Abstract
Tracheal stenosis caused by congenital anomalies, tumors, trauma, or intubation-related damage can cause severe breathing issues, diminishing the quality of life, and potentially becoming fatal. Current treatment methods include laryngotracheal reconstruction or slide tracheoplasty. Laryngotracheal reconstruction utilizes rib cartilage harvested from the patient, requiring a second surgical site. Slide tracheoplasty involves a complex surgical procedure to splay open the trachea and reconnect both segments to widen the lumen. A clear need exists for new and innovative approaches that can be easily adopted by surgeons, and to avoid harvesting autologous tissue from the patient. This study evaluated the use of an electrospun patch, consisting of randomly layered polycaprolactone (PCL) nanofibers enveloping 3D-printed PCL rings, to create a mechanically robust, suturable, air-tight, and bioresorbable graft for the treatment of tracheal defects. The study design incorporated two distinct uses of PCL: electrospun fibers to promote tissue integration, while remaining air-tight when wet, and 3D-printed rings to hold the airway open and provide external support and protection during the healing process. Electrospun, reinforced tracheal patches were evaluated in an ovine model, in which all sheep survived for 10 weeks, although an overgrowth of fibrous tissue surrounding the patch was observed to significantly narrow the airway. Minimal tissue integration of the surrounding tissue and the electrospun fibers suggested the need for further improvement. Potential areas for further improvement include a faster degradation rate, agents to increase cellular adhesion, and/or an antibacterial coating to reduce the initial bacterial load.
Keywords: : airway stenosis/reconstruction, trachea, wound healing
Introduction
Obstruction of the airway due to tracheal stenosis (i.e., narrowing of the airway) makes breathing difficult, especially during physical activity. If the condition worsens, it reduces the quality of life and can even become fatal. The main causes of tracheal stenosis are congenital anomalies, tumors, trauma, intubation-related damage, or infection, and the condition affects both children and adults. For example, it is estimated that 1 in 200,000 people develop tracheal stenosis due to intubation-related trauma each year.1 The affected region of trachea can be short, less than 50% of the trachea length, or long, greater than 50% of the trachea length.2 Current surgical treatment methods for short segment defects are tracheal resection with end-to-end anastomosis and/or laryngotracheal reconstruction.3,4
Laryngotracheal reconstruction involves making a superoinferior incision on the anterior larynx and/or trachea, spreading open the cartilaginous tissue mediolaterally, and inserting autologous tissue to increase the tracheal lumen diameter. Slide tracheoplasty, another reconstructive alternative, is a complex surgical procedure typically used to treat long segment defects. The procedure involves dividing the trachea and fitting the two ends together once they have been incised lengthwise, in such a way as to shorten the trachea, while increasing the circumference.5,6 Trachea reconstruction, and occasionally slide tracheoplasty, relies on autologous donor tissue (e.g., buccal mucosa graft, fascia, pericardium, and rib cartilage) to augment the airway.2,7 Not only is the collection of an autograft painful for the patient but the graft can also be challenging to harvest and manipulate into the required shape to properly fit the tracheal defect.
Using a tissue-engineered tracheal patch would be an off-the-shelf solution that is easily customized to fit a patient's trachea and would eliminate donor-site morbidity. Such a construct could circumvent many of the limitations of harvesting autologous tissue in a patient needing tracheal reconstruction. Requirements for a tissue-engineered tracheal “patch” include a biomaterial that needs to be biocompatible and vascularized, create an air-tight seal, readily mucosalize the luminal surface, and maintain mechanical integrity of the native airway.8,9
The literature describes the use of various biomaterials as scaffolds, ranging from decellularized extracellular matrix to synthetic scaffolds, incorporating one or more cell sources, and occasionally using specialized techniques such as bioreactor culture and 3D printing.9 Since the clinical implantation of a tissue-engineered trachea in 2008, multiple groups have implanted tissue-engineered tracheas into patients. Elliott et al.10,11 designed and implanted a three-part construct, composed of a decellularized scaffold seeded with bone marrow and epithelial patches, omental wrap, and transforming growth factor-β and erythropoietin added to induce chondrogenesis and angiogenesis, respectively. The three-part construct was implanted into a 12-year-old boy born with a long-segment congenital tracheal stenosis and the surgery took place in the United Kingdom.
Zopf et al.12 3D printed a synthetic splint composed of polycaprolactone (PCL) to place around a collapsing bronchus in a 2-month-old child. The 3D-printed splint was implanted in the United States after receiving approval from the Food and Drug Administration (FDA) for the use of the device under the emergency-use exemption. Many of the approaches have been met with complications due to failed mechanical properties, lack of vascularization, or poor incorporation of the construct into the native trachea.13,14
In the aforementioned examples, and in much of the literature, the focus has been on replacing an entire full-circumferential segment of the trachea, although the patient population requiring treatment for segmental tracheal stenosis is far larger than the patient population requiring replacement for an entire segment of the trachea. With the tracheal stenosis patient population in mind, to provide an alternative solution to autografting and slide tracheoplasty, the overall objective of this study was to develop a synthetic off-the-shelf tracheal patch to treat tracheal stenosis. Successful creation of an off-the-shelf tracheal patch would benefit other areas such as idiopathic subglottic stenosis and posttracheostomy reconstruction. This study evaluated the use of an electrospun patch consisting of randomly layered fibers of PCL enveloping 3D-printed PCL rings to create a mechanically robust, suturable, air-tight, and bioresorbable graft for the treatment of tracheal defects.
The study design incorporated two distinct uses of PCL: electrospun fibers to promote tissue integration, while remaining air-tight when wet, and 3D-printed rings to hold the airway open and provide external strength and protection during the healing process. PCL is a slowly resorbable polyester that has previously been approved by the FDA in multiple applications, and potentially offers ideal properties for use in tracheal regenerative medicine.15 The PCL patches were implanted into an in vivo ovine tracheal defect model and evaluated after 10 weeks of recovery time. Explanted tracheas were assessed using microcomputed tomography (μCT) to quantify patency volumes, and histologically to evaluate tissue regeneration and scaffold integration. The reinforced PCL tracheal patch used in this study represents a next-generation material from our previously published work16,17 using a co-electrospun PCL-poly(lactic-co-glycolic acid) gradient scaffold. We hypothesized that the reinforced electrospun PCL patches would allow sheep to survive after closing an elliptical tracheal defect, with minimal stenosis.
Materials and Methods
3D-printed ring fabrication
3D-printed ring reinforcements for the tracheal scaffolds were fabricated with PCL filament (MakerBot, Brooklyn, NY) using a RepRapPro Tricolor Mendel 3D Printer (Briston, United Kingdom). The rings were designed with 3D computer-aided design software (Autodesk Inventor, San Rafael, CA) to have inner diameters of 30 mm and circular cross-sections, 3 mm in diameter. The 3D model was then converted into G-code using an open-source slicing software (Slic3r) and sent to the 3D printer through the host software, Pronterface, for layer-by-layer construction.
Electrospun patch fabrication
A custom mandrel was machined from stainless steel to match the dimensional measurements obtained from native juvenile sheep specimens (lumen diameter: 17 mm). Polymer nanofiber precursor solutions were prepared by dissolving 5% PCL (Mw = 80 kDa, Cat# 440744; Sigma-Aldrich, St. Louis, MO) in 1,1,1,3,3,3-hexafluoroisopropanol (wt/wt) and heating the solution to 60°C, followed by continuous stirring until the PCL was completely dissolved. Once cooled, the PCL solution was electrospun in a custom-designed electrospinning apparatus utilizing 20-gauge blunt tip needles, a high voltage DC power supply set to +14 kV, and a 20 cm tip-to-substrate distance. Midway through the fiber deposition process, 3D-printed PCL support rings were applied to the tracheal graft and fiber deposition resumed until the final desired thickness was achieved. All tracheal scaffolds were removed from the mandrel and placed in a vacuum overnight to ensure removal of residual solvent (typically less than 10 ppm). The scaffolds were packaged in Tyvek pouches, terminally sterilized with 30 kGy of gamma irradiation, and shipped standard at ambient conditions to Colorado State University.
Animal model and surgical method
Five skeletally mature (>3.5 years of age) female Rambouillet-Columbia sheep were approved by the Institutional Animal Care and Use Committee of Colorado State University (protocol #16-6467) to be used in this study. The wool around the throat region and along the neck was clipped. Sheep were induced for anesthesia with a combination of ketamine and diazepam and maintained in a surgical plane of anesthesia with isoflurane. The sheep were then placed in the dorsal recumbent position, and the surgical site was scrubbed and draped for aseptic surgery using povidone-iodine and 70% alcohol. A ventral midline cervical incision was made over the trachea, including identification and division of the strap muscles, to gain access to the cervical trachea. An elliptical defect was marked on the wall of the trachea with an approximate size of 1.5 × 2.5 cm, with the long axis oriented along the length of the trachea. The defect was centered ∼4 cm below the cricoid cartilage.
The marked area of tracheal wall was removed using a scalpel, and the resulting defect was patched with the reinforced electrospun scaffold patch (2.5 × 3.5 cm). The patch was cut to be slightly larger than the defect itself to create an airtight seal with ∼5 mm of overlap around the periphery. The scaffold was cut with surgical scissors in the operating room immediately before placement. Extensions of the PCL rings were intentionally designed to provide external support to the sheep tracheal walls. The surgical process is illustrated in Figure 1. Absorbable polydioxanone sutures (size 4-0) were used to suture the scaffold to the trachea in all animals. The cervical incision was then closed in 3 layers using running polyglactin 910 suture (3-0 Vicryl; Ethicon, Sommerville, NJ) for the muscular and subcutaneous layers and nylon for the skin closure layer.
FIG. 1.
(A) Electrospun polycaprolactone (PCL) tracheal patch after fabrication. (B) Tracheal patches were cut to the shape of the defect in the operating room. (C) A 2.5 by 1.5 cm elliptical shape incision was created in the center of each trachea. (D) Tracheal patches were sutured into place over the tracheal defect. Note the sutures placed around the PCL ring extensions. Color images available online at www.liebertpub.com/tea
Postoperative tracheal endoscopy was conducted at the end of each surgery to ensure patch integrity. After each procedure was finished, the sheep recovered from anesthesia and were returned to their community pen and monitored closely for any signs of breathing difficulty. At the conclusion of the study, the sheep were humanely euthanized with an overdose of pentobarbital (88 mg/Kg-intravenous) in accordance with AVMA guidelines. Euthanasia took place 10 weeks after implantation, 2 weeks before the scheduled euthanasia date due to observed breathing concerns during physical exertion. Following euthanasia, the sheep tracheas were retrieved and prepared for analysis.
Microcomputed tomography
μCT was performed on explanted sheep tracheas to quantify patency. μCT scans were also conducted on healthy control tracheas from age-, breed-, and gender-matched ovine tracheas for comparison. Before imaging, harvested samples were cut to an approximate length of 10 cm and fixed in 10% phosphate-buffered formalin for 48 h. Samples were then incubated in a diluted contrast agent composed of 40% Optiray 320 (Mallinckrodt Pharmaceuticals, Staines-upon-Thames, United Kingdom) and 60% phosphate-buffered saline for 24 h. After incubation in contrast agent, a Quantum FX imaging system (PerkinElmer, Waltham, MA) with a 50-kV X-ray source at 160 μA was used to obtain the μCT images of the tracheas. Samples were situated such that the center of the μCT scan was aligned with the defect of the trachea.
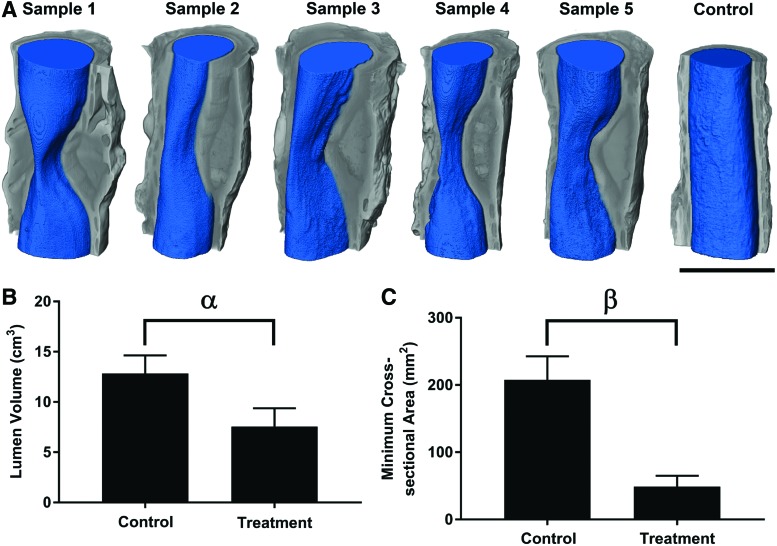
Avizo computational software (FEI Company, Hillsboro, OR) was used to reconstruct μCT scans and quantify luminal volumes inside each trachea sample, and the minimum cross-sectional area. Blue coloring represents the open space within the trachea, and gray coloring represents the tissue of the trachea. Luminal volumes are reported as the total volume (cm3) across a 5.5 cm length of trachea.
Histology
After μCT imaging, explanted tracheas were placed in 70% ethanol for long-term storage. Tissue embedding, sectioning, and staining were performed by the Stephenson Cancer Tissue Pathology Core at the University of Oklahoma Health Sciences Center. Briefly, transverse trachea sections were cut from the middle of the defect, and 1 cm above and below the midline. Tissue sections were embedded in paraffin wax following a standard protocol. Tissue blocks were sectioned at a thickness of 4 μm and affixed to glass slides. The SelecTech hematoxylin and eosin staining system (Leica Biosystems, Wetzlar, Germany), Masson's trichrome (MT) stain (Cat# HT15; Sigma-Aldrich), and Verhoeff-Van Gieson (VG) stain (Cat# HT25A; Sigma-Aldrich) were performed following the manufacturer's protocol. All staining was performed using a Leica ST5020 multistainer (Leica Biosystems).
Statistical methods
Statistical analysis was conducted using the GraphPad Prism statistical software (GraphPad Software, Inc., La Jolla, CA). An unpaired t-test (two tailed) was used to analyze μCT results. μCT testing had n = 5 samples in each group, and data are presented as the mean ± standard deviation.
Results
Animal survival
At ∼8 weeks postsurgery, one of the animals began to develop stridulous breathing that resolved during minimal physical activity (i.e., standing or walking). The sheep was immediately moved indoors to an air-conditioned region of the barn under 24-h monitoring. A second sheep developed similar signs at ∼9 weeks, followed by two more sheep at 10 weeks. Based on the respiratory clinical signs that four of the five sheep were showing and concern that respiratory effort would increase, the decision was made to humanely euthanize all animals 2 weeks before the scheduled euthanasia time.
Microcomputed tomography
Noticeable new tissue formation surrounding the electrospun patch was detected by gross examination and μCT; however, obvious tissue ingrowth toward the luminal side of the trachea was observed in all samples (Fig. 2A). Tissue formation covering the electrospun patch narrowed the trachea, causing partial stenosis in all samples, where the smallest tracheal cross-section was observed near the center of each implanted patch. The average lumen volume of the control trachea (12.8 cm3) was 1.7 times larger than the treatment group (Fig. 2B, p < 0.05). The average minimum cross-sectional area of the control trachea (207.9 mm2) was 4.3 times larger compared with the treatment group (Fig. 2C, p < 0.0001).
FIG. 2.
Microcomputed tomography (μCT) reconstructions and analysis of control and treatment groups using Avizo software. (A) Visualized μCT reconstructions where gray coloring indicates the tissue of the trachea and blue coloring indicates the lumen space. All five experimental tracheas are shown, and one representative healthy control trachea is shown. Note the decrease in cross-sectional area at the apex of the stenosis. Scale bar = 25 mm. (B) Quantified lumen volume calculated from reconstructed μCT scans. α = significant difference in lumen volume (p < 0.05). (C) Minimum lumen cross-sectional area calculated from reconstructed μCT scans. β = significant difference in lumen cross-sectional area (p < 0.05). Values represent the mean ± standard deviation. n = 5. Color images available online at www.liebertpub.com/tea
Histology
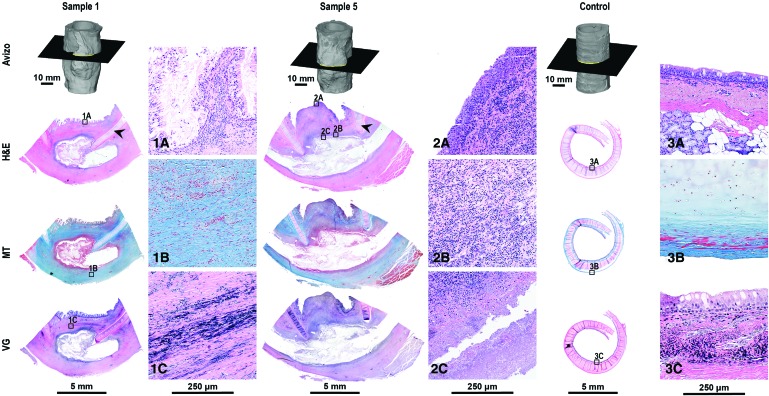
PCL rings did not adhere to the microscope slides, leaving an empty space (Fig. 3). On the upper portion of the scaffold toward the mucosal side, reepithelialization by ciliated epithelium was noted (Fig. 3.1A); however, regenerating ciliated epithelium was not observed in sections taken from the middle of the defect. Midline sections showed reepithelialization with metaplastic squamous epithelium rather than ciliated epithelium (Fig. 3.2A). In general, a substantial amount of reactive changes composed of a mixture of inflammatory changes and fibrosis was noted on the luminal side and around the PCL rings.
FIG. 3.
Histological analysis of sheep tracheas after 10 weeks postsurgery for two representative samples from the experimental group and one representative sample from the healthy control group. Sections were taken in the transverse plane illustrated by the cross-section through the Avizo reconstruction. Sections were stained using hematoxylin and eosin (H&E), Masson's Trichrome (MT), and Verhoeff-Van Gieson (VG) stain to visualize tissue regeneration. Arrows indicate native cartilage rings of the trachea, and sections have been oriented such that the lumen is at the top of the image. Magnified images correspond to the small window with the same image identifier shown in the bottom left corner (1A–3C). Note fibrous tissue formation surrounding the electrospun scaffolds; however, the tissue did not integrate into the patch. Color images available online at www.liebertpub.com/tea
The proportion of fibrosis and inflammatory changes varied among different samples. A substantial amount of fibrotic reactive changes, best demonstrated by MT staining (Fig. 3.1B), was noted in comparison to healthy control tissue (Fig. 3.3B). A substantial amount of fibrosis around the PCL ring and on the luminal side of the trachea was observed (Fig. 3.1B). Midline sections showed a nodule of tissue protruded into the lumen and were composed predominantly of inflammatory cells without significant deposition of collagen compared to sections taken further from the defect midline, as demonstrated by MT stain. The inflammatory cells were a mixture of acute and chronic inflammatory cells with no foreign body-type giant cell presence (Fig. 3.2B).
The amount of fibrosis on the abluminal side of the patch was comparable between sample 1 and sample 5, as demonstrated by MT staining. Portions of the scaffold were observed inside the mass of tissue forming toward the luminal side of the tracheal patch. Necrotic tissue was observed at the interface of the scaffold material and the native tissue (Fig. 3.2C), with no tissue integration into the scaffold material. Elastic fibers were observed in the treatment groups, as demonstrated by VG staining (Fig. 3.1C).
Discussion
This study was the first to attempt a completely synthetic, acellular, air-tight, and suturable tracheal patch for the treatment of tracheal stenosis, which was accomplished in a sheep model. In addition, this study was the first to implement 3D-printed PCL rings to provide mechanical reinforcement and hold the trachea open during the healing process.17 The electrospun tracheal patch described has been developed with clinical translation in mind, focusing on an off-the-shelf approach to provide an affordable and accessible treatment option. The surgeon was able to reseal the wall of the trachea using the developed electrospun patch. Immediately after surgery, and after complete recovery from anesthesia, the sheep were able to immediately breathe normally and return to physical activity.
The survival of all sheep after creation and closure of the tracheal defect, immediate return to normalcy following implantation of the patch, and survival for 10 weeks are considered successes. Nevertheless, there is clear need for future improvement as the study was prematurely ended due to observed breathing concerns during physical exertion. Fibrotic tissue growth surrounding the implanted patch resulted in a desirable outcome in terms of tissue type, but the observed overgrowth was problematic in our model. In humans, fibrotic tissue overgrowth could lead to tracheal stenosis due to contracture of the fibrotic scar and potentially requires follow-up procedures. The degree of tissue inflammation on the luminal side of the electrospun patch that caused a decrease in cross-sectional area in our sheep and prompted early euthanasia could potentially be attributed to poor integration of the bioengineered construct with the surrounding native tissue.
We believe that the necrotic tissue observed at the periphery of the patch and the lack of cellular presence in the patch itself were the primary limitations. The lack of giant cells suggested that the patch was not inducing a foreign body response, prompting further research into avenues to increase cellular infiltration for future trials. Although prior cell seeding was considered to remedy the tissue integration issues observed in this study, our previous studies have demonstrated an increased accumulation of inflammatory cells.16 Due to the increase in inflammatory cells using prior cell seeding, acellular approaches are still the overall focus. Ultimately, the correct balance between structural support and polymer degradation for tissue ingrowth can be tuned to create a suitable mix of tissues to permanently treat the tracheal defect.
Previous groups have attempted various materials for tracheal replacement including Dacron, Marlex, Teflon, cadaveric grafts, tissue-engineered constructs, and many others.8,18 Omori et al. in 2005 used a Marlex mesh tube covered by a collagen sponge to treat a defect created after a hemithyroidectomy procedure.19 Recent tracheal tissue engineering work by other groups has relied on a combination of cells and scaffolds, sometimes including growth factors as well. Another study attempting to create an implant for tracheal reconstruction was put forth by Delaere et al.,20 but that case involved a rather complex process using a decellularized donor trachea and several procedures to utilize the recipient's own tissues to build upon the skeletal framework. Since 2011, there have been no new publications on patients who have undergone tracheal tissue repair or replacement by means of tissue engineering. To the best of our knowledge, the last synthetic trachea surgery was performed on a pediatric patient in Peoria, Illinois, by Macchiarini and Holterman in 2013. The complexity of the patient's care and the patient's eventual death from the use of a nonbiodegradable polymeric tracheal implant led to a cessation of further human trials.
A recent (2017) summary report was published speculating on the reasons why the gap since 2011 exists.21 Although the trachea was one of the first targets of tissue engineering because of the perceived lack of complexity, the trachea is still a challenging organ to engineer. One potential reason may be that too much emphasis has been placed on the matrix material and not enough on recellularization. Despite this open question, our team continues to pursue a more straightforward approach: to create and develop a biomaterial that allows for tissue regeneration in situ. There is merit to a simplified approach by avoiding issues with cell handling, antigenicity, timing, regulatory strategy, reproducibility, cost, and ease of use. If a bioresorbable product could be simply created and implanted, with few ingredients, the approval process could potentially be expedited for use in humans, providing variously sized and shaped options for tracheal reconstruction.
Limitations exist with this material design, the major limitation speculated to be poor tissue integration into the electrospun patch effecting tissue overgrowth and mucosalization. Poor tissue integration may be attributed to the polymer degradation time, lack of initial cellular adhesion, or an initial bacterial load due to the open environment of the trachea delaying cellular proliferation. Previous work has identified an altered microbiome associated with tracheal stenosis.22,23 In addition, in another study, airway stents recovered from patients were observed to have bacterial colonization.24 Although this study was not applied to a stenosed trachea sheep model, antibacterial materials may potentially aid in the overall healing in either situation. The bacterial burden associated with an open environment applies to all trachea regeneration approaches, but is not exclusive to the trachea.
Our trachea tissue engineering community may benefit from lessons learned in working in open environments, including gastrointestinal,25,26 oral,27 esophageal,28 and skin regeneration.29 In addressing the aforementioned concerns, numerous potential options exist. Different polymer blends or co-electrospinning could be used to modulate the degradation time to improve cellular infiltration.30–35 Alternatively, cell adhesion peptides, such as RGD (Arg-Gly-Asp), could be incorporated onto the surface of the material to increase initial cellular adhesion.36–40 Antibacterial approaches to prevent the initial formation of undesirable bacteria on the patch may be an attractive option to avoid delay in regeneration or any potential biofilm-related complications.41–45 Future studies attempting to develop an off-the-shelf patch for tracheal repair may incorporate one or more of the these strategies to increase tissue integration.
Conclusion
This study reported on the first use of PCL nanofibers enveloping 3D-printed PCL rings to create a mechanically robust, suturable, air-tight, and bioresorbable graft for use in tracheal reconstruction. There is a need to improve upon the current design; however, successful and encouraging findings were present in this ovine study. The material is easy to implant, provides external protection during healing, and did not incite a foreign body reaction evident at 10 weeks of implantation in an in vivo ovine tracheal defect model. Minimal tissue integration into the patch suggests the need for faster material degradation, increased cellular adhesion, and/or antibacterial coatings to reduce initial bacterial burden.
Acknowledgments
The authors would like to thank Dr. Cory Berkland for his assistance and guidance in completing the project, and Dr. Hong Liu and Dr. Di Wu for assistance with the μCT. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award no. R43 HL131367 (to J.K.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nouraei S.A., Ma E., Patel A., Howard D.J., and Sandhu G.S. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol 32, 411, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Den Hondt M., and Vranckx J.J. Reconstruction of defects of the trachea. J Mater Sci Mater Med 28, 24, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Cotton R.T., Gray S.D., and Miller R.P. Update of the Cincinnati experience in pediatric laryngotracheal reconstruction. Laryngoscope 99, 1111, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Grillo H.C. Tracheal replacement. Ann Thorac Surg 49, 864, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Elliott M., Hartley B.E., Wallis C., and Roebuck D. Slide tracheoplasty. Curr Opin Otolaryngol Head Neck Surg 16, 75, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Tsang V., Murday A., Gillbe C., and Goldstraw P. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg 48, 632, 1989 [DOI] [PubMed] [Google Scholar]
- 7.de Trey L.A., and Morrison G.A. Buccal mucosa graft for laryngotracheal reconstruction in severe laryngeal stenosis. Int J Pediatr Otorhinolaryngol 77, 1643 [DOI] [PubMed] [Google Scholar]
- 8.Grillo H.C. Tracheal replacement: a critical review. Ann Thorac Surg 73, 1995, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Ott L.M., Weatherly R.A., and Detamore M.S. Overview of tracheal tissue engineering: clinical need drives the laboratory approach. Ann Biomed Eng 39, 2091, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Elliott M.J., De Coppi P., Speggiorin S., et al. . Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380, 994, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton N.J., Kanani M., Roebuck D.J., et al. . Tissue-engineered tracheal replacement in a child: a 4-year follow-up study. Am J Transplant 15, 2750, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zopf D.A., Hollister S.J., Nelson M.E., Ohye R.G., and Green G.E. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med 368, 2043, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Vogel G. Trachea transplants test the limits. Science 340, 266, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Weiss D.J., Elliott M., Jang Q., Poole B., Birchall M.; and International Society of Cell Therapy Pulmonary Scientific Committee. Tracheal bioengineering: the next steps. Proceeds of an International Society of cell therapy pulmonary cellular therapy signature series workshop, Paris, France, April 22, 2014. Cytotherapy 16, 1601, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Yeo A., Rai B., Sju E., Cheong J.J., and Teoh S.H. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: an in vitro and in vivo study. J Biomed Mater Res A 84, 208, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Ott L.M., Vu C.H., Farris A.L., et al. . Functional reconstruction of tracheal defects by protein-loaded, cell-seeded, fibrous constructs in rabbits. Tissue Eng Part A 21, 2390, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott L.M., Zabel T.A., Walker N.K., et al. . Mechanical evaluation of gradient electrospun scaffolds with 3D printed ring reinforcements for tracheal defect repair. Biomed Mater 11, 025020, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Haykal S., Salna M., Waddell T.K., and Hofer S.O. Advances in tracheal reconstruction. Plast Reconstr Surg Glob Open 2, e178, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omori K., Nakamura T., Kanemaru S., et al. . Regenerative medicine of the trachea: the first human case. Ann Otol Rhinol Laryngol 114, 429, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Delaere P., Vranckx J., Verleden G., De Leyn P., Van Raemdonck D., and Leuven Tracheal Transplant G. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 362, 138, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Siddiqi S., Wit R., Timman S., Oosterwijk E., Morshuis W., and Verhagen A. Tissue engineering of the trachea: what is the Hold-up? MOJ Cell Sci Rep 4, 00076, 2017 [Google Scholar]
- 22.Gelbard A., Katsantonis N.G., Mizuta M., et al. . Molecular analysis of idiopathic subglottic stenosis for Mycobacterium species. Laryngoscope 127, 179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazhar K., Gunawardana M., Webster P., et al. . Bacterial biofilms and increased bacterial counts are associated with airway stenosis. Otolaryngol Head Neck Surg 150, 834, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Nouraei S.A., Petrou M.A., Randhawa P.S., Singh A., Howard D.J., and Sandhu G.S. Bacterial colonization of airway stents: a promoter of granulation tissue formation following laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg 132, 1086, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Chang H.-M., Huang C.-C., Parasuraman V.R., et al. . In vivo degradation of poly (ɛ-caprolactone) films in Gastro Intestinal (GI) tract. Mater Today Commun 11, 18, 2017 [Google Scholar]
- 26.Spurrier R.G., and Grikscheit T.C. Tissue engineering the small intestine. Clin Gastroenterol Hepatol 11, 354, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Wang Z., Shen Y., and Haapasalo M. Antibiofilm peptides against oral biofilms. J Oral Microbiol 9, 1327308, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maghsoudlou P., Eaton S., and De Coppi P. Tissue engineering of the esophagus. Semin Pediatr Surg 23, 127, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Chaudhari A.A., Vig K., Baganizi D.R., et al. . Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci 17, 1974, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsawy M.A., Kim K.-H., Park J.-W., and Deep A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew Sustain Energy Reviews 79, 1346, 2017 [Google Scholar]
- 31.Gámiz-González M.A., Vidaurre A., and Gómez Ribelles J.L. Biodegradable chitosan-poly(ɛ-caprolactone) dialdehyde copolymer networks for soft tissue engineering. Polym Degradation Stability 138, 47, 2017 [Google Scholar]
- 32.Grossen P., Witzigmann D., Sieber S., and Huwyler J. PEG-PCL-based nanomedicines: a biodegradable drug delivery system and its application. J Control Release 260, 46, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Li C., Wang F., Chen P., Zhang Z., Guidoin R., and Wang L. Preventing collapsing of vascular scaffolds: the mechanical behavior of PLA/PCL composite structure prostheses during in vitro degradation. J Mech Behav Biomed Mater 75, 455, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Sun X., Lang Q., Zhang H., et al. . Electrospun photocrosslinkable hydrogel fibrous scaffolds for rapid in vivo vascularized skin flap regeneration. Adv Funct Mater 27, 1604617, 2017 [Google Scholar]
- 35.Yao Q., Cosme J.G., Xu T., et al. . Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 115, 115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee P., Yeo G.C., and Weiss A.S. A cell adhesive peptide from tropoelastin promotes sequential cell attachment and spreading via distinct receptors. FEBS J 284, 2216, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Li J., Yu Y., Myungwoong K., et al. . Manipulation of cell adhesion and dynamics using RGD functionalized polymers. J Mater Chem B 5, 6307, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Mobasseri R., Tian L., Soleimani M., Ramakrishna S., and Naderi-Manesh H. Bio-active molecules modified surfaces enhanced mesenchymal stem cell adhesion and proliferation. Biochem Biophys Res Commun 483, 312, 2017 [DOI] [PubMed] [Google Scholar]
- 39.van Dijk I.A., Beker A.F., Jellema W., et al. . Histatin 1 enhances cell adhesion to titanium in an implant integration model. J Dent Res 96, 430, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Xu Q., Zhang Z., Xiao C., He C., and Chen X. Injectable polypeptide hydrogel as biomimetic scaffolds with tunable bioactivity and controllable cell adhesion. Biomacromolecules 18, 1411, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Epand R.F., Pollard J.E., Wright J.O., Savage P.B., and Epand R.M. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob Agents Chemother 54, 3708, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epand R.F., Savage P.B., and Epand R.M. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). Biochim Biophys Acta 1768, 2500, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Guyomard A., Dé E., Jouenne T., Malandain J.-J., Muller G., and Glinel K. Incorporation of a hydrophobic antibacterial peptide into amphiphilic polyelectrolyte multilayers: a bioinspired approach to prepare biocidal thin coatings. Adv Funct Mater 18, 758, 2008 [Google Scholar]
- 44.Vila-Farres X., Callarisa A.E., Gu X., Savage P.B., Giralt E., and Vila J. CSA-131, a ceragenin active against colistin-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates. Int J Antimicrob Agents 46, 568, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Yu K., Lo J.C., Yan M., et al. . Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 116, 69, 2017 [DOI] [PubMed] [Google Scholar]