Abstract
The lower cloud layer of Venus (47.5–50.5 km) is an exceptional target for exploration due to the favorable conditions for microbial life, including moderate temperatures and pressures (∼60°C and 1 atm), and the presence of micron-sized sulfuric acid aerosols. Nearly a century after the ultraviolet (UV) contrasts of Venus' cloud layer were discovered with Earth-based photographs, the substances and mechanisms responsible for the changes in Venus' contrasts and albedo are still unknown. While current models include sulfur dioxide and iron chloride as the UV absorbers, the temporal and spatial changes in contrasts, and albedo, between 330 and 500 nm, remain to be fully explained. Within this context, we present a discussion regarding the potential for microorganisms to survive in Venus' lower clouds and contribute to the observed bulk spectra. In this article, we provide an overview of relevant Venus observations, compare the spectral and physical properties of Venus' clouds to terrestrial biological materials, review the potential for an iron- and sulfur-centered metabolism in the clouds, discuss conceivable mechanisms of transport from the surface toward a more habitable zone in the clouds, and identify spectral and biological experiments that could measure the habitability of Venus' clouds and terrestrial analogues. Together, our lines of reasoning suggest that particles in Venus' lower clouds contain sufficient mass balance to harbor microorganisms, water, and solutes, and potentially sufficient biomass to be detected by optical methods. As such, the comparisons presented in this article warrant further investigations into the prospect of biosignatures in Venus' clouds.
Key Words: : Venus, Clouds, Life, Habitability, Microorganism, Albedo, Spectroscopy, Biosignatures, Aerosol, Sulfuric Acid
1. Introduction
The habitability of Venus' clouds has been a subject of discussion for several decades (Morowitz and Sagan, 1967; Grinspoon, 1997) yet has gained limited traction as a popular target in astrobiology research. Initially stirring excitement, Cockell (1999) concluded that the conditions between the lower and middle atmosphere were conducive to (terrestrial) biology, and that conditions at higher altitudes would freeze but not necessarily kill microorganisms. Since then, subsequent studies, such as those by Schulze-Makuch et al. (2004), have highlighted the potential for life in Venus' cloud layers due to favorable chemical and physical conditions, including the presence of sulfur compounds, carbon dioxide (CO2), and water, and moderate temperatures (0–60°C) and pressures (∼0.4–2 atm).
In this hypothesis article, we further consider these conditions and examine the potential for terrestrial microorganisms to both survive within and contribute to the bulk spectral properties of Venus' clouds. Herein, we provide a short review of relevant Venus observations, compare the properties of Venus' clouds to terrestrial biological materials, expand on the hypothesis of a coupled iron- and sulfur-centered metabolism in the clouds, and present conceivable mechanisms of transport from the surface to the clouds. Finally, we identify spectral and biological experiments, including instruments, which can address the habitability of Venus' clouds through use of ground-based terrestrial analogues and in situ measurements at Venus.
2. Overview of Venus' Spectral Observations
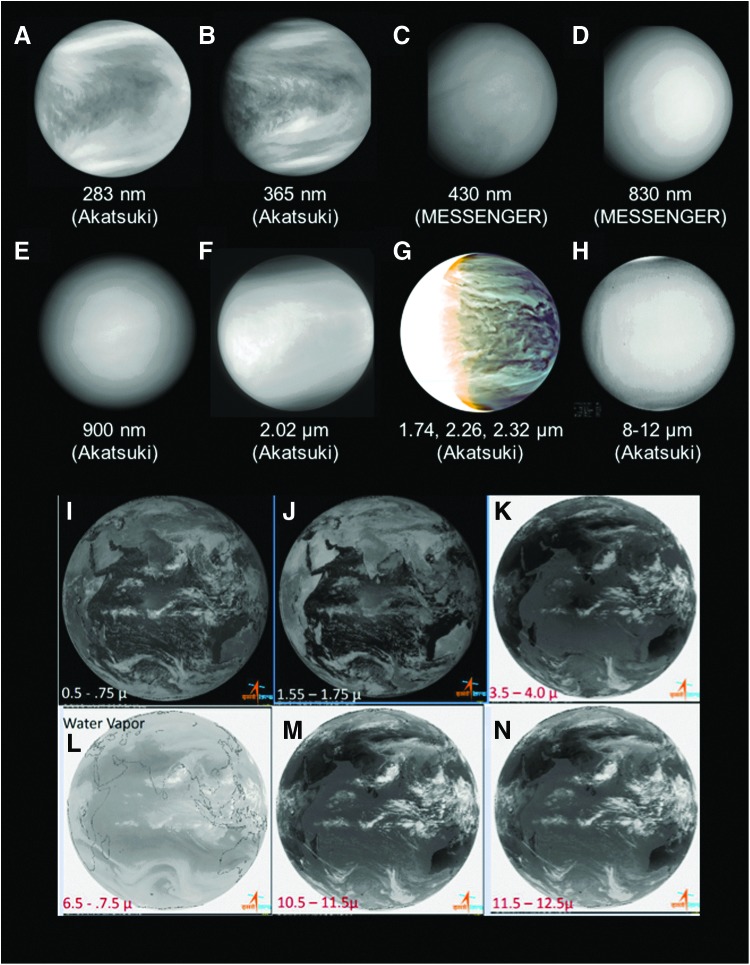
Comparisons of spectral measurements (Fig. 1) obtained from the Akatsuki and MESSENGER missions show several differences in albedo across the spectrum in the ultraviolet (UV) (images A and B), visible (image C), near-infrared (IR) (images D–G), and mid-IR wavelengths (image H). Venus is globally covered in clouds and devoid (or nearly devoid) of contrasts in the visible and IR wavelengths in dayside images (images C–F). Rather, contrasts in the cloud cover are observed only at wavelengths shorter than blue in reflected sunlight (images A and B), and at near-IR wavelengths (1.7–2.4 μm) on the nightside (image G). Despite spacecraft investigations from orbit and entry probes, the chemical and physical properties of these contrasts are still unknown, including the identities of the contrasting substances, the sources of these substances, the lack of mixing, and any potential sinks.
FIG. 1.
Images of clouds on Venus (A–H) and Earth (I–N) demonstrating the relationships of contrast with wavelength. The images were obtained at (A) 283 nm, (B) 365 nm, (C) 430 nm, (D) 830 nm, (E) 900 nm, (F) 2.02 μm, (G) 1.74, 2.26, and 2.32 μm, and (H) 8–12 μm. Images A, B, E, and F were taken by the Akatsuki orbiter using filters with central wavelengths equal to the aforementioned wavelengths on May 6, 2016. Images C and D were taken by the MDIS camera on the MESSENGER spacecraft (Hawkins et al., 2009) on June 6, 2007. Images G (March 25, 2016) and H (May 6, 2016) show the nightside of Venus and were, respectively, taken from the Akatsuki orbiter with the IR2 and LIR cameras; the bandwidth of the Akatsuki dayside filter was much wider (14 nm) than that of the MESSENGER MDIS filters (5 nm), and the orientation of the MESSENGER images is somewhat more tilted, compared with the Akatsuki images (rotation axis ∼45°). For Earth's clouds, images were obtained between (I) 0.5–0.75 μm, (J) 1.55–1.75 μm, (K) 3.5–4.0 μm, (L) 6.5–7.5 μm, (M) 10.5–11.5 μm, and (N) 11.5–12.5 μm. Akatsuki data are available at: https://www.darts.isas.jaxa.jp/planet/project/akatsuki/; Earth images were obtained by the INSAT-3D weather satellite (Katti et al., 2006) operated by ISRO/Space Applications Center.
Thermal IR images (8–12 μm) show small-scale (∼50 km) contrasts of <2 K in brightness temperature on the day and night hemispheres at all latitudes (image H), except poleward of ∼65° latitude in both hemispheres. At these latitudes, Hadley circulation is presumed to lower the cloud tops, due to downwelling in the polar regions, as observed near the top and bottom of the Venus images (images F and H). In contrast, clouds on Earth are often observed in satellite images as discrete features, with clear air in between, at visible and (short to thermal) IR wavelengths (images I–N). Unlike on Earth, where the contrasts are independent of wavelength, the observed contrasts in Venus' global clouds vary in morphology and magnitude at wavelengths from visible to IR (Limaye et al., 2018), as seen from images A–H in Figure 1.
The Venus UV contrasts were first observed in Earth-based photographs (Ross, 1928) and subsequently characterized by ground-based polarimetry (Hansen and Hovenier, 1974), spectroscopy (Barker, 1978), remote spacecraft observations (Kawabata et al., 1980; Titov et al., 2008), and entry probes (Knollenberg and Hunten, 1980; Knollenberg et al., 1980; Esposito et al., 1983; Knollenberg, 1984). Together, these studies indicate that the global cloud cover is composed of sulfuric acid droplets (∼1.1 μm equivalent radius) in a mixture consisting mostly of small particles (∼0.2–0.3 μm equivalent radius), with larger particles (∼2–8 μm diameter) present at lower altitudes (Knollenberg and Hunten, 1979). In addition, slight differences in cloud particle properties at the polar regions have been inferred from the Venus Express data (Wilson et al., 2008).
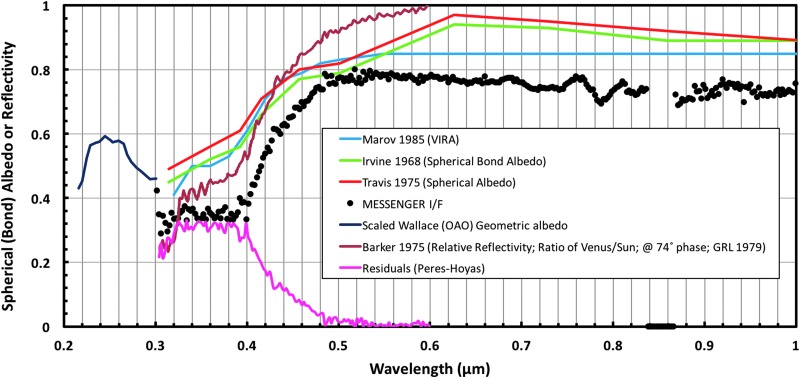
Provided in Figure 2 is a collective summary of Venus' spectra between 200 and 1000 nm, including global geometric or spherical albedo estimates (Irvine, 1968; Travis, 1975; Moroz et al., 1985) and measurements from ground-based telescopes (Barker et al., 1975) and the MESSENGER spacecraft during the second Venus fly-by (Perez-Hoyos et al., 2013; Pérez-Hoyos et al., 2017). Also shown is the calculated difference between the VIRA cloud model and the MESSENGER spectra as reported by Perez-Hoyos et al. (2013), which gives an indication of the spectral absorption by the unknown materials in the clouds of Venus.
FIG. 2.
Venus' spectra as measured by Moroz et al. (1985), Irvine (1968), Travis (1975), Wallace et al. (1972) (scaled geometric albedo), MESSENGER (Perez-Hoyos et al., 2013; Pérez-Hoyos et al., 2017), and Barker et al. (1975), including the unexplained absorption, as calculated from the difference between the VIRA cloud model and the MESSENGER spectra. The real Venus spectrum varies with location and time, so the residual curve is illustrative and not definitive.
We note here that the original identification of the sulfuric acid composition of Venus' cloud particles was derived by matching the index of refraction, required for matching the phase dependence of disk-integrated polarization at different optical wavelengths (Hansen and Hovenier, 1974), and not by spectral identification. Interpretation of Venus' UV and IR spectra, along with questions about the cloud composition and UV absorbers, are summarized by Krasnopolsky (2006) and in chapters within the review books on Venus (Hunten et al., 1983) and Venus II (Bougher et al., 1997).
Herein, we briefly summarize the pertinent cloud properties, which must play a part in the absorption of incident sunlight and the observed contrasts. Travis (1975) pointed out the differences in spectral dependence of albedo and cloud contrasts, thus indicating at least two different absorbers. Pollack et al. (1980) identified gaseous sulfur dioxide (SO2) as a potential absorber and ruled out many other suggested candidates due to insufficient spectral overlap. Esposito and Travis (1982) suggested from analysis of the polarization data obtained from the Pioneer Venus orbiter that the differential polarization between bright and dark UV features could not be explained by haze abundance variations, which favored a chemical model where water vapor and molecular oxygen are depleted at the cloud tops.
Zasova et al. (1981) pursued a suggestion by Kuiper (1969) to propose the presence of incompletely hydrated iron chloride in the clouds, and offered that SO2 (<330 nm) along with ferric chloride (FeCl3) (>330 nm) could explain the observed lowered albedo <500 nm. Partial contribution of SO2 to the UV absorption of incident solar radiation has been inferred through observations by Venus Express (Lee et al., 2015b), as well as those from the Hubble Space Telescope (Jessup et al., 2015), and can also be discerned from differences in the 283 and 365 nm appearance of Venus (images A and B of Fig. 1) taken by Akatsuki at 283 nm (where there is some absorption by SO2) and 365 nm (where the contrast peaks, and SO2 does not absorb).
Based on Venus Express measurements, however, analysis of the glory feature, as observed in unpolarized (Markiewicz et al., 2014) and polarized light (Rossi et al., 2015), yielded values for the index of refraction that were larger than those inferred by Hansen and Hovenier (1974). In fact, Markiewicz et al. (2014) found that their measured indices of refraction exceeded the values expected from sulfuric acid cloud particles, thereby suggesting the presence of FeCl3 attached to the sulfuric acid droplets, or within the droplets to serve as cloud condensation nuclei. Furthermore, Krasnopolsky (2017) concluded that sulfur aerosols cannot be the UV absorber since the required abundance and vertical profile were incompatible with Venera 14 observations; however, the presence of FeCl3 was compatible with contributions toward the higher indices of refraction inferred by Markiewicz et al. (2014). Nevertheless, some doubt remains as to whether the analysis of glory features can provide accurate inferences of the index of refraction between 1.07 and 1.7 (Laven, 2008), which encompasses the range of values for Venus.
3. Spatial Contrasts in the UV Spectrum
From ground-based and spacecraft observations, it is widely accepted that Venus' clouds contain micron-sized particles (Hansen and Hovenier, 1974; Knollenberg et al., 1980) consisting of sulfuric acid solutions (75–98%). In fact, all UV and blue images of Venus (Belton et al., 1992) show small-scale (10–100 km) contrasts at 270 nm (Pioneer Venus OCPP, polarimetry mode; Limaye, 1984), 283 nm (Akatsuki), 365 nm (Mariner 10, Venus Monitoring Camera [VMC] on Venus Express), 410 nm (Galileo), and 430 nm (MESSENGER MDIS; Peralta et al., 2017). These contrast features have been observed to evolve over time scales ranging from minutes (Limaye et al., 2018), on small scales (∼5 km), to days and weeks (del Genio and Rossow, 1982) on larger spatial scales (∼1000 km). Temporal changes in cloud contrasts have additionally been noted by Ross (1928) and in spacecraft images (Murray et al., 1974; Rossow et al., 1980; Titov et al., 2012).
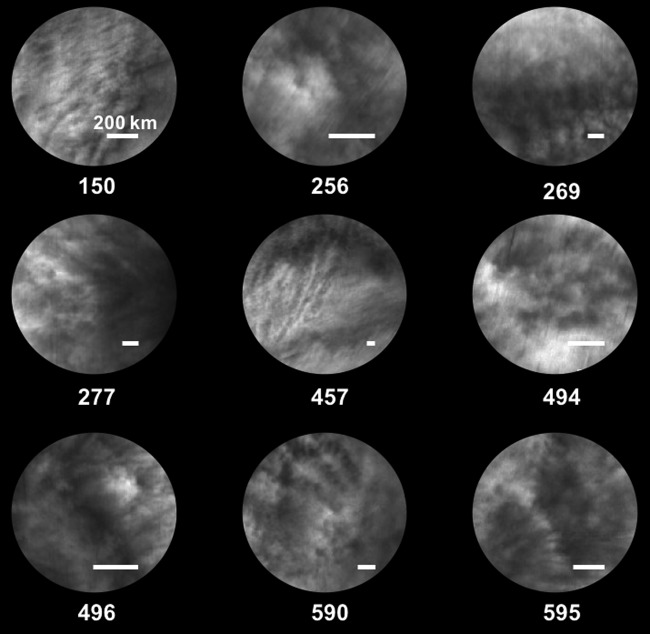
Figure 3 provides exemplar images of the equatorial region of Venus, which illustrate the variability of the UV contrasts, over a variety of scales as captured by the VMC on the Venus Express orbiter (Titov et al., 2012). Similarly, Figure 4 provides two mapped UV images from the VMC on Venus Express taken only 12 min apart. The short-term evolution (growth and decay in terms of areal extent and contrast) of these features has been challenging to explain in terms of cloud structure, cloud top altitude differences (Ignatiev et al., 2009), and/or purely dynamical processes. Nevertheless, absorption <330 nm has been attributed to SO2 and sulfur monoxide (SO) as a result of ground-based observations (Barker, 1979), and spacecraft (Conway et al., 1979; Stewart et al., 1979; Pérez-Hoyos et al., 2017) and entry probe measurements (Surkov et al., 1978; Oyama et al., 1980).
FIG. 3.
Views of the equatorial region of Venus from the Venus Monitoring Camera obtained using a 365 nm filter; numbers below each view indicate the orbit number of Venus Express (nominal period of 24 h), while the white bar in the lower right of each image indicates a 200 km scale. Figure adapted from Titov et al. (2012).
FIG. 4.
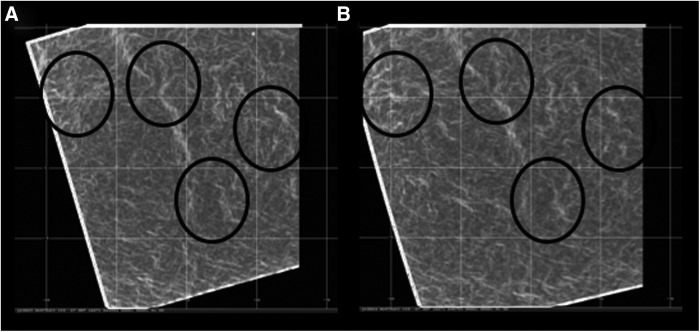
Rapid changes in the shape, size, and magnitude of the UV contrasts, as observed through distribution and intensity of the white thread-like lines, with specific examples highlighted by the black ovals. Images show Venus' clouds at low latitudes (where the absorption of incident solar radiation is greater) and were obtained 12 min apart, on a scale of 5 m/pixel, on September 27, 2016, at 04:45:58 (A) and at 04:57:53 UT (B). Mapped views include grid lines 5° apart in latitude and longitude. These images were obtained by the Akatsuki mission from the UVI camera (Yamazaki et al., 2017) and used the 365 nm filter. They were processed to bring out subtle contrasts by using ratios of local brightness deviations and average brightness. UV, ultraviolet; UVI, Ultraviolet Imager.
Between 200 and 500 nm, other proposed absorbing candidates (besides SO2) include fine graphite grains (Shimizu, 1977), elementary sulfur polymers (Young, 1973; Hapke and Nelson, 1975; Toon et al., 1982), octasulfur (Schulze-Makuch and Irwin, 2006), nitric oxide (Shaya and Caldwell, 1976), croconic acid (Hartley et al., 1989), hydrated FeCl3 (Kuiper, 1969), hydrobromic acid (Sill, 1975), and chlorine (Pollack et al., 1980). Besides SO2 and SO, other compounds absorbing <330 nm, but not between 330 and 600 nm (Mills et al. (2007), include carbon sulfide (Barker, 1978; Young, 1978) and carbonyl sulfide (Bezard et al., 1990). Esposito et al. (1983) and Krasnopolsky (2006) have also published discussions regarding the unknown “ultraviolet absorber.” Furthermore, Zasova et al. (2007) point out that the spectrally active compound/s may absorb in both the visible and near IR regions.
Sulfur aerosols have also been postulated as the UV absorbers (Krasnopolsky, 2016, 2017), where analysis of the (limited) data from in situ measurements has suggested a role for FeCl3. In addition, a sulfur oxide isomer (OSSO) has been recently proposed as an alternative UV absorber (between 320 and 400 nm) and a potential sulfur reservoir. However, the lifetimes of the two isomers of OSSO are very short (a few seconds), and the estimates of opacity are uncertain (Frandsen et al., 2016). Nevertheless, recent studies (Pérez-Hoyos et al., 2017) have concluded that the identity of the UV absorber in the clouds is still unresolved, even after considering the newly proposed isomers of disulfur dioxide (as inferred from analysis of spectroscopic observations from the MESSENGER spacecraft made during the Venus fly-by).
On the dayside, the UV component of the incident sunlight is progressively absorbed, as the radiation penetrates the cloud tops and travels downward, and is almost immeasurable at an altitude of 57 km (Tomasko et al., 1980), thereby preventing detection of the absorber by using sunlight. However, the absorber is present below this altitude, as inferred from spectroscopic measurements by the VeGa 1 and VeGa 2 landers. Using a xenon lamp, these probes descended on the nightside and established that the UV absorbers are present at the highest altitudes measureable (64 km), down to the base of the clouds at 47 km (Bertaux et al., 1996). In terms of spatial and temporal variability of the absorbers, studies of the cloud tops support such fluctuations for SO2 (Encrenaz et al., 2016); however, the reasons or causes remain unknown, as detailed investigations of spatial and temporal variability of contrast features are yet to be conducted. Similarly, near-simultaneous Akatsuki observations of Venus at 283 and 365 nm also indicate (Lee et al., 2017) that SO2 variations can partly explain the differences in contrasts; however, as noted earlier, there are a number of other trace species that may contribute to the observed variations.
Based on observations of the glory feature in Venus' images, FeCl3 has also been reproposed as a candidate (Markiewicz et al., 2014) and remains the most likely contender as a UV contrasting agent (Krasnopolsky, 2017), as FeCl3 in the clouds has also been detected by X-ray fluorescence data (Krasnopolsky, 1985; Andreychikov et al., 1987). However, Zasova et al. (1981) note that FeCl3 is not stable in the presence of sulfuric acid, presumably due to formation of Fe2(SO4)3. As such, a continuous resupply of FeCl3 would be required to support the observed contrasts. The most logical source for resupply of FeCl3 would be the Venus' surface, since on Earth, FeCl3 is commonly found in volcanic regions as the mineral molysite (which is brownish or reddish in color, and soluble in water). This assessment, however, introduces several questions as to how FeCl3 particles are transported, on the timescales of the contrasts, to ∼50 km above the Venus' surface, and whether FeCl3 particles act as cloud condensation nuclei.
In summary, the identities of the absorber(s) in the 330–600 nm region remain uncertain. In addition, current cloud models (and observations) do not adequately explain the origin of the contrasts and the spatial and temporal changes in opacity (i.e., lack of mixing of the absorbers and spatial variations in abundances over time). Therefore, in this absence of cohesive physical and chemical explanations, we present a discussion of Venus' clouds serving as a favorable habitat for life, where biological sources may contribute to the observed spectral contrasts.
4. Can Biology Contribute to Venus' Spectral Signatures?
The possibility of Venus life in the clouds was initially discussed by Morowitz and Sagan (1967), Grinspoon (1997) and followed up by Cockell (1999), Schulze-Makuch and Irwin (2002), Schulze-Makuch et al. (2004), and considered by Grinspoon and Bullock (2007). These reports introduced the premise that acid-resistant terrestrial bacteria could potentially tolerate the Venus' cloud environment, and metabolize through phototrophic and chemotrophic means. In his book “Venus Revealed,” Grinspoon (1997) proposed that a photosynthetic pigment may serve as the “unknown ultraviolet absorber.” In fact, Grinspoon posited that the “unknown ultraviolet absorber” may represent one of four possible signs of life, with the remaining signs, including absorption of solar energy by (micro)organisms as a driving force for super-rotation, the presence of larger and irregularly shaped cloud particles (mode 3) that may be “creatures,” and the presence of bright radar signatures on the mountain tops, which may be covered with life. Grinspoon and Bullock (2007) also explored life in the clouds and discuss Venus life in the context of comparative planetary astrobiology. In fact, numerous studies have contributed to the current understanding of Venus' clouds, including analysis of data collected by the Galileo orbiter, during its fly-by of Venus (Carlson et al., 1991; Grinspoon et al., 1993), and by the Venus Express mission (Ignatiev et al., 2009; Tsang et al., 2010; Barstow et al., 2012; Cottini et al., 2012; Parkinson et al., 2013a, 2013b, 2015).
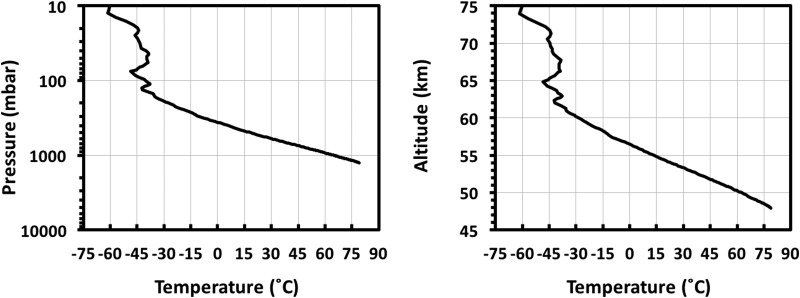
As is the case for any discussion of extremotolerant biology, parameters such as temperatures, radiation levels, and the presence of available water serve as major limitations for habitability. However, in the context of Venus' clouds, these specific issues are likely nonlimiting. As displayed in Figure 5, the bottom cloud layer of Venus at ∼48–50 km possesses rather favorable conditions, with temperatures of ∼60°C and pressures of ∼1000 mbar (∼1 atm). Furthermore, as described by Cockell (1999), UV radiation in Venus' clouds is likely not prohibitive to life, as UV flux in the upper levels of the atmosphere of Venus is comparable with the surface flux of Archean Earth, when photosynthetic life was considered to be present (Olson, 2006), and substantially attenuated within the Venus' cloud layer due to atmospheric CO2 and the aforementioned UV absorbers.
FIG. 5.
Representative atmospheric temperature variation with pressure (left) and with altitude (right) in the Venus' atmosphere. The profile shows a Magellan radio occultation profile (Jenkins et al., 1994) taken on orbit 3212.
In terms of water availability, water vapor values (mixing ratios) at altitudes of 40 km and higher are thought to vary widely from 20 to 50 ppm at latitudes of 60°, to >500 ppm near the equator, with global averages suggesting mixing ratios of 40–200 ppm (Donahue and Hodges, 1992; Barstow et al., 2012). However, desiccation in the Venus' atmosphere may be avoidable, despite these low water abundances, due to the hygroscopic nature of sulfuric acid [which would likely yield droplets or aerosols containing liquid water (Carleton et al., 1997; McGouldrick et al., 2011), even at high altitudes due to the freezing point depression]. Nonetheless, any inferences regarding the cloud particles, either near the cloud tops or at high altitudes, are the result of remotely sensed observations and not conclusive with respect to particle states as liquid or solid. However, if the Venus' cloud particles are spherical, then the prevailing theory is that the droplets must be liquid (Hansen and Hovenier, 1974).
Across the cloud layers, the sulfuric acid aerosols are described in roughly three size modes ranging in diameter between ∼0.4–0.6 μm (mode 1), ∼2–2.8 μm (modes 2 and 2′), and ∼7.3–8 μm (mode 3), with a small number of particles as large as ∼35 μm in diameter, as measured by the Venera missions and inferred from Galileo data (Knollenberg et al., 1980; Grinspoon et al., 1993). For the total cloud layer (lower, middle, and upper clouds), ∼70% of the columnar mass loading (∼32 mg·m−3, assuming a total 12.5 km column) arises from particles in the lower clouds (∼21 mg·m−3), where ∼94% of this mass is associated with mode 3 particles (∼20 mg·m−3), ranging in diameter of 8.0 ± 2.5 μm. Assuming that these particles are indeed suspensions (or possibly heterogeneous mixtures similar to terrestrial aerosols), then the majority of the observed UV contrasting materials and/or the major biomass are likely to be found in the lower clouds. In comparison, the middle cloud layer comprises only 24% of the total cloud columnar mass loading (assuming a 6 km column), despite the potential habitability of this region, where temperatures and pressures range from 10–50°C and 400–800 mbar.
For the lower cloud layer (47.5–50.5 km), particle densities are reported (Knollenberg and Hunten, 1980; Ragent et al., 1985) to be ∼50 particles·cm−3 for the larger sizes (∼2–8 μm diameter) and 600 particles·cm−3 for the smallest sizes (∼0.4 μm diameter). In the middle and upper cloud region (∼50–70 km), the respective particle densities are 10–50 particles·cm−3 (∼2–8 μm diameter) and 300–800 particles·cm−3 (∼0.3–0.4 μm diameter), with the largest particle densities of ∼800 particles·cm−3 (∼0.4 μm diameter) being detected at the highest altitudes. Among these particle distributions, the largest masses are associated with the ∼5–15-μm-sized particles in the lower clouds, and ∼2–15-μm-sized particles in the middle clouds; with mass loading estimates ranging ∼0.1–100 and ∼0.01–10 mg·m−3 for the lower and middle cloud regions (50.5–56.5 km), respectively, as reported by Knollenberg et al. (1980).
In comparison, the primary biological aerosols in Earth's atmosphere range in particle size from nanometer to submillimeter, and are composed of differing biological materials, including bacteria, fungal spores, fungal hyphen fragments, pollen, plant spores, plant debris, algae, and viral particles (Morris et al., 2011; Fröhlich-Nowoisky et al., 2016). A majority of these materials are associated with dust particles and black carbon, while a minority is suspended in water vapor or sea spray (Smith et al., 2013). Global estimates of the total primary biological aerosols indicate ∼104 particles·m−3, where the median diameters of particles containing cultivable bacteria are reported to be ∼4 μm at continental sites, and ∼2 μm at coastal sites (Després et al., 2012). Global measurements show that primary biological aerosols are dominated by bacteria at ∼104 cells·m−3 (Fröhlich-Nowoisky et al., 2016), thus amounting to a biomass of ∼5 ng·m−3 when assuming a buoyant cell density of 1.041 g·cm−3 (Bakken and Olsen, 1983). However, localized measurements in the cloud-forming regions in the lower troposphere reveal much higher abundances of 8.1 × 104 cells·mL−1, or ∼1011 cells·m−3 (Amato et al., 2007), amounting to a theoretical cloud biomass of ∼44 mg·m−3.
Thus, for Venus' lower clouds, the mass loading estimates (∼0.1–100 mg·m−3) are comparable to the upper biomass value for terrestrial biological aerosols (∼44 mg·m−3), while the particle size regime (≤8 μm) opens the possibility that the clouds may similarly harbor suspensions of single cells or aggregated microbial communities. In theory, the 2- and 8-μm-sized particles (modes 2′ and 3) could harbor a maximum of ∼108 and 1010 cells·m−3, respectively; these estimates assume spherical cloud particles, spherical microorganisms with a mean diameter of 1 μm, and particle densities of 50 particles·cm−3 (5 × 107 particles·m−3). Using these assumptions (including buoyant cell density), the theoretical and maximum biomass loadings for these particles amount to 0.2 and 14 mg·m−3, respectively. Again, these values are comparable to the upper biomass levels of bacterial aerosols on Earth (∼44 mg·m−3). Moreover, when compared with Venus, these values are respectively ∼6- and ∼1.5-fold lower than the columnar mass loadings for the mode 2′ and 3 particles (1.3 and 20 mg·m−3) from the lower cloud region (when assuming a 3 km column depth), and well within the aforementioned range of total mass loading estimates. These calculations and comparisons suggest that the mode 2′ and 3 particles, from Venus' lower cloud layer, possess sufficient mass balance to harbor microorganisms, solutes, and water.
To date, there are no in-depth studies focusing on the spectroscopy of aerosolized microorganisms or biomolecules under Venus conditions. Under terrestrial conditions, there are limited reports on the passive detection of aerosolized Bacillus spores using IR spectroscopy (FT-IR), with measurements on ∼104 to 109 cells·m−3 providing (mass) extinction coefficients (at ∼1100 cm−1) in the range of ∼720–1400 cm2·g−1 (Gurton et al., 2001; Ben-David, 2003; Ben-David and Ren, 2003; Blecka et al., 2012). Furthermore, turbidity (or optical density) measurements (at 540 nm) on concentrated aqueous suspensions of bacteria (∼1015 cells·m−3) provide extinction coefficients of ∼4000 cm2·g−1 (Spaun, 1962). In addition, there are multiple reports on the remote sensing of microbial blooms in fresh and ocean waters, where the intense absorption and/or fluorescence properties of photosynthetic pigments (e.g., chlorophyll a and phycocyanin) yield very large extinction coefficients, with ∼2 × 105 cm2·g−1 (at ∼640 nm) representing the terrestrial global average of chlorophyll a in the oceans (Bidigare et al., 1990; Schalles, 2006; Hunter et al., 2010).
The lower clouds of Venus exhibit comparable extinction coefficients ranging from ∼500 to 5000 cm2·g−1, as estimated from size particle spectrometer (LCPS) measurements at 600 nm, and calculated using a density of 2 mg·cm−3, as indicated by Knollenberg and Hunten (1980). These total values suggest that Venus' lower cloud region could harbor sufficient biomass to be characterizable and quantifiable through optical techniques. Moreover, comparison of Venus and Earth extinction coefficients supports the plausibility of the presence of high cell densities and/or appreciable concentrations of chromogenic pigments in Venus' lower clouds (as inferred from measurements at 600 nm).
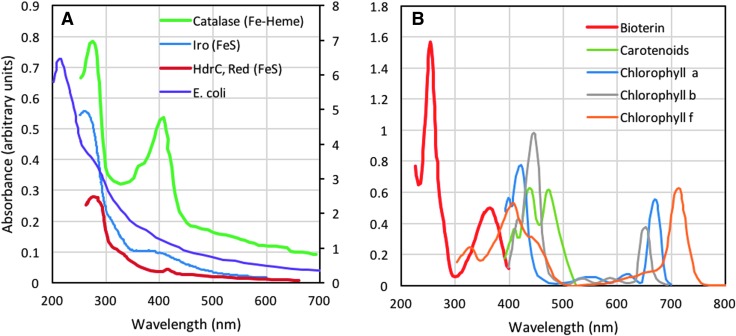
If Venus' clouds indeed harbor biology, then these biotic materials could potentially exhibit spectral signatures that overlap with those of Venus' clouds. For example, the observed contrasts at 270, 283, 365, 410, and 430 nm (Pioneer, Akatsuki, Galileo, and MESSENGER) are tantalizingly similar to the absorption properties of terrestrial biological molecules, which have peak absorptions at wavelengths across the UV and visible regions of the electromagnetic spectrum. Examples include nucleic acids and proteins, which have respective λmax values of 260 and 280 nm, where absorbances from the molecules often overlap, as is shown in Figure 6A for cellular extracts of Escherichia coli. Acidithiobacillus ferrooxidans has a UV spectrum that is very similar to that of Venus (Więckowski et al., 1999). Typical absorbances of iron-containing proteins (which would presumably be high in abundance in an Fe-rich environment) are also shown in Figure 6A, with Fe–heme and iron–sulfur (Fe–S) cluster proteins displaying λmax values between 350 and 450 nm (for the coordinated iron complex within the protein) and at ∼280 nm (for the aromatic amino acids within the protein). Across the visible spectrum, many organic cofactors (or biochemicals) such as pterins, carotenoids, and chlorophylls also strongly absorb between 300 and 500 nm (Fig. 6B), with the photosynthetic pigments additionally absorbing in the far visible and near IR regions (Fig. 6B).
FIG. 6.
Absorbance spectra for (A) whole cells of Escherichia coli and purified iron-containing proteins of Iro and HdrC, which are Fe–S proteins from Acidithiobacillus ferrooxidans, and catalase, an Fe–heme protein from Acinetobacter gyllenbergii 2P01AA; and (B) various cofactors and biochemicals, including biopterin, carotenoids, and chlorophylls a, b, and f; plots are adapted from (A) Derecho et al. (2014), Ossa et al. (2011), and Zeng et al. (2007); and (B) Airs et al. (2014) and http://hyperphysics.phy-astr.gsu.edu/hbase/Biology/ligabs.html.
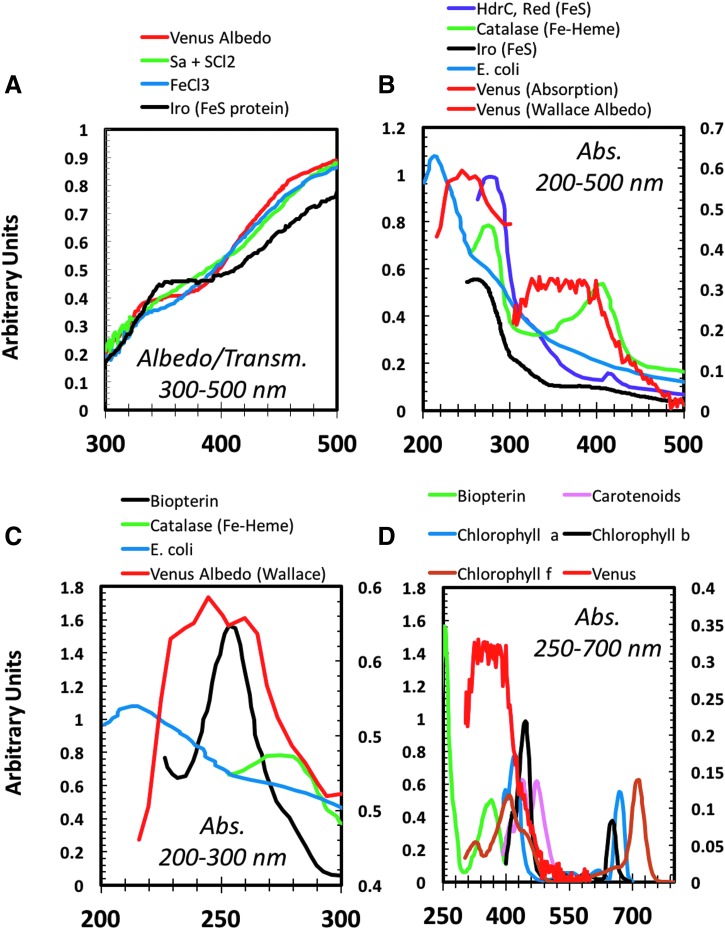
As described in the preceding section, however, there are several abiotic candidates that show reasonable spectral overlap with Venus, including aerosolized elemental sulfur and FeCl3. Comparisons of the transmission spectra for these compounds, along with the dayside Venus albedo, are displayed in Figure 7A and collectively show similar transmission properties between 300 and 500 nm (Krasnopolsky, 2017). Similarly, as also shown in Figure 7A, the transmission spectra for the Iro protein (which contains an Fe–S cofactor) additionally show reasonable overlap with the Venus albedo. In the context of Venus' survival, these spectral similarities are perhaps important, as the Fe–S-containing Iro protein is believed to be involved in iron respiration in the acidophilic and sulfur-metabolizing bacterium, Acidithiobacillus ferrooxidans (Zeng et al., 2007). As shown in Figure 7B, comparisons of both the Venus scaled geometric albedo (Wallace) and absorption residuals (MESSENGER) to differing Fe–S- and Fe–heme-containing proteins (Iro, HdrC, and catalase) also reveal several similarities between ∼215–290 and ∼300–480 nm, while whole cells of E. coli showed limited overlap. Although speculative, these spectral comparisons are consistent with the potential presence of cloud-based microorganisms containing high abundances of iron-based cofactors (relative to E. coli).
FIG. 7.
Comparison of the spectra for Venus (red) with terrestrial biological molecules and Escherichia coli: (A) comparison of the visible Venus albedo (Barker et al., 1975) with the transmission spectra for aerosolized elemental sulfur (Sa) + sulfur dichloride (SCl2) (green), ferric chloride (FeCl3) (blue), and the Fe–S-containing Iro protein isolated from Acidithiobacillus ferrooxidans (black); (B) comparison of the Venus scaled geometric albedo (Wallace) and absorption residuals (MESSENGER) with the absorbance spectra for the Fe–S-containing HdrC protein isolated from A. ferrooxidans (purple), the Fe–heme-containing catalase protein from Acinetobacter gyllenbergii 2P01AA (green), the Fe–S-containing Iro protein isolated from A. ferrooxidans (black), and whole cell preparations of Escherichia coli (blue); (C) comparison of the Venus scaled geometric albedo (Wallace) with the absorption spectra for biopterin (black), the Fe–heme-containing catalase protein from Acinetobacter gyllenbergii 2P01AA (green), and whole cell preparations of E. coli (blue); and (D) comparison of the Venus' absorption residuals (MESSENGER) with the absorption spectra for biopterin (green), carotenoids (purple), chlorophyll a (blue), chlorophyll b (black), and chlorophyll f (brown); spectra for the biological molecules were adapted from Alupoaei and García-Rubio (2004), Barker et al. (1975), Derecho et al. (2014), Durairaju Nisshanthini et al. (2015),Ossa et al. (2011), and Zeng et al. (2007). Fe–S proteins derived from Thiobacillus ferrooxidans also have a UV spectrum that is very similar to that of Venus (Więckowski et al., 1999).
In Figure 7C and D, the Venus scaled geometric albedo (Wallace) and absorption residuals (MESSENGER) are additionally compared to the organic cofactors of biopterin, carotenoids, and chlorophylls a, b, and f. As shown in Figure 7C, especially when considering the impacts of relative abundance, the Venus absorption shares several overlapping regions with biopterin (∼255 nm) and proteins (∼280 nm, catalase). Again, in terms of speculation, this is interesting, as bacterial pterin cofactors are involved in the metabolism of sulfur compounds such as sulfite and dimethyl sulfoxide. Finally, as displayed in Figure 7D, the Venus absorption residuals (MESSENGER) share significant overlaps with the aforementioned organic cofactors between ∼300 and 480 nm. Together, these preliminary spectral comparisons demonstrate that overlaps may be obtained from abiotic and biotic sources, thereby illuminating the need for in-depth ground-based studies focusing on the spectroscopy of chromogenic microorganisms (unlike E. coli) under Venus' cloud conditions.
Of course, these discussions must also consider the nightside opacity contrasts, which are observed vividly at 2.3 μm (Fig. 1G, H) and revealed in spectacular detail by the Akatsuki orbiter (Limaye et al., 2018). Similar to the UV contrasts, the 2.3 μm contrasts are not well understood, with potential causes including the presence of CO and the effects of differential opacities in the upper clouds, which may impede the transmission of radiation emitted by the surface of Venus or lower atmosphere (Carlson, 1993; Grinspoon, 1993; Pollack et al., 1993). Interestingly, IR studies on biological and organic molecules show that reflectance and absorption at ∼2.3 μm are clearly associated with C–H groups (C–H stretch) (Dalton et al., 2003; Clark et al., 2009), found in high abundance in lipid molecules (the primary constituent of cellular membranes). While the cloud layer of Venus has zonal flows, which circle the planet in 4–6 days in the cloud layer (∼50–65 km), the atmosphere is very stably stratified, within and above the cloud layer, and should be well mixed. However, this expectation is inconsistent with the vertical gradients in nitrogen abundance, as detected between 22 and 60 km (Peplowski and Lawrence, 2016). Hence, in the context of Venus' global contrasts, the planetary winds may potentially carry indigenous microorganisms around the planet, where these biological sources may additionally contribute to the nightside contrasts.
5. Survival in Venus' Clouds
Several terrestrial microorganisms could serve as relevant analogues for life in Venus' clouds, which are sulfuric acid-enriched, anaerobic (CO2 dominated), and iron-containing environments. On Earth, airborne and cultivable microorganisms have been found with specialized aircraft and balloons at altitudes ranging from 15 to 42 km (Narlikar et al., 2003; Smith et al., 2013). As mentioned, cell counts of terrestrial primary biological aerosols range from 104 to 1011 cells·m−3, with active spectral techniques, such as laser-induced fluorescence, providing measures of 104 to 105 particles·m−3 (fluorescent biological particle aerosols of >1 μm) (Després et al., 2012; Huffman et al., 2012).
For Venus' clouds, however, any potential biomass would clearly be dependent on available water, carbon, and other biogenic nutrients (e.g., sulfur, nitrogen, phosphorous, boron, and transition metals). The phototropic reduction of atmospheric CO2 would likely be a major source for carbon acquisition, with an attenuated UV flux within the cloud layer providing the driving energy source. Furthermore, both phosphorus and sulfur (along with iron) have been detected by the X-ray fluorescent radiometer on VeGa 1 and VeGa 2 landers (Andreychikov et al., 1987), with the most abundant phosphorus compound in the lower cloud layer possibly being partially hydrated phosphoric anhydride P2O5+H3PO4 (Krasnopolsky, 2006). For water availability, the low vapor pressure in the clouds is likely offset by the aerosols composed of aqueous sulfuric acid (75–98% and pH of −1.5 to 0.5 between 48 and 65 km; Grinspoon and Bullock, 2007), where the aforementioned 2 and 8 μm spherical particles (modes 2′ and 3) equate to suspension volumes of ∼4 and 260 pL, respectively.
In terms of survival in sulfur-rich and low pH environments, A. ferrooxidans serves as an exemplar terrestrial analogue for life in Venus' clouds, as this bacterium thrives at extremely low pH values (pH 1 to 2), fixes both CO2 and nitrogen gas from the atmosphere (Valdes et al., 2008), and obtains its energy for growth from the oxidation of hydrogen, ferrous iron, elemental sulfur, or partially oxidized sulfur compounds (Vera et al., 2008). This chemolithoautotrophic and acidophilic γ-proteobacterium also thrives at temperatures of 50–60°C, similar to those found in the lower clouds of Venus (Fig. 3). Moreover, under low pH and anaerobic conditions, this bacterium produces sulfuric acid, and possibly other oxidized forms of sulfur by metabolically oxidizing elemental sulfur and using Fe3+ as a terminal electron acceptor (Pronk et al., 1992).
Members of the archaeal Stygiolobus genus of the order Sulfolobales also anaerobically oxidize elemental sulfur to yield sulfuric acid under acidic conditions, and optimal growth temperatures of ∼80°C, and also utilize Fe3+ as a terminal electron acceptor (Segerer et al., 1991). Additional terrestrial analogues include green sulfur bacteria, which couple the oxidation of elemental sulfur to the anoxygenic phototrophic reduction of CO2 (Frigaard and Dahl, 2009), and the sulfate-reducing bacteria, which couple the oxidation of low-molecular-weight organics and hydrogen gas to the reduction of sulfuric acid (and other oxidized forms of sulfur) to form compounds, including sulfite and hydrogen sulfide (Muyzer and Stams, 2008).
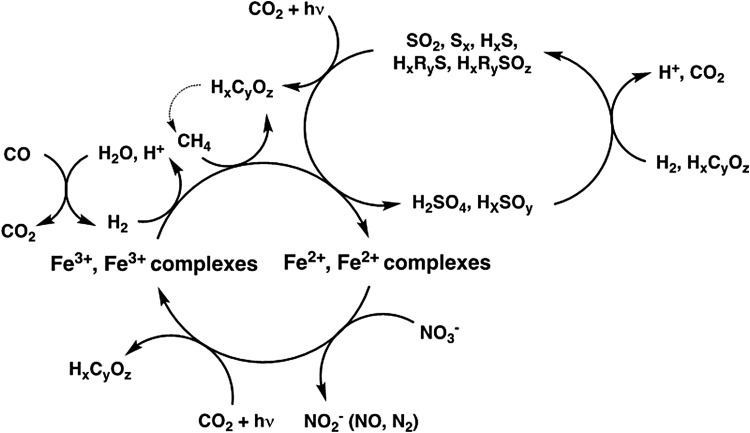
Together, these terrestrial analogues assist in framing the biochemical potential for an iron- and sulfur-centered metabolism in Venus' clouds, where oxidation of Fe2+ and sulfur compounds would be intrinsically coupled to the anoxic photosynthetic reduction of CO2. As summarized in Figure 8, these respective iron and sulfur redox cycles could also be sustained by coupling to the redox-reactive constituents within Venus' clouds and atmosphere. For instance, Fe2+ oxidation could additionally be coupled to the reduction of nitrate, while completion of the Fe3+/Fe2+ redox cycle could be afforded by coupling the reduction of Fe3+ to the oxidation of hydrogen, methane, and/or differing low oxidation state sulfur compounds (e.g., Sx, HS−, SOx, HxRyS, and HxRySOz). In parallel, redox cycling between the differing sulfur oxidation states could be afforded by coupling the reduction of polyatomic sulfur compounds to the oxidation of hydrogen and/or low-molecular-weight organics.
FIG. 8.
Diagram of iron- and sulfur-focused metabolic redox reactions that could occur in the Venus' clouds, where Fe3+/2+ complexes refer to inorganic and organic ligands and dotted arrows refer to possible redox cycles.
6. Transport from the Surface to the Clouds
Numerical simulations have suggested that Venus had a habitable climate for at least 750 million years, with liquid water on its surface for perhaps as long as 2 billion years (Grinspoon and Bullock, 2007; Way et al., 2016). The presence of past liquid water is supported by comparisons of atmospheric deuterium/hydrogen ratios between Venus and Earth (Donahue et al., 1982; Donahue and Hodges, 1992). In context, this suggests a geological time frame that is sufficient for life to have evolved in the Venus' environment, especially when the time estimates required for the evolution of life on Earth are considered (Lazcano and Miller, 1994; Des Marais, 1998; Nisbet and Sleep, 2001). As conditions on Venus' surface warmed and became increasingly inhospitable, life could have migrated to the clouds (Grinspoon and Bullock, 2007) as the surface water evaporated, with multiple possible mechanisms transporting microorganisms from the surface to the clouds. Ultimately, these microorganisms could have adapted to the cloud environments due to selective pressures (in the biological sense) arising from surface transport, aerosolization, limited water availability, and low pH environments (Cockell, 1999; Schulze-Makuch et al., 2004; Grinspoon and Bullock, 2007).
Within the context of terrestrial biology, surface-to-atmosphere transport of microorganisms is reasonably well accepted, as is the atmospheric transport of biologically relevant elements and low-molecular-weight metabolites (Burrows et al., 2009; Morris et al., 2011; Fröhlich-Nowoisky et al., 2016). The movement of water, organics, and other life-essential nutrients in the upper atmosphere on Venus is likely similarly regulated by surface topography, diurnal cycles, strong storms, and a variety of other conceivable physical weathering forces. On Earth, all evidence to date indicates that airborne microbes do not remain perpetually aloft. Instead, biological aerosols are continuously swept into the atmosphere through strong convections that emanate from diverse marine and surface sources, and eventually fall out of the atmosphere through gravitational settling or precipitation.
On Venus therefore, any cloud-based microbial population would need to remain aloft for long periods and replenished on relatively fast timescales. Plausible atmospheric nutrient transport mechanisms can be inferred from the experience of VeGa 1 and VeGa 2 balloons, which occasionally experienced very strong updrafts and downdrafts (some triggered by underlying topography) at their nominal float level (∼54 km). Blamont et al. (1986) reported that the typical vertical motions (up and down) encountered by the two balloons were 1 to 2 m·s−1. Furthermore, residence times of the cloud particles on Venus are quite long, and are comparable to the Hadley circulation times of 2 to 3 months, which are several orders of magnitude greater than the division time of bacteria (Grinspoon and Bullock, 2007).
The recent discovery, from Akatsuki measurements (Fukuhara et al., 2017), of stationary gravity waves at the cloud tops, which are considered to be the result of surface topography, indicates that vertical motions are possible even within the very stable cloud layer. These measurements suggest that ambient winds blowing over mountains and hills on the surface appear to trigger vertical motions, which can reach the bottom of the clouds without any impediment and extend up to the middle cloud layer. The very stable lapse rates in the Venus' clouds could also maintain airborne particles of essential nutrient-rich minerals aloft for long periods. To a large extent, the atmosphere below the clouds down to the surface is close to being neutrally stable, with at least two layers having superadiabatic lapse rates; one layer ∼4 km above the surface and another layer at ∼15–17 km, as based on the VeGa 2 lander data (Pioneer Venus probe sensors did not provide any data <12 km due to an electrical problem and published values are extrapolated using an adiabatic lapse rate for pure CO2; Seiff et al., 1995).
Therefore, and by extension, the observed UV contrasts of Venus are perhaps best compared to phytoplankton blooms (bacteria, algae, diatoms, etc.), which sustain high cell abundances and experience both temporal and spatial variabilities over large areas. For instance, phytoplankton blooms in the Barents Sea in Norway and Ross Sea are reported to cover areas of 10,000 and >106,000 km2, respectively (Arrigo and McClain, 1994; Signorini et al., 1999). On Earth, phytoplankton blooms are triggered by differing environmental conditions (i.e., wind, rain, and water currents) and periodic bursts in nutrients (such as iron, phosphorous, and nitrogen), which ultimately lead to broad fluctuations in metabolite composition, cell aggregation, and/or cell abundances (with division rates ranging from 0.5 to 3 d−1) (Coale et al., 1996; Valiela et al., 1997). Spectrally, these fluctuations are associated with optical changes in color, opacity, and/or turbidity, occur on timescales ranging from hours, days, to months, and are often associated with seasonal and diurnal cycles (Kanoshina et al., 2003; Egli et al., 2004; Blondeau-Patissier et al., 2014).
In Venus' clouds, atmospheric cycling may similarly promote rapid fluctuations in bloom densities, consistent with the images in Figure 4, which show detectable changes in contrasts on a 12-min timescale. Moreover, as observed in Figures 1 and 3, the global cloud contrasts of Venus clearly persist and evolve over thousands of hours, consistent with the persistent microbial blooms on Earth (Phlips et al., 1999; Buskey et al., 2001, 2003), which can last for months to years. Thus, over this time frame, the metabolism of sulfur compounds (e.g., dimethyl sulfide, polyatomic sulfur compounds, and alkyl sulfoxides) could potentially account for the persistent UV contrasts on Venus.
In summary, evaporation and atmospheric circulation could account for the initial transport of biomass from the surface, and vertically (and currently) through the cloud layer, with recycling between the bottom and the top layers. Figure 9 shows our ideas in a schematic diagram. Biological material at the higher altitudes would then likely be partly or fully degraded through photochemical means, where the resulting organic products could be recycled through the cloud layer, potentially serving as carbon sources for any cloud-based biology. Such a scenario would be consistent with the conclusions of Knollenberg and Hunten (1980) that there is a source for the smaller particles in the upper cloud layer. Alternatively, Venus' clouds could have been seeded by interplanetary exchange of rocks (harboring terrestrial bacteria or the building blocks of life) resulting from large impacts on Earth (Melosh, 1988; Grinspoon and Bullock, 2007; Gao et al., 2014).
FIG. 9.
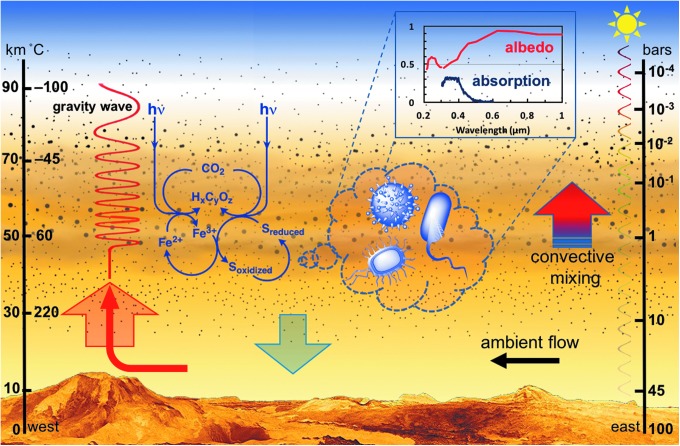
A schematic representation summarizing the ideas presented in this hypothesis paper regarding the potential for microorganisms to survive in Venus' lower clouds and contribute to the observed bulk spectra. In this scheme, the approximate altitude and temperatures are shown on the left axis, the approximate pressure on the right axis, while the surface topography represents an exaggerated perspective view of Venus. The cloud layer is depicted by a yellow-tinted hazy region between an altitude of ∼47 and 72 km, where the varying opacities and thicknesses represent differences in mass loading. The black dots within the cloud layer depict the sulfuric acid aerosols with diameters ranging from 0.2 μm (which are found as high as 90 km) to 2.5 μm and to as large as 36 μm (in smaller quantities) near the bottom of the cloud layer (Knollenberg and Hunten, 1980); aerosols below the cloud base have also been reported by the Venera probes. The hypothetical microbial contents of particles from the lower cloud layer are depicted in a magnified view using the dashed-line callout bubble, which shows differing possible microbial morphologies. These microorganisms may potentially survive by fixation of carbon dioxide (CO2) through the phototrophic or chemolithotrophic oxidation of iron and sulfur compounds, and by a coupled iron-sulfur metabolism (depicted by the blue reaction scheme). The cloud-based microbial communities may remain afloat through gravity waves (red wavy line), which propagate up, and are triggered by westward ambient flows over the elevated topographies; gravity waves have been detected at the cloud tops in thermal infrared in the Akatsuki data (Fukuhara et al., 2017). Additionally, the convective activity of the lower cloud region may persist on the night side, thereby leading to opacity variations and differing thermal emissions through the cloud layer, as is observed in the near infrared in the Akatsuki and Venus Express data. Consequently, the spectra of Venus may include contributions from the cloud-based microorganisms, as is depicted by the dashed-line callout originating from the magnified view of the particles; the inset spectral plot shows the albedo of Venus compiled from differing observations (red) and the sunlight absorption estimated by a singular measurement on the dayside (at one location), as calculated from the difference between the VIRA cloud model and the MESSENGER spectra (Perez-Hoyos et al., 2017). The absorption of sunlight may actually extend to much longer wavelengths based on muted contrasts observed by the Akatsuki orbiter (Limaye et al., 2018), which is consistent with the albedo variation with wavelength.
7. Conclusions and Future Studies
Our comparative analyses support the blended hypotheses that terrestrial-type biology can survive within and contribute to the spectral signatures of Venus' clouds (Fig. 9). To test the ideas presented here, we propose the need for an integrated chemical, biochemical, and microbiological study focusing on the survival and spectroscopy of terrestrial microorganisms under Venus' cloud conditions. To accomplish this task, specialized chambers, such as the Glenn Extreme Environment Rig at the NASA Glenn Research Center (https://geer.grc.nasa.gov), would be required to simulate the atmospheric and physical conditions of the clouds, while simultaneously allowing for spectral analysis of aerosolized chemical, biochemical, and microbial samples (via IR, visible, and other means). These studies would also need to address the multiple sources of variation associated with the spectroscopy of bioaerosols, including cell morphology (cocci, bacilli, or spirilla), cell state (vegetative or sporulated), presence of abiotic materials (e.g., dust, salts, and polymer matrices), states of hydration, and emission source/type and location. Crucially, the persistence of terrestrial microorganisms and the potential for metabolism, under these conditions, would serve as positive indicators for habitability. Potential biology-related experiments therefore include measurements of viable plate counts, adenosine triphosphate abundances, and intracellular enzyme activities of sulfur-metabolizing, acid-tolerant, and/or radiation-tolerant microorganisms after exposures to the Venus' cloud conditions.
Looking forward, investigations into the actual habitability of Venus' clouds would ideally benefit from a mixture of orbiter, lander, airplane/balloons, and sample return missions as proposed by Schulze-Makuch and Irwin (2002) and Grinspoon (1997). Long-lived aerial platforms are capable of observing the temporal changes in spectral, physical, and chemical properties of the cloud layer aerosols. Example platforms include Aerobots (van den Berg et al., 2006) and the Venus Atmospheric Mobile Platform, a concept being developed by Northrop Grumman Aerospace (Lee et al., 2015a). In fact, a mid-sized version of this flying aerial platform has been suggested for inclusion in the Venera-D mission, which is under joint study between ROSCOSMOS and NASA (https://solarsystem.nasa.gov/docs/Venera-D_Final_Report_170213.pdf; Zasova et al., 2017). Indeed, if microbes are actively metabolizing or dividing in Earth's atmosphere, then the search for life (or evidence of life) should be broadened to include planetary atmospheres. For Venus' clouds therefore, potential in situ interrogation strategies include compact Raman LIDAR (Abedin et al., 2018), fluorescence LIDAR, and a life detection microscope (Yamagishi et al., 2016), which could be used to measure organic/inorganic composition, organic (or biomolecular) fluorescence, and the presence of liposome-like particles (or live or dead microbial cells), respectively. For future flagship endeavors to Venus, these types of instruments could potentially be incorporated into missions focused on planetary geology or atmospheric chemistry, while simultaneously providing key insights into the potential habitability of the clouds.
Abbreviations Used
- CO2
carbon dioxide
- FeCl3
ferric chloride
- Fe–S
iron–sulfur
- IR
infrared
- OSSO
sulfur oxide isomer
- SO
sulfur monoxide
- SO2
sulfur dioxide
- UV
ultraviolet
- VMC
Venus Monitoring Camera
Acknowledgments
Funding from NASA Grants NNX09AE85G and NNX16AC79G supported the development of ideas presented in this article. We acknowledge editing contributions from Amanda Evans and Rosalyn Pertzborn, and motivating discussions with many members of the Venus research community. We are grateful to the three reviewers for their very constructive comments and acknowledge David Grinspoon for his feedback. We thank Pat Fry for image processing and Santiago Pèrez-Hoyos for providing the data for MESSENGER and the difference from the computed spectrum for the clouds. This article was partly inspired by the Spaceward Bound: India expedition in Ladakh, India (August 2016), where the continual lifting of salt deposits by wind gusts from the shores of Tso Kar, a lake at ∼4500 m, provoked comparisons to early Venus, when life may have been swept from the surface into the clouds.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Abedin M.N., Bradley A.T., Misra A.K., Bai Y., Hines G.D., and Sharma S.K. (2018) Standoff ultracompact micro-Raman sensor for planetary surface explorations. Appl Opt 57:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airs R.L., Temperton B., Sambles C., Farnham G., Skill S.C., and Llewellyn C.A. (2014) Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation. FEBS Lett 588:3770–3777 [DOI] [PubMed] [Google Scholar]
- Alupoaei E.C., and García-Rubio H.L. (2004) Growth behavior of microorganisms using UV-Vis spectroscopy: Escherichia coli. Biotechnol Bioeng 2004;86:163–167 [DOI] [PubMed] [Google Scholar]
- Amato P., Parazols M., Sancelme M., Mailhot G., Laj P., and Delort A.-M. (2007) An important oceanic source of micro-organisms for cloud water at the Puy de Dôme (France). Atmos Environ 41:8253–8263 [Google Scholar]
- Andreychikov B.M., Akhmetshin I.K., Korchuganov B.N., Mukhin L.M., Ogorodnikov B.I, Petryanov I.V., and Skitovich V.I. (1987) X-ray radiometric analysis of the cloud aerosol of Venus by the Vega 1 and 2 probes. Cosmic Res 25:16 [Google Scholar]
- Arrigo K.R., and McClain C.R. (1994) Spring phytoplankton production in the western Ross Sea. Science 266:261–263 [DOI] [PubMed] [Google Scholar]
- Bakken L.R., and Olsen R.A. (1983) Buoyant densities and dry-matter contents of microorganisms: conversion of a measured biovolume into biomass. Appl Environ Microbiol 45:1188–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E.S. (1978) Detection of CS2 in the UV spectrum of Venus. Bull Am Astron Soc 10:548 [Google Scholar]
- Barker E.S. (1979) Detection of SO2 in the UV spectrum of Venus. Geophys Res Lett 6:117–120 [Google Scholar]
- Barker E.S., Woodman J.H., Perry M.A., Hapke B.A., and Nelson R. (1975) Relative spectrophotometry of Venus from 3067 to 5960 A. J Atmos Sci 32:1205–1211 [Google Scholar]
- Barstow J.K., Tsang C.C.C., Wilson C.F., Irwin P.G.J., Taylor F.W., McGouldrick K., Drossart P., Piccioni G., and Tellmann S. (2012) Models of the global cloud structure on Venus derived from Venus Express observations. Icarus 217:542–560 [Google Scholar]
- Belton M.J.S., Gierasch P., Klaasen K.P., Anger C.D., Carr M.H., Chapman C.R., Davies M.E., Greeley R., Greenberg R., and Head J.W. (1992) Imaging of Venus from Galileo—Early results and camera performance. Adv Space Res 12:91–103 [Google Scholar]
- Ben-David A. (2003) Remote detection of biological aerosols at a distance of 3 km with a passive Fourier transform infrared (FTIR) sensor. Opt Express 11:418–429 [DOI] [PubMed] [Google Scholar]
- Ben-David A. and Ren H. (2003) Detection, identification, and estimation of biological aerosols and vapors with a Fourier-transform infrared spectrometer. Appl Opt 42:4887–4900. 10.1364/AO.42.004887. [DOI] [PubMed] [Google Scholar]
- Bertaux J.-L., Widemann T., Hauchecorne A., Moroz V.I., and Ekonomov A.P. (1996) VEGA 1 and VEGA 2 entry probes: an investigation of local UV absorption (220–400 nm) in the atmosphere of Venus (SO2, aerosols, cloud structure). J Geophys Res 101:12709–12746 [Google Scholar]
- Bezard B., de Bergh C., Crisp D., and Maillard J.-P. (1990) The deep atmosphere of Venus revealed by high-resolution nightside spectra. Nature 345:508–511 [Google Scholar]
- Bidigare R.R., Kennicutt M.C., Ondrusek M.E., Keller M.D., and Guillard R.R.L. (1990) Novel chlorophyll-related compounds in marine phytoplankton: distributions and geochemical implications. Energy & Fuels 4:653–657 [Google Scholar]
- Blamont J.E., Young R.E., Seiff A., Ragent B., Sagdeev R., Linkin V.M., Kerzhanovich V.V., Ingersoll A.P., Crisp D., Elson L.S., Preston R.A., Golitsyn G.S., and Ivanov V.N. (1986) Implications of the VEGA balloon results for Venus atmospheric dynamics. Science 231:1422–1425 [DOI] [PubMed] [Google Scholar]
- Blecka M.I., Rataj M., and Szymanski G. (2012) Passive detection of biological aerosols in the atmosphere with a Fourier Transform Instrument (FTIR)—the results of the measurements in the laboratory and in the field. Orig Life Evol Biosph 42:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau-Patissier D., Gower J.F., Dekker A.G., Phinn S.R., and Brando V.E. (2014) A review of ocean color remote sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Progress in Oceanography 123:123–144 [Google Scholar]
- Bougher S.W., Hunten D.M., and Phillips R.J., (eds) (1997) Venus II. Geology, geophysics, atmosphere, and solar wind environment. University of Arizona Press, Tucson [Google Scholar]
- Bullock M.A., and Grinspoon D.H. (2013) The atmosphere and climate of Venus. In: Comparative Climatology of Terrestrial Planets, edited by Mackwell S.J., Simon-Miller A.A., Harder J.W., and Bullock M.A. University of Arizona Press, pp 19–54. doi: 10.2458/azu_uapress_9780816530595-ch002 [DOI] [Google Scholar]
- Burrows S.M., Butler T., Jöckel P., Tost H., Kerkweg A., Pöschl U., and Lawrence M.G. (2009) Bacteria in the global atmosphere - Part 2: modeling of emissions and transport between different ecosystems. Atmos Chem Phys 9:9281–9297 [Google Scholar]
- Buskey E.J., Liu H., Collumb C., and Bersano J.G.F. (2001) The decline and recovery of a persistent Texas brown tide algal bloom in the Laguna Madre (Texas, USA). Estuaries 24:337–346 [Google Scholar]
- Buskey E.J., Deyoe H., Jochem F.J., and Villareal T.A. (2003) Effects of mesozooplankton removal and ammonium addition on planktonic trophic structure during a bloom of the Texas ‘brown tide’: a mesocosm study. Journal of Plankton Research 25:215–228 [Google Scholar]
- Carleton K.L., Sonnenfroh D.M., Rawlins W.T., Wyslouzil B.E., and Arnold S. (1997) Freezing behavior of single sulfuric acid aerosols suspended in a quadrupole trap. J Geophys Res 102:6025–6033 [Google Scholar]
- Carlson R.W. (1993) Variations in Venus cloud particle properties: a new view of Venus's cloud morphology as observed by the Galileo near-infrared mapping spectrometer. Planet Space Sci 41:477–485 [Google Scholar]
- Carlson R.W., Baines K.H., Encrenaz T., Taylor F.W., Drossart P., Kamp L.W., Pollack J.B., Lellouch E., Collard A.D., Calcutt S.B., Grinspoon D., Weissman P.R., Smythe W.D., Ocampo A.C., Danielson G.E., Fanale F.P., Johnson T.V., Kieffer H.H., Matson D.L., McCord T.B., and Soderblom L.A. (1991) Galileo infrared imaging spectroscopy measurements at venus. Science 253:1541–1548 [DOI] [PubMed] [Google Scholar]
- Clark R.N., Curchin J.M., Hoefen T.M., and Swayze G.A. (2009) Reflectance spectroscopy of organic compounds: 1. Alkanes. J Geophys Res 114:n./a. [Google Scholar]
- Coale K.H., Johnson K.S., Fitzwater S.E., Gordon R.M., Tanner S., Chavez F.P., Ferioli L., Sakamoto C., Rogers P., Millero F., Steinberg P., Nightingale P., Cooper D., Cochlan W.P., Landry M.R., Constantinou J., Rollwagen G., Trasvina A., and Kudela R. (1996) A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383:495–501 [DOI] [PubMed] [Google Scholar]
- Cockell C.S. (1999) Life on venus. Planet Space Sci 47:1487–1501 [Google Scholar]
- Conway R.R., McCoy R.P., Barth C.A., and Lane A.L. (1979) IUE detection of sulfur dioxide in the atmosphere of Venus. Geophys Res Lett 6 629–631 [Google Scholar]
- Cottini V., Ignatiev N.I., Piccioni G., Drossart P., Grassi D., and Markiewicz W.J. (2012) Water vapor near the cloud tops of Venus from Venus Express/VIRTIS dayside data. Icarus 217:561–569 [Google Scholar]
- Dalton J.B., Mogul R., Kagawa H.K., Chan S.L., and Jamieson C.S. (2003) Near-infrared detection of potential evidence for microscopic organisms on Europa. Astrobiology 3:505–529 [DOI] [PubMed] [Google Scholar]
- del Genio A.D., and Rossow W.B. (1982) Temporal variability of ultraviolet cloud features in the Venus stratosphere. Icarus 51:391–415 [Google Scholar]
- Derecho I., McCoy K.B., Vaishampayan P., Venkateswaran K., and Mogul R. (2014) Characterization of hydrogen peroxide—resistant acinetobacter species isolated during the Mars Phoenix Spacecraft Assembly. Astrobiology 14:837–847 [DOI] [PubMed] [Google Scholar]
- Des Marais D.J. (1998) Earth's early biosphere. Gravit Space Biol Bull 11:23–30 [PubMed] [Google Scholar]
- Després V., Huffman J.A., Burrows S.M., Hoose C., Safatov A., Buryak G., Fröhlich-Nowoisky J., Elbert W., Andreae M., Pöschl U., and Jaenicke R. (2012) Primary biological aerosol particles in the atmosphere: a review. Tellus B: chemical and Physical Meteorology 64:15598 [Google Scholar]
- Donahue T.M., and Hodges R.R., Jr (1992) Past and present water budget of Venus. J Geophys Res 97:6083–6091 [Google Scholar]
- Donahue T.M., Hoffman J.H., Hodges R.R., and Watson A.J. (1982) Venus was wet—A measurement of the ratio of deuterium to hydrogen. Science 216:630–633 [DOI] [PubMed] [Google Scholar]
- Durairaju Nisshanthini S., Teresa Infanta S.A.K., Raja D.S., Natarajan K., Palaniswamy M., and Angayarkanni J. (2015) Spectral characterization of a pteridine derivative from cyanide-utilizing bacterium Bacillus subtilis—JN989651. J Microbiol 53:262–271 [DOI] [PubMed] [Google Scholar]
- Egli K., Wiggli M., Fritz M., Klug J., Gerss J., and Bachofen R. (2004) Spatial and temporal dynamics of a plume of phototrophic microorganisms in a meromictic alpine lake using turbidity as a measure of cell density. Aquat Microb Ecol 35:105–113 [Google Scholar]
- Encrenaz T., Greathouse T.K., Richter M.J., DeWitt C., Widemann T., Bézard B., Fouchet T., Atreya S.K., and Sagawa H. (2016) HDO and SO2 thermal mapping on Venus. III. Short-term and long-term variations between 2012 and 2016. Astronomy and Astrophysics 595:A74 [Google Scholar]
- Esposito L.W. and Travis L.D. (1982) Polarization studies of the Venus UV contrasts—Cloud height and haze variability. Icarus 51:374–390 [Google Scholar]
- Esposito L.W., Knollenberg R.G., Marov M.I., Toon O.B., Turco R.P., Colin L., Donahue T.M., and Moroz V.I. (1983) The clouds are hazes of Venus. In: Venus (A83-37401 17-91), edited by D.M. Hunten, University of Arizona Press, Tucson, AZ, pp. 484–564 [Google Scholar]
- Frandsen B.N., Wennberg P.O., and Kjaergaard H.G. (2016) Identification of OSSO as a near-UV absorber in the Venusian atmosphere. Geophys Res Lett 43:11146–11155 [Google Scholar]
- Frigaard N.U. and Dahl C. (2009) Sulfur metabolism in phototrophic sulfur bacteria. Adv Microb Physiol 54:103–200 [DOI] [PubMed] [Google Scholar]
- Fröhlich-Nowoisky J., Kampf C.J., Weber B., Huffman J.A., Pöhlker C., Andreae M.O., Lang-Yona N., Burrows S.M., Gunthe S.S., Elbert W., Su H., Hoor P., Thines E., Hoffmann T., Després V.R., and Pöschl U. (2016) Bioaerosols in the Earth system: climate, health, and ecosystem interactions. Atmos Res 182:346–376 [Google Scholar]
- Fukuhara T., Futaguchi M., Hashimoto G.L., Horinouchi T., Imamura T., Iwagaimi N., Kouyama T., Murakami S.Y., Nakamura M., Ogohara K., Sato M., Sato T.M., Suzuki M., Taguchi M., Takagi S., Ueno M., Watanabe S., Yamada M., and Yamazaki A. (2017) Large stationary gravity wave in the atmosphere of Venus. Nat Geosci 10:85–88 [Google Scholar]
- Gao P., Zhang X., Crisp D., Bardeen C.G., and Yung Y.L. (2014) Bimodal distribution of sulfuric acid aerosols in the upper haze of Venus. Icarus 231:83–98 [Google Scholar]
- Grinspoon D.H. (1993) Probing Venus's cloud structure with Galileo NIMS. Planet Space Sci 41:515–542 [Google Scholar]
- Grinspoon D.H. (1997) Venus Revealed: A New Look Below the Clouds of Our Mysterious Twin Planet. Addison Wesley, Reading, MA, USA, 355 pp [Google Scholar]
- Grinspoon D.H. and Bullock M.A. (2007) Astrobiology and Venus exploration. In: Exploring Venus as a Terrestrial Planet, edited by L.W. Esposito, E.R. Stafan, and T.E. Cravens, American Geophysical Union, pp. 191–206. doi: 10.1029/176GM12 [DOI] [Google Scholar]
- Grinspoon D.H., Pollack J.B., Sitton B.R., Carlson R.W., Kamp L.W., Baines K.H., Encrenaz T., and Taylor F.W. (1993) Probing Venus's cloud structure with Galileo NIMS. Planet Space Sci 41:515–542 [Google Scholar]
- Gurton K.P., David L., and Kvavilashvii R. (2001) Extinction, Absorption, Scattering, and Backscatter for Aerosolized Bacillus Subtilis Var. Niger Endospores from 3 to 13 Micrometers. Army Research Lab, Adelphi, MD, p. 23 [Google Scholar]
- Hansen J.E. and Hovenier J.W. (1974) Interpretation of the polarization of Venus. J Atmos Sci 31:1137–1160 [Google Scholar]
- Hapke B. and Nelson R. (1975) Evidence for an elemental sulfur component of the clouds from Venus spectrophotometry. J Atmos Sci 32:1212–1218 [Google Scholar]
- Hartley K.K., Wolff A.R., and Travis L.D. (1989) Croconic acid: an absorber in the Venus clouds? Icarus 77:382–390 [Google Scholar]
- Huffman J.A., Sinha B., Garland R.M., Snee-Pollmann A., Gunthe S.S., Artaxo P., Martin S.T., Andreae M.O., and Pöschl U. (2012) Size distributions and temporal variations of biological aerosol particles in the Amazon rainforest characterized by microscopy and real-time UV-APS fluorescence techniques during AMAZE-08. Atmos Chem Phys 12:11997–12019 [Google Scholar]
- Hunten D.M., Colin L., Donahue T.M., Moroz V.I., Colin L., Donahue T.M., and Moroz V.I. (1983) Venus. University of Arizona Press, Tucson, Arizona [Google Scholar]
- Hunter P.D., Tyler A.N., Carvalho L., Codd G.A., and Maberly S.C. (2010) Hyperspectral remote sensing of cyanobacterial pigments as indicators for cell populations and toxins in eutrophic lakes. Remote Sens Environ 114:2705–2718 [Google Scholar]
- Ignatiev N.I., Titov D.V., Piccioni G., Drossart P., Markiewicz W.J., Cottini V., Roatsch T., Almeida M., and Manoel N. (2009) Altimetry of the Venus cloud tops from the Venus Express observations. J Geophys Res 114:E00B43 [Google Scholar]
- Irvine W.M. (1968) Monochromatic phase curves and albedos for Venus. J Atmos Sci 25:610–616 [Google Scholar]
- Jenkins J.M., Steffes P.G., Hinson D.P., Twicken J.D., and Tyler G.L. (1994) Radio occultation studies of the Venus atmosphere with the Magellan spacecraft. 2: results from the October 1991 experiments. Icarus 110:79–94 [Google Scholar]
- Jessup K.L., Marcq E., Mills F., Mahieux A., Limaye S., Wilson C., Allen M., Bertaux J.-L., Markiewicz W., Roman T., Vandaele A.C., Wilquet V., and Yung Y. (2015) Coordinated Hubble Space Telescope and Venus Express Observations of Venus' upper cloud deck. Icarus 258:309–336 [Google Scholar]
- Kanoshina I., Lips U., and Leppänen J.-M. (2003) The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2:29–41 [Google Scholar]
- Katti V.R., Pratap V.R., Dave R.K., and Mankad K. (2006) INSAT-3D: an advanced meteorological mission over Indian Ocean. Proc SPIE 6407:12 [Google Scholar]
- Kawabata K., Coffeen D.L., Hansen J.E., Lane W.A., Sato M., and Travis L.D. (1980) Cloud and haze properties from Pioneer Venus polarimetry. J Geophys Res 85:8129–8140 [Google Scholar]
- Knollenberg R.G. (1984) A reexamination of the evidence for large, solid particles in the clouds of Venus. Icarus 57:161–183 [Google Scholar]
- Knollenberg R.G. and Hunten D.M. (1979) Clouds of Venus—A preliminary assessment of microstructure. Science 205:70–74 [DOI] [PubMed] [Google Scholar]
- Knollenberg R.G. and Hunten D.M. (1980) The microphysics of the clouds of Venus—Results of the Pioneer Venus particle size spectrometer experiment. J Geophys Res 85:8039–8058 [Google Scholar]
- Knollenberg R., Travis L., Tomasko M., Smith P., Ragent B., Esposito L., McCleese D., Martonchik J., and Beer R. (1980) The clouds of Venus—A synthesis report. J Geophys Res 85:8059–8081 [Google Scholar]
- Krasnopolsky V. (1985) Chemical composition of Venus clouds. Planet Space Sci 33:109–117 [Google Scholar]
- Krasnopolsky V.A. (2006) Chemical composition of Venus atmosphere and clouds: some unsolved problems. Planet Space Sci 54:1352–1359 [Google Scholar]
- Krasnopolsky V.A. (2016) Sulfur aerosol in the clouds of Venus. Icarus 274:33–36 [Google Scholar]
- Krasnopolsky V.A. (2017) On the iron chloride aerosol in the clouds of Venus. Icarus 286:134–137 [Google Scholar]
- Kuiper G.P. (1969) Identification of the Venus cloud layers. Commun Lunar Planet Lab 6:229–245 [Google Scholar]
- Laven P. (2008) Effects of refractive index on glories. Appl Opt 47:H133. [DOI] [PubMed] [Google Scholar]
- Lazcano A. and Miller S.L. (1994) How long did it take for life to begin and evolve to cyanobacteria? J Mol Evol 39:546–554 [DOI] [PubMed] [Google Scholar]
- Lee G., Polidan R.S., and Ross F. (2015a) Venus Atmospheric Maneuverable Platform (VAMP)—A Low Cost Venus Exploration Concept [abstract id.P23A-2109]. American Geophysical Union, Fall Meeting
- Lee Y.J., Imamura T., Schröder S.E., and Marcq E. (2015b) Long-term variations of the UV contrast on Venus observed by the Venus Monitoring Camera on board Venus Express. Icarus 253:1–15 [Google Scholar]
- Lee Y.J., Yamazaki A., Imamura T., Yamada M., Watanabe S., Sato T.M., Ogohara K., Hashimoto G.L., and Murakami S. (2017) Scattering Properties of the Venusian Clouds Observed by the UV Imager on board Akatsuki. Astron J 154:44 [Google Scholar]
- Limaye S.S. (1984) Morphology and movements of polarization features on Venus as seen in the Pioneer Orbiter Cloud Photopolarimeter data. Icarus 57:362–385 [Google Scholar]
- Limaye S.S., Watanabe S., Yamazaki A., Yamada M., Satoh T., Sato T.M., Nakamura M., Taguchi M., Fukuhara T., Imamura T., Kouyama T., Lee Y.J., Horinouchi T., Peralta J., Iwagami N., Hashimoto G.L., Takagi S., Ohtsuki S., Murakami S., Yamamoto Y., Ogohara K., Ando H., Sugiyama K., Ishii N., Abe T., Hirose C., Suzuki M., Hirata N., Young E.F., and Ocampo A.C. (2018) Venus Looks Different from Day to Night Across Wavelengths: Morphology from Akatsuki Multispectral Images. Earth Planets Space 70:24. doi: 10.1186/s40623-018-0789-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz W.J., Petrova E., Shalygina O., Almeida M., Titov D.V., Limaye S.S., Ignatiev N., Roatsch T., and Matz K.D. (2014) Glory on Venus cloud tops and the unknown UV absorber. Icarus 234:200–203 [Google Scholar]
- McGouldrick K., Toon O.B., and Grinspoon D.H. (2011) Sulfuric acid aerosols in the atmospheres of the terrestrial planets. Planet Space Sci 59:934–941 [Google Scholar]
- Melosh H.J. (1988) The rocky road to panspermia. Nature 332:687–688 [DOI] [PubMed] [Google Scholar]
- Mills F.P., Esposito L.W., and Yung Y.L. (2007) Atmospheric composition, chemistry, and clouds. In: Exploring Venus as a Terrestrial Planet (Geophysical Monograph Series, Volume 176), edited by L.W. Esposito, E.R. Stofan, and T.E. Cravens, American Geophysical Union, pp. 73–100. doi: 10.1029/176GM06 [DOI] [Google Scholar]
- Morowitz H.A. and Sagan C. (1967) Life in the Clouds of Venus? Nature 215:1259–1260 [Google Scholar]
- Moroz V.I., Ekonomov A.P., Moshkin B.E., Revercomb H.E., Sromovsky L.A., and Schofield J.T. (1985) Solar and thermal radiation in the Venus atmosphere. Adv Space Res 5:197–232 [Google Scholar]
- Morris C.E., Sands D.C., Bardin M., Jaenicke R., Vogel B., Leyronas C., Ariya P.A., and Psenner R. (2011) Microbiology and atmospheric processes: research challenges concerning the impact of airborne micro-organisms on the atmosphere and climate. Biogeosciences 8:17–25 [Google Scholar]
- Murray B.C., Belton M.J.S., Danielson G.E., Davies M.E., Gault D., Hapke B., O'Leary B., Strom R.G., Suomi V., and Trask N. (1974) Venus: atmospheric Motion and Structure from Mariner 10 Pictures. Science 183:1307–1315 [DOI] [PubMed] [Google Scholar]
- Muyzer G. and Stams A.J.M. (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454 [DOI] [PubMed] [Google Scholar]
- Narlikar J.V., Lloyd D., Wickramasinghe N.C., Harris M.J., Turner M.P., Al-Mufti S., Wallis M.K., Wainwright M., Rajaratnam P., Shivaji S., Reddy G.S.N., Ramadurai S., and Hoyle F. (2003) A balloon experiment to detect microorganisms in the outer space. Astrophys Space Sci 285:555–562 [Google Scholar]
- Nisbet E.G. and Sleep N.H. (2001) The habitat and nature of early life. Nature 409:1083–1091 [DOI] [PubMed] [Google Scholar]
- Olson J.M. (2006) Photosynthesis in the Archean Era. Photosynth Res 88:109–117 [DOI] [PubMed] [Google Scholar]
- Ossa D.M.H., Oliveira R.R., Murakami M.T., Vicentini R., Costa-Filho A.J., Alexandrino F., Ottoboni L.M.M., and Garcia Jr O. (2011) Expression, purification and spectroscopic analysis of an HdrC: an iron–sulfur cluster-containing protein from Acidithiobacillus ferrooxidans. Process Biochem 46:1335–1341 [Google Scholar]
- Oyama V.I., Carle G.C., Woeller F., Pollack J.B., Reynolds R.T., and Craig R.A. (1980) Pioneer Venus gas chromatography of the lower atmosphere of Venus. J Geophys Res 85:7891–7902 [Google Scholar]
- Parkinson C., Bougher S., Yung Y., and Brecht A. (2013a) Photochemical distribution of Venusian sulfur and halogen species [#45, id.118.05]. In: AAS/Division for Planetary Sciences Meeting Abstracts
- Parkinson C.D., Bougher S.W., Schulte R., Gao P., Yung Y.L., Vandaele A., Wilquet V., Mahieux A., and Tellmann S. (2013b) Analysis of Venus Express optical extinction due to aerosols in the upper haze of Venus [abstract id.P41D-1949]. In: American Geophysical Union, Fall Meeting [Google Scholar]
- Parkinson C.D., Gao P., Schulte R., Bougher S.W., Yung Y.L., Bardeen C.G., Wilquet V., Vandaele A.C., Mahieux A., Tellmann S., and Pätzold M. (2015) Distribution of sulphuric acid aerosols in the clouds and upper haze of Venus using Venus Express VAST and VeRa temperature profiles. Planet Space Sci 113:205–218 [Google Scholar]
- Peplowski P.N. and Lawrence D.J. (2016) Nitrogen Content of Venus' Upper Atmosphere from the MESSENGER Neutron Spectrometer. In: 47th Lunar and Planetary Science Conference The Woodlands, TX, LPI Contribution No. 1903, p. 1177 [Google Scholar]
- Peralta J., Lee Y.J., Hueso R., Clancy R.T., Sandor B.J., Sánchez-Lavega A., Lellouch E., Rengel M., Machado P., Omino M., Piccialli A., Imamura T., Horinouchi T., Murakami S., Ogohara K., Luz D., and Peach D. (2017) Venus's winds and temperatures during the MESSENGER's flyby: an approximation to a three-dimensional instantaneous state of the atmosphere. Geophys Res Lett 44:3907–3915 [Google Scholar]
- Perez-Hoyos S., Garcia-Muñoz A., Sánchez-Lavega A., and McClintock W.M. (2013) Analysis of MESSENGER/MASCS data during second Venus flyby [id.EPSC2013-156]. In: European Planetary Science Congress 2013, held 8–13 September in London, UK, p. 8
- Pérez-Hoyos S., Sánchez-Lavega A., García-Muñoz A., Irwin P.G.J., Peralta J., Holsclaw G., McClintock W.M., and Sanz-Requena J.F. (2017) Venus upper clouds and the UV-absorber from MESSENGER/MASCS observations. J Geophys Res Planets 123:43 [Google Scholar]
- Phlips E.J., Badylak S., and Lynch T.C. (1999) Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a subtropical inner-shelf lagoon. Limnol Oceanogr 44:1166–1175 [Google Scholar]
- Pollack J.B., Toon O.B., Whitten R.C., Boese R., Ragent B., Tomasko M., Eposito L., Travis L., and Wiedman D. (1980) Distribution and source of the UV absorption in Venus' atmosphere. J Geophys Res 85:8141–8150 [Google Scholar]
- Pollack J.B., Dalton J.B., Grinspoon D., Wattson R.B., Freedman R., Crisp D., Allen D.A., Bezard B., de Bergh C., Giver L.P., Ma Q., and Tipping R. (1993) Near-infrared light from Venus' nightside—A spectroscopic analysis. Icarus 103:1–42 [Google Scholar]
- Pronk J.T., de Bruyn J.C., Bos P., and Kuenen J.G. (1992) Anaerobic growth of Thiobacillus ferrooxidans. Appl Environ Microbiol 58:2227–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragent B., Esposito L.W., Tomasko M.G., Marov M.I., and Shari V.P. (1985) Particulate matter in the Venus atmosphere. Adv Space Res 5:85–115 [Google Scholar]
- Ross F.E. (1928) Photographs of Venus. Astrophys J 68:57 [Google Scholar]
- Rossi L., Marcq E., Montmessin F., Fedorova A., Stam D., Bertaux J.-L., and Korablev O. (2015) Preliminary study of Venus cloud layers with polarimetric data from SPICAV/VEx Planet. Space Sci 113-114 (2015):159–168. 10.1016/j.pss.2014.11.011 [DOI]
- Rossow W.B., del Genio A.D., Limaye S.S., and Travis L.D. (1980) Cloud morphology and motions from Pioneer Venus images. J Geophys Res 85:8107–8128 [Google Scholar]
- Schalles J.F. (2006) Optical remote sensing techniques to estimate phytoplankton chlorophyll and concentratoons in coastal watres with varying suspended matter and CDOM concentrations. In: Remote Sensing of Aquatic Coastal Ecosystem Processes. edited by L.L. Richardson and E.F. LeDrews, Springer, Dordrecht, Netherlands, pp. 27–79 [Google Scholar]
- Schulze-Makuch D. and Irwin L.N. (2002) Reassessing the possibility of life on Venus: proposal for an astrobiology mission. Astrobiology 2:197–202 [DOI] [PubMed] [Google Scholar]
- Schulze-Makuch D. and Irwin L.N. (2006) The prospect of alien life in exotic forms on other worlds. Naturwissenschaften 93:155–172 [DOI] [PubMed] [Google Scholar]
- Schulze-Makuch D., Grinspoon D.H., Abbas O., Irwin L.N., and Bullock M.A. (2004) A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 4:11–18 [DOI] [PubMed] [Google Scholar]
- Segerer A.H., Trincone A., Gahrtz M., and Stetter K.O. (1991) Stygiolobus azoricus gen. nov., sp. nov. represents a novel genus of anaerobic, extremely thermoacidophilic archaebacteria of the order sulfolobales. Int J Syst Evol Microbiol 41:495–501 [Google Scholar]
- Seiff A., Sromovsky L., Borucki W., Craig R., Juergens D., Young R.E., and Ragent B. (1995) Pioneer Venus 12.5 KM Anomaly Workshop Report, volume 1. In: NASA STI/Recon Technical Report N 95. NASA Ames Research Center; Moffett Field, CA [Google Scholar]
- Shaya E. and Caldwell J. (1976) Photometry of Venus below 2200 A from the Orbiting Astronomical Observatory-2. Icarus 27:255–264 [Google Scholar]
- Shimizu M. (1977) Ultraviolet absorbers in the Venus clouds. Astrophys Space Sci 51:497–499 [Google Scholar]
- Signorini S.R., McClain C.R., and Dandonneau Y. (1999) Mixing and phytoplankton bloom in the wake of the Marquesas Islands. Geophys Res Lett 26:3121–3124 [Google Scholar]
- Sill G.T. (1975) The composition of the ultraviolet dark markings on Venus. J Atmos Sci 32:1201–1204 [Google Scholar]
- Smith D., Timonen H., Jaffe D., Griffin D.W., Birmele M., Perry K.D., Ward P.D., and Roberts M. (2013) Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl Environ Microbiol 79:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaun J. (1962) Problems in standardization of turbidity determinations on bacterial suspensions. Bull World Health Organ 26:219–225 [PMC free article] [PubMed] [Google Scholar]
- Stewart A.I., Anderson D.E., Esposito L.W., and Barth C.A. (1979) Ultraviolet spectroscopy of Venus—Initial results from the Pioneer Venus orbiter. Science 203:777–779 [DOI] [PubMed] [Google Scholar]
- Surkov Y.A., Ivanova V.F., Pudov A.N., Verkin B.I., Bagrov N.N., and Philipenko A.P. (1978) Mass-spectral study of the chemical composition of the Venus atmosphere by Venera 9 and Venera 10 space probes. Geokhimiia 4:506–513 [Google Scholar]
- Titov D.V., Markiewicz W.J., Moissl R., Ignatiev N., Limaye S., Khatuntsev I., Roatsch T., and Almeida M. (2008) Morphology of the Venus clouds from the imaging by Venus Monitoring Camera onboard Venus Express [EPSC2008-A-00225]. In: European Planetary Science Congress 2008, p. 225 [Google Scholar]
- Titov D.V., Markiewicz W.J., Ignatiev N.I., Song L., Limaye S.S., Sanchez-Lavega A., Hesemann J., Almeida M., Roatsch T., Matz K.-D., Scholten F., Crisp D., Esposito L.W., Hviid S.F., Jaumann R., Keller H.U., and Moissl R. (2012) Morphology of the cloud tops as observed by the Venus Express Monitoring Camera. Icarus 217:682–701 [Google Scholar]
- Tomasko M.G., Doose L.R., Smith P.H., and Odell A.P. (1980) Measurements of the flux of sunlight in the atmosphere of Venus. J Geophys Res 85:8167–8186 [Google Scholar]
- Toon O.B., Pollack J.B., and Turco R.P. (1982) The ultraviolet absorber on Venus—Amorphous sulfur. Icarus 51:358–373 [Google Scholar]
- Travis L.D. (1975) On the origin of ultraviolet constrasts on Venus. J Atmos Sci 32:1190–1200 [Google Scholar]
- Tsang C.C.C., Wilson C.F., Barstow J.K., Irwin P.G.J., Taylor F.W., McGouldrick K., Piccioni G., Drossart P., and Svedhem H. (2010) Correlations between cloud thickness and sub-cloud water abundance on Venus. Geophys Res Lett 37:L0220 [Google Scholar]
- Valdes J., Pedroso I., Quatrini R., Dodson R.J., Tettelin H., Blake R., 2nd, Eisen J.A., and Holmes D.S. (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiela I., McClelland J., Hauxwell J., Behr P.J., Hersh D., and Foreman K. (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118 [Google Scholar]
- van den Berg M.L., Falkner P., Atzei A.C., and Peacock A. (2006) Venus microsat explorer programme, an ESA technology reference study. Acta Astronaut 59:593–597 [Google Scholar]
- Vera M., Pagliai F., Guiliani N., and Jerez C.A. (2008) The chemolithoautotroph Acidithiobacillus ferrooxidans can survive under phosphate-limiting conditions by expressing a C-P lyase operon that allows it to grow on phosphonates. Appl Environ Microbiol 74:1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L., Caldwell J.J., and Savage B.D. (1972) Ultraviolet photometry from the Orbiting Astronomical Observatory. III. Observations of Venus, Mars, Jupiter, and Saturn longward of 2000 A. Astrophys J 172:755–769 [Google Scholar]
- Way M.J., Del Genio A.D., Kiang N.Y., Sohl L.E., Grinspoon D.H., Aleinov I., Kelley M., and Clune T. (2016) Was Venus the first habitable world of our solar system? Geophys Res Lett 43:8376–8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Więckowski A.B., Słowik G.P., Gąsiorek J.A., Gąsiorek P., Domka F., and Perkowska A. (1999) EPR study and structural aspects of ferredoxins obtained from Thiobacillus ferrooxidans. Appl Microbiol Biotechnol 52:96–98 [Google Scholar]
- Wilson C.F., Guerlet S., Irwin P.G.J., Tsang C.C.C., Taylor F.W., Carlson R.W., Drossart P., and Piccioni G. (2008) Evidence for anomalous cloud particles at the poles of Venus. J Geophys Res 113 [Google Scholar]
- Yamagishi A., Satoh T., Enya K., Miyakawa A., Yoshimura Y., Honda H., Imai E., Sasaki S., Ishigami G., Fujita K., and Miyamoto H. (2016) LDM (Life Detection Microscope): in situ imaging of living cells on surface of Mars. Trans. JSASS Aerospace Tech. Japan 14:Pk_117-Pk_124 [Google Scholar]
- Yamazaki A.M.Y., Lee Y.J., Watanabe S., Horinouchi T., Murakami S., Kouyama T., Ogohara K., Imamura T., Sato T.M., Yamamoto Y., Fukuhara T., Ando H., Sugiyama K., Takagi S., Ohtsuki S., Hirata N., Hashimoto G.L., Suzuki M., Hirose C., Ueno M., Satoh T., Abe T., Ishii N., and Nakamura M. (2017) Ultraviolet imager on Venus orbiter Akatsuki and its initial results. Earth Planets Space 70:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.T. (1973) Are the clouds of Venus sulfuric acid? Icarus 18:564–582 [Google Scholar]
- Young A.T. (1978) Carbon disulfide on Venus. Bull Am Astron Soc 10:548 [Google Scholar]
- Zasova L.V., Krasnopolskii V.A., and Moroz V.I. (1981) Vertical distribution of SO2 in upper cloud layer of Venus and origin of U.V.-absorption. Adv Space Res 1:13–16 [Google Scholar]
- Zasova L.V., Ignatiev N., Khatuntsev I., and Linkin V. (2007) Structure of the Venus atmosphere. Planet Space Sci 55:1712–1728 [Google Scholar]
- Zasova L., Senske D., Economou T., Eismont N., Esposito L., Gerasimov M., Gorinov D., Ignatiev N., Ivanov M., Jessup K. L.ea Khatuntsev, I., Korablev O., Kremic T., Limaye S., Lomakin I., Martynov A., Ocampo A., Vaisberg O., and Burdanov A. (2017) Joint IKI/ROSCOSMOS - NASA Science Definition Team and concept mission to Venus based on Venera-D, European Planetary Science Congress held 17–22 September, 2017 in Riga Latvia, id. EPSC2017-296
- Zeng J., Geng M., Liu Y., Zhao W., Xia L., Liu J., and Qiu G. (2007) Expression, purification and molecular modelling of the Iro protein from Acidithiobacillus ferrooxidans Fe-1. Protein Expr Purif 52:146–152 [DOI] [PubMed] [Google Scholar]