Abstract
We examined associations of 5 plasma choline metabolites with carotid plaque among 520 HIV-infected and 217 HIV-uninfected participants (112 incident plaque cases) over 7 years. After multivariable adjustment, higher gut microbiota-related metabolite trimethylamine-N-oxide (TMAO) was associated with an increased risk of carotid plaque in HIV-infected participants (risk ratio = 1.25 per standard deviation increment; 95% confidence interval, 1.05–1.50; P = .01). TMAO was positively correlated with biomarkers of monocyte activation and inflammation (sCD14, sCD163). Further adjustment for these biomarkers attenuated the association between TMAO and carotid plaque (P = .08). Among HIV-infected individuals, plasma TMAO was associated with carotid atherosclerosis progression, partially through immune activation and inflammation.
Keywords: trimethylamine-N-oxide, choline metabolites, carotid atherosclerosis, HIV infection, risk factors
Higher plasma TMAO, a gut microbial-related choline metabolite, was associated with greater progression of carotid artery atherosclerosis in HIV-infected individuals, suggesting a potential role of gut microbiota and related TMAO in the development of cardiovascular disease in HIV-infected individuals.
Choline, an essential nutrient, plays important roles in neurotransmitter synthesis, cell-membrane signaling, lipid transport, and methyl-group metabolism [1]. Recent studies suggested that the gut microbiota-dependent choline metabolite trimethylamine-N-oxide (TMAO) may promote atherosclerosis and cardiovascular disease (CVD) [2, 3]. Other choline metabolites have been also reported to be associated with CVD risk, though results remain controversial [4]. Alterations in gut microbiota have been observed among human immunodeficiency virus (HIV)-infected individuals [5], but previous studies with limited sample sizes have yielded various results regarding the relationship between TMAO and CVD in HIV-infected individuals [6–8]. Other nongut microbiota-dependent choline metabolites have not been comprehensively investigated in HIV-infected individuals in previous studies.
Our previous work in 2 prospective HIV cohorts, the Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS), has shown greater progression of carotid artery atherosclerosis in HIV-infected individuals compared to those without HIV infection [9]. In addition, we have previously reported that serum soluble (s)CD14 [10] and plasma tryptophan-kynurenine metabolites [11] were associated with carotid artery atherosclerosis in this population. In the present study, we examined the associations of plasma TMAO and other choline metabolites with risk of incident carotid artery plaque, HIV infection parameters, immune activation and inflammation markers, and traditional CVD risk factors among participants from the WIHS and MACS.
METHODS
Study Participants
The present study included WIHS and MACS participants who underwent carotid artery imaging for plaque assessment at a baseline visit (2004–2006) and at a follow-up visit (2011–2013), as described in our previous study [9]. In 2015, we initiated a metabolomics study to identify plasma metabolites in different pathways associated with cardiometabolic risk among participants without prevalent carotid artery plaques and prevalent diabetes at baseline [11], and 737 women and men were included in the current analysis. All individuals provided informed consent, and the studies were approved by each site’s Institutional Review Board.
Carotid Artery Plaque Ascertainment
Participants underwent high-resolution B-mode carotid artery ultrasound to image 6 locations in the right carotid artery: the near and far walls of the common carotid artery, carotid bifurcation, and internal carotid artery. Focal plaque measures were obtained at a centralized reading center (University of Southern California). We defined a focal plaque as an area with localized intima-media thickness >1.5 mm in any of the 6 imaged carotid artery locations.
Plasma Choline Metabolite Measurement
Choline metabolites were measured, from stored frozen (−80°C) plasma specimens that had been collected at the core visit closest to the baseline carotid artery imaging study visit, using liquid chromatography–tandem mass spectrometry at the Broad Institute Metabolomics Platform (Cambridge, MA). Briefly, metabolites were profiled using Nexera X2 ultra-high performance liquid chromatography (Shimadzu Corp., Marlborough, MA) coupled to an Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Metabolite peaks were identified and confirmed using authentic reference standards and values of metabolites were quantified using area-under-the-curve of the peaks. As there were no standard curves used to quantify metabolites in our untargeted metabolomics method, all metabolites were measured as relative values across study samples without any units. Raw data were processed using TraceFinder software (Thermo Fisher Scientific, Waltham, MA) and Progenesis QI (Nonlinear Dynamics, Newcastle upon Tyne, UK). Detailed methods of plasma metabolite measurement have been described previously [11].
Assessments of HIV Infection and Other Variables
HIV infection was ascertained by enzyme-linked immunosorbent assay (ELISA) and confirmed by western blot. CD4+ T-cell counts were determined by flow cytometry in AIDS Clinical Trials Group-certified laboratories. Quantification of HIV-1 viral load was performed using the isothermal nucleic acid sequence-based amplification method. Detailed information on antiretroviral therapy exposure and virologic suppression status was assessed using a standard method [9]. Hepatitis C virus (HCV) infection status was based on antibody or viral RNA testing. Measurements of serum levels of soluble (s)CD14, sCD163, galectin-3, galectin-3 binding protein, C-reactive protein, and interleukin-6 have been previously described [10].
Statistical Analysis
Baseline characteristics of participants were compared by HIV serostatus in women and men separately. Raw values of choline metabolites were natural-log transformed to approximate a normal distribution before analysis. Linear regression was used to compare metabolite levels by sex and by HIV serostatus, adjusting for age. Poisson regression models with robust variance estimates were used to calculate risk ratios (RRs) and 95% confidence intervals (CIs) of carotid artery plaque per standard deviation (SD) increment in log-transformed metabolites and across tertiles of metabolites in HIV-infected and HIV-uninfected groups separately. We adjusted for age, sex, race/ethnicity, education, study site, current smoking status, HIV serostatus, antiretroviral therapy (ART) use and baseline viral load (in HIV-infected individuals only), body mass index (BMI), systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medication use, and lipid lowering medication use in regression models. We further adjusted for plasma kynurenic acid-to-tryptophan (KYNA/TRP) ratio and immune activation and inflammation markers, which have been associated with progression of carotid artery atherosclerosis in our previous studies [10, 11]. We examined the association between plasma TMAO and carotid artery plaque in HIV-infected individuals stratified by several dichotomous factors, including sex (women, men), age (≤45, >45 years old), BMI (<25, ≥25 kg/m2), CD4+ T-cell count (≤500, >500 cells/mm3), viral load (≤80, >80 copies/mL), ART use (no, yes), and virologic suppression status during follow-up (persistent virologically suppressed, virologically nonsuppressed). Partial Spearman correlations of choline metabolites with CD4+ T-cell counts, viral load, inflammation, and immune activation markers, and conventional CVD risk factors were estimated in HIV-infected participants, adjusted for age and sex. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC) and a 2-sided P value < .05 was considered statistically significant.
RESULTS
Participant Characteristics
There were 520 HIV-infected (291 women, 229 men) and 217 HIV-uninfected (107 women, 110 men) participants included in this study, identical to those in our previous study [11]. HIV-infected and HIV-uninfected participants were generally similar in terms of demographic and behavioral variables, although HIV-infected participants were more likely to have a history of HCV infection, as described in our previous study [11]. Compared to men, women were younger, more likely to be of black race or Hispanic ethnicity and current smokers, and have a history of HCV infection and higher BMI.
All plasma choline metabolites were positively correlated with age (P < .01). Plasma TMAO was weakly correlated with choline (r = 0.15; P < .01) and dimethylglycine (r = 0.10; P = .02) (Supplementary Table 1), lower in the younger group (≤45 years) than in the older group (>45 years) (P < .01), and higher in men than in women (P < .01) (Supplementary Table 2). After adjustment for age and sex, no significant differences in plasma levels of choline metabolites were observed between HIV-infected and HIV-uninfected participants (all P > .05).
TMAO and Risk of Incident Carotid Artery Plaque
Among 520 HIV-infected and 217 HIV-uninfected participants, 90 HIV-infected and 22 HIV-uninfected participants developed carotid artery plaque over a median 7-year follow-up. Among HIV-infected participants, after multivariate adjustment, higher plasma TMAO was significantly associated with an increased risk of incident carotid artery plaque (RR = 1.25 per SD increment; 95% CI, 1.05–1.50; P = .02) (Table 1). We also observed a significant trend of increased risk of carotid artery plaque across tertiles of plasma TMAO (RRs = 1.00, 1.39 [95% CI, 0.83–2.32], and 1.69 [95% CI, 1.03–2.78]; P for trend = .04). The association between plasma TMAO and incident carotid artery plaque was consistent across different subgroups (all P for interaction ≥.05) (Supplementary Table 3). No other significant associations were observed among HIV-infected or HIV-uninfected individuals (all P ≥ .05).
Table 1.
Associations of Plasma Choline Metabolites With Risk of Incident Carotid Artery Plaque in HIV-Infected and HIV-Uninfected Individuals
| HIV-Infected Participants | HIV-Uninfected Participants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertiles of Metabolites | Continuous (per SD) | Tertiles of Metabolites | Continuous (per SD) | |||||||||
| T1 | T2 | T3 | P trend | P | T1 | T2 | T3 | P trend | P | |||
| TMAO | ||||||||||||
| N, cases/total | 22/173 | 30/174 | 38/173 | 90/520 | 7/71 | 6/73 | 9/73 | 22/217 | ||||
| Model 1 | 1.0 (Ref) | 1.33 (0.81–2.19) | 1.61 (1.00–2.57) | .04 | 1.21 (1.01–1.45) | .04 | 1.0 (Ref) | 0.67 (0.22–1.99) | 1.04 (0.38–2.84) | .88 | 1.05 (0.66–1.67) | .85 |
| Model 2 | 1.0 (Ref) | 1.45 (0.87–2.41) | 1.67 (1.01–2.75) | .04 | 1.22 (1.03–1.45) | .02 | 1.0 (Ref) | 0.77 (0.23–2.52) | 0.94 (0.35–2.52) | .94 | 0.93 (0.59–1.47) | .77 |
| Model 3 | 1.0 (Ref) | 1.39 (0.83–2.32) | 1.69 (1.03–2.78) | .04 | 1.25 (1.05–1.50) | .01 | 1.0 (Ref) | 0.98 (0.32–3.02) | 0.64 (0.18–2.26) | .46 | 0.87 (0.52–1.47) | .62 |
| Choline | ||||||||||||
| N, cases/total | 24/173 | 29/174 | 37/173 | 90/520 | 7/71 | 6/73 | 9/73 | 22/217 | ||||
| Model 1 | 1.0 (Ref) | 1.22 (0.74–2.01) | 1.44 (0.88–2.37) | .15 | 1.32 (0.97–1.79) | .08 | 1.0 (Ref) | 0.84 (0.28–2.56) | 1.17 (0.46–3.02) | .72 | 0.91 (0.55–1.50) | .70 |
| Model 2 | 1.0 (Ref) | 1.13 (0.69–1.87) | 1.50 (0.92–2.47) | .10 | 1.31 (0.97–1.77) | .08 | 1.0 (Ref) | 1.05 (0.38–2.94) | 1.22 (0.50–2.98) | .66 | 0.91 (0.58–1.42) | .67 |
| Model 3 | 1.0 (Ref) | 1.17 (0.70–1.96) | 1.52 (0.92–2.52) | .10 | 1.32 (0.97–1.80) | .08 | 1.0 (Ref) | 1.23 (0.43–3.50) | 1.10 (0.43–2.79) | .86 | 0.98 (0.62–1.54) | .92 |
| Betaine | ||||||||||||
| N, cases/total | 26/173 | 29/174 | 35/173 | 90/520 | 7/71 | 5/73 | 10/73 | |||||
| Model 1 | 1.0 (Ref) | 1.06 (0.66–1.71) | 1.18 (0.76–1.85) | .45 | 1.03 (0.82–1.3) | .80 | 1.0 (Ref) | 0.68 (0.23–2.02) | 1.63 (0.62–4.25) | .36 | 1.33 (0.75–2.36) | .34 |
| Model 2 | 1.0 (Ref) | 1.25 (0.76–2.06) | 1.27 (0.80–2.02) | .30 | 1.02 (0.80–1.30) | .85 | 1.0 (Ref) | 0.64 (0.22–1.80) | 1.80 (0.73–4.46) | .27 | 1.35 (0.82–2.23) | .24 |
| Model 3 | 1.0 (Ref) | 1.11 (0.66–1.85) | 1.27 (0.81–1.97) | .29 | 1.02 (0.80–1.30) | .87 | 1.0 (Ref) | 1.13 (0.36–3.58) | 2.41 (0.90–6.46) | .08 | 1.36 (0.81–2.29) | .25 |
| Dimethylglycine | ||||||||||||
| N, cases/total | 23/173 | 27/174 | 40/173 | 90/520 | 5/71 | 4/73 | 13/73 | 22/217 | ||||
| Model 1 | 1.0 (Ref) | 1.12 (0.67–1.87) | 1.46 (0.90–2.37) | .12 | 1.11 (0.93–1.33) | .23 | 1.0 (Ref) | 0.81 (0.23–2.81) | 2.74 (1.01–7.47) | .05 | 1.33 (0.89–1.99) | .16 |
| Model 2 | 1.0 (Ref) | 1.06 (0.61–1.82) | 1.55 (0.94–2.56) | .08 | 1.12 (0.93–1.35) | .21 | 1.0 (Ref) | 0.75 (0.21–2.66) | 3.05 (1.02–9.10) | .06 | 1.37 (0.89–2.10) | .15 |
| Model 3 | 1.0 (Ref) | 1.12 (0.66–1.91) | 1.55 (0.94–2.57) | .09 | 1.12 (0.93–1.36) | .23 | 1.0 (Ref) | 0.64 (0.17–2.38) | 2.42 (0.84–6.94) | .13 | 1.36 (0.89–2.09) | .16 |
| Sarcosine | ||||||||||||
| N, cases/total | 24/173 | 36/174 | 30/173 | 90/520 | 7/71 | 7/73 | 8/73 | 22/217 | ||||
| Model 1 | 1.0 (Ref) | 1.40 (0.88–2.23) | 1.28 (0.78–2.08) | .34 | 1.19 (0.92–1.53) | .19 | 1.0 (Ref) | 0.93 (0.32–2.66) | 1.25 (0.50–3.15) | .65 | 1.21 (0.80–1.83) | .36 |
| Model 2 | 1.0 (Ref) | 1.37 (0.87–2.17) | 1.21 (0.73–2.01) | .49 | 1.15 (0.88–1.49) | .30 | 1.0 (Ref) | 0.98 (0.32–3.02) | 1.06 (0.41–2.72) | .91 | 1.21 (0.82–1.78) | .33 |
| Model 3 | 1.0 (Ref) | 1.53 (0.94–2.51) | 1.21 (0.70–2.09) | .58 | 1.14 (0.88–1.49) | .31 | 1.0 (Ref) | 0.62 (0.19–2.01) | 0.99 (0.42–2.29) | .94 | 1.21 (0.84–1.74) | .31 |
Data are risk ratios (95% confidence intervals) of incident carotid artery plaque per SD increment of metabolites or across tertiles of metabolites, adjusted for age, sex, race/ethnicity, education, study site, current smoking, and history of hepatitis C virus (Model 1); and further adjusted for human immunodeficiency virus (HIV) serostatus, antiretroviral therapy use, and baseline viral load level, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medication use, lipid lowering medication use, and body mass index (Model 2); and further adjusted for plasma kynurenic acid-to-tryptophan ratio (Model 3).
Abbreviation: TMAO, trimethylamine-N-oxide.
TMAO, Immune Activation and Inflammation Markers, and CVD-Related Factors
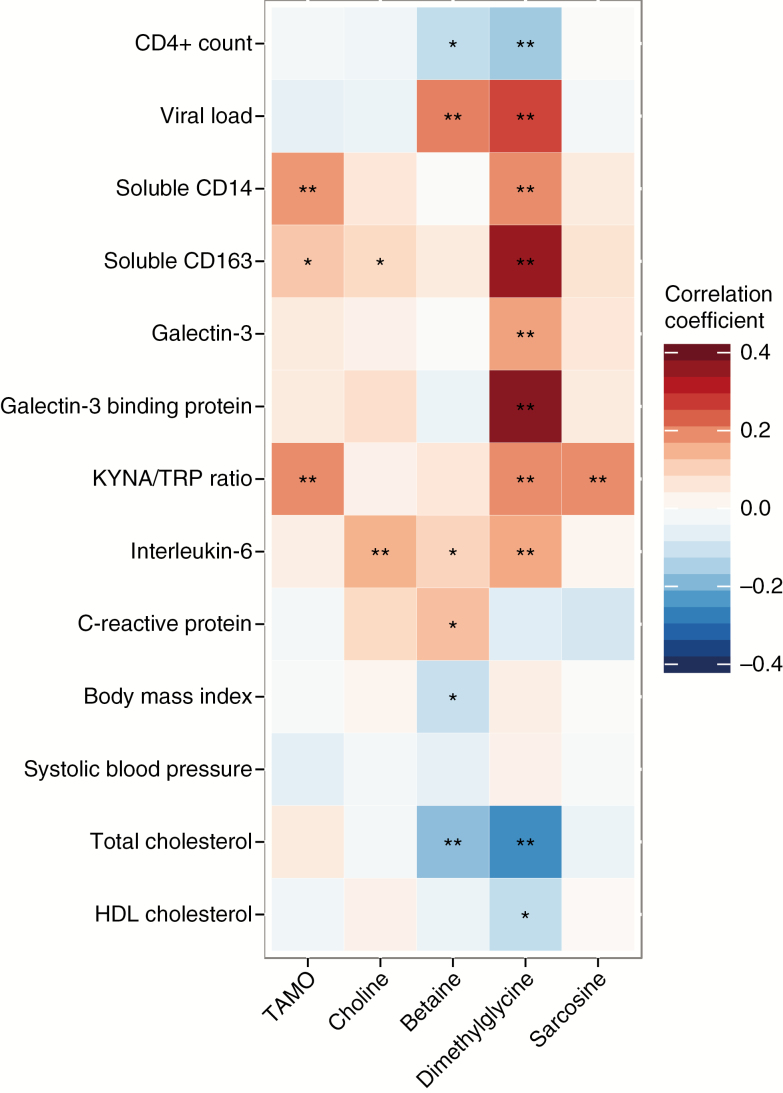
After adjustment for age and sex, plasma TMAO was significantly positively correlated with plasma KYNA/TRP ratio (r = 0.19, P < .01), serum sCD14 (r = 0.18, P < .01), and sCD163 (r = 0.12, P = .01), but not with HIV infection parameters, traditional CVD risk factors, or global inflammation markers (Figure 1).
Figure 1.
Spearmen correlation of plasma choline metabolites with human immunodeficiency virus (HIV) infection parameter, inflammation and immune activation markers, and cardiovascular disease risk factors in HIV-infected participants. Data are partial Spearmen correlation coefficients (r), adjusted for age and sex. *P < .05; **P < .01. Abbreviations: HDL, high-density lipoprotein; KYNA/TRP, kynurenic acid-to-tryptophan ratio; TMAO, trimethylamine-N-oxide.
In a subset of HIV-infected participants with available data on serum sCD14 and sCD163 (505 HIV-infected participants with 85 incident cases), plasma TMAO was significantly associated with risk of carotid artery plaque (RR = 1.27 per SD increment; 95% CI, 1.05–1.53; P = .01) after multivariate adjustment. After further adjustment for sCD14 and sCD163, the association was attenuated (RR = 1.18 per SD increment; 95% CI, 0.98–1.42; P = .08) (Supplementary Table 4).
DISCUSSION
In 2 prospective HIV cohorts, we found that higher levels of plasma TMAO were associated with increased risk of carotid artery plaque in HIV-infected individuals. The association pattern was consistent between HIV-infected women and men, and across different subgroups stratified by HIV infection-related parameters. However, we did not observe significant association between TMAO and carotid artery plaque in HIV-uninfected participants.
Our results were partially consistent with a previous cross-sectional analysis of 100 men from the MACS, which reported an inverted U-shaped association between TMAO and coronary artery stenosis (higher odds of stenosis in the third quartile, but not in the fourth quartile, compared to the first quartile of TMAO) among HIV-infected men, but not those without HIV infection [8]. In another cross-sectional study, Srinivasa et al reported a link between serum TMA (precursor to TMAO), but not TMAO, and coronary plaque burden in a symptomatic HIV-infected population [7]. Additionally, plasma TMAO was elevated in symptomatic HIV-infected persons with myocardial perfusion defects but was not associated with coronary artery calcium score, intima media thickness, or first-time myocardial infarction [6]. To the best of our knowledge, this is the first study to report a significant association between plasma TMAO and progression of carotid artery atherosclerosis among individuals with chronic HIV infection in a prospective manner. In addition to plasma TMAO, we also examined other choline metabolites, but we did not find clear evidence linking these metabolites with risk of subclinical atherosclerosis. The results, especially in HIV-uninfected individuals, should be interpreted with caution, and further studies are warranted due to the limited sample size.
Data from this study support an important role of gut microbiota-related TMAO, rather than other choline metabolites, in atherosclerosis among HIV-infected individuals, but the underlying mechanisms remain unclear. Gut microbiota alteration has been observed during HIV infection [12], but no significant difference was found in plasma TMAO levels between HIV-infected and uninfected individuals in this study. It remains unknown whether HIV-related gut microbiota alteration may influence plasma TMAO levels and subsequently contribute to increases risk of atherosclerosis and CVD. Interestingly, we found that plasma TMAO was positively correlated with serum sCD14 and sCD163 levels, which are biomarkers of monocyte activation and macrophage inflammation [13] and have been reported to be associated with carotid artery atherosclerosis and CVD risk in HIV-infected individuals [10, 14]. The association between plasma TMAO and progression of carotid artery atherosclerosis was attenuated after further adjustment for serum sCD14 and sCD163 levels. This is in line with one suggested pathway that links TMAO and atherosclerosis through macrophage inflammation [3]. However, it should be noted that the observed correlations of TMAO with these 2 biomarkers were very modest, and other potential pathways need further investigation.
The strengths of this study include 2 well-characterized HIV cohorts with similar prospective study designs, identical metabolomics assays for TMAO and other choline metabolites, and a uniform imaging protocol on longitudinal measurements of carotid artery atherosclerosis. Our results should be interpreted in the context of several limitations. Firstly, we did not have information regarding clinical CVD events, though our subclinical measure of carotid artery atherosclerosis has been validated as an important predictor of clinical CVD events [15]. Secondly, although our results are highly consistent in HIV-infected women and men, differences with respect to age and some sociodemographic characteristics between the 2 cohorts limited our ability to fully test for sex differences. Replication of our findings in other HIV cohorts would provide further validation. Thirdly, our untargeted metabolomics method can only measure relative values of plasma TMAO levels across study samples without a unit, and future studies with targeted measurement data are warranted to determine normal physiological ranges of circulating TMAO levels. Finally, we did not have information regarding dietary choline intake, which could directly influence plasma levels of choline metabolites.
In conclusion, our study found that higher plasma levels of TMAO were associated with greater progression of carotid artery atherosclerosis in HIV-infected individuals. Further studies are needed to clarify underlying mechanisms and examine whether interventions targeting gut microbiota and TMAO may help prevent atherosclerosis and CVD in HIV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Z. S., R. C. K. and Q. Q. designed the research; S. H., C. B. C., J. M. S., D. B. H., R. D. B., S. A. H., S. J. S., J. B. M., C. L. S., W. S. P., A. L. L., J. M. L., H. N. H., K. A., R. C. K., and Q. Q. contributed to the data collection and edited the manuscript; Z. S. and S. H. analyzed the data; Z. S. wrote the manuscript. Q. Q. was the guarantor of this work, had full access to all of the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis; and all authors read and approved the final manuscript.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Heart, Lung, and Blood Institute (grant numbers K01HL129892 and R01HL140976 to Q. Q.; R01 HL126543, R01 HL132794, R01HL083760, R01HL095140 to R. C. K.; R01HL095129 to W. S. P.; and K01HL137557 to D. B. H.); and Feldstein Medical Foundation research grant to Q. Q.
The WIHS is supported by the National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute (NCI), the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by University of Southern California Clinical and Translational Science Award (grant number UL1-TR000004), Atlanta Clinical and Translational Science Award (grant number UL1-TR000454), and University of North Carolina Center for AIDS Research (grant number P30-AI-050410). The MACS is supported by the NIAID and NCI. MACS data collection is also supported by Johns Hopkins University Clinical and Translational Science Award (grant number UL1-TR000424).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Study participants and NIH grants. WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (IghovwerhaOfotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I–WIHS IV). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick, Todd Brown), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Amber d’Souza), UM1-AI35043.
References
- 1. Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009; 67:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roe AJ, Zhang S, Bhadelia RA, et al. Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am J Clin Nutr 2017; 105:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 6. Haissman JM, Knudsen A, Hoel H, et al. Microbiota-dependent marker TMAO is elevated in silent ischemia but is not associated with first-time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr 2016; 71:130–6. [DOI] [PubMed] [Google Scholar]
- 7. Srinivasa S, Fitch KV, Lo J, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS 2015; 29:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller PE, Haberlen SA, Brown TT, et al. Brief report: intestinal microbiota-produced trimethylamine-N-oxide and its association with coronary stenosis and HIV serostatus. J Acquir Immune Defic Syndr 2016; 72:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanna DB, Lin J, Post WS, et al. Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis 2017; 215:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qi Q, Hua S, Clish CB, et al. Plasma tryptophan-kynurenine metabolites are altered in HIV infection and associated with progression of carotid artery atherosclerosis [published online ahead of print 3 February, 2018]. Clin Infect Dis doi: 10.1093/cid/ciy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes 2014; 5:562–70. [DOI] [PubMed] [Google Scholar]
- 13. Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128:262–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.