Abstract
Background
The standard protocol to achieve haemostasis during total hip arthroplasty (THA) is uncertain. Tranexamic acid plus diluted epinephrine (DEP) and tranexamic acid (TXA) alone are the two most common alternatives. The purpose of this study was to compare the efficacy and safety of TXA plus DEP to treat blood loss in THA patients.
Methods
Published randomized controlled trials (RCTs) were identified from the following electronic databases: PubMed, Embase, Web of Science, Cochrane Library and Google from inception to July 10, 2018. Studies comparing TXA plus DEP with TXA alone to treat blood loss were included. Either a random-effects model or a fixed-effects model was used for meta-analysis depending on the heterogeneity. We used the need for transfusion as the primary outcome. Stata 12.0 was used for meta-analysis.
Results
Six studies involving 703 patients were included in the present meta-analysis. The pooled results demonstrated that TXA plus DEP was associated with a lower transfusion rate than TXA alone (RR = 0.57, 95% CI 0.38–0.86, P = 0.006). Furthermore, TXA plus DEP was associated with less total blood loss and hidden blood loss by approximately 209.79 ml and 297.74 ml, respectively, than TXA alone. There was no significant difference in terms of intraoperative blood loss or the occurrence of deep venous thrombosis or haematoma between the TXA plus DEP and TXA alone groups (P > 0.05).
Conclusions
Our meta-analysis suggested that TXA plus DEP significantly decreased the need for transfusion, total blood loss and hidden blood loss among THA patients. Furthermore, TXA plus DEP did not increase the occurrence of DVT or haemostasis. Additional long-term follow-up RCTs are needed to identify the optimal doses of TXA and DEP.
Electronic supplementary material
The online version of this article (10.1186/s13018-018-0948-1) contains supplementary material, which is available to authorized users.
Keywords: Tranexamic acid, Blood loss, Total hip arthroplasty, Meta-analysis
Introduction
Total hip arthroplasty (THA) is an effective treatment for end-stage hip osteoarthritis (OA) [1]. By 2030, the demand for primary THA is estimated to increase to 572,000 [2]. THA is associated with a large amount of intraoperative blood loss and hidden blood loss [3]. Extensive blood loss results in cardiovascular complications and the need for a blood transfusion [4, 5]. Blood transfusion carries the risk of hepatitis virus transmission and immunomodulation, increasing economic costs and prolonging the length of hospital stay [6]. Therefore, there is an urgent need to identify a safe, effective method of reducing blood loss and blood transfusions after THA.
Several alternatives are available for minimizing blood loss after THA. These include topical fibrin sealants, topical or intravenous tranexamic acid (TXA) [7, 8], aminocaproic acid [3, 9] or diluted epinephrine (DEP) [10]. Recently, administration TXA plus DEP has become popular for THA patients [11]. DEP enhances coagulation by several mechanisms [12]. Nevertheless, whether TXA plus DEP is superior to TXA alone remains unclear. To further explore these issues and to identify the best haemostatic techniques for THA, we performed a meta-analysis of all the available randomized controlled trials (RCTs) of patients with THA.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2011 [13]. Ethical approval was not necessary for this study, as only de-identified pooled data from individual studies were analysed.
Search strategies
We searched PubMed, Embase and Cochrane CENTRAL for relevant studies from the time of inception of these databases to July 10, 2018. The following groups of keywords and medical terms were used for the literature search: “tranexamic acid” AND “epinephrine” (OR “total hip arthroplasty” OR “total hip replacement” OR arthroplasty OR “THA” “THR”) AND (random* OR prospective* OR trial*). The language was not restricted to English. We also conducted an additional search by screening the references of eligible studies.
Study eligibility
We evaluated each identified RCT against the following predetermined selection criteria:
-
i.
Study population: adults with hip OA eligible for primary THA.
-
ii.
Interventions: the review focused on topical or intravenous TXA plus topical DEP, which are commonly used in the management of blood loss after THA, as commonly reported in the literature.
-
iii.
Comparator: direct comparisons among any of the four core therapeutic interventions (i.e. DEP alone, topical or intravenous TXA alone and a control group).
-
iv.
Outcome measures: the primary outcomes for this review were the need for transfusion, total blood loss, blood loss in drainage and the occurrence of deep venous thrombosis (DVT).
Data extraction
Two authors independently extracted the general characteristics and outcomes from the included studies. The following data were extracted from each study: first author, publication year, location, age and number of patients in the intervention and control groups, doses of TXA and DEP, outcomes, transfusion threshold and follow-up. The differences in the extracted data were discussed by a panel of all the reviewers. When there were no clear data or missing data from the included studies, we tried to contact the corresponding author to obtain the relevant data.
Quality assessment
Two reviewers independently evaluated the risk of bias using the Cochrane risk-of-bias tool. Seven major domains of bias (selection bias (random sequence generation), selection bias (allocation concealment), performance bias, detection bias, attrition bias, reporting bias and other bias) in each trial were reviewed. Disagreements between the reviewers were resolved by discussion.
Statistically analysis
The risk ratios (RRs) with 95% confidence intervals (CIs) were calculated for the need for transfusion and the occurrence of DVT. The weighted mean difference (WMD) and corresponding CIs were calculated for continuous data (total blood loss, blood loss in drainage). Heterogeneity was explored for all the meta-analyses and quantified using I2 statistics. When I2 value was > 50%, this was considered substantial heterogeneity between studies. If there was a large clinical heterogeneity, a random-effects model was applied to pool the outcome data. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 12.0 (Stata Corp., College Station, TX). Subgroup analysis was further performed according to the following variables: risk of bias (low or unclear/high), IV TXA dose (≥ 2 g or < 2 g), topical dose (≥ 2 g or < 2 g) and transfusion protocol (strict or loose). We categorized the TXA dose of 30 mg/kg into the subgroup of ≥ 2 g. Sensitivity analysis was also performed by omitting each of the studies in turn.
Quality of evidence assessment
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to assess the quality of evidence. The assessment includes five items: risk of bias, inconsistency, indirectness, imprecision and publication bias. Each outcome was rated as high, moderate, low or very low. Summary tables were constructed using GRADE Pro version 3.6 (GRADE Working Group).
Results
Search results
A flowchart of study search and selection is presented in Fig. 1. We identified 320 references (PubMed = 185, Embase = 20, Web of Science = 80, Cochrane Library = 35) in our initial literature search. There were no additional records identified through other sources. After removing duplicates using Endnote X7 software, there were 206 studies remaining. Subsequently, 200 studies were excluded according to the inclusion criteria. Finally, 6 trials with 703 patients met our inclusion criteria and were included in the meta-analysis [14–19]. The general characteristics of the included studies can be seen in Table 1. All trials were published after the year 2015. Five studies were performed in China, and one was performed in Denmark. The mean age of the patients ranged from 50.0 to 69 years. Patients’ ages ranged from 21 to 65 years, and all were less than 100 years old.
Fig. 1.
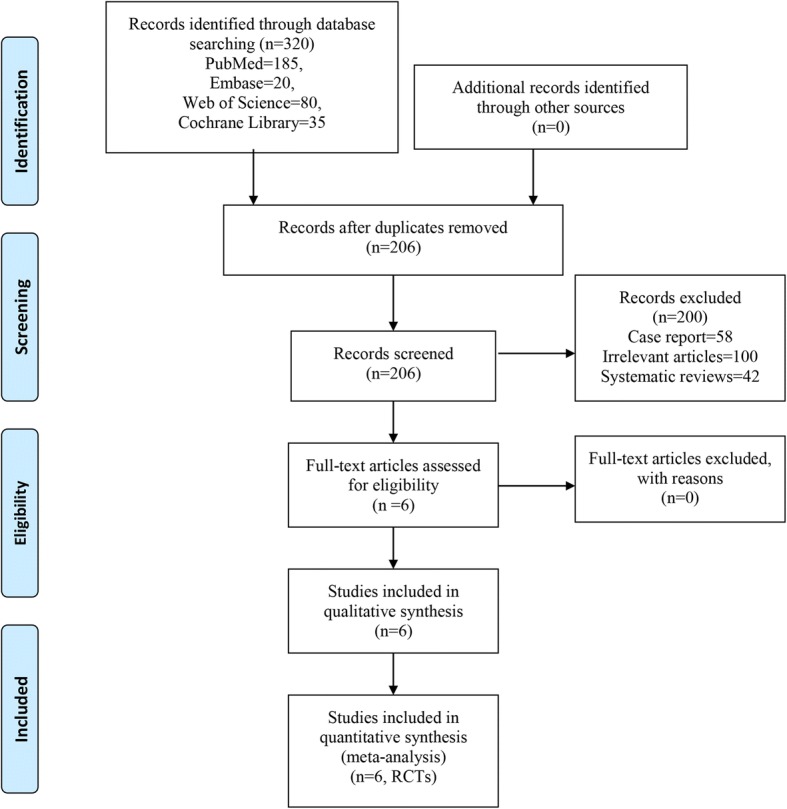
Flow diagram of the study selection process
Table 1.
General characteristic of the included studies. 1, need for transfusion; 2, total blood loss; 3, hidden blood loss; 4, intraoperative blood loss; 5, the occurrence of DVT; 6, the occurrence of hematoma
| Author | Country | Age (years, I/C) | No. of patients (n) | Interventions | Dose of intervention | Outcomes | Transfusion threshold | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Gao F 2015 | China | 58.6 vs 61.7 | 53 vs 54 | Topical TXA + topical DEP vs topical TXA | TXA (3 g), DEP (0.25 mg, 1:200000) | 1, 2, 3, 4, 5, 6 | Hb < 70 g/l | 3 months |
| Jans O 2016 | Denmark | 67 vs 69 | 50 vs 50 | Intravenous TXA + intravenous DEP vs intravenous TXA | TXA (1 g), DEP (0.05 μg kg−1 min−1) | 2,3, | Hb < 80 g/l | At discharge |
| Liu JL 2018 | China | 50.0 vs 50.2 vs 51.8 | 65 vs 65 vs 65 | Intravenous TXA + DEP vs intravenous TXA + topical DEP vs intravenous TXA | IV DEP (1 mg), IV TXA (10 mg/kg), topical DEP (0.25 mg, 1:200000) | 1, 2, 3, 4, 5, 6 | Hb < 70 g/l | 2 weeks |
| Wang JW 2017 | China | 67 vs 69 | 45 vs 45 | Topical TXA + topical DEP vs topical TXA | TXA (3 g), DEP (0.25 mg, 1:200000) | 1, 2, 3, 4, 5, 6 | Hb < 80 g/l | 2 months |
| Zhang JK 2017 | China | 62.5 vs 63.1 | 21 vs 34 | Topical TXA + topical DEP vs topical TXA | TXA (3 g), DEP (0.25 mg, 1:200000) | 1, 2, 3, 4, 5, 6 | Hb < 80 g/l | 6 months |
| Zhang JZ 2017 | China | 59.8 vs 60.3 vs 58.6 | 52 vs 52 vs 52 | Topical 1 g TXA + topical low dose DEP vs topical 1 g TXA + topical high dose DEP vs topical TXA | TXA (1 g), DEP (0.125 mg, 0.25 mg, 1:200000) | 1, 2, 3, 4, 5 | Hb < 70 g/l | 3 months |
Quality assessment
Data regarding the risk of bias summary and risk of bias graphs for each study are presented in Figs. 2 and 3, respectively. Three studies had a low risk of bias. The other studies were considered to have an unclear risk of bias.
Fig. 2.
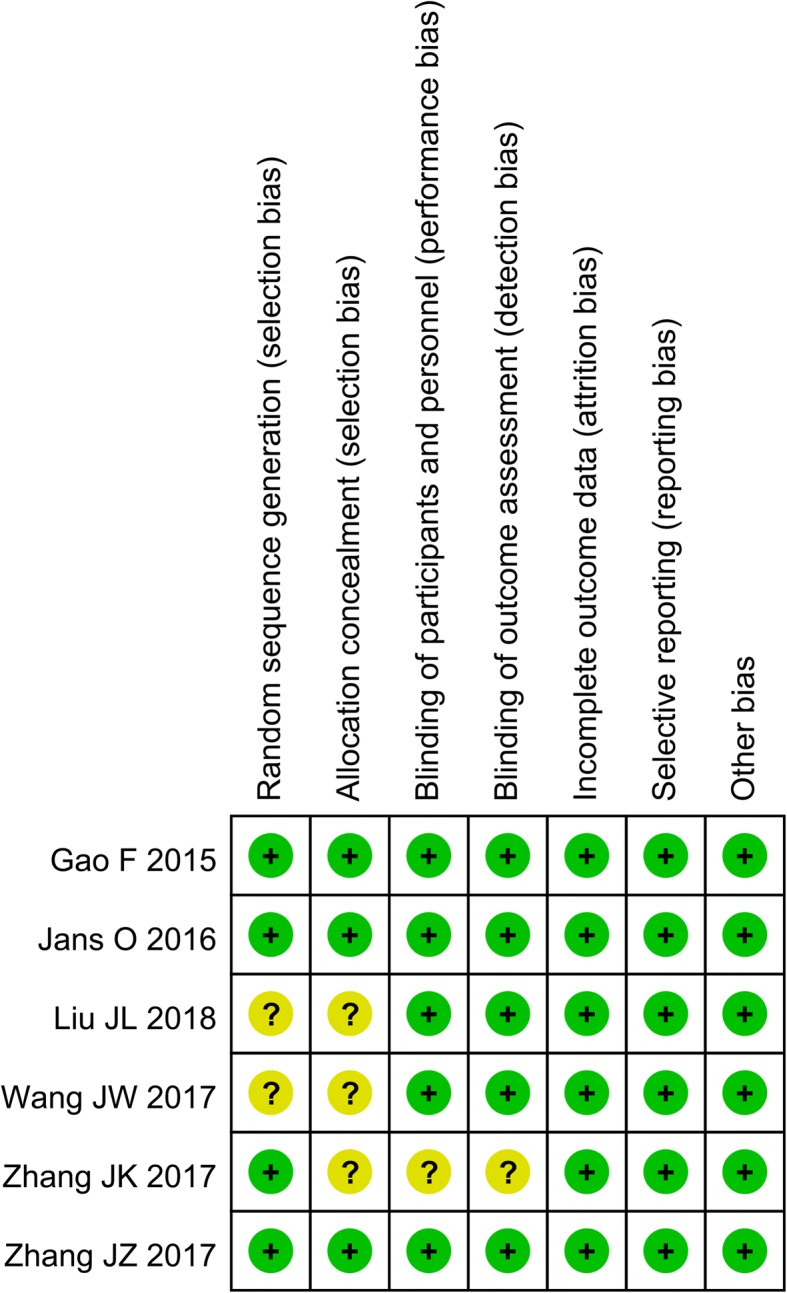
Risk of bias summary for the included RCTs. +, low risk of bias; −, high risk of bias; ?, unclear risk of bias
Fig. 3.
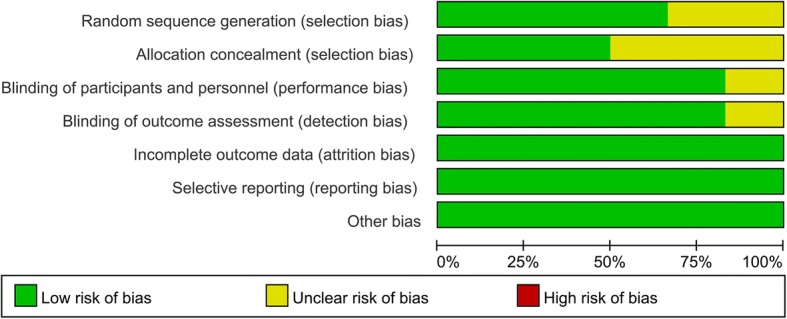
Risk of bias graph for the included RCTs
Quality of evidence assessment
The GRADE evidence profiles are presented in Additional file 1. The GRADE level of evidence was low for total blood loss, hidden blood loss and intraoperative blood loss; it was moderate for the need for transfusion and the occurrence of DVT and haematoma.
Results of the meta-analysis
Need for transfusion
Five studies were available with information regarding transfusion rate. The pooled results demonstrated that TXA plus DEP was associated with a lower transfusion rate than TXA alone (RR = 0.57, 95% CI 0.38–0.86, P = 0.006, Fig. 4). No heterogeneity was detected (I2 = 0%, P = 0.441), and thus, a fixed-effects model was used. The results of the subgroup analysis are shown in Table 2. The findings of a decreased need for transfusion were consistent for different doses of TXA except for the risk of bias and transfusion protocol.
Fig. 4.
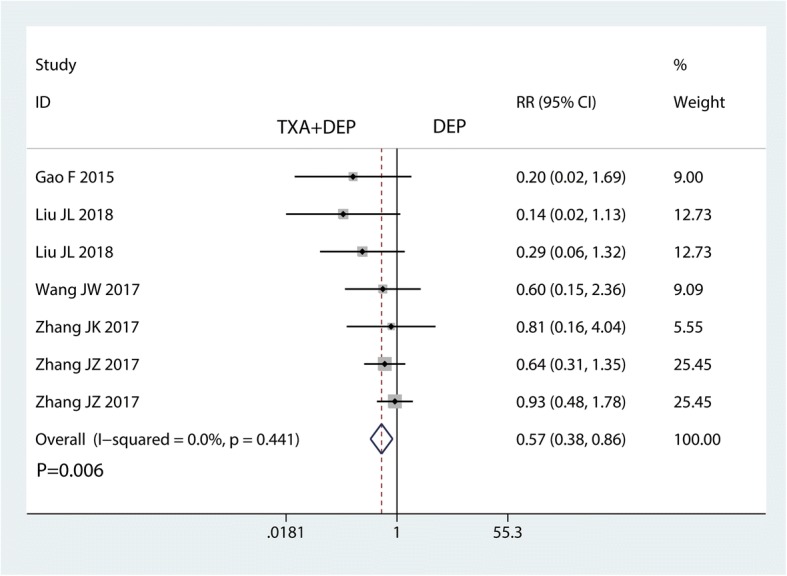
Forest plot for the comparison of the need for transfusion between the TXA plus DEP and TXA alone groups
Table 2.
Subgroup analysis for the need for transfusion
| Subgroup | No. trials | RR (95% CI) | P value | I2 (%) | Test of interaction, P |
|---|---|---|---|---|---|
| Risk of bias | |||||
| Low | 4 | 0.60 (0.38, 0.95) | 0.028 | 35.5 | 0.047 |
| Unclear/high | 3 | 0.50 (0.21, 1.16) | 0.105 | 0 | |
| Dose of TXA | |||||
| Low | 4 | 0.60 (0.38, 0.93) | 0.014 | 34.1 | 0.125 |
| High | 3 | 0.50 (0.16, 0.84) | 0.023 | 0 | |
| Transfusion protocol | |||||
| Strict | 3 | 0.43 (0.18, 1.05) | 0.064 | 0 | 0.043 |
| Loose | 4 | 0.63 (0.40, 0.98) | 0.041 | 14.5 | |
Total blood loss
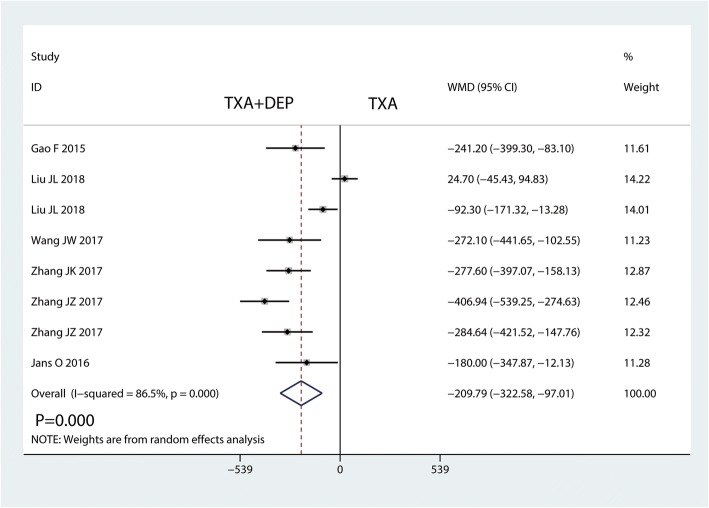
Five studies were available for analysis of total blood loss. TXA plus DEP led to significantly less total blood loss than TXA alone (WMD = − 209.79, 95% CI − 322.58 to − 97.02, P = 0.000; I2 = 86.5%, P = 0.000, Fig. 5). Thus, we used a random-effects model to pool the relevant data.
Fig. 5.
Forest plot for the comparison of total blood loss between the TXA plus DEP and TXA alone groups
Hidden blood loss
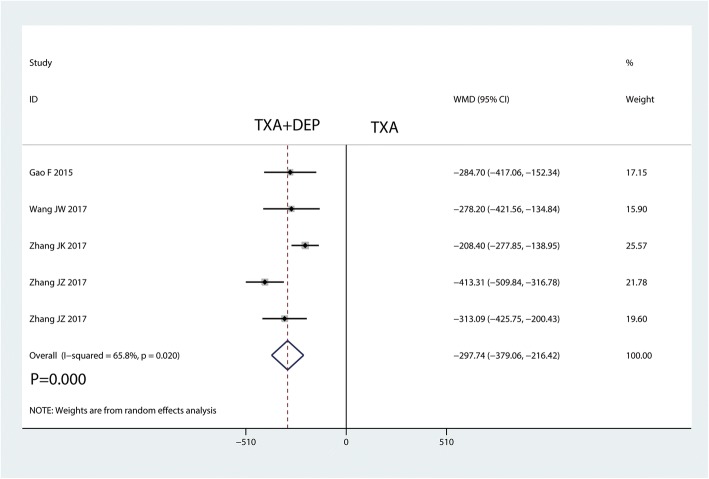
Five studies were available for analysing hidden blood loss. TXA plus DEP led to significantly less hidden blood loss than TXA alone (WMD = − 297.74, 95% CI − 379.06 to − 216.42, P = 0.000; I2 = 65.8%, P = 0.020, Fig. 6). Thus, we used a random-effects model to pool the relevant data.
Fig. 6.
Forest plot for the comparison of hidden blood loss between the TXA plus DEP and TXA alone groups
Intraoperative blood loss
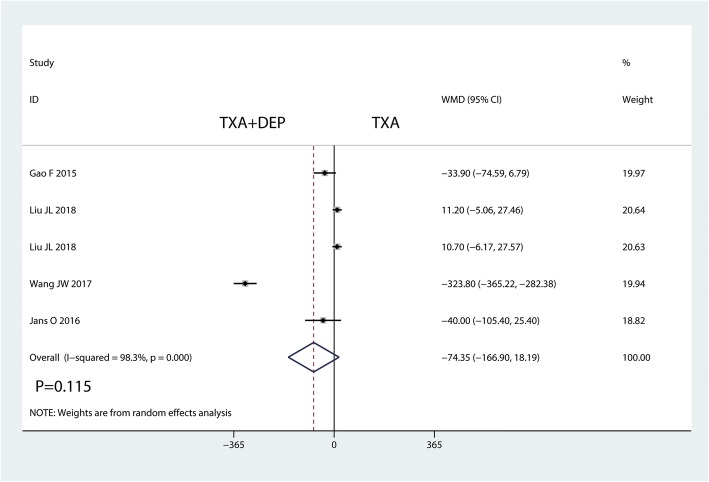
Four studies were available for analysis of intraoperative blood loss. TXA plus DEP led to significantly less hidden blood loss than TXA alone (WMD = − 74.35, 95% CI − 166.90 to 18.19, P = 0.115; I2 = 98.3%, P = 0.000, Fig. 7). Thus, we used a random-effects model to pool the relevant data.
Fig. 7.
Forest plot for the comparison of intraoperative blood loss between the TXA plus DEP and TXA alone groups
The occurrence of DVT and haematoma
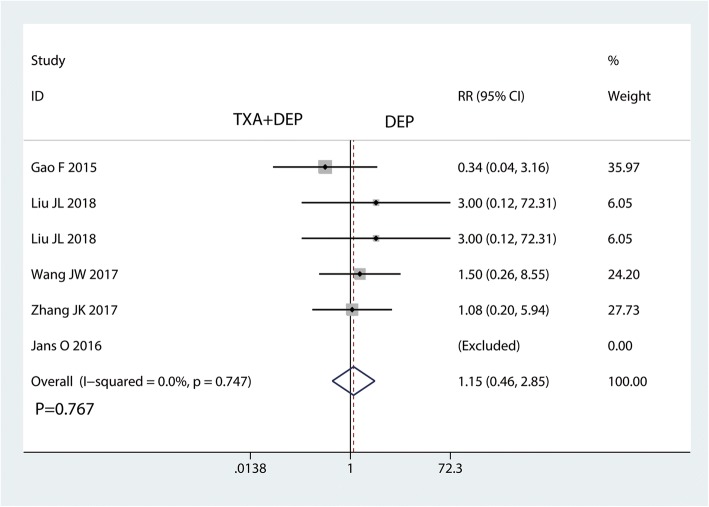
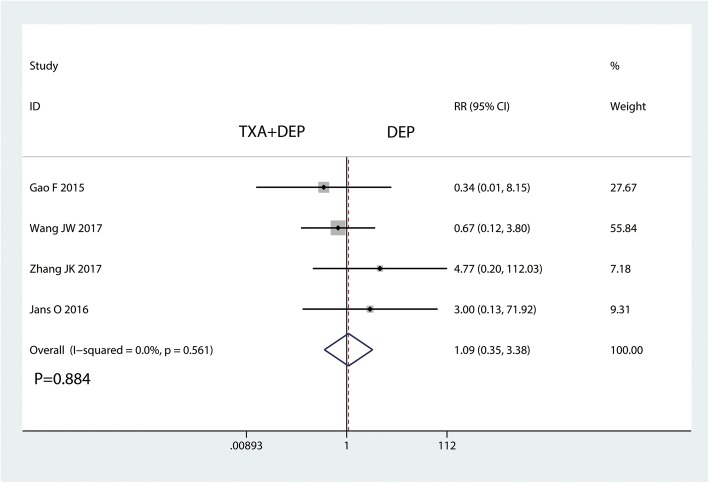
Five studies reported the occurrence of DVT. There was no significant difference in the occurrence of DVT between the TXA plus DEP and TXA alone groups (RR = 1.15, 95% CI 0.46–2.85, P = 0.767, Fig. 8). No heterogeneity was detected (I2 = 0%, P = 0.747); thus, a fixed-effects model was used. Four studies reported the occurrence of haematoma. There was no significant difference in the occurrence of DVT between the TXA plus DEP and the TXA alone groups in terms of the occurrence of haematoma (RR = 1.09, 95% CI 0.35–3.38, P = 0.884, Fig. 9). No heterogeneity was detected (I2 = 0%, P = 0.561); thus, a fixed-effects model was sued.
Fig. 8.
Forest plot for the comparison of the occurrence of DVT between the TXA plus DEP and TXA alone groups
Fig. 9.
Forest plot for the comparison of the occurrence of haematoma between the TXA plus DEP and TXA alone groups
Sensitivity analysis, publication bias
We performed a sensitivity analysis for the need for transfusion (Fig. 10. The results showed that after omitting the included studies, in turn, the overall effects did not change. The funnel plots were visually assessed and revealed no asymmetry (Fig. 11); no evidence of publication bias was determined by the Egger linear regression test for the need for transfusion (P = 0.72, Fig. 12),
Fig. 10.
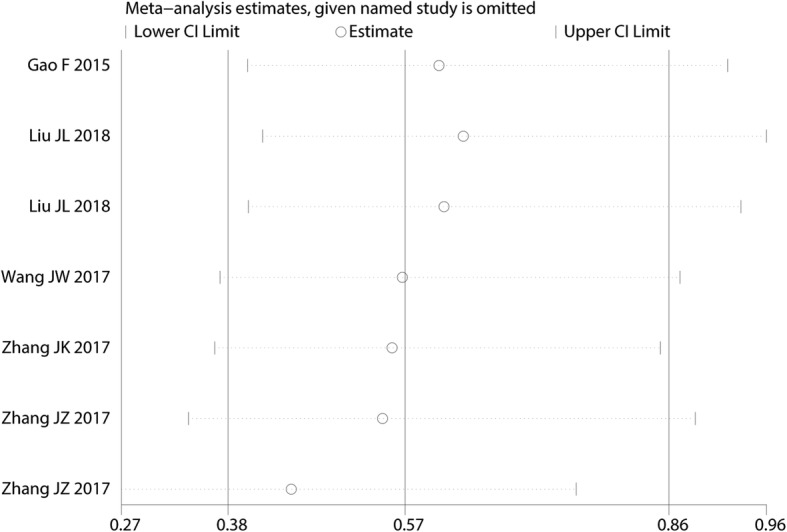
Sensitivity analysis for the need for transfusion after omitting each study in turn
Fig. 11.
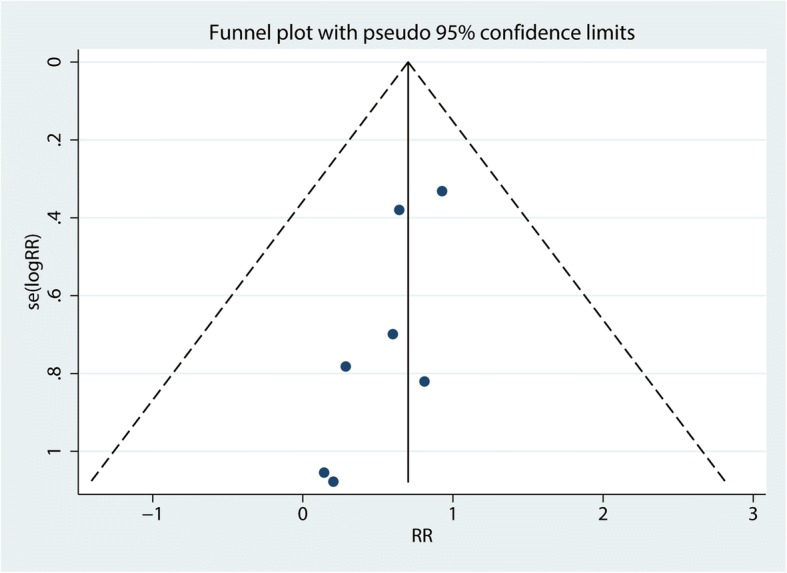
Funnel plot of the need for transfusion
Fig. 12.
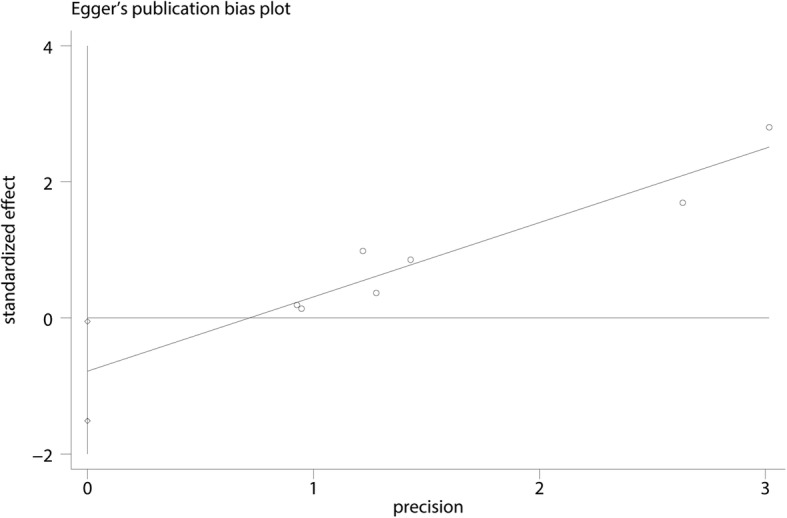
Egger’s test for publication bias for the need for transfusion
Discussion
In the current meta-analysis, we evaluated the efficacy and safety of TXA plus DEP for patients with THA. On the basis of the pooled estimates, TXA plus DEP was associated with significantly less total blood loss and subsequent need for transfusion than TXA alone. The use of tanezumab was not associated with a significantly increased risk of DVT or haematoma.
This was not the first meta-analysis. Yu et al. [20] conducted a meta-analysis comparing TXA plus DEP for blood loss after total joint arthroplasty (THA and total knee arthroplasty). Thus, we could not determine whether TXA plus DEP was certain to have a significant influence on controlling blood loss among patients undergoing THA alone. Moreover, Yu et al. [20] only included two studies that focused on THA. In this meta-analysis, we ultimately included six studies totalling 703 patients, adding the statistical power of at least 535 cases. Our meta-analysis was the latest and the most comprehensive one, and it generally concurs and further reinforces the results of the previous meta-analysis. Finally, we performed a subgroup-analysis and evaluated the quality of evidence using GRADE to help healthcare professionals make clinical decisions.
The current meta-analysis demonstrated that TXA plus DEP has a beneficial effect on total blood loss. TXA plus DEP was associated with less total blood loss by 209.79 ml than TXA alone. Several meta-analyses have found that TXA has a beneficial role in reducing blood loss in THA patients without increasing DVT occurrence [21, 22]. TXA can be administered by several routes including topical [23], intravenous [24] and oral [25]. Studies have shown that there was no significant difference among these routes in terms of the total blood loss. Concerning DEP, Jans O et al. [15] strongly suggested that intravenous DEP could be beneficial for reducing blood loss after THA. The administration of low-dose DEP could act as a procoagulant by increasing platelet aggregation, resulting in an instant 20–30% increase in platelet count [26]. Furthermore, DEP could activate α-adrenergic and β-adrenergic receptors; therefore, DEP could stimulate the release of several coagulation factors [27].
We measured hidden blood loss between the TXA plus DEP and TXA alone groups. We found that TXA plus DEP significantly reduced hidden blood loss by 297.74 ml compared with TXA alone. In THA patients, significant blood loss can occur after wound closure, and the proportion of this blood loss is called hidden blood loss. Hidden blood loss accounts for as much as 60% of the total perioperative blood loss [28]. With the administration of DEP, the procoagulant effects could last for 1–2 h; therefore, oozing could be decreased.
Regarding complications, we measured the occurrences of DVT and haematoma formation. We found that there was no significant difference between the occurrences of DVT and haematoma formation. Regarding the administration of TXA, DVT was the major concern. Several meta-analyses have identified that administration with TXA does not increase the occurrence of DVT [29]. Due to the incidence rate being relatively small, there is a need for studies to further clarify the risk [30].
There were several limitations in this meta-analysis: (1) the doses of TXA and DEP varied among the included studies, and the optimal doses of TXA and DEP require further exploration; (2) heterogeneity was large in terms of total blood loss and hidden blood loss, and these two outcomes should be interpreted cautiously; (3) the follow-up period varied among the included studies; thus, complications of TXA plus DEP may have been underestimated; and (4) the sample size was relatively small in the included studies; therefore, high-quality large-scale sample RCTs are needed.
Conclusion
This meta-analysis suggests that TXA plus DEP has benefits in terms of total blood loss, hidden blood loss and the need for transfusion. Furthermore, TXA plus DEP had no influence on the occurrence of DVT or haematoma formation. Given all the shortcomings of this meta-analysis, further research and analysis are required to draw more reliable conclusions.
Additional file
Grade evidence for the outcomes. (DOCX 14 kb)
Availability of data and materials
Supporting data is available.
Abbreviations
- CIs
Confidence intervals
- DEP
Diluted epinephrine
- DVT
Deep venous thrombosis
- OA
Osteoarthritis
- RCTs
Randomized controlled trials
- RR
Risk ratio
- THA
Total hip arthroplasty
- TXA
Tranexamic acid
- WMD
Weighted mean difference
Authors’ contributions
ZW designed the study and developed the retrieval strategy. ZW and HJZ searched and screened the summaries and titles. HJZ and ZW drafted the article. Both authors read and approved the final draft.
Ethics approval and consent to participate
This is a meta-analysis; no relevant problems exist.
Consent for publication
Not applicable.
Competing interests
Both authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhao Wang, Email: wangzhao090@qq.com.
Hao-jie Zhang, Email: 2046293417@qq.com.
References
- 1.Guo H, Wang C, He Y. A meta-analysis evaluates the efficacy of intravenous acetaminophen for pain management in knee or hip arthroplasty. J Orthop Sci. 2018;23(5):793–800. [DOI] [PubMed]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q, Geng P, Shi L, Wang Q, Wang P. Tranexamic acid versus aminocaproic acid for blood management after total knee and total hip arthroplasty: a systematic review and meta-analysis. Int J Surg. 2018;54(Pt A):105–112. doi: 10.1016/j.ijsu.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 4.Li JF, Li H, Zhao H, Wang J, Liu S, Song Y, Wu HF. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. J Orthop Surg Res. 2017;12(1):22. doi: 10.1186/s13018-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li YJ, Xu BS, Bai SP, Guo XJ, Yan XY. The efficacy of intravenous aminocaproic acid in primary total hip and knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2018;13(1):89. doi: 10.1186/s13018-018-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloch EM, Ingram C, Hull J, Fawcus S, Anthony J, Green-Thompson R, Crookes RL, Ngcobo S, V Creel D, Courtney L, et al. Risk factors for peripartum blood transfusion in South Africa: a case-control study. Transfusion. 2018;[Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 7.Gianakos AL, Hurley ET, Haring RS, Yoon RS, Liporace FA. Reduction of blood loss by tranexamic acid following total hip and knee arthroplasty: a meta-analysis. JBJS reviews. 2018;6(5):e1. doi: 10.2106/JBJS.RVW.17.00103. [DOI] [PubMed] [Google Scholar]
- 8.Sridharan K, Sivaramakrishnan G. Tranexamic acid in total hip arthroplasty: mixed treatment comparisons of randomized controlled trials and cohort studies. J Orthop. 2018;15(1):81–88. doi: 10.1016/j.jor.2018.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang F, Wu Y, Yin Z, Ma G, Chang J. A systematic review and meta-analysis of the use of antifibrinolytic agents in total hip arthroplasty. Hip Int. 2015;25(6):502–509. doi: 10.5301/hipint.5000285. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Zeng Y, Bao X, Xiong H, Fan X, Shen B. Application of tranexamic acid and diluted epinephrine in primary total hip arthroplasty. Blood Coagul Fibrinolysis. 2018;29(5):451–7. [DOI] [PubMed]
- 11.Teng Y, Ma J, Ma X, Wang Y, Lu B, Guo C. The efficacy and safety of epinephrine for postoperative bleeding in total joint arthroplasty: a PRISMA-compliant meta-analysis. Medicine. 2017;96(17):e6763. doi: 10.1097/MD.0000000000006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tullavardhana T, Akranurakkul P, Ungkitphaiboon W, Songtish D. Efficacy of submucosal epinephrine injection for the prevention of postpolypectomy bleeding: a meta-analysis of randomized controlled studies. Annals of Medicine and Surgery. 2012;2017(19):65–73. doi: 10.1016/j.amsu.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Sun W, Guo W, Li Z, Wang W, Cheng L. Topical application of tranexamic acid plus diluted epinephrine reduces postoperative hidden blood loss in total hip arthroplasty. J Arthroplast. 2015;30(12):2196–2200. doi: 10.1016/j.arth.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Jans O, Grevstad U, Mandoe H, Kehlet H, Johansson PI. A randomized trial of the effect of low dose epinephrine infusion in addition to tranexamic acid on blood loss during total hip arthroplasty. Br J Anaesth. 2016;116(3):357–362. doi: 10.1093/bja/aev408. [DOI] [PubMed] [Google Scholar]
- 16.Liu JL, Zeng WN, Wang FY, Chen C, Gong XY, Yang H, Tan ZJ, Jia XL, Yang L. Effects of low-dose epinephrine on perioperative hemostasis and inflammatory reaction in major surgical operations: a randomized clinical trial. J. Thromb. Haemost. 2018;16(1):74–82. doi: 10.1111/jth.13896. [DOI] [PubMed] [Google Scholar]
- 17.Wang JW, Ren SH. The effects of topical tranexamic acid plus epinephrine for blood loss in total hip arthroplasty patients. J Pract Med. 2017;33(2):279–281. [Google Scholar]
- 18.Zhang JK, Liang PX, Liu Z. Effects of topical application of tranexamic acid plus adrenaline in total hip arthroplasty. Journal of Clinical Orthopaedics. 2017;20(1):58–61. [Google Scholar]
- 19.Zhang JZ, Zhu K. Hemostasis effect of local application of tranexamic acid and epinephrine for total hip arthroplasty. Journal of Clinical Orthopaedics. 2017;20(3):318–321. [Google Scholar]
- 20.Yu Z, Yao L, Yang Q. Tranexamic acid plus diluted-epinephrine versus tranexamic acid alone for blood loss in total joint arthroplasty: a meta-analysis. Medicine. 2017;96(24):e7095. doi: 10.1097/MD.0000000000007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-b(8):1005–1015. doi: 10.1302/0301-620X.96B8.33745. [DOI] [PubMed] [Google Scholar]
- 22.Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151–162. doi: 10.1111/tme.12212. [DOI] [PubMed] [Google Scholar]
- 23.Abdel MP, Chalmers BP, Taunton MJ, Pagnano MW, Trousdale RT, Sierra RJ, Lee YY, Boettner F, Su EP, Haas SB, et al. Intravenous versus topical tranexamic acid in total knee arthroplasty: both effective in a randomized clinical trial of 640 patients. J Bone Joint Surg Am. 2018;100(12):1023–1029. doi: 10.2106/JBJS.17.00908. [DOI] [PubMed] [Google Scholar]
- 24.Wei W, Dang S, Duan D, Wei L. Comparison of intravenous and topical tranexamic acid in total knee arthroplasty. BMC Musculoskelet Disord. 2018;19(1):191. doi: 10.1186/s12891-018-2122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo ZY, Wang D, Meng WK, Wang HY, Pan H, Pei FX, Zhou ZK. Oral tranexamic acid is equivalent to topical tranexamic acid without drainage in primary total hip arthroplasty: a double-blind randomized clinical trial. Thromb Res. 2018;167:1–5. doi: 10.1016/j.thromres.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 26.von Kanel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000;65(6):357–369. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- 27.Bakovic D, Pivac N, Eterovic D, Breskovic T, Zubin P, Obad A, Dujic Z. The effects of low-dose epinephrine infusion on spleen size, central and hepatic circulation and circulating platelets. Clin Physiol Funct Imaging. 2013;33(1):30–37. doi: 10.1111/j.1475-097X.2012.01156.x. [DOI] [PubMed] [Google Scholar]
- 28.Lei Y, Huang Q, Huang Z, Xie J, Chen G, Pei F. Multiple-dose intravenous tranexamic acid further reduces hidden blood loss after total hip arthroplasty: a randomized controlled trial. J Arthroplast. 2018;33(9):2940–5. [DOI] [PubMed]
- 29.Xie J, Hu Q, Ma J, Huang Q, Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss and the inflammatory response following enhanced-recovery primary total hip arthroplasty: a randomised clinical trial. Bone Joint J. 2017;99-b(11):1442–1449. doi: 10.1302/0301-620X.99B11.BJJ-2017-0488.R1. [DOI] [PubMed] [Google Scholar]
- 30.Wu XD, Hu KJ, Sun YY, Chen Y, Huang W. Letter to the editor on “the safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis”. J Arthroplast. 2018;[Epub ahead of print]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Grade evidence for the outcomes. (DOCX 14 kb)