Abstract
The long-term goal of this work is to develop a potassium (K+)-based intra-articular (IA) injection for osteoarthritis treatment. Within this context, the objectives of this study were to (1) demonstrate that hyperosmolar K+ solutions can suppress proinflammatory macrophage activation and (2) evaluate the therapeutic potential of a hyperosmolar K+ solution relative to a clinically utilized drug-based (methylprednisolone acetate [MPA]—a corticosteroid) or cell-based (human mesenchymal stem cell [hMSC]) IA injectable. A 3D in vitro model with poly(ethylene glycol) diacrylate hydrogels encapsulated with proinflammatory interferon-gamma (IFN)-stimulated macrophages (M(IFN)s) was utilized. Long-term changes in cell phenotype in response to short-term stimulation (i.e., mimicking an IA injection) were assessed following treatment with 80 mM K+ gluconate, hMSCs, or MPA. Addition of 80 mM K+ gluconate to culture media significantly reduced iNOS and TNF protein levels in M(IFN)s. Furthermore, short-term stimulation with K+ gluconate elicited a significant increase in the anti/proinflammatory cytokine profile in M(IFN)s, a response that is not noticed with either clinically utilized MPA or an hMSC injectable. Hyperosmolar K+ solutions are capable of attenuating proinflammatory macrophage activation. Moreover, when evaluated in an in vitro setting mimicking an IA injection, K+ performed significantly better than hMSCs or the corticosteroid MPA. Cumulatively, these results support further development and application of a K+-based IA injection toward osteoarthritis research.
Keywords: : macrophage activation, osteoarthritis, potassium, intra-articular injection
Introduction
Osteoarthritis (OA) is a major cause of disability worldwide,1 yet no disease-modifying treatments exist. Inflammation coupled with heightened cell and tissue activation results in an imbalance between anabolic and catabolic processes characteristic of OA disease progression.2,3 Separate from OA, ions are emerging as fundamental regulators of the immune system, acting distinctly from well-studied chemical factors.4,5 Similarly, bioelectrical signals, manifested as changes in cellular transmembrane potential (Vmem), are emerging as powerful mediators of cell phenotype, separate from known chemical factors.6 The working hypothesis of this work is that potassium (K+), either through Vmem depolarization or chemical effects from the ion, is capable of limiting chondrocyte and macrophage (MΦ) activation. In theory, a K+-based intra-articular (IA) injection treatment would be cheap, widely available, and easy to implement.
Whether K+ limits MΦ activation is unknown. As such, demonstrating this proof of concept was one of the main objectives of this study. In our first experiment, gene and protein level analyses revealed that treatment of M(IFN)s with 80 mM K+ gluconate significantly reduced the activation of these cells, assessed by gene and protein levels of NOS-2 and TNF (Supplementary Fig. S1 and Table S1; Supplementary Data are available online at www.liebertpub.com/tea).
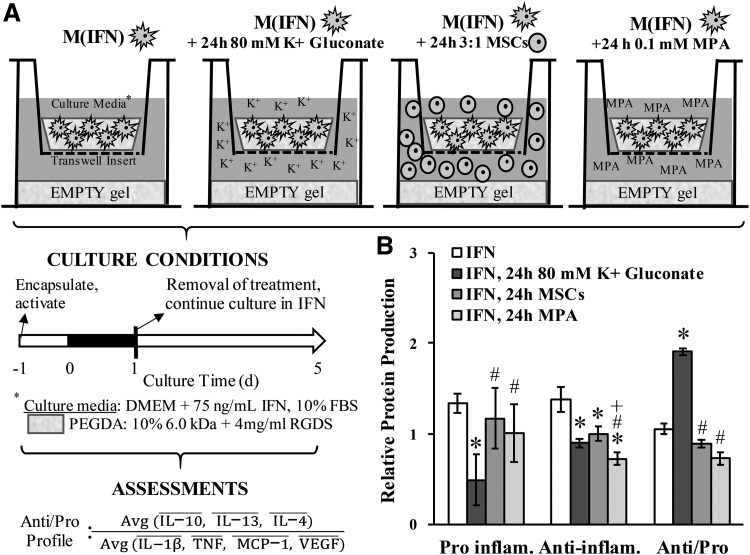
Another goal of this study was to assess long-term effects elicited in response to short-term K+ stimulation (i.e., mimicking an IA injection; Fig. 1A). In contrast to MSCs and MPA, treatment with 80 mM K+ gluconate significantly enhanced the anti/proinflammatory cytokine ratio (Fig. 1B and Supplementary Fig. S2). The extended benefit from a short-term K+ treatment was also noted for IL-8, MCP-1, and MMP-13 production in OACs (Supplementary Fig. S3). From an OA disease perspective, the overall decreased levels of cytokines, the enhanced ratio of anti/proinflammatory cytokines, and the reduced production of MMP-13 noted herein would seem desirable for a potential disease-modifying treatment and supports further development of K+-based solutions.
FIG. 1.
(A) Experimental design mimicking an IA injection in vitro. M(IFN)s were cultured for 5 days in the continued presence of IFN, with 80 mM K+ applied only during the first day and then removed. Other IA injection formulations—human MSCs and MPA—were also included to gain a sense of efficacy and benchmark against current OA treatments. An empty PEGDA hydrogel (∼50 kPa stiffness) was provided at the bottom of the culture well to create a surface that more closely matches the stiffness of articular cartilage (aggregate modulus ∼500 kPa12) than traditional plastic 2D surfaces (∼1 × 106 kPa). MSC behavior is known to respond to substrate stiffness. Assessments for treatment efficacy included cell lysate levels of several proinflammatory markers, anti-inflammatory markers, and the ratio between anti/proinflammatory profiles. A bar above the protein denotes normalization. (B) Relative protein production of pooled proinflammatory and pooled anti-inflammatory molecules and the ratio between anti/proinflammatory profiles in Raw 264.7 MΦs after 5 days in culture. Treatments were applied only during the first day of culture. *Denotes a significant difference relative to IFN controls. #Denotes a significant difference relative to 24-h 80 mM K+ gluconate. +Denotes a significant difference relative to 24-h MSCs. PEGDA, poly(ethylene glycol) diacrylate; MSCs, mesenchymal stem cells; MPA, methylprednisolone acetate.
From a mechanistic perspective, suppression of M(IFN)s in addition to the shift in anti/proinflammatory ratio would suggest that 80 mM K+ gluconate drives MΦ polarization toward an anti-inflammatory/proresolving phenotype. This is consistent with K+ treatment enhancing the generation of Foxp3+ Treg cells,7,8 reducing T cell effector function8 and inflammatory protein production in OACs.9 As the extracellular K+ concentration is elevated, ∼40 mM in necrotic cancerous tumors,7 this work may help explain previous findings of the tumor-associated macrophage phenotype.10 Interestingly, the anti/proinflammatory profile was not significantly different between 40 and 80-mM treatments (Supplementary Fig. S4). These additional data suggest that (1) the underlying cell response is primarily a function of the ionic content and (2) the therapeutic dose of K+-based salts could be broad. Overall, these findings are also consistent with recent T cell literature demonstrating that increased intracellular K+ is the main mediator of the cell response7 and that even small amounts of K+ (i.e., 2 mM) are capable of suppressing inflammatory cytokine production.11 Future work will seek to definitively decouple contributions from osmolarity, the K+ ions, and membrane potential.
Methods
Detailed methods and associated references can be found online in Supplementary Data.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the NIH (R03 AG056168-02 and R01 DC013508) for funding this research.
Disclosure Statement
J.E.M. and M.H. have a provisional patent (U.S Provisional Patent, Application Number 62/474,891) for this work.
References
- 1.Mobasheri A., Barrett-Jolley R., Staunton C.A., Ford C., and Henrotin Y. Nutrigenomics, inflammaging, and osteoarthritis: a review. In: Bagchi D., Swaroop A., and Bagchi M., eds. Genomics, Proteomics and Metabolomics in Nutraceuticals and Functional Foods. West Sussex, UK: Wiley, 2015, pp. 71–84 [Google Scholar]
- 2.Berenbaum F., and van den Berg W.B. Inflammation in osteoarthritis: changing views. Osteoarthritis Cartilage 23, 1823, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Goldring M.B., Otero M., Plumb D.A., et al. . Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cells Mater 21, 202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurusamy D., Clever D., Eil R., and Restifo N.P. Novel “Elements” of Immune 1ression within the Tumor Microenvironment. Cancer Immunol Res 5, 426, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schatz V., Neubert P., Schroder A., et al. . Elementary immunology: Na+ as a regulator of immunity. Pediatr Nephrol 32, 201, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin M., and Stevenson C.G. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu Rev Biomed Eng 14, 295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eil R., Vodnala S.K., Clever D., et al. . Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 537, 539, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalili H., Malik S., Ananthakrishnan A.N., et al. . Identification and characterization of a novel association between dietary potassium and risk of Crohn's Disease and Ulcerative Colitis. Front Immunol 7, 554, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erndt-Marino J., Trinkle E., and Hahn M.S. Hyperosmolar Potassium (K+) treatment suppresses osteoarthritic chondrocyte catabolic and inflammatory protein production in a 3-dimensional in vitro model. Cartilage 1947603517734028, 2017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanmee T., Ontong P., Konno K., and Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 6, 1670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen W., Wan Z.F., Ren K.Y., et al. . Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp Mol Pathol 100, 370, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Athanasiou K.A., Rosenwasser M.P., Buckwalter J.A., Malinin T.I., and Mow V.C. Interspecies comparisons of in situ intrinsic mechanical-properties of distal femoral cartilage. J Orthop Res 9, 330, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.