Abstract
Varicella-zoster virus (VZV) causes clinically significant illness during acute and recurrent infection accompanied by robust innate and acquired immune responses. Innate immune cells in skin and ganglion secrete type I interferon (IFN-I) and proinflammatory cytokines to control VZV. Varicella-zoster virus subverts pattern recognition receptor sensing to modulate antigen presentation and IFN-I production. During primary infection, VZV hijacks T cells to disseminate to the skin and establishes latency in ganglia. Durable T- and B-cell memory formed within a few weeks of infection is boosted by reactivation or re-exposure. Antigen-specific T cells are recruited and potentially retained in VZV-infected skin to counteract reactivation. In latently VZV-infected ganglia, however, virus-specific T cells have not been recovered, suggesting that local innate immune responses control VZV latency. Antibodies prevent primary VZV infection, whereas T cells are fundamental to resolving disease, limiting severity, and preventing reactivation. In this study, we review current knowledge on the interactions between VZV and the human immune system.
Keywords: adaptive immunity, chickenpox, innate immunity, shingles, varicella-zoster virus
Varicella-zoster virus (VZV) is a human alphaherpesvirus that infects >90% of people worldwide. Primary VZV infection, typically acquired during early childhood, leads to varicella (chickenpox) and establishment of a lifelong latent infection in neurons of trigeminal ganglia (TG) and dorsal root ganglia (DRG). Later in life, VZV reactivates in approximately one third of infected individuals to cause herpes zoster ([HZ] shingles) [1]. Whereas primary VZV infection is generally benign, HZ is frequently accompanied by postherpetic neuralgia (PHN), and it is an important cause of uveitis and vasculitic stroke [2, 3]. Both the highly cell-associated nature of VZV and the strict virus species barrier has hampered studies on VZV pathogenesis. Consequently, the immunobiology of VZV infection is mainly studied in human clinical specimens, experimental VZV infection of human fetal skin and DRG xenografts transplanted in severe combined immunodeficient (SCID-hu) mice [4], and the simian varicella virus (SVV) nonhuman primate model for VZV infection [5]. Host innate and adaptive immune responses are essential to limit disease severity and to prevent VZV reactivation. Vaccines based on the live-attenuated VZV strain vOka, and recently the VZV glycoprotein E (gE)-based subunit vaccine (HZ/su), provide variable protection against VZV disease by harnessing VZV-specific immunity [6–8]. During long-term cospeciation, VZV has evolved to dodge immunity and utilize lymphocytes for intrahost dissemination [4, 9]. In this review, we will summarize and discuss current understanding of the immunobiology of VZV infection.
T CELLS DISSEMINATE VARICELLA-ZOSTER VIRUS DURING PRIMARY INFECTION
During primary infection, VZV replicates in respiratory epithelial cells and is transferred to T cells within the tonsillar lymphoid tissue [10, 11], either directly or via virus-infected dendritic cells (DCs) [12]. Varicella-zoster virus infection reconfigures the T cells to become activated memory T cells with enhanced skin-homing capacity and reduced immune functions [13]. Consequently, VZV-infected T cells transport the virus to skin and possibly ganglia during primary infection [14, 15]. Detection of VZV-infected T cells in blood during acute infection, and observations in the SVV model support the role of T cells for intrahost dissemination of the virus [5, 16, 17]. Although HZ patients may develop a low viremia [18], the analogous role of T cells in VZV reactivation has not been described.
INNATE IMMUNE RESPONSE TO VARICELLA-ZOSTER VIRUS INFECTION
Varicella-zoster virus is sensed by the innate immune system via pattern recognition receptors that recognize viral pathogen-associated molecular patterns. Cell surface Toll-like receptors (TLRs) 2 and TLR1/2 recognize VZV particles and the viral dUTPase, and viral double-stranded deoxyribonucleic acid (dsDNA) is recognized by endosomal TLR9. Varicella-zoster virus is sensed via unknown mechanisms by nucleotide-binding oligomerization domain-like receptor NLRP3 [19–22]. By analogy to related herpesviruses, both viral double-stranded ribonucleic acid (dsRNA) intermediates and single-stranded RNA with a 5’-triphosphate group are most likely detected by the cytosolic RNA receptors melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene (RIG-I), whereas viral dsDNA is recognized by the DNA receptors such as RNA polymerase III and cyclic GMP-AMP synthase [23, 24]. These interactions activate transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB), interferon (IFN) regulatory factor 3 (IRF3), and IRF7 to induce the expression of type I IFNs ([IFN-I] IFN-α and IFN-β) and proinflammatory cytokines that inhibit viral replication and recruit inflammatory cells to the site of infection [23].
Innate Immune Cells
The innate immune cells comprise mast cells, granulocytes, monocytes/macrophages, DCs, and the more recently identified innate lymphoid cells ([ILCs] that include natural killer [NK] cells) and “innate-like” T cells such as invariant NKT (iNKT) cells, MAIT cells, and γδ T cells [25, 26]. The contribution of innate immune cells to VZV pathogenesis is ill-defined. Cultured monocytes and monocyte-derived macrophages produce proinflammatory cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) in response to VZV infection [20]. Plasmacytoid DCs (pDCs) produce abundant IFN-α in response to VZV-infected peripheral blood mononuclear cells (PBMCs) [21]. Natural killer cells restrict VZV replication by secreting antiviral molecules (IFN-γ and granulysin) or by direct cytolysis [27, 28]. Genetic defects that impair NK immunity are associated with more severe varicella [29], although not all individuals with NK cell deficiencies will develop severe VZV disease, implicating variable host and possible virus genetic involvement. Likewise, disease severity of primary VZV infection is increased in individuals with compromised iNKT immune responses [30]. More recently, long-lived NK cells with immunological memory have been identified that contribute to antiherpesvirus immunity [31]. Overall, recent discoveries of novel innate immune cells and memory NK cells warrant future studies on their role in VZV pathogenesis.
Innate Immune Response in Skin
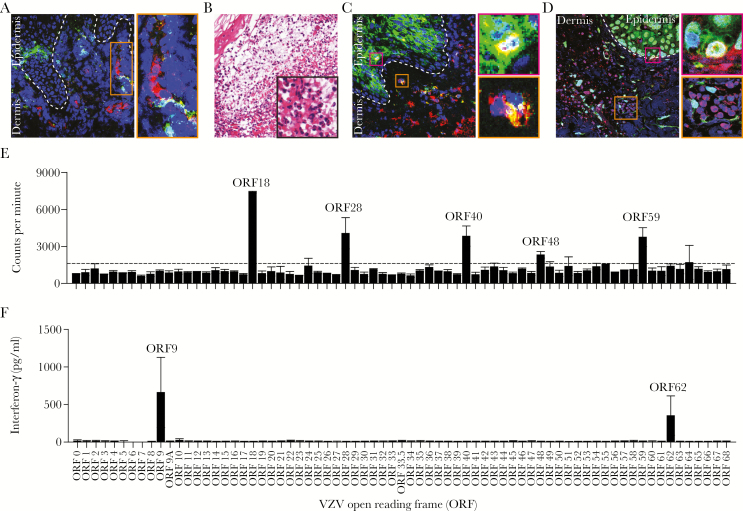
Upon arrival in skin, VZV needs to counteract local innate immune responses to produce the characteristic vesicular skin rash. Interferon-α production by epidermal epithelial cells and high numbers of infiltrating pDCs restrict VZV replication [14, 32] (Figure 1A). In contrast to pDCs, a dramatic loss of Langerhans cells is observed in the epidermis of both varicella and HZ biopsies, most likely due to their efflux to regional lymph nodes [32]. Leukocyte infiltrates in early varicella and HZ skin lesions contain mainly epidermal neutrophils and dermal perivascular and perineural macrophages [33, 34], followed by the influx of a low number of NK cells (Figure 1B–D) [33]. Although the SCID-hu mouse model provided essential insights into VZV skin pathogenesis [4], the model’s chimeric make-up precludes detailed analyses on the role of innate immune cells in controlling VZV infection in human skin. Future studies are warranted to address this issue by combining bone marrow, liver, and thymus (BLT)-humanized mice with VZV replication in human fetal skin xenografts [35] or by using the SVV model [5, 36].
Figure 1.
Immunopathology of herpes zoster lesions and systemic virus-specific T-cell immune responses in latently varicella-zoster virus (VZV)-infected adults. (A–D) Sections of formalin-fixed and paraffin-embedded skin biopsy obtained from a herpes zoster patient were analyzed by immunofluorescence ([IF] A, C, and D) or stained with hematoxylin and eosin (B). (A) Dual IF staining for VZV glycoprotein E ([gE] green) and CD123 (plasmacytoid dendritic cell [pDC]; red) reveals dermal pDC adjacent to VZV-infected cells. Inset: magnification of indicated area. (B) Hematoxylin and eosin staining showing polymorphonuclear cell infiltrates in a zoster vesicle. Magnification: ×100; inset: ×400. (C) Dual IF staining for VZV gE (green) and CD68 (macrophages; red) showing high numbers of dermal macrophages adjacent to VZV-infected epidermis. Insets: magnification of the indicated areas showing VZV gE-positive epidermal (pink inset) and dermal (orange inset) macrophages. (D) Dual IF staining for VZV open reading frame (ORF)63 (green) and CD3 (T cells; red) showing dermal T-cell infiltrates in proximity to VZV-infected cells. Pink inset: magnification showing VZV ORF63-positive T cell in epidermis. Orange inset: magnification showing dermal T cells adjacent to VZV ORF63-positive cells. (A, C, and D) Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (blue), and dashed lines indicate epidermal—dermal junction and images are shown at ×200 magnification. (E–F) Bulk in vitro VZV-enriched CD4 (E) and CD8 (F) T-cell lines (TCLs) obtained from different representative latently VZV-infected healthy adults were assayed for reactivity to proteins encoded by individual VZV ORFs (indicated on the x-axis). (E) CD4 T-cell reactivity was determined using [3H]-thymidine incorporation, as described previously (Supplementary Data Ref. 77). Bars and error bars indicate mean ± standard deviation of duplicate measurements. Dashed horizontal line indicates threshold for positivity [(median of all negative control antigens) + (2.33 times median absolute deviation of negative control antigens)]. Both duplicates needed to exceed the threshold for the ORF to be determined as positive. (F) Interferon-γ secretion levels determined by enzyme-linked immunosorbent assay in conditioned medium of CD8 TCL cocultured with Cos-7 cells cotransfected with the responsive human leukocyte antigen (HLA)-I allele (ie, HLA-B*0802) and the individual VZV ORFs, as described previously (Supplementary Data Ref. 76). The VZV ORFs specifically recognized by T cells in the individuals analyzed are indicated.
Innate Immune Response in Ganglia
Both neurons and nonneuronal cells contribute to anti-VZV innate immune responses in sensory ganglia. Varicella-zoster virus infection of fetal DRG in SCID-hu mice produces transient virus replication, followed by persistent lower levels of viral DNA and limited viral transcription, suggesting that local innate immune responses control VZV replication in ganglia [15]. Alternatively, the differential susceptibility of neuronal subtypes to productive VZV infection, and subsequent depletion of susceptible neurons and retention of quiescently infected neurons, may be involved [37]. Although sensory neurons produce little IFN-I upon alphaherpesvirus infection, exposure to IFN-I at the nerve endings or ganglion provides local protection [38, 39]. The IFN-I induction in VZV-infected human DRG and upregulation of IFN-I-stimulated genes in acutely SVV-infected monkey ganglia support the latter option [37, 40, 41]. Neuron-interacting satellite glial cells (SGCs) support neuron functionality, but they are also involved in local immune responses [42, 43]. The SGCs upregulate major histocompatibility complex type I (MHC-I) and MHC-II and produce TNF-α and IL-6 in response to herpesvirus infection [44–46]. In addition, macrophages and NK cells infiltrate ganglia after HZ [46]. Overall, these findings suggest that both resident and infiltrating cells contribute to innate immunity to VZV in ganglia. Although newly developed in vitro models may identify intrinsic neuronal antiviral responses [47, 48], detailed analyses on the role of innate immune cells, particularly SGCs, in controlling VZV infection in ganglia are warranted.
ADAPTIVE IMMUNITY TO VARICELLA-ZOSTER VIRUS INFECTION
Functional roles for adaptive immunity are proven by the resistance to secondary varicella, the efficacy of preventative and therapeutic VZV vaccines, and the severity of HZ in CD4-lymphopenic persons [49]. Low VZV-specific CD4 T-cell responses, but not antibodies, correlate with more severe HZ and PHN [50] (Supplementary Data Ref. 51). CD8 T-cell depletion may also be associated with increased VZV reactivation (Supplementary Data Ref. 52). Age-related decrease in VZV-specific T-cell frequencies in blood and increased levels of regulatory T cells (Tregs) are also associated with increased risk of HZ (Supplementary Data Ref. 53). In this section, we review data on systemic and local VZV-specific B- and T-cell immunity during primary infection, latency, and exogenous or endogenous VZV antigen re-exposure.
Humoral Immunity
Antibodies are involved in controlling both primary VZV infection and reactivation. Passively transferred varicella zoster immunoglobulin (Ig) ameliorates or prevents primary varicella if given to seronegative individuals (Supplementary Data Ref. 54). Similarly, transplacental IgG protects infants against primary infection (Supplementary Data Ref. 55). Functional activities include antibody-dependent cell-mediated cytotoxicity (ADCC), neutralization of cell-free virus, and inhibition of cell-to-cell spread (Supplementary Data Ref. 56). It is interesting to note that antibody immunodeficiency (eg, agammaglobulinemia) is not associated with severe primary varicella (Supplementary Data Ref. 57), which indicates that innate immunity and/or acquired cellular immunity is sufficient to prevent prolonged or disseminate primary varicella in these patients. Anti-VZV Ig appears 1 week after varicella rash and peaks at 1 month. Prompt antibody responses do not correspond with varicella severity (Supplementary Data Ref. 58). Whereas VZV-specific IgM and IgA wane after a few months, VZV-specific IgG levels have a functional half-life of 50 years in the absence of boosting, implying the presence of long-lived plasma cells(Supplementary Data Ref. 59). Immunoglobulin G boosts observed in the absence of HZ are attributed to subclinical VZV reactivation or re-exposure to VZV (Supplementary Data Ref. 59). Serum VZV-specific antibodies and memory B-cell numbers do not correlate (Supplementary Data Ref. 59), reflecting the bone marrow residency of plasma cells. Varicella-zoster virus IgG titers at 3 weeks post-HZ are higher in subjects with severe HZ or PHN, potentially due to a higher antigen load [50]. Varicella-zoster virus-specific IgA and IgM titers are rarely increased after HZ (Supplementary Data Ref. 60). However, this may be an underestimation given the anticipated narrow time frame of production after VZV reactivation, which warrants future studies on their expression, specificity, and potential contribution in modulating HZ.
Sera from VZV-seropositive persons react to approximately one third of the VZV proteome, including all viral glycoproteins, and various tegument proteins, capsid proteins, and nonstructural proteins (Supplementary Data Ref. 61). Antibodies from varicella or HZ patients preferentially bind to specific regions of VZV proteins involved in cell entry: gB (ORF31) and gE (ORF68). The latter is used for the candidate HZ/su subunit vaccine discussed at length elsewhere in this issue. Human monoclonal antibodies against several conformational epitopes on gH (ORF37, also important for cell entry), gB, or gE neutralize VZV (Supplementary Data Refs. 62 and 63). Varicella-zoster virus-specific IgG can cross-react between herpes simplex virus (HSV) (Supplementary Data Ref. 64) with unknown clinical significance. Although VZV-specific CD4 T cells with a T follicular helper phenotype have not been formally demonstrated, they are of interest in determining (1) the mechanism of anti-VZV IgG persistence and (2) the unique potency of the HZ/su vaccine.
CD4 T-Cell Response
The majority of T-cell studies concern CD4 T cells. Early studies predated knowledge of T-cell subsets, but they used techniques now understood to reflect CD4 responses, and contemporary studies mostly continue the CD4 T-cell focus. The SVV model highlights functional roles for CD4 T cells. Depletion of CD4 T cells results in higher viral loads, prolonged viremia, and more severe varicella than CD8 T-cell depletion, but it does not increase latent viral loads after recovery. Given the helper functions of CD4 T cells, depletion is accompanied with delayed and reduced VZV-specific antibody and CD8 T-cell responses (Supplementary Data Ref. 65). Therefore, it is difficult to definitively isolate the functional role of CD4 T cells in the experimental SVV model.
Varicella-zoster virus-specific CD4 and CD8 T cells are detectable before seroconversion, starting approximately 3 days after varicella onset (Supplementary Data Refs. 58 and 66). Early, high magnitude T-cell responses correlate with mild varicella (Supplementary Data Ref. 66), whereas T-cell immunodeficiency leads to severe varicella (Supplementary Data Ref. 58). Circulating VZV-specific CD4 T cells with an activated phenotype express the skin-homing receptor cutaneous leucocyte-associated antigen (CLA) early during varicella (Supplementary Data Ref. 66), in accordance with functional trafficking of effector T cells to clear VZV from the skin, and separate from the role of CLA in virus dissemination [13]. These cells peak in temporal association with viral clearance (Supplementary Data Ref. 67).
Studies of systemic memory T-cell responses in latently infected individuals reveal antigen-specific Th1 cells (expressing IFN-γ, IL-2, and TNF-α), cytotoxic T-lymphocytes within both CD4 and CD8 subsets, and cells with a skin-homing (CLAhiCXCR3pos) phenotype (Supplementary Data Refs. 68–70). The skin-homing ability of VZV-specific T cells measured by intradermal challenge with VZV antigen is demonstrated by the enrichment of VZV-specific T cells at levels of up to 5% of total local CD4 T cells (Supplementary Data Ref. 71). Th1 cells predominated the skin infiltrate, with 8%–15% having a Treg phenotype. Postvaricella circulating VZV-specific CD4 T-cell memory is long-lived and detectable at typical levels of ~0.1% CD4 T cells decades after primary infection. Boosting via re-exposure or subclinical reactivation may occur (Supplementary Data Ref. 72).
During latency, VZV transcription in ganglia is limited to ORF63 and no viral proteins are detectable (Supplementary Data Refs. 73 and 74). Little is known concerning the CD4 T-cell response in latently infected ganglia. No VZV-specific T cells have been identified in latently infected human TG collected at autopsy, but, because only a few VZV proteins were assayed, full VZV ORFeome T-cell screening is warranted (Supplementary Data Ref. 75). In contrast, latently HSV-1-infected human TG contain lymphocytic infiltrates and virus-specific CD4 and CD8 T cells (Supplementary Data Ref. 76). It is presently unclear whether the mechanism of HZ vaccines includes T-cell activity in latent ganglia.
With reactivation, CD4 T cells participate in inflammation and resolution. Blood VZV-specific CD4 T cells elevate ~4-fold above baseline at approximately 2 weeks post-HZ, before declining at 3–6 weeks to modestly elevated levels that persist for many years (Supplementary Data Ref. 77). During HZ, VZV-specific CD4 T cells transiently adopt a terminal effector phenotype, deficient TNF-α, and IL-2 secretion but maintain IFN-γ (Supplementary Data Ref. 78) and gain high levels of T-cell inhibitory markers CTLA-4 and PD-1. After resolution, these T cells revert to a pre-HZ phenotype (Supplementary Data Ref. 78).
T cells are attracted to skin and ganglia during acute HZ (Supplementary Data Ref. 75). Keratinocytes that border VZV-infected cells in HZ skin lesions upregulate MHC-II, permitting activation of VZV-specific CD4 T cells [33]. T-cell cytokine profiles of HZ blister fluid reflect transient increases in IL-10 and IL-4 that potentially reflect Th2 and/or Treg cell involvement (Supplementary Data Ref. 79). It is less clear how CD4 T cells could act in ganglia, where neurons have limited MHC expression. CD4 T cells do infiltrate ganglia during HZ, and SGC are known to be competent for antigen presentation to CD4 T cells [43]. The eye is an additional anatomic site with clinical significance of VZV reactivation. Varicella-zoster virus-specific CD4 T cells are detectable in ocular fluids of patients with VZV uveitis. These T cells have Th1/Th0 cytokine pattern and can lyse VZV-infected retinal pigment epithelial cells, suggesting their role in a detrimental local immune response (Supplementary Data Ref. 80).
Early studies on VZV-specific CD4 T-cell specificity tested neutralizing antibody targets (above) and ORFs 62 and 63 proteins. More recently, genome-wide studies using blood sampled decades after childhood varicella detected memory CD4 T cells specific for 28 of 70 unique VZV proteins (Figure 1E) (Supplementary Data Ref. 81). Population-prevalent antigens included both glycoproteins (ORFs 31, 67, and 68) and other viral antigens (ORFs 4, 62, 63, 9, 18, and 40). Like antibodies, CD4 T cells can cross-recognize VZV and HSV (Supplementary Data Refs. 82 and 83). Most cross-reactive T-cell epitopes display 1–3 amino acid sequence variation between viruses, which is tolerated by MHC and T-cell receptor binding (Supplementary Data Ref. 82). Although there are no studies of the fine specificity of CD4 T cells in varicella or HZ lesions, ORF63-specific T cells infiltrate skin sites of VZV antigen challenge (Supplementary Data Ref. 71). The fine specificity of VZV-specific CD4 T cells from ocular and blood specimens overlaps (Supplementary Data Refs. 82 and 83).
CD8 T-Cell Response
CD8 T cells potentially recognize a wider variety of VZV-infected cells due to their restriction by the broadly expressed MHC-I and are typically equipped with cytolytic machinery. Limited studies have addressed VZV-specific CD8 T-cell reactivity using appropriate antigen-presenting cell (APC) platforms. One study, using autologous VZV-infected monocyte-derived DCs as APCs, showed that the frequency of both VZV-specific CD4 and CD8 effector memory T cells was inversely correlated with age, whereas the naive and central memory T-cell subsets were not (Supplementary Data Ref. 84). Studies of the dynamics and significance of VZV-specific CD8 T-cell responses during varicella, latency, and reactivation lag behind CD4 T-cell studies. Limited information about circulating VZV-specific CD8 T cells comes from tetramer stains of blood that unfortunately identify cells that cross-recognize an epitope in Epstein-Barr virus, HSV, and VZV (Supplementary Data Ref. 85). In some individuals, these cells have a central memory phenotype including cytokine polyfunctionality and low cytolytic machinery (Supplementary Data Ref. 85). In others, the cells are terminal effector-like, expressing granzyme B, perforin, and inhibitory receptors and have limited cytokine function (Supplementary Data Ref. 85). As yet, “clean” tetramer studies of noncross-reactive VZV-specific CD8 T cells have not been reported.
Similarly, little is known concerning CD8 T-cell dynamics or tissue localization during HZ. Boosting is suggested in allogeneic stem cell transplant studies because the frequencies of tetramer-positive CD8 T cells, and IFN-γ responses in PBMCs, increase during HZ (Supplementary Data Ref. 86). A boost in ORF9-specific CD8 T cells in response to the live-attenuated zoster vaccine is observed in healthy VZV-experienced individuals (Supplementary Data Ref. 87). Antigenic stimulation is presumably stronger during HZ, but it is not yet detailed at the T-cell epitope level. CD8 T cells can be detected histologically in HZ skin lesions (Supplementary Data Ref. 88). Autopsies also confirm cytotoxic CD8 T cells in necrotic ganglia of HZ patients [46]. There is no formal demonstration that these cells are VZV-specific.
Early CD8 T-cell fine specificity surveys focused on selected VZV proteins. ORF62 and ORF9 are CD8 T-cell targets, albeit their detection requires ex vivo amplification (Supplementary Data Refs. 87 and 88). We adapted DCs cross-presentation to expand polyclonal cultures of VZV-reactive blood-derived CD8 T cells and conducted VZV proteome-wide screens, demonstrating that ORF9 is an immunoprevalent CD8 T-cell target in latently infected individuals independent of their HLA genotype (Figure 1F) (G. M. G. M. V., 2017). This screen platform holds promise to identify population-prevalent CD8 T-cell antigens and yield tetramer reagents for detailed kinetic, phenotypic, and genetic studies. A significant amount of CD8 T-cell cross-reactivity has been observed between VZV and HSV (Supplementary Data Ref. 82). Cross-reactive CD8 T-cell epitopes observed to date are nonidentical between herpesviruses. Individual T-cell clonotypes recognizing a specific HSV epitope can vary in their ability to cross-recognize the sequence-related VZV peptide homolog (Supplementary Data Ref. 82).
Tissue Resident Memory T Cells
Tissue resident memory (TRM) T cells are left behind at multiple anatomic sites of cleared infection or vaccination. Tissue resident memory T cells traffic minimally into the circulation and have distinctive protein, transcription factor, and metabolic phenotypes (Supplementary Data Ref. 90). Recent studies of VZV-specific CD4 TRM T cells in skin show aging-associated declines in density and IFN-γ (but not IL-2) secretion and increases in PD-1 expression, possibly related to increased skin-resident Tregs (Supplementary Data Ref. 53). Essentially nothing is known about VZV-specific CD8 TRM T cells.
Evasion of Immune Responses by Varicella-Zoster Virus
Varicella-zoster virus has developed multiple mechanisms to block the induction and perpetuation of both innate and adaptive immune responses. Pattern recognition receptor signaling is inhibited by at least 3 VZV proteins (ORF47, ORF61, and ORF62) that prevent IRF3 phosphorylation and inhibit the NF-κB pathway (Supplementary Data Refs. 91–94). Varicella-zoster virus infection impairs TLR9 responses in pDCs [32]. Varicella-zoster virus blocks IFN-I signaling by inhibiting phosphorylation of STAT1 and STAT2 and by reducing Janus kinase 2 (JAK2) and IRF9 (via ORF63) protein levels (Supplementary Data Ref. 95–97). Varicella-zoster virus-induced STAT3 phosphorylation induces expression of survivin, which promotes virus replication and spread in skin (Supplementary Data Ref. 98). Finally, VZV modulates expression of NK cell activator receptor NKG2D ligands to escape NK cell killing (Supplementary Data Ref. 99).
Varicella-zoster virus infection of DCs impairs their ability to induce T-cell responses by downregulating surface expression of costimulatory molecules (CD80, CD83, and CD86) and MHC-I and -II (Supplementary Data Refs. 100 and 101). In contrast to related varicelloviruses, the VZV homolog of HSV UL49.5 (ORF9A) does not impair peptide loading onto MHC-I in the endoplasmic reticulum (Supplementary Data Ref. 102). Although VZV ORF66 prevents MHC-I transport to the cell surface by Golgi retention (Supplementary Data Ref. 101), VZV-infected fibroblasts remain susceptible to CD8 T-cell cytolysis (Supplementary Data Ref. 82). In addition, VZV blocks IFN-γ signaling by downregulation of STAT1 and JAK2 proteins, thereby preventing IFN-γ-induced expression of MHC-II and intercellular adhesion molecule 1 [33] (Supplementary Data Ref. 95). Finally, the VZV gE/gI heterodimer functions as a viral Fc receptor, which, by analogy to HSV-1, may contribute to evasion of ADCC (Supplementary Data Ref. 103). Overall, the delicate balance between VZV immune evasion and host immune responses enables the virus to replicate and spread to naive individuals in a controlled manner.
CONCLUDING REMARKS
Studies of innate and adaptive immunity to VZV have benefited from SCID mouse and SVV models, but they remain limited due to the host restriction of VZV and the difficulty of accessing human ganglia during latent or lytic infection. There are several outstanding questions in VZV immunology that will likely require detailed human studies (see Table 1). Little is known concerning the cell-intrinsic receptors for VZV nucleic acids. We are largely ignorant concerning VZV transcripts or proteins expressed during latency that could modulate immune responses. There is still much to learn about the anatomic sites and cell types involved in the increased susceptibility to HZ with age. For example, do SGC change with regards to innate containment or competence to present antigen to ganglionic VZV-specific TRM? Do ganglionic, skin-resident, or mobile inflammation-responsive levels of VZV-specific CD4 or CD8 T cells change with regards to numbers, activation threshold, or influence by regulatory cells or other countervailing factors? Perhaps acquired immune responses serve as surrogates for changes in innate immune cell populations (eg, ILC, MAIT, and NK cells), which we have yet to detect. Fortunately, potent vaccines can prevent (with variable efficacy) primary and recurrent VZV infection, albeit their discovery and development have been largely empiric rather than driven by knowledge of protective mechanisms. Antibody appears to be important for protection against primary varicella. The efficacy of the adjuvanted gE subunit candidate vaccine is undiminished in elderly vaccinees >70 years old [8], indicating that memory immune responder cells, despite their low abundance in the blood, remain competent for boosting to clinically protective levels in this population. With vaccine control of both acute and recurrent VZV disease on the horizon, lessons from VZV immunology hold the promise of yielding clinically meaningful insights into control strategies for other chronic viral pathogens, especially those such as HSV associated with a neuronal site of latency.
Table 1.
Outstanding Questions on the Immune Response Controlling Varicella-Zoster Virus Infection
| • Which pattern recognition receptors are involved in innate immune control of VZV? • How is VZV latency controlled: innate versus adaptive immunity? • How does aging affect the immune control of VZV latency: what is waning of VZV immunity? • How does vaccination improve VZV-specific immunity: what are correlates of protection? • Does VZV infection or vaccination lead to the protective virus-specific TRM in the skin and ganglia? |
Abbreviations: TRM, tissue resident memory; VZV, varicella-zoster virus.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded in part by National Institutes of Health (NIH) contract HHSN272201400049C and grant AI030731 (to D. M. K.) and NIH grant AG032958 (to W. J. D. O. and G. M. G. M. V.).
Supplement sponsorship. This work is part of a supplement sponsored by the Royal Society of Medicine (Royal Charter number RC000525) funded through unrestricted educational grants from Merck, Sanofi Pasteur MSD, The Research Foundation for Microbial Diseases of Osaka University, Seqirus and GlaxoSmithKline.
Potential conflicts of interest. D. M. K. has served as a consultant to GlaxoSmithKline and Biomedical Research Models and has received research funding from Merck, Admedus Immunotherapy, Sanofi Pasteur, Vical, and Immune Design Corporation. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Advances and Controversies in Our Understanding of Herpes Zoster Meeting, Royal Society of Medicine, March 20–21, 2017, London, United Kingdom.
References
- 1. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers 2015; 1:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med 2000; 342:635–45. [DOI] [PubMed] [Google Scholar]
- 3. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008; 115:S3–12. [DOI] [PubMed] [Google Scholar]
- 4. Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol 2014; 12:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ouwendijk WJ, Mahalingam R, de Swart RL, et al. T-cell tropism of simian varicella virus during primary infection. PLoS Pathog 2013; 9:e1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 1974; 2:1288–90. [DOI] [PubMed] [Google Scholar]
- 7. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 8. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 9. Abendroth A, Arvin A. Varicella-zoster virus immune evasion. Immunol Rev 1999; 168:143–56. [DOI] [PubMed] [Google Scholar]
- 10. Arvin AM, Moffat JF, Sommer M, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol 2010; 342:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asano Y, Itakura N, Kajita Y, et al. Severity of viremia and clinical findings in children with varicella. J Infect Dis 1990; 161:1095–8. [DOI] [PubMed] [Google Scholar]
- 12. Abendroth A, Morrow G, Cunningham AL, Slobedman B. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol 2001; 75:6183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sen N, Mukherjee G, Sen A, et al. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Rep 2014; 8:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med 2004; 200:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci U S A 2005; 102:6490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asano Y, Itakura N, Kajita Y, et al. Severity of viremia and clinical findings in children with varicella. J Infect Dis 1990; 161:1095–8. [DOI] [PubMed] [Google Scholar]
- 17. Ozaki T, Ichikawa T, Matsui Y, et al. Lymphocyte-associated viremia in varicella. J Med Virol 1986; 19:249–53. [DOI] [PubMed] [Google Scholar]
- 18. Levin MJ. Varicella-zoster virus and virus DNA in the blood and oropharynx of people with latent or active varicella-zoster virus infections. J Clin Virol 2014; 61:487–95. [DOI] [PubMed] [Google Scholar]
- 19. Nour AM, Reichelt M, Ku CC, Ho MY, Heineman TC, Arvin AM. Varicella-zoster virus infection triggers formation of an interleukin-1β (IL-1β)-processing inflammasome complex. J Biol Chem 2011; 286:17921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol 2005; 79:12658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu HR, Huang HC, Kuo HC, et al. IFN-α production by human mononuclear cells infected with varicella-zoster virus through TLR9-dependent and -independent pathways. Cell Mol Immunol 2011; 8:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ariza ME, Glaser R, Williams MV. Human herpesviruses-encoded dUTPases: a family of proteins that modulate dendritic cell function and innate immunity. Front Microbiol 2014; 5:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 2011; 11:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogunjimi B, Zhang SY, Sørensen KB, et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J Clin Invest 2017; 127:3543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vandevenne P, Sadzot-Delvaux C, Piette J. Innate immune response and viral interference strategies developed by human herpesviruses. Biochem Pharmacol 2010; 80:1955–72. [DOI] [PubMed] [Google Scholar]
- 26. Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell 2016; 164:1198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hata A, Zerboni L, Sommer M, et al. Granulysin blocks replication of varicella-zoster virus and triggers apoptosis of infected cells. Viral Immunol 2001; 14:125–33. [DOI] [PubMed] [Google Scholar]
- 28. Ihara T, Kamiya H, Starr SE, Arbeter AM, Lange B. Natural killing of varicella-zoster virus (VZV)-infected fibroblasts in normal children, children with VZV infections, and children with Hodgkin’s disease. Acta Paediatr Jpn 1989; 31:523–8. [DOI] [PubMed] [Google Scholar]
- 29. Vossen MT, Biezeveld MH, de Jong MD, et al. Absence of circulating natural killer and primed CD8+ cells in life-threatening varicella. J Infect Dis 2005; 191:198–206. [DOI] [PubMed] [Google Scholar]
- 30. Banovic T, Yanilla M, Simmons R, et al. Disseminated varicella infection caused by varicella vaccine strain in a child with low invariant natural killer T cells and diminished CD1d expression. J Infect Dis 2011; 204:1893–901. [DOI] [PubMed] [Google Scholar]
- 31. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huch JH, Cunningham AL, Arvin AM, et al. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol 2010; 84:4060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nikkels AF, Sadzot-Delvaux C, Piérard GE. Absence of intercellular adhesion molecule 1 expression in varicella zoster virus-infected keratinocytes during herpes zoster: another immune evasion strategy?Am J Dermatopathol 2004; 26:27–32. [DOI] [PubMed] [Google Scholar]
- 34. Stevens DA, Ferrington RA, Jordan GW, Merigan TC. Cellular events in zoster vesicles: relation to clinical course and immune parameters. J Infect Dis 1975; 131:509–15. [DOI] [PubMed] [Google Scholar]
- 35. Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system?Nat Rev Immunol 2011; 11:558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Messaoudi I, Barron A, Wellish M, et al. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog 2009; 5:e1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zerboni L, Arvin A. Neuronal subtype and satellite cell tropism are determinants of varicella-zoster virus virulence in human dorsal root ganglia xenografts in vivo. PLoS Pathog 2015; 11:e1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Enquist LW, Leib DA. Intrinsic and innate defenses of neurons: detente with the herpesviruses. J Virol 2017; 91:1e01200–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yordy B, Iijima N, Huttner A, Leib D, Iwasaki A. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe 2012; 12:334–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella-zoster virus infection. J Virol 2011; 85:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnold N, Girke T, Sureshchandra S, Messaoudi I. Acute simian varicella virus infection causes robust and sustained changes in gene expression in the sensory Ganglia. J Virol 2016; 90:10823–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitterreiter JG, Ouwendijk WJ, van Velzen M, van Nierop GP, Osterhaus AD, Verjans GM. Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors. Eur J Immunol 2017; 47:1181–7. [DOI] [PubMed] [Google Scholar]
- 43. van Velzen M, Laman JD, Kleinjan A, Poot A, Osterhaus AD, Verjans GM. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol 2009; 183:2456–61. [DOI] [PubMed] [Google Scholar]
- 44. Shimeld C, Easty DL, Hill TJ. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and cytokines. J Virol 1999; 73:1767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ouwendijk WJ, Getu S, Mahalingam R, Gilden D, Osterhaus AD, Verjans GM. Characterization of the immune response in ganglia after primary simian Varicella virus infection. J Neurovirol 2016; 22:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol 2014; 88:2704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sadaoka T, Serada S, Kato J, et al. Varicella-zoster virus ORF49 functions in the efficient production of progeny virus through its interaction with essential tegument protein ORF44. J Virol 2014; 88:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Markus A, Lebenthal-Loinger I, Yang IH, Kinchington PR, Goldstein RS. An in vitro model of latency and reactivation of varicella zoster virus in human stem cell-derived neurons. PLoS Pathog 2015; 11:e1004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takahashi M, Asano Y, Kamiya H, et al. Development of varicella vaccine. J Infect Dis 2008; 197Suppl 2:S41–4. [DOI] [PubMed] [Google Scholar]
- 50. Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis 2009; 200:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.