed] [Google Scholar]
- 5.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. European urology. 2007;51:1175–1184. [DOI] [PubMed] [Google Scholar]
- 6.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA : the journal of the American Medical Association. 1999;281:1591–1597. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA : the journal of the American Medical Association. 2005;294:433–439. [DOI] [PubMed] [Google Scholar]
- 8.Partin AW, Oesterling JE. The clinical usefulness of prostate specific antigen: update 1994. The Journal of urology. 1994;152:1358–1368. [DOI] [PubMed] [Google Scholar]
- 9.Moreira DM, Presti JC, Aronson WJ Jr., et al. Natural History of Persistently Elevated Prostate Specific Antigen After Radical Prostatectomy: Results From the SEARCH Database. The Journal of urology. 2009;182:2250–2255. [DOI] [PubMed] [Google Scholar]
- 10.Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. International journal of radiation oncology, biology, physics. 2006;64:1140–1150. [DOI] [PubMed] [Google Scholar]
- 11.Chung CS, Chen MH, Cullen J, McLeod D, Carroll P, D’Amico AV. Time to prostate-specific antigen nadir after androgen suppression therapy for postoperative or postradiation PSA failure and risk of prostate cancer-specific mortality. Urology. 2008;71:136–140. [DOI] [PubMed] [Google Scholar]
- 12.Vesely S, Jarolim L, Schmidt M, Minarik I, Dusek P, Babjuk M. Parameters derived from the postoperative decline in ultrasensitive PSA improve the prediction of radical prostatectomy outcome. World J Urol. 2013;31:299–304. [DOI] [PubMed] [Google Scholar]
- 13.Wadhwa H, Terris MK, Aronson WJ, et al. Long-term oncological outcomes of apical positive surgical margins at radical prostatectomy in the Shared Equal Access Regional Cancer Hospital cohort. Prostate cancer and prostatic diseases. 2016;19:423–428. [DOI] [PubMed] [Google Scholar]
- 14.Moreira DM, Presti JC, Aronson WJ, et al. Definition and preoperative predictors of persistently elevated prostate-specific antigen after radical prostatectomy: Results from the SEARCH Database. BJU Int. 2009. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:365–369. [DOI] [PubMed] [Google Scholar]
- 16.Rogers CG, Khan MA, Craig Miller M, Veltri RW, Partin AW. Natural history of disease progression in patients who fail to achieve an undetectable prostate-specific antigen level after undergoing radical prostatectomy. Cancer. 2004;101:2549–2556. [DOI] [PubMed] [Google Scholar]
- 17.Taylor JA, Koff SG, Dauser DA, McLeod DG. The relationship of ultrasensitive measurements of prostate-specific antigen levels to prostate cancer recurrence after radical prostatectomy. BJU international. 2006;98:540–543. [DOI] [PubMed] [Google Scholar]
- 18.Keto CJ, Aronson WJ, Terris MK, et al. Detectable Prostate-Specific Antigen Nadir During Androgen-Deprivation Therapy Predicts Adverse Prostate Cancer-Specific Outcomes: Results from the SEARCH Database. European urology. 2014;65:620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Amico AV, McLeod DG, Carroll PR, Cullen J, Chen MH. Time to an undetectable prostate-specific antigen (PSA) after androgen suppression therapy for postoperative or postradiation PSA recurrence and prostate cancer-specific mortality. Cancer. 2007;109:1290–1295. [DOI] [PubMed] [Google Scholar]
- 20.Hanlon AL, Diratzouian H, Hanks GE. Posttreatment prostate-specific antigen nadir highly predictive of distant failure and death from prostate cancer. International journal of radiation oncology, biology, physics. 2002;53:297–303. [DOI] [PubMed] [Google Scholar]
- 21.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. The Journal of urology. 2009;181:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong SK, Park HZ, Lee WK, et al. Prognostic significance of undetectable ultrasensitive prostate-specific antigen nadir after radical prostatectomy. Urology. 2010;76:723–727. [DOI] [PubMed] [Google Scholar]