Abstract
Background
Despite concerns that antimicrobial treatment of prevalent infections may select for drug-resistant bacteria, the effects of antimicrobial treatment on colonization dynamics have not been well quantified.
Methods
We measured impacts of antimicrobial treatment on nasopharyngeal carriage of penicillin-susceptible Streptococcus pneumoniae (PSSP) and penicillin–nonsusceptible (PNSP) lineages at the end of treatment and 15, 30, and 60 days after treatment in a previously conducted randomized, double-blinded, placebo-controlled trial of amoxicillin-clavulanate for stringently defined acute otitis media.
Results
In intention-to-treat analyses, immediate treatment with amoxicillin-clavulanate reduced PSSP carriage prevalence by 88% (95% confidence interval [CI], 76%–96%) at the end of treatment and by 27% (−3%–49%) after 60 days but did not alter PNSP carriage prevalence. By the end of treatment, 7% of children who carried PSSP at enrollment remained colonized in the amoxicillin-clavulanate arm, compared with 61% of PSSP carriers who received placebo; impacts of amoxicillin-clavulanate persisted at least 60 days after treatment among children who carried PSSP at enrollment. Amoxicillin-clavulanate therapy reduced PSSP acquisition by >80% over 15 days. Among children who carried PNSP at enrollment, no impacts on carriage prevalence of S. pneumoniae, PSSP, or PNSP were evident at follow-up visits.
Conclusions
Although the absolute risk of carrying PNSP was unaffected by treatment, antimicrobial therapy conferred a selective impact on colonizing pneumococci by accelerating clearance and delaying acquisition of PSSP.
Keywords: Streptococcus pneumoniae, antimicrobial resistance, acute otitis media, colonization, randomized controlled trial
In a randomized, controlled trial, antimicrobial treatment for acute otitis media contributed to resistance selection by accelerating clearance and preventing acquisition of penicillin-susceptible Streptococcus pneumoniae colonization, without impacting dynamics of nonsusceptible S. pneumoniae. Emergence of resistance was not observed.
(See the Editorial Commentary Flasche and Atkins, on pages 1351–3.)
The problem of antimicrobial resistance has raised concerns about routine antimicrobial treatment of prevalent infections [1]. Acute otitis media (AOM) is the leading cause of antimicrobial prescribing among children in high-income countries [2]. Although many infections are self-limiting, antimicrobial therapy accelerates the resolution of otoscopic signs and symptoms [3, 4]. At present, there are conflicting guidelines about the optimal clinical management of AOM [5]. While certain countries recommend “watchful waiting” as a strategy to limit unnecessary antimicrobial prescribing for infections that may resolve spontaneously [6, 7], the limited impact of such recommendations on real-world prescribing practices [8–10] underscores the persisting need to determine how antimicrobial consumption influences selection of antimicrobial-resistant bacterial lineages.
Uncertainty about epidemiological dynamics underlying the relationship between antimicrobial consumption and antimicrobial resistance [11, 12] has hindered assessments of how resistance risks may offset clinical benefits of treatment for AOM [5], among other prevalent conditions. Associations between previous antimicrobial treatment and carriage of resistant bacteria have been reported in observational studies [13, 14]. However, end points of drug-susceptible and drug-resistant bacterial carriage have been measured in few trials, and incompatible reporting of such trial end points has made outcomes difficult to interpret [4, 15–17]. The effects of antimicrobial treatment on the clearance, replacement, or acquisition of bacterial strains and the persistence of these effects over time have not been well quantified. Because such data are integral to the parameterization of mathematical models of selection and transmission dynamics [12], this gap has hindered our ability to assess the impacts of alternative treatment protocols on the spread of drug-resistant bacteria [6, 7] and to evaluate interventions combating antimicrobial resistance [18, 19].
A previously conducted trial of immediate initiation of amoxicillin-clavulanate therapy for AOM provided an opportunity to assess how antimicrobial treatment influences nasopharyngeal carriage of Streptococcus pneumoniae with varying susceptibility to penicillin [3]. We revisited data from this study to measure treatment effects on carriage of susceptible and resistant lineages.
METHODS
Study Design and Patients
Nasopharyngeal carriage of S. pneumoniae was assessed as a secondary outcome in a randomized, double-blinded, placebo-controlled trial of amoxicillin-clavulanate for stringently defined AOM in Turku, Finland (clinical trials registration NCT00299455). Primary clinical end points, adverse events, and the original study protocol were reported previously [3]. Briefly, children 6–35 months old seeking care were screened for AOM diagnosis, defined as fulfilling 3 criteria: presence of middle ear fluid, acute inflammatory signs in the tympanic membrane, and acute symptoms such as fever, ear pain, or respiratory symptoms. Enrollment ran from March 2006 through December 2008.
Upon enrollment, children were randomly assigned to receive immediate receive antimicrobial therapy or placebo and attended scheduled follow-up visits with study physicians. Rescue therapy with open-label antimicrobial drugs was initiated if a child’s condition did not improve or worsened during treatment, based on parental report and clinical assessment of symptoms and otoscopic signs. We compared outcomes within the intention-to-treat populations of the treatment and placebo arms to measure the effect of prescribing strategies resembling immediate antimicrobial therapy and watchful waiting, respectively; children were thus analyzed according to the randomized assignment, regardless of whether they received rescue therapy.
Nasopharyngeal swabbing occurred in the clinic at enrollment, at scheduled visits, and when children sought care for new episodes. We analyzed carriage outcomes for scheduled visits occurring at the end of treatment (day 8) and at 15, 30, and 60 days. So that follow-up visits occurred with equal time after end of treatment for all children, study days were counted from the enrollment visit, for children who did not require rescue treatment, or from the day of initiating rescue treatment. Susceptibility of isolates to penicillin was determined using standard breakpoints [20], as detailed below.
Ethics
Parents provided written informed consent on behalf of children. The ethics committee of the Hospital District of Southwest Finland Ethical approved the original study. Secondary analyses were exempted by the institutional review board at Harvard T. H. Chan School of Public Health.
Random Assignment
Patients were enrolled by study physicians and randomly assigned at a ratio of 1:1 to receive amoxicillin-clavulanate or placebo, using computer-generated allocation numbers, which were provided over the telephone by nurses in the Pediatric Infectious Disease Ward of Turku University Hospital. Patients, study physicians, and laboratory personnel were blinded to the randomization codes for the duration of the trial. The allocation list was generated as a block of 10 and kept by the hospital pharmacy. Pharmacists concealed assignments by labeling identical study drug containers with allocation numbers. Placebo treatments (capsules containing lactose monohydrate) were identical to active treatment in appearance, taste, and smell. The biostatistician performing analyses presented in this article was not blinded to treatment assignments.
Procedures
Eligible patients were randomly assigned to receive amoxicillin-clavulanate (40 and 5.7 mg, respectively, per kilogram of body weight daily, divided into 2 doses) or placebo for 7 days. Study physicians encouraged use of analgesic and antipyretic agents and allowed use of decongestant nose drops and sprays. To enable comparisons of immediate versus delayed antimicrobial treatment, the main rescue treatment was open-label amoxicillin-clavulanate (40 and 5.7 mg, respectively, per kilogram of body weight per day, divided into 2 doses) for 7 days. However, study physicians could also prescribe high-dose amoxicillin-clavulanate or intramuscular ceftriaxone as rescue treatment, based on clinical assessment, consistent with Finnish treatment guidelines.
Nasopharyngeal specimens were collected with polyethylene terephthalate–tipped swabs (Copan Diagnostics, Corona, CA) and plated on selective agar immediately after the study visit, to determine pneumococcal carriage. Plates were incubated in 5% CO2 at ≥35°C and examined at 18–24 hours and 36–48 hours. Penicillin susceptibility was determined via a 2-step approach. Samples were first screened for reduced susceptibility, using oxacillin disks. For samples with a decreased (diameter, <20 mm) zone of inhibition, minimal inhibitory concentrations (MICs) were determined using penicillin G Etests (bioMérieux, Cambridge, MA). All samples with a zone of exclusion of ≥20 mm or both a zone of exclusion of <20 mm and an MIC of ≤0.064 µg/mL were classed as penicillin susceptible. Intermediate resistance was defined as a zone of exclusion of 20 mm and an MIC of either >0.064 µg/mL or ≤2 µg/mL, and resistance was defined as a zone of exclusion of <20 mm and an MIC of >2 µg/mL.
Outcomes
The end points of interest were carriage of any pneumococci, carriage of penicillin-susceptible S. pneumoniae (PSSP), and carriage of penicillin-nonsusceptible S. pneumoniae (PNSP), defined as an intermediately resistant or resistant phenotype, at 8, 15, 30, and 60 days after beginning treatment with the study drug or rescue therapy.
Analysis
Children were analyzed in the study arms to which they were randomly assigned, in an intention-to-treat framework. We calculated the risk ratio (RR) of carriage in the 2 arms via the prevalence ratio of carriage of any pneumococci, PSSP, and PNSP. We measured the impact of immediate antimicrobial prescribing against each colonization end point at follow-up visits as [1 − RR] × 100. To understand effects of treatment on acquisition and clearance of S. pneumoniae, we also performed subgroup analyses comparing the prevalence of carrying any pneumococci, PSSP, and PNSP among children who, at enrollment, were not colonized or carried PSSP or PNSP.
To determine the selective impact of antimicrobial treatment on pneumococci carried by children treated for AOM, we next assessed the susceptibility of strains carried by children who had been assigned to receive immediate treatment with amoxicillin-clavulanate or placebo [17]. We measured the conditional RR for strains carried at follow-up visits to show reduced susceptibility as the ratio, between the study arms, of the proportions of pneumococcal isolates tested that were classified as PNSP.
Last, we calculated risk differences to measure the prevalence of carriage attributable to or avertable by immediate antimicrobial prescribing. For each scheduled visit, we calculated differences in pneumococcal carriage prevalence—including all pneumococci, PSSP, and PNSP—between the study arms. We again performed subgroup analyses according to carriage status at enrollment.
We conducted statistical inference over 100000 bootstrap-resampled data sets to measure medians and 95% confidence intervals (CIs) around prevalence, RR, and risk difference estimates. As detailed in the Supplementary Materials, the bootstrap approach allowed us to generate unbiased estimates of PSSP and PNSP carriage prevalence in the presence of missing observations that would otherwise bias estimates.
RESULTS
Enrollment and Sample Processing
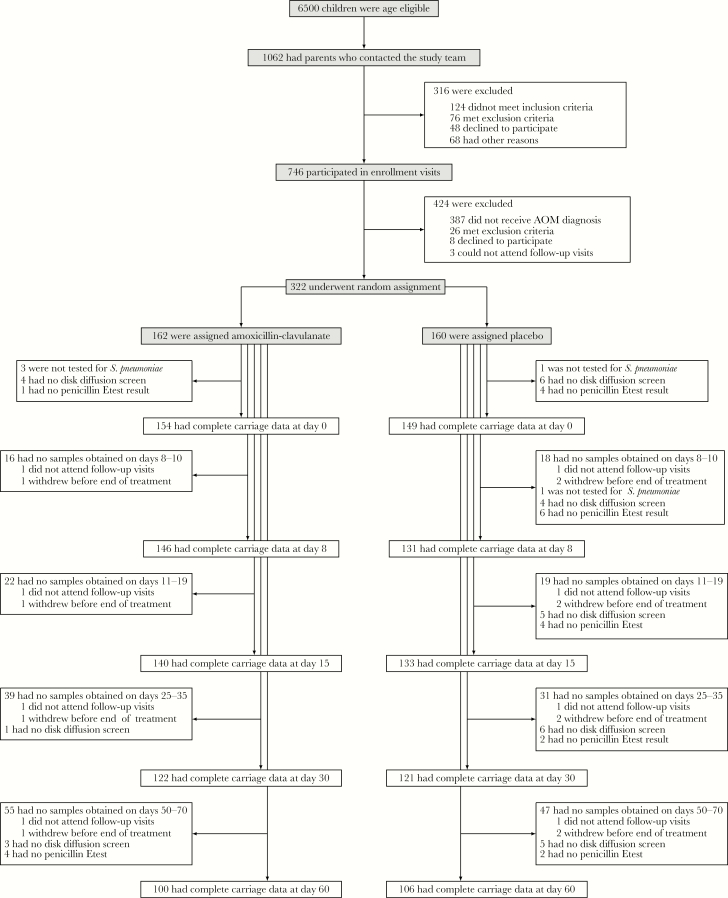
Of 162 children initially assigned amoxicillin-clavulanate and 160 initially assigned placebo, 2 and 3, respectively, left the study before the end of treatment (Figure 1). Rescue therapy was initiated for 11 children (7%) assigned amoxicillin-clavulanate and for 53 (33%) assigned placebo.
Figure 1.
Trial outline, illustrating recruitment, enrollment, and random assignment of patients, together with the completion of follow-up visits as scheduled and processing of nasopharyngeal specimens. Samples were not obtained if children did not attend a visit within the specified intervals of scheduled visits at days 8, 15, 30, and 60 or if nasopharyngeal swabbing was refused by children (or by parents on behalf of their children).
At enrollment, 193 of 318 (61%) children carried pneumococci (Table 1); the most frequently carried serotypes were 6B (n = 60), 19F (n = 56), 14 (n = 35), 23F (n = 27), 6A (n = 15), 6C (n = 11), and 35B (n = 10). In total, pneumococcal carriage was determined at 675 of 810 scheduled visits (83%) among children assigned amoxicillin-clavulanate and at 683 of 800 (85%) among children assigned placebo (Figure 1). Resistance determinations for pneumococcal isolates were available for 662 (98%) and 640 (94%) assigned amoxicillin-clavulanate and placebo, respectively. Baseline characteristics of children with complete follow-up data to day 60 did not differ from baseline characteristics of children missing data from any scheduled visit (Supplementary Table 1).
Table 1.
Baseline Characteristics of Patients Assigned to Receive Amoxicillin-Clavulanate (Amox-Clav) or Placebo, Overall and by Streptococcus pneumoniae Colonization Status at Enrollment
| Characteristic | Overall | Colonized | Not Colonized | |||
|---|---|---|---|---|---|---|
| Amox-Clav (n = 162) | Placebo (n = 160) | Amox-Clav (n = 102) | Placebo (n = 91) | Amox-Clav (n = 57) | Placebo (n = 68) | |
| Age, mo | 15.6 ± 7.3 | 16.0 ± 7.9 | 15.7 ± 7.3 | 16.4 ± 8.2 | 15.5 ± 7.4 | 15.5 ± 7.5 |
| Male sex | 92 (57) | 91 (57) | 59 (58) | 46 (51) | 31 (54) | 45 (66) |
| Received antimicrobials within past mo | 37 (23) | 34 (21) | 21 (21) | 14 (15) | 15 (26) | 20 (29) |
| Siblings, no. | ||||||
| 0 | 73 (45) | 66 (41) | 46 (45) | 34 (37) | 24 (42) | 31 (46) |
| 1–2 | 79 (49) | 89 (56) | 51 (50) | 53 (58) | 28 (49) | 36 (53) |
| ≥3 | 10 (6) | 5 (3) | 5 (5) | 4 (4) | 5 (9) | 1 (1) |
| Daycare attendance | 87 (54) | 87 (54) | 63 (47) | 49 (34) | 23 (30) | 37 (43) |
| Parental smokinga | 57 (35) | 48 (30) | 33 (32) | 24 (26) | 23 (40) | 24 (35) |
| Uses pacifier | 80 (49) | 86 (54) | 50 (49) | 42 (46) | 30 (53) | 43 (63) |
| Duration of breastfeeding, mob | 7.5 ± 4.7 | 7.1 ± 4.5 | 7.4 ± 4.4 | 6.8 ± 4.1 | 7.7 ± 5.3 | 7.5 ± 4.9 |
| Received ≥1 dose of PCV | 3 (2) | 4 (3) | 2 (2) | 4 (4) | 1 (2) | 0 (0) |
| Severe bulging | 45 (28) | 41 (26) | 33 (32) | 30 (33) | 12 (21) | 10 (15) |
| Bilateral AOM | 61/160 (38) | 68/158 (43) | 41/100 (41) | 34/90 (38) | 19/57 (33) | 34/67 (51) |
| Fever ≥39°C in past 24 h | 24 (15) | 18 (11) | 16 (16) | 13 (14) | 8 (14) | 5 (7) |
| Pneumococcal carriage | ||||||
| Any | 102/159 (64) | 91/159 (57) | … | … | … | … |
| PSSPc | 78/154 (52) | 63/149 (45) | 78/97 (81) | 63/81 (78) | … | … |
| PNSPc | 19/154 (13) | 18/149 (13) | 19/97 (19) | 18/81 (22) | … | … |
Data are no. (%) or proportion (%) of patients or mean value ± SD.
Abbreviations: PCV, pneumococcal conjugate vaccine.
aNo parents reported smoking inside the household.
bDuration of any breastfeeding at the time of the enrollment visit.
cPrevalence estimates (in parentheses) are corrected for missing data from isolates without resistance tests (see Supplemental Materials).
Impact Against Pneumococcal Colonization
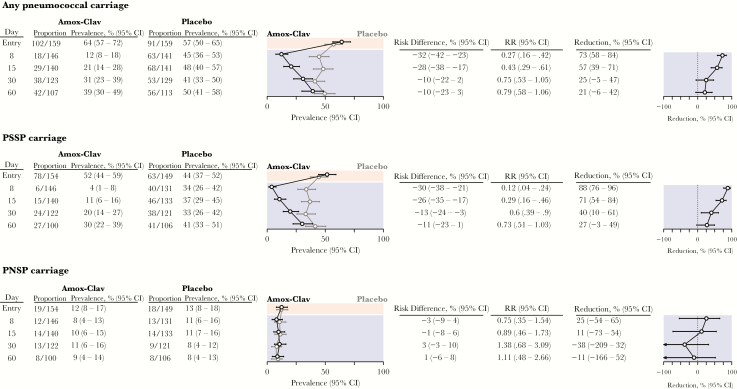
Among children from whom swab specimens were obtained as scheduled at the end of treatment visit, we detected pneumococcal carriage in 18 of 146 (12%) assigned amoxicillin-clavulanate and in 63 of 141 (45%) assigned placebo (Figure 2), indicating an amoxicillin-clavulanate–attributable 73% (95% CI, 58%–84%) decrease in the prevalence of pneumococcal colonization at the end of treatment. Only 4% of children assigned amoxicillin-clavulanate, compared with 34% assigned placebo, carried PSSP at the end of treatment. In contrast, 8% and 11% assigned amoxicillin-clavulanate and placebo, respectively, carried PNSP at the end of treatment. These findings signified amoxicillin-clavulanate–attributable reductions of 88% (95% CI, 76%–96%) and 25% (95% CI, −54%–65%) in the prevalence of PSSP and PNSP carriage, respectively, at the end of treatment. In total, a 32% (95% CI, 23%–42%) prevalence of pneumococcal carriage among children treated for AOM could be averted by immediate treatment with amoxicillin-clavulanate, largely by preventing PSSP carriage in 30% (95% CI, 21%–38%) of children who would otherwise be expected to carry PSSP.
Figure 2.
Impact of immediate treatment with amoxicillin-clavulanate on carriage of Streptococcus pneumoniae with varying susceptibility to penicillin. Tables and plots indicate the proportion of carrying S. pneumoniae, penicillin-susceptible S. pneumoniae (PSSP), and penicillin-nonsusceptible S. pneumoniae (PNSP), measured among all swab specimens with microbiological determinations available (carriage presence/absence, or carriage presence/absence and penicillin susceptibility, given carriage). Prevalence estimates are presented as medians (95% confidence intervals [CIs]), based on the bootstrap approach (Methods and Supplementary Materials), to correct for bias from incomplete testing of specimens. Consequently, median estimates may not equal proportions. Amox-Clav, amoxicillin-clavulanate; RR, risk ratio.
Amoxicillin-clavulanate-attributable reductions in pneumococal carriage and PSSP carriage declined over successive visits to 21% (95% CI, −6%–42%) and 27% (95% CI, −3%–49%), respectively, by 60 days after treatment (Figure 2). We did not detect strong statistical evidence (defined by a 95% CI entirely above 0) that immediate treatment with amoxicillin-clavulanate influenced the prevalence of PNSP carriage; only 8%–11% of children in either arm carried PNSP at any follow-up visit.
Clearance and Acquisition Dynamics
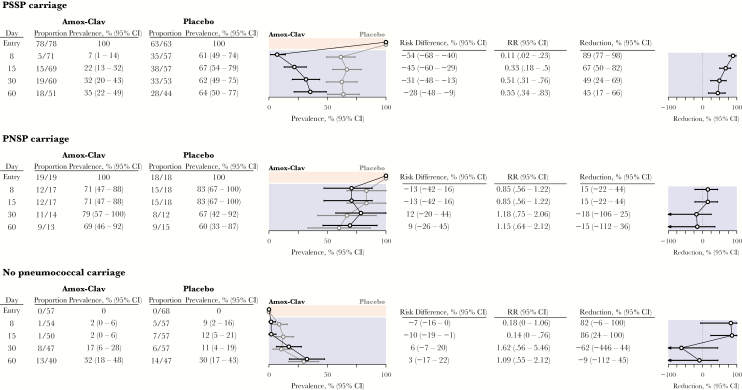
Stratifying analyses by carriage status at the enrollment visit afforded a view into how antimicrobial therapy for AOM differentially influenced the clearance and acquisition of PSSP and PNSP (Figure 3 and Table 2). Among children carrying PSSP at enrollment, 61%–67% of those assigned placebo carried pneumococci at follow-up visits, whereas the prevalence among children assigned amoxicillin-clavulanate dropped to 7% by the end of treatment and reached only 35% after 60 days. This signified an amoxicillin-clavulanate–attributable 89% (95% CI, 77%–98%) lower prevalence of pneumococcal carriage at the end of treatment, with a 45% (95% CI, 17%–66%) lower prevalence in the amoxicillin-clavulanate arm persisting up to 60 days after treatment. No children who carried PSSP at enrollment were observed to carry PNSP at any follow-up visit (Table 2). In absolute terms, immediate treatment with amoxicillin-clavulanate prevented 54% (95% CI, 40%–68%) of baseline PSSP carriers from carrying PSSP at the end of treatment; the averted PSSP carriage prevalence 60 days after treatment was 28% (95% CI, 9%–48%).
Figure 3.
Impact of immediate treatment with amoxicillin-clavulanate on carriage of Streptococcus pneumoniae, according to carriage at enrollment. Tables and plots indicate the number carrying S. pneumoniae at follow-up visits, according to carriage at enrollment, measured among all swab specimens with microbiological determinations available (carriage presence/absence, or carriage presence/absence and penicillin susceptibility, given carriage). Prevalence estimates are presented as medians (95% confidence intervals [CIs]), based on the bootstrap approach (Methods and Supplementary Materials), to correct for bias from incomplete testing of specimens. We expand on results presented in this figure—detailing susceptibility of isolates carried at follow-up—in Table 2. Amox-Clav, amoxicillin-clavulanate; RR, risk ratio.
Table 2.
Prevalence of Pneumococcal Carriage Averted by Immediate Treatment With Amoxicillin-Clavulanate (Amox-Clav), According to Streptococcus pneumoniae Carriage Status at Enrollment
| Carriage Status, End Point, Study Day | Amox-Clav | Placebo | Risk Difference, % (Bootstrap)a | ||
|---|---|---|---|---|---|
| Proportion | Prevalence, % (Bootstrap)a | Proportion | Prevalence, % (Bootstrap)a | ||
| PSSP carriers | |||||
| Any carriage | |||||
| 8 | 5/71 | 7 (1–14) | 35/57 | 61 (49–74) | −54 (−68 to −40) |
| 15 | 15/69 | 22 (13–32) | 38/57 | 67 (54–79) | −45 (−60 to −29) |
| 30 | 19/60 | 32 (20–43) | 33/53 | 62 (49–76) | −31 (−48 to −13) |
| 60 | 18/51 | 35 (22–49) | 28/44 | 64 (50–77) | −28 (−48 to −9) |
| PSSP carriage | |||||
| 8 | 5/71 | 7 (1–14) | 35/57 | 61 (49–74) | −54 (−68 to −40) |
| 15 | 15/69 | 22 (13–32) | 37/56 | 67 (54–79) | −45 (−60 to −29) |
| 30 | 19/60 | 32 (20–43) | 31/51 | 62 (49–75) | −31 (−48 to −13) |
| 60 | 17/50 | 35 (22–49) | 26/42 | 64 (50–77) | −28 (−48 to −9) |
| PNSP carriage | |||||
| 8 | 0/71 | 0 | 0/57 | 0 | 0 |
| 15 | 0/69 | 0 | 0/56 | 0 | 0 |
| 30 | 0/60 | 0 | 0/51 | 0 | 0 |
| 60 | 0/51 | 0 | 0/42 | 0 | 0 |
| PNSP carriers | |||||
| Any carriage | |||||
| 8 | 12/17 | 71 (47–88) | 15/18 | 83 (67–100) | −13 (−42–16) |
| 15 | 12/17 | 71 (47–88) | 15/18 | 83 (67–100) | −13 (−42–16) |
| 30 | 11/14 | 79 (57–100) | 8/12 | 67 (42–92) | 12 (−20–44) |
| 60 | 9/13 | 69 (46–92) | 9/15 | 60 (33–87) | 9 (−26–45) |
| PSSP carriage | |||||
| 8 | 0/17 | 0 | 1/17 | 5 (0–17) | −5 (−17–0) |
| 15 | 0/17 | 0 | 1/17 | 5 (0–17) | −5 (−17–0) |
| 30 | 0/14 | 0 | 1/11 | 8 (0–25) | −8 (−25–0) |
| 60 | 2/11 | 19 (0–39) | 2/15 | 12 (0–28) | 6 (−17–30) |
| PNSP carriage | |||||
| 8 | 12/17 | 71 (47–88) | 13/17 | 78 (58–94) | −7 (−36–21) |
| 15 | 12/17 | 71 (47–88) | 13/17 | 78 (58–94) | −7 (−36–21) |
| 30 | 11/14 | 79 (57–100) | 6/11 | 58 (32–83) | 20 (−13–5) |
| 60 | 5/11 | 49 (27–77) | 7/15 | 47 (26–73) | 3 (−31–38) |
| No pneumococcus carriage | |||||
| Any carriage | |||||
| 8 | 1/54 | 2 (0–6) | 5/57 | 9 (2–18) | −7 (−16–0) |
| 15 | 1/50 | 2 (0–6) | 7/57 | 12 (5–21) | −10 (−19 to −1) |
| 30 | 8/47 | 17 (6–28) | 6/57 | 11 (4–19) | 7 (−7–20) |
| 60 | 13/40 | 33 (18–48) | 14/47 | 30 (17–43) | 3 (−17–22) |
| PSSP carriage | |||||
| 8 | 1/54 | 2 (0–6) | 4/56 | 9 (2–18) | −7 (−16–0) |
| 15 | 0/50 | 0 | 7/57 | 12 (5–21) | −12 (−21 to −5) |
| 30 | 5/46 | 12 (4–24) | 6/57 | 11 (4–19) | 2 (−10–15) |
| 60 | 8/37 | 26 (14–41) | 13/46 | 30 (17–43) | −4 (−22–15) |
| PNSP carriage | |||||
| 8 | 0/54 | 0 | 0/56 | 0 | 0 |
| 15 | 1/50 | 2 (0–6) | 0/57 | 0 | 2 (0–6) |
| 30 | 2/46 | 4 (0–13) | 0/57 | 0 | 4 (0–13) |
| 60 | 2/37 | 6 (0–15) | 0/46 | 0 | 6 (0–15) |
Abbreviations: PNSP, penicillin-susceptible S. pneumoniae; PSSP, penicillin-susceptible S. pneumoniae.
aData are presented as medians (95% confidence intervals), based on the bootstrap approach (see Methods and Supplementary Materials), to correct for bias from incomplete testing of specimens.
Among children carrying PNSP at enrollment, 71% and 83% assigned amoxicillin-clavulanate and placebo, respectively, carried pneumococci at the end of treatment, indicating an amoxicillin-clavulanate–attributable 15% (95% CI, −22%–44%) lower colonization prevalence (Figure 3). Estimates of the impact of treatment at subsequent follow-up visits remained statistically indistinguishable from 0. By 60 days after treatment, 69% and 60% of children assigned amoxicillin-clavulanate and placebo, respectively, carried pneumococci. Of children assigned amoxicillin-clavulanate who were carrying PNSP at enrollment, none carried PSSP until 60 days after treatment (Table 2), whereas a 5%–8% prevalence of PSSP carriage was detected at follow-up visits among children assigned placebo.
Among children who were not colonized at enrollment, we estimated that immediate treatment with amoxicillin-clavulanate resulted in 82% (95% CI, −6%–100%) and 86% (95% CI, 24%–100%) lower prevalences of pneumococcal colonization at the end of treatment and 15 days after treatment, respectively (Figure 3). However, the prevalence of colonization among such children was low; the averted prevalence 15 days after treatment amounted to only 10% (95% CI, 1%–19%). We did not identify strong statistical evidence that treatment influenced carriage prevalence at subsequent follow-up visits. The majority of pneumococcal isolates collected after treatment from children who were not colonized at enrollment were penicillin susceptible (Table 2). Among children who were not colonized at enrollment, no PNSP carriage was detected among those assigned placebo, whereas the PNSP prevalence reached 6% (95% CI, 0%–15%) by 60 days after treatment among those assigned amoxicillin-clavulanate. However, this reflected acquisition of PNSP by only 3 children, with 0–2 simultaneously carrying PNSP at any visit.
Selective Impact
Owing to the differential impacts of antimicrobial therapy on clearance and acquisition of PSSP and PNSP, the proportion of carried strains showing reduced susceptibility to penicillin at the end of treatment was 2.74 (95% CI, 1.62–4.88) times higher among children assigned amoxicillin-clavulanate (12 of 18 [67%]) than among children assigned placebo (13 of 53 [25%]; Table 3). This effect persisted for at least 30 days after treatment, when the proportion of carried strains showing reduced susceptibility to penicillin was 2.28 (95% CI, 1.18–5.53) times higher among children assigned amoxicillin-clavulanate (35%) than among those assigned placebo (15%). By 60 days after treatment, the proportion of pneumococcal isolates with reduced susceptibility to penicillin remained higher among children assigned amoxicillin-clavulanate (conditional RR, 1.40; 95% CI, .64–3.20), although this difference no longer reached conventional thresholds for statistical significance.
Table 3.
Conditional Risk Ratios (RRs) for Carried Streptococcus pneumoniae to Show Reduced Susceptibility to Penicillin, Given Immediate Treatment With Amoxicillin-Clavulanate (Amox-Clav)
| Study Day | Amox-Clav | Placebo | Conditional RR | ||
|---|---|---|---|---|---|
| Proportion | Percentage (95% CI) | Proportion | Percentage (95% CI) | ||
| 8 | 12/18 | 67 (45–87) | 13/53 | 25 (15–35) | 2.74 (1.62–4.88) |
| 15 | 14/29 | 48 (32–65) | 14/60 | 23 (14–33) | 2.08 (1.22–3.69) |
| 30 | 13/37 | 35 (22–48) | 7/45 | 15 (7–25) | 2.28 (1.18–5.53) |
| 60 | 8/35 | 23 (12–34) | 8/49 | 16 (8–25) | 1.40 (.64–3.20) |
Abbreviation: CI, confidence interval.
Penicillin-Resistant Pneumococcal Carriage
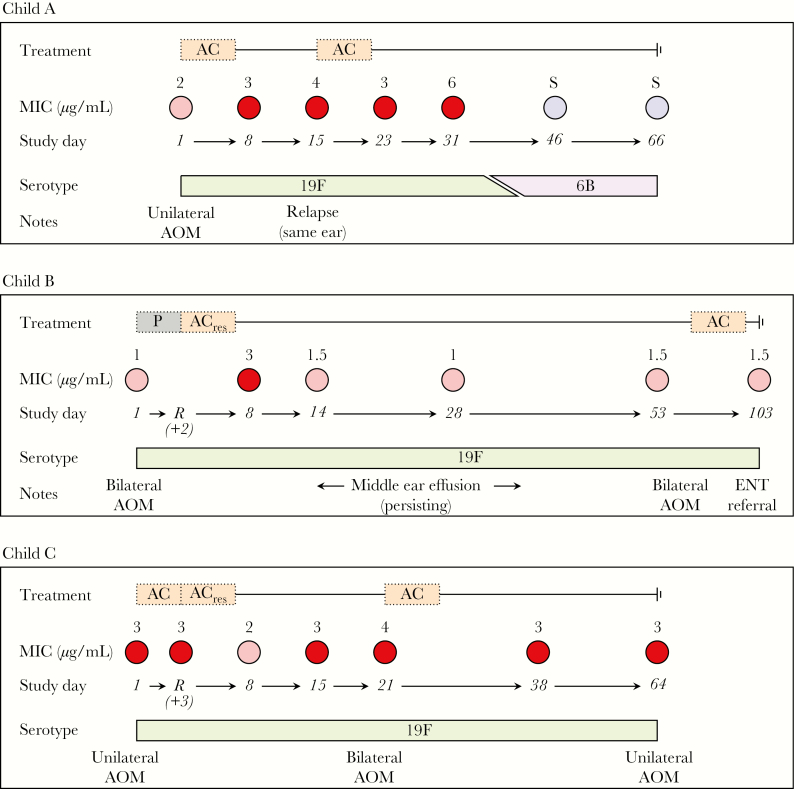
Three children carried pneumococcal strains that were classed as fully resistant to penicillin (defined by an oxacillin disk diameter of <20 mm and an MIC of >2 µg/mL; Figure 4). Two of these children carried strains registering an intermediate MIC at enrollment and yielded penicillin-resistant isolates of the same serotype at least once after treatment with amoxicillin-clavulanate as the study drug or rescue therapy. A third child who received amoxicillin-clavulanate carried a strain registering a resistant MIC at all study visits, with the exception of the end of treatment visit, when intermediate resistance was detected. We detail the clinical course of these children in the Supplementary Materials.
Figure 4.
Observations among children who carried resistant isolates during the study. Study drug and rescue therapy assignments are illustrated together with isolates (with minimum inhibitory concentrations [MICs]) collected from children from the time of the enrollment visit through the time of the last visit). Study days are numbered from the first day of treatment with study drug or rescue therapy. Colors indicate penicillin susceptibility of the carried strain: blue, penicillin susceptible; pink, intermediate penicillin resistance; red, penicillin resistance. AC, amoxicillin-clavulanate; AOM, acute otitis media; P, placebo.
DISCUSSION
We report a sustained, selective impact of antimicrobial treatment for AOM on pneumococcal carriage among children 6–35 months old in a high-income country. Children assigned amoxicillin-clavulanate were less likely than those assigned placebo to carry PSSP up to 60 day after treatment. By the end of treatment, PNSP constituted 67% of carried strains among children assigned amoxicillin-clavulanate, compared with 25% of carried strains among children assigned placebo; differences between the amoxicillin-clavulanate and placebo arms in the susceptibility of carried strains persisted at least 30 days after treatment. Nonetheless, amoxicillin-clavulanate did not measurably influence the absolute prevalence of PNSP carriage. These findings clarify the magnitude and duration of the impact of immediate antimicrobial therapy, compared with watchful waiting, on carriage of pneumococci with varying susceptibility to penicillin.
Repeated sampling of nasopharyngeal carriage at enrollment and follow-up visits illustrated how differential clearance and acquisition of PSSP and PNSP mediate resistance selection. Under circumstances resembling watchful waiting, 39% of PSSP carriers assigned placebo cleared colonization by the end of treatment, either naturally or because of rescue therapy with antimicrobial drugs, compared with 18% of PNSP carriers. Immediate receipt of amoxicillin-clavulanate cleared PSSP colonization by the end of treatment in an additional 54% of children but had no measurable impact on colonization among PNSP carriers, despite the majority of PNSP lineages showing only intermediate resistance in our trial. We also observed no instances of resistance emerging after treatment among children who initially carried PSSP. Among children who were not colonized at enrollment, immediate treatment with amoxicillin-clavulanate lowered the risk of acquiring PSSP by >80% over the first 2 weeks after treatment.
Among placebo-controlled trials of antimicrobial treatment for AOM, this study enrolled the largest sample of children and included the longest continuous monitoring of carriage [21]. By stratifying according to carriage at enrollment, our analysis is the first to assess effects of antimicrobial treatment for AOM on clearance and acquisition dynamics of PSSP and PNSP. Several other trials of antimicrobial therapy for AOM in high-income countries provided complementary insights. In the United States, no difference in the absolute prevalence of PNSP was reported at the end of treatment or 21–25 days after treatment among children assigned 10 days of high-dose amoxicillin-clavulanate versus placebo [4], or at the end of a 10-day versus 5-day course of high-dose amoxicillin-clavulanate [15]. In both trials, the proportions of pneumococcal isolates showing reduced susceptibility to penicillin at the end of treatment were higher among children assigned a 10-day course of amoxicillin-clavulanate than among children assigned either placebo or a 5-day course of amoxicillin-clavulanate, suggesting selection was mediated by reductions in PSSP persistence or acquisition. Similar changes in the proportion of isolates showing reduced susceptibility were evident in a trial comparing immediate antimicrobial prescribing and watchful waiting [16], although the effects on the absolute prevalence of PSSP and PNSP were not reported, and carriage was assessed only 12 days after treatment. Unified reporting of effects on bacterial carriage and susceptibility will facilitate direct comparison of results from future trials [17]. In our study, comparing colonization outcomes among children who initially carried or did not carry PSSP and PNSP provided a basis for measuring selective effects of antimicrobial treatment, even in the absence of differences between arms in PNSP prevalence. Such analyses should be routinely undertaken in future studies to understand drivers of antimicrobial resistance. Our finding that reductions in PSSP carriage persist up to 60 days after treatment also suggests future studies should monitor carriage over longer follow-up windows than in previous trials.
Whereas we did not detect increases in PNSP carriage prevalence due to amoxicillin-clavulanate, this observation has been reported in several studies undertaken in lower-income countries with higher PNSP prevalence. In the Dominican Republic, children with respiratory tract infections randomly assigned to receive a low-dose, 10-day amoxicillin regimen had higher prevalence of PNSP carriage after treatment than children randomly assigned to receive a high-dose, 5-day regimen [22]. In Malawi, increases in PNSP carriage prevalence were reported after treatment of malaria with sulfadoxine-pyrimethamine and treatment of suspected bacterial infections with trimethoprim-sulfamethoxazole [23]. Whereas most assessments of antimicrobial therapy for AOM have been conducted in the United States and Europe [21], trials and observational studies are warranted in low- and middle-income countries to guide appropriate treatment practices for such settings.
It is important to note that our analysis measured only the direct effects of antimicrobial therapy on carriage dynamics [24]. Large-scale changes in treatment practices may spur indirect effects on carriage of PSSP and PNSP within populations, even if direct effects of treatment are small at the individual level. Mass azithromycin administration for the prevention of trachoma caused community-wide increases in the prevalence of macrolide-resistant pneumococcal carriage in a cluster-randomized trial in Ethiopia [25]. In ecological studies in higher-income settings, outpatient antibiotic prescribing predicts the proportion of pneumococcal isolates showing reduced penicillin susceptibility at geographic scales ranging from municipalities to countries [26, 27].
This study has several limitations. It is important to note that the study treatment (40 mg of amoxicillin and 5.7 mg of clavulanate per kilogram of body weight daily) differs from the high-dose amoxicillin-clavulanate regimen recommended in the United States (90 and 6.4 mg/kg, respectively). Notably, high-dose amoxicillin-clavulanate did not deliver larger reductions in carriage or lower rates of clinical failure in the United States than 40 and 5.7 mg/kg of amoxicillin and clavulanate, respectively, delivered in this trial in Finland [3, 4]. Because our trial was designed to assess clinical failure as a primary end point, stratified analyses addressing PNSP carriage had limited statistical power; nonsignificant between-group differences may thus signify where effect sizes were too small to be measured reliably, rather than the absence of effects. Although data from 4 time points up to 60 days after treatment provided a more detailed view of pneumococcal carriage dynamics than other trials have afforded [4, 15, 16], swab specimens were not obtained from 22% and 32% of children from follow-up visits 30 and 60 days, respectively, after treatment. While our analysis addresses treatment effects on pneumococci, effects on other pathogens and commensal flora within the microbiome are also of importance [28]. Last, our analysis does not address the association of carriage end points with antimicrobial prescribing for secondary AOM episodes or other causes during follow-up. An advantage of the modified intention-to-treat framework we use is that this circumstance does not hinder our interpretation of carriage outcomes as causal effects of the initial randomized assignment.
Because this trial was undertaken before the introduction of pneumococcal conjugate vaccination in the Finnish national immunization program, the distribution of circulating pneumococcal lineages has changed substantially. Nonetheless, stratifying analyses by resistance of the colonizing isolate yields transportable effect-size estimates for predicting treatment effects in settings where the resistance profile is known. Whereas a higher proportion of vaccine-targeted serotypes than nonvaccine serotypes tended to be resistant to antimicrobial drugs in the prevaccine era, increases in the proportion of resistant nonvaccine serotypes have been reported amid serotype replacement, generally mitigating any net change in the prevalence of resistance [29–31].
In conclusion, we find that immediate treatment with amoxicillin-clavulanate for AOM facilitates clearance and delays acquisition of PSSP in the nasopharynx, indirectly increasing the proportion of carried pneumococcal strains showing reduced susceptibility to penicillin. We detected no increase in absolute prevalence of PNSP among children assigned amoxicillin-clavulanate as compared to children assigned placebo. These findings demonstrate the possible selective impacts of treating AOM with antimicrobial drugs, even in the absence of measurable increases in risk for individual children to carry PNSP. Quantifying the effects of different AOM treatment strategies on carriage of PSSP and PNSP helps to weigh resistance risks against clinical benefits of antimicrobial therapy for AOM.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of General Medical Sciences, National Institutes of Health (grant U54GM088558 to M. L.); the European Society for Paediatric Infectious Diseases (fellowship to P. A. T.); the Orion Foundation (fellowship to P. A. T.); the Foundation for Paediatric Research; the Jenny and Antti Wihuri Foundation; the Paulo Foundation; the Maud Kuistila Memorial Foundation; and the Emil Aaltonen Foundation (grant to P. A. T., M. K. L., and A. R.).
Potential conflicts of interest. J. A. L. has received research funds from Pfizer (to Harvard University) and consulting fees from Pfizer for unrelated studies. M. L. has received consulting fees/honoraria from Merck, Pfizer, Affinivax, and Antigen Discovery and grant support from Pfizer and PATH Vaccine Solutions (both to Harvard University) for unrelated studies. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 11th International Symposium on Pneumococci and Pneumococcal Diseases, Melbourne, Australia, 15–19 April 2018.
References
- 1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. . Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 2. Rovers MM. The burden of otitis media. Vaccine 2008; 26(Suppl 7):G2–4. [DOI] [PubMed] [Google Scholar]
- 3. Tähtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med 2011; 364:116–26. [DOI] [PubMed] [Google Scholar]
- 4. Hoberman A, Paradise JL, Rockette HE, et al. . Treatment of acute otitis media in children under 2 years of age. N Engl J Med 2011; 364:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vergison A, Dagan R, Arguedas A, et al. . Otitis media and its consequences: beyond the earache. Lancet Infect Dis 2010; 10:195–203. [DOI] [PubMed] [Google Scholar]
- 6. Lieberthal AS, Carroll AE, Chonmaitree T, et al. . The diagnosis and management of acute otitis media. Pediatrics 2013; 131:e964–99. [DOI] [PubMed] [Google Scholar]
- 7. Ovnat Tamir S, Shemesh S, Oron Y, Marom T. Acute otitis media guidelines in selected developed and developing countries: uniformity and diversity. Arch Dis Child 2017; 102:450–7. [DOI] [PubMed] [Google Scholar]
- 8. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009; 302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plasschaert AIO. Trends in doctor consultations, antibiotic prescription, and specialist referrals for otitis media in children: 1995–2003. Pediatrics 2006; 117:1879–86. [DOI] [PubMed] [Google Scholar]
- 10. Coco A, Vernacchio L, Horst M, Anderson A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics 2010; 125:214–20. [DOI] [PubMed] [Google Scholar]
- 11. Atkins KE, Flasche S. Vaccination to reduce antimicrobial resistance. Lancet Glob Health 2018; 6:e252. [DOI] [PubMed] [Google Scholar]
- 12. Lipsitch M, Colijn C, Cohen T, Hanage WP, Fraser C. No coexistence for free: neutral null models for multistrain pathogens. Epidemics 2009; 1:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guillemot D, Carbon C, Balkau B, et al. . Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998; 279:365–70. [DOI] [PubMed] [Google Scholar]
- 14. Ghaffar F, Muniz LS, Katz K, et al. . Effects of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children with acute otitis media. Clin Infect Dis 2000; 31:875–80. [DOI] [PubMed] [Google Scholar]
- 15. Hoberman A, Paradise JL, Rockette HE, et al. . Shortened antimicrobial treatment for acute otitis media in young children. N Engl J Med 2016; 375:2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCormick DP, Chonmaitree T, Pittman C, et al. . Nonsevere acute otitis media: a clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics 2005; 115:1455–65. [DOI] [PubMed] [Google Scholar]
- 17. Lipsitch M. Measuring and interpreting associations between antibiotic use and penicillin resistance in Streptococcus pneumoniae. Clin Infect Dis 2001; 32:1044–54. [DOI] [PubMed] [Google Scholar]
- 18. Atkins KE, Lafferty EI, Deeny SR, Davies NG, Robotham JV, Jit M. Use of mathematical modelling to assess the impact of vaccines on antibiotic resistance. Lancet Infect Dis 2017; pii:S1473-3099(17)30478-4. [DOI] [PubMed] [Google Scholar]
- 19. Ginsburg AS, Klugman KP. Vaccination to reduce antimicrobial resistance. Lancet Glob Health 2017; 5:e1176–7. [DOI] [PubMed] [Google Scholar]
- 20. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 7.0, 2017. 2017. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf. Accessed 1 March 2018. [Google Scholar]
- 21. Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Databse Syst Rev 2015; CD000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schrag SJ, Peña C, Fernández J, et al. . Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA 2001; 286:49–56. [DOI] [PubMed] [Google Scholar]
- 23. Feikin DR, Dowell SF, Nwanyanwu OC, et al. . Increased carriage of trimethoprim/sulfamethoxazole-resistant Streptococcus pneumoniae in Malawian children after treatment for malaria with sulfadoxine/pyrimethamine. J Infect Dis 2000; 181:1501–5. [DOI] [PubMed] [Google Scholar]
- 24. Halloran ME, Struchiner CJ, Longini IM Jr. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol 1997; 146:789–803. [DOI] [PubMed] [Google Scholar]
- 25. Skalet AH, Cevallos V, Ayele B, et al. . Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med 2010; 7:e1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melander E, Ekdahl K, Jönsson G, Mölstad S. Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr Infect Dis J 2000; 19:1172–7. [DOI] [PubMed] [Google Scholar]
- 27. Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365:579–87. [DOI] [PubMed] [Google Scholar]
- 28. Sommer MO, Dantas G. Antibiotics and the resistant microbiome. Curr Opin Microbiol 2011; 14:556–63. [DOI] [PubMed] [Google Scholar]
- 29. Lewnard JA, Givon-Lavi N, Huppert A, et al. . Epidemiological markers for interactions among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in upper respiratory tract carriage. J Infect Dis 2016; 213:1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang SS, Hinrichsen VL, Stevenson AE, et al. . Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 2009; 124:e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andam CP, Worby CJ, Gierke R, McGee L, Pilishvili T, Hanage WP. Penicillin resistance of nonvaccine type pneumococcus before and after PCV13 introduction, United States. Emerg Infect Dis 2017; 23:1012–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.