Abstract
Varicella zoster virus (VZV) infects and becomes latent in sensory, enteric, and other autonomic neurons during the viremia of varicella. Reactivation of VZV in neurons that project to the skin causes the rash of zoster; however, reactivation of VZV in enteric neurons can cause a painful gastrointestinal disorder (“enteric zoster”) without cutaneous manifestations. Detection of VZV DNA in saliva of patients with gastrointestinal symptoms may suggest enteric zoster. This diagnosis is reinforced by observing a response to antiviral therapy and can be confirmed by detecting VZV gene products in intestinal mucosal biopsies. We developed an in vivo guinea pig model that may be useful in studies of VZV latency and reactivation. VZV-infected lymphocytes are used to induce latent infection in sensory and enteric neurons; evidence suggests that exosomes and stimulator of interferon genes (STING) may, by preventing proliferation play roles in the establishment of neuronal latency.
Keywords: enteric zoster, guinea pig model, latency, reactivation, saliva
It has long been recognized that varicella-zoster virus (VZV) infects and becomes latent in neurons in ganglia of the cranial nerves (CNG) and dorsal roots (DRG) [1, 2]. That VZV also establishes latency in autonomic ganglia was only recently appreciated [3]. Reactivation of VZV in neurons of the CNG and DRG causes zoster (shingles) when virions are conducted down the processes of the neurons to infect their cutaneous targets. However, cutaneous manifestations may not occur when VZV reactivates in autonomic neurons that do not project to the skin [4]. Giant cell arteritis is an example of an occult form that zoster takes when latent VZV reactivates in sympathetic ganglia [5]. Another set of autonomic ganglia in which VZV latency occurs and virus can reactivate is the enteric nervous system (ENS) [6–9]. The ENS is by far the largest group of ganglia in the peripheral nervous system and the only one that can control the behavior of an organ, even in the absence of input from the brain or spinal cord [10, 11]. The ENS provides the bowel with its intrinsic innervation; however, the gut also receives an extrinsic innervation from neurons that reside in CNG, DRG, and sympathetic ganglia. Reactivation of VZV, within the ENS or in any of the extrinsic neurons that project to the bowel, can infect gastrointestinal (GI) targets and cause “enteric zoster.”
Before latency of VZV in the ENS was known, the association of VZV with GI disorders was made serendipitously when patients with a serious GI disease, such as pseudoobstruction (Ogilvie’s syndrome), required surgical intervention and VZV was discovered in resected tissue screened for infective agents [12–18]. Varicella-zoster virus has also been associated similarly with inflammatory bowel disease [19, 20] and perforated ulcers [21]. Enteric zoster from reactivation of vaccine-type virus (vOka) has been found to cause perforating gastric ulcers without cutaneous manifestations [8]. Because the neurons that convey active VZV to visceral and vascular targets do not innervate the skin, reactivation of VZV in autonomic neurons may cause serious disease of the vasculature or the viscera without rash, making it difficult to diagnose.
MECHANISMS BY WHICH LATENCY IS ESTABLISHED IN NEURONS
When VZV establishes latency in neurons of DRG and CNG, the virus may reach nerve cell bodies via retrograde transport from sensory nerve terminals during varicella (chickenpox). Sensory nerve terminals are intraepidermal and thus become bathed with infectious virions released from infected neighboring epithelial cells [22]. Alternatively, a T-cell viremia, which occurs during varicella [23], might transport VZV to neurons of DRG and CNG [2]. After its inhalation, VZV proliferates in the tonsils and is carried in skin-homing T cells to the epidermis [24–26], where VZV slowly overcomes innate immunity to cause epidermal disruption [27]. Cutaneous zoster constitutes a return of VZV to skin, this time via anterograde transport [28]. Although sites on the skin where varicella vesicles were most numerous are often those where zoster appears [1, 29], zoster can occur even in skin sites that were unaffected during varicella. Studies with live-attenuated varicella vaccine compellingly demonstrate this phenomenon. It was originally thought that zoster in vaccinees mainly involved the vaccinated area of the skin [30]. However, it is now apparent that vaccinees may develop vOka-caused zoster in locations far from the vaccination site [31, 32]. It is likely that a viremia, which has been detected in immunocompromised patients [33], occurs after vaccination and infects DRG, CNG, sympathetic ganglia, and/or the ENS. Although it may seem counterintuitive to postulate a viremia in the absence of systemic symptoms, VZV deoxyribonucleic acid (DNA) has been demonstrated in DRG removed at autopsy from vaccinated children who never had a pre-mortem VZV rash [6]. There is no reason for T cells transporting VZV during a viremia to limit their infectivity to neurons of DRG and CNG. Thus, it is ikely that the mechanism responsible for initial infection of autonomic ganglia, which do not project to the skin, is the viremia of varicella or that after varicella vaccination.
The mechanism by which T lymphocytes carrying VZV during the viremia of varicella transmit VZV to neurons enabling the virus to establish latent and not lytic infection is unknown. We found that exposure of isolated guinea pig enteric neurons to cell-associated VZV in fibroblasts results in a lytic and lethal infection [34]. Before their death, the neurons express immunocytochemically detectable late proteins, including gE, gI, and gB, as well as immediate early proteins, including ORFs 29p, 62p, and 63p, each of which are intranuclear. In contrast, exposure of isolated neurons to cell-free VZV resulted in a latent infection in which the neurons survived for weeks. Neurons with latent infection failed to express late proteins; however, low levels of transcripts and immunocytochemically detectable immediate early proteins, especially ORF63p, were found in their cytoplasm. Strikingly, the inclusion of uninfected fibroblasts with cell-free VZV in the inoculum caused a lytic infection the neurons. Evidence that infection with only cell-free VZV in isolated enteric neurons was latent and not abortive is that expression of ORF61 (or its herpes simplex virus [HSV] orthologue, ICP0) in the guinea pig enteric neurons reactivated VZV [6, 35]. Evidence of reactivation included expression of late proteins, nuclear translocation of immunofluorescence of the immediate early proteins, production of electron microscopically visible virions, transmission of infection to cocultured MeWo cells, and death of the neurons within 72 hours of expression of ORF61. More importantly, a mutant VZV lacking ORF61 was able to infect isolated guinea pig enteric neurons, but, in contrast to wild-type (WT) VZV, the ORF61 null VZV failed to produce lytic infection of neurons, even in the presence of fibroblasts [36]. ORF61p is not a structural protein; therefore, cells infected with cell-free VZV receive an inoculum that lacks ORF61p. Therefore, we initially postulated that VZV might only establish latency in neurons when infection occurs in the absence of ORF61p, that is, when cell free virions, rather than infected cells, transmit VZV to neurons.
Therefore, we tested the hypothesis that infected lymphocytes release intact varicella virions, which, because they lack ORF61p, establish latency in neurons. We found that both guinea pig and human peripheral blood mononuclear cells (PBMCs) could be infected in vitro with VZV [37]. Immunocytochemical and electron microscopic studies showed that the VZV-infected cells in the PBMC population were primarily CD3-immunoreactive T lymphocytes. When VZV-infected PBMCs were coincubated with isolated guinea pig enteric neurons, the VZV-infected lymphocytes in the PBMC population were able to transfer infection to the neurons, which was invariably latent (Figure 1). Thus, lymphocytes differ from other VZV-infected cells, such as fibroblasts. To determine whether VZV-infected lymphocytes in PBMC populations release infectious VZV, the infected PBMCs were grown in Transwell assemblies over a filter with a pore diameter of 0.4 µm [37]. A reporter layer of HELF cells was grown below the filters, and plaques were assayed to assess the release of cell-free VZV from the infected PBMCs. Although infectious virions readily passed through the filters when cell-free VZV was placed above the filter as a positive control, there was essentially no transfilter passage of infectious material from VZV-infected PBMCs grown over the filters. Thus, infected lymphocytes do not appear to release infectious virions.
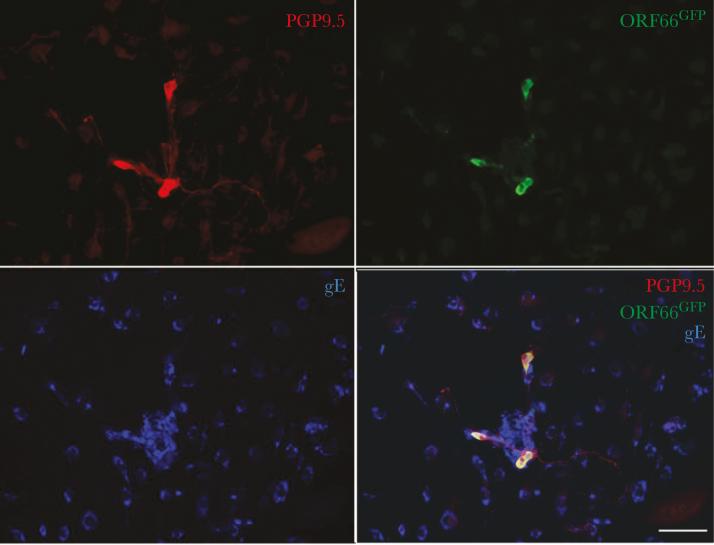
Figure 1.
VZVORF66.GFP infection of isolated enteric neurons. The longitudinal muscle and adherent myenteric plexus were dissected from the guinea pig small intestine and dissociated with collagenase. The ganglia were manually selected under microscopic control, isolated, and grown in culture. Peripheral blood mononuclear cells (PBMCs) were obtained from a donor guinea pig and cocultured with VZVORF66.GFP to infect T cells in the population. The VZVORF66.GFP-infected PBMCs were then cocultured with the infected neurons. After 1 week in culture, the cells were fixed and immunocytochemically examined as whole mounts. The immunoreactivities of varicella-zoster virus (VZV) glycoprotein E ([gE] blue), a neuronal marker, PGP9.5 (red), and green fluorescent protein (GFP) were visualized. Coincident immunostaining of PGP9.5 and GFP is found in neurons, which do not contain gE immunoreactivity. Many gE-immunoreactive nonneuronal cells are seen, many of which cluster around the neurons. The bar = 100 µm.
If VZV-infected lymphocytes are not a source of cell-free VZV, why then is the infection they transmit to neurons different from that transmitted by fibroblasts, which is lytic and rapidly lethal? Fusion of infected lymphocytes with neuronal targets might occur and would be a likely mechanism of neuronal infection; however, fusion of VZV-infected lymphocytes with neurons remains to be demonstrated, and an additional lymphocyte-specific intervention would have to occur to assure that the resulting VZV infection is latent. Therefore, we tested the hypotheses that VZV-infected lymphocytes secrete exosomes with content that inhibits VZV proliferation in neurons, and that this inhibition allows VZV to establish latency. Exosomes are extracellular vesicles (30- to 129-nm diameter) [38]. They are derived either from multivesicular bodies (MVBs) or plasma membrane (PM); MVBs are a subset of endosomes within which vesicles bud into the MVB lumen. If MVBs fuse (exocytosis) with the PM, they release their intraluminal vesicles, including exosomes, to the extracellular space. After release, exosomes can fuse with the plasma membrane of target cells and deliver their contents (protein, micro-ribonucleic acid [RNA], messenger RNA) to the target’s cytosol. Cells infected with HSV1 have recently been shown to export stimulator of interferon (IFN) genes (STING) in exosomes, which in turn deliver STING to noninfected cells [39]. STING is a dimeric 379 amino acid endoplasmic reticulum protein that acts as a DNA sensor [40, 41]. It is interesting to note that HSV-1 enables STING export but does not induce STING or an exosome marker (CD9) to accumulate in infected cells [39]. STING initiates transcription of innate immune genes, including type I IFNs, which inhibit viral proliferation. It seems curious that cells, in which HSV1 is produced, would export a molecule such as STING for delivery to uninfected cells. Most products of herpesvirus genes foster viral growth, but STING is an innate immune sensor that is inimical to viral propagation. Possible explanations include modulating viral proliferation to prevent the host from becoming overwhelmed [42], but, also, if transferred from VZV-infected lymphocytes to neurons in exosomes, STING might prevent VZV from proliferating [43, 44] and thereby promote latency. This would be analogous to the ability of acyclovir and IFN-α, which interfere with viral proliferation, to facilitate establishment of HSV latency [45].
In support of the STING-induced latency hypothesis, we have found that VZV infection greatly increases STING immunoreactivity in human, guinea pig, and mouse lymphocytes; moreover, transcripts encoding STING are selectively concentrated (~100-fold) in exosomes that VZV-infected lymphocytes secrete. STING is not similarly concentrated in exosomes that noninfected lymphocytes secrete. In vivo, guinea pig enteric neurons do not express STING immunoreactivity; however, these neurons become STING-immunoreactive when VZV establishes latency (see below). Thus far, the data are consistent with the hypothesis that exosomal transfer of STING transcripts from lymphocytes to neurons causes the neurons to accumulate STING and express type 1 IFNs, which inhibit VZV proliferation and thus promote latency. To confirm the hypothesis, it will be necessary to prevent the transfer of STING from VZV-infected lymphocytes to neurons and then to show that the VZV infection that lymphocytes transfer to neurons becomes lytic, rather than latent.
STUDIES OF VARICELLA-ZOSTER VIRUS IN HUMANS
Our determination that latent infection can be established in guinea pig enteric neurons in vitro [34], raised the question of whether VZV can establish latent infection in the human ENS. More importantly, the ENS can readily be obtained from living subjects and, for studies of VZV, ought to be preferable to DRG and CNG, which can only be obtained post-mortem. Therefore, we obtained full-thickness GI biopsies from normal regions of the bowel of patients who were undergoing surgery for reasons unrelated to VZV infection. Biopsies were obtained from 13 children who had either experienced natural varicella (n = 6) or who had received varicella vaccine (n = 7). Controls consisted of similar GI biopsies from infants who had not experienced either varicella or immunization (n = 7). The DNA and transcripts encoding at least 1 VZV gene product were detected in 12 of the 13 patients that had been exposed to VZV (through varicella or vaccination) and in none of the 7 controls. Transcripts encoding ORF63 (11 of 13) were found most frequently, followed by those encoding ORF4 (8 of 13) and ORF66 (2 of 13). Transcripts encoding the late gene products gB and gE were not detected, suggesting that VZV is latent in human bowel [6, 7]. These data are consistent with the idea that VZV establishes latency in enteric neurons after episodes of varicella or vaccination against varicella. Varicella-zoster virus DNA has also been detected in autopsied human gut, which confirms these data [46]. It is not clear why VZV transcription in human trigeminal ganglia obtained from autopsies carried out 3.7–9 hours after death appears to be more restricted than that occurring in biopsies of pediatric bowel [47]. It is conceivable that transcription during VZV latency in enteric and trigeminal ganglia is different. Alternatively, patterns of VZV transcription during latency in children and adults are not the same; adult neurons in autopsy specimens, for example, presumably have harbored latent VZV for a much longer time than neurons in intestinal biopsies of children. Vesicular gastritis has been observed during episodes of varicella [48], which demonstrates that the ENS is a target of VZV; however, lytic infection of the ENS of this type is rare and latency evidently is much more commonly established. The more common serendipitous observations of VZV-induced bowel disease discussed above all occurred in adults and were probably instances of enteric zoster, the reactivation of VZV within enteric neurons. In 3 patients with disseminated zoster with high blood levels of viral DNA and severe abdominal pain, VZV DNA has been detected in stool, confirming the occurrence of enteric zoster [9]. There is also evidence of early reactivation of VZV in the esophagus in a case of congenital varicella syndrome [49].
The hidden nature of VZV infection of the viscera or vasculature makes the development of a noninvasive diagnostic test to identify VZV a high priority. The appearance of DNA encoding VZV gene products in saliva of patients in which a VZV infection is active has recently emerged as such a diagnostic tool. The discovery of this phenomenon followed from the observation that salivary VZV DNA was detected in astronauts who experienced asymptomatic reactivations of VZV as a consequence of space travel [50, 51]. The stress and/or immunosuppression associated with space travel also seems to provoke the asymptomatic reactivation of other herpesviruses, indicated by the presence of DNA encoding their viral gene products in saliva [52]. The observations on astronauts led to the investigation of the utility of using saliva to diagnose VZV infections. Salivary VZV DNA was found both in patients with varicella [53] and zoster [54]. Moreover, VZV DNA persists in saliva of patients with zoster long after the rash has cleared, although the presence of salivary VZV DNA was found not to be related to the development of post herpetic neuralgia [55]. Subsequently, the appearance of salivary VZV DNA was found to occur transiently without evidence of VZV infection in children experiencing the severe stress of hospitalization [56]; moreover, no VZV DNA was observed in random samples of saliva of 80 adult controls [57]. These studies suggested that any active VZV infection, even one that is occult, might lead to the appearance of VZV DNA in saliva. Therefore, we tested the hypothesis that GI reactivation of VZV can be revealed by finding salivary VZV DNA in patients with GI symptoms, such as unexplained persistent abdominal pain [8]. Although we found that 0 of 20 healthy controls had VZV DNA in saliva, salivary VZV DNA was present in 13 of 18 (72%; P < .0001) positive controls, consisting of patients in whom the diagnoses of zoster, zoster sine herpete, or varicella had been made clinically. Surprisingly, salivary VZV DNA was found in 11 of 24 (46%; P < .001) subjects with unexplained abdominal pain but in none of 5 subjects (0%) with unrelated GI disorders. In 8 of 8 subjects with abdominal pain, both the pain and salivary VZV DNA disappeared within 1 week on treatment with valacyclovir. Salivary VZV DNA also disappeared after the pain resolved in all patients.
One of the patients with abdominal pain and salivary VZV DNA was a 16-year-old boy who presented with the sudden development of perforated gastric ulcers. This necessitated a wedge gastrectomy to close the bowel and stop the bleeding. Helicobacter was not detected; however, the presence of salivary VZV DNA led to an examination of the surgically removed tissue. Varicella-zoster virus DNA (vaccine-type; vOka) was discovered in the resected stomach; immediate early (ORF63p) and late (gE) VZV proteins were immunocytochemically detected in the patient’s gastric epithelium. More importantly, no surviving enteric neurons were found despite the use of multiple neural markers, suggesting that the tissue had become denervated due to the acute illness. After recovery, VZV DNA and proteins were not detected in either gastric biopsies or saliva. Because this patient had been vaccinated and the virus was vaccine-type, he clearly had enteric zoster (reactivation). Despite the evident reactivation of vOka to cause the patient’s disease, no obvious immunological abnormalities were detected. Still, these data are consistent with the idea that salivary VZV DNA can be used as a diagnostic marker, suggesting enteric zoster in patients with severe unexplained abdominal pain [58]. Tests of stool [9] in patients found to have VZV DNA in saliva may help to confirm the diagnosis.
Testing of saliva for VZV DNA to find potential cases of enteric zoster has recently revealed 2 additional patients in whom analyses of GI tissue confirmed the diagnosis. One was a 9-year-old boy who presented with an immunological abnormality of dysfunctional natural killer (NK) cells, acute zoster on his buttock, and unexplained severe abdominal pain. Varicella-zoster virus DNA and transcripts encoding ORF62, ORF63, ORF67, and ORF68 were detected in colonic mucosal biopsies. The immunoreactivities of VZV gB and the neuronal marker, PGP9.5, were coincident in terminal axons apparently innervating gB-immunoreactive neuroendocrine cells in crypts of the colonic mucosal epithelium. Sequencing of VZV ORF62 revealed that the responsible virus was vaccine type. The patient’s abdominal pain abated, and salivary VZV DNA disappeared after treatment with acyclovir. Because the virus involved was vOka, reactivation of VZV must have been responsible for the colonic disease; moreover, the relationship of VZV-infected nerve terminals to infected enteroendocrine cells suggests that VZV-infected nerves transmit VZV to epithelial cells they innervate. Another patient was a young adult male with Crohn’s disease receiving prednisone who developed severe abdominal pain. He had a history of varicella at 18 months of age and was not vaccinated. His previous history included “several” rashes thought to be recurrent zoster. Salivary VZV DNA was found during the investigation of his abdominal pain, which led to treatment with valaciclovir, after which salivary VZV DNA disappeared. A colonic mucosal biopsy was obtained but unfortunately not before valacyclovir was administered. Although VZV DNA was found in the colonic mucosa, no transcripts encoding any VZV gene products were detected. The presence of VZV DNA without transcripts in the colonic mucosa is consistent with the idea that an active infection with VZV had been present but that the virus was no longer active and transcribing gene products. Viral DNA, perhaps even in fragments, may persist in phagocytes that have ingested the remains of infected cells. The enteric zoster in this patient was presumably due to WT VZV because he had a history of varicella. The relationship of VZV and the recurrent zoster to the patient’s Crohn’s disease merits further investigation.
GUINEA PIG MODEL OF VARICELLA-ZOSTER VIRUS INFECTION, LATENCY, AND REACTIVATION
Although the burden of VZV-induced GI disease cannot yet be assessed, our observations made using salivary VZV DNA as a noninvasive marker suggest that enteric zoster may be far more common than currently realized. It is difficult to investigate mechanisms of VZV latency and reactivation in the human ENS. Therefore, we developed a guinea pig model of VZV latency and reactivation to learn how VZV establishes and maintains latency in enteric neurons, what provokes its reactivation, and the manifestations of enteric zoster [37]. Intravenous injection of human or guinea pig VZV-infected PBMCs (106 cells in 200 mL), primarily CD3+ T lymphocytes, leads in recipient guinea pigs, first to transient infection of macrophages in lung and liver, and then to latent infection of virtually all enteric and DRG/CNG neurons. In these experiments, in situ hybridization with a probe designed to detect DNA encoding VZV ORF54 was used to identify cells harboring latent VZV. Immunologically intact hairless guinea pigs were used to facilitate detection of rash. In addition to the systemic infection with intravenous VZV, we restricted infection by injecting VZV directly into the wall of the gut during laparotomy or into multiple (~100) cutaneous sites. Under these conditions, latent infection was restricted, respectively, to the injected region of the bowel or to the region of the gut innervated by the DRGs that project to the injected skin. Small numbers of DRG neurons of both guinea pig and mouse were found (with 2 retrograde tracers) to project both to the skin and to the gut [7].
We used the systemic model of VZV latency in guinea pigs to test the hypothesis that stress and/or immunosuppression reactivates VZV. To produce latent infection, PBMCs were isolated, infected with VZV expressing green fluorescent protein (GFP) under the control of the promoter for ORF66 (VZVORF66.GFP), and injected intravenously into hairless guinea pigs [59]. The immunoreactivities of VZV ORF63, gE, or GFP were used to identify VZV-infected cells in recipient guinea pigs. Animals remained asymptomatic for up to 4 months, although almost all enteric and DRG neurons expressed the immunoreactivities of ORF63 (Figure 2) and GFP but not that of gE. Immunosuppression was induced with tacrolimus (1 mg/kg per day), after which intravenous corticotrophin-releasing hormone (4 g/kg) was given to mimic stress. Controls included (1) uninfected animals receiving tacrolimus + corticotrophin releasing hormone (CRH) and (2) VZV-infected animals treated only with tacrolimus or (3) only with CRH. One week later, 5 of 5 VZVORF66.GFP-infected animals that had received both tacrolimus + CRH (but no controls) became anorectic, lost up to 20% body weight, and acquired a segmental green fluorescent rash. Varicella-zoster virus DNA was detected in saliva and transcripts encoding gE and gI, were found in blood, colon, ileum, DRG, liver, pancreas, heart, skin, and kidney. Blood chemistries were abnormal, suggesting multiorgan disease and carditis. Scattered GFP-expressing enteric and DRG neurons coexpressed gE as did cardiomyocytes and cells of the region of the epidermis with rash. These observations suggest that immunosuppression, combined with CRH, induces VZV to reactivate in guinea pigs. The resulting syndrome resembled disseminated zoster. The intravenous injection of VZVORF66.GFP-infected lymphocytes produced a latent infection in almost all DRG and enteric neurons. To our knowledge, this is the first animal in which latent infection and reactivation of VZV has been induced. In a recent study, simian varicella virus has also been found to establish latency in the monkey ENS [46].
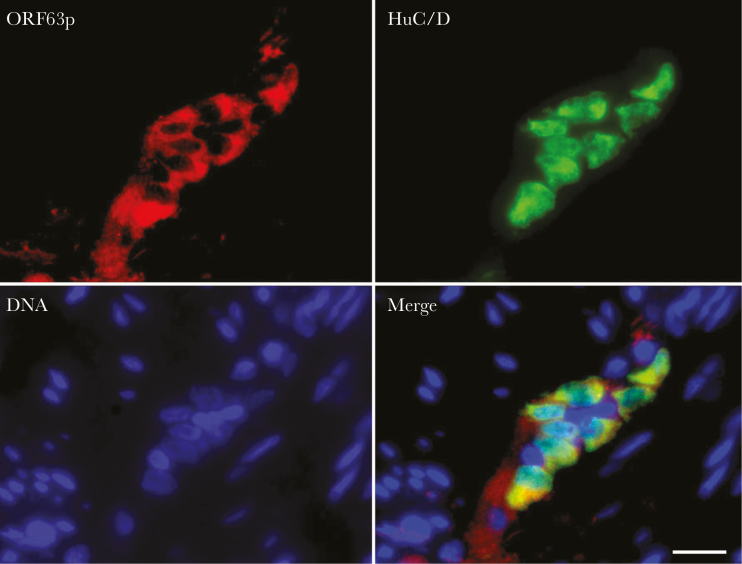
Figure 2.
Intravenous injection of varicella-zoster virus (VZV)-infected peripheral blood mononuclear cells (PBMCs) leads to latent infection of the guinea pig enteric nervous system (ENS). Four weeks after injection of VZV-infected PBMCs, the gut was removed and the longitudinal muscle and adherent myenteric plexus were dissected from the guinea pig small intestine, fixed, and prepared as whole mounts for immunocytochemistry. The immunoreactivity of VZV ORF63 (red) was detected in the cytoplasm of myenteric neurons identified with antibodies to the neuronal marker HuC/D. The bar = 36 µm.
CONCLUSIONS
The viremia of varicella [23], in which VZV is transported in T lymphocytes, carries VZV to the ENS [6, 7] and other autonomic neurons [3], which lack cutaneous projections. Severe pain and GI involvement is a dire sign when it occurs in varicella [48, 60–65]. Thus, our observation that VZV-infected CD3+ lymphocytes of guinea pigs and humans transmit latent infection to enteric neurons is highly significant. This still-to-be explained predilection of lymphocytes to deliver only latent VZV to neurons, including those of the ENS, is an adaptation that fosters survival of host and virus. The ENS is a bystander that becomes secondarily enmeshed in the viral web because of the viremia of varicella. Once in the ENS, however, VZV can reactivate. Small reactivations in the bowel might be controllable because the gut is a major immune organ and might even help to maintain long-term immunity to varicella. However, enteric zoster is not always a small reactivation; it can be devastating, as when it causes pseudoobstruction or perforating ulcers. The occult and internal nature of enteric zoster and its low index of suspicion have limited what is currently known about it. Our observations with salivary VZV DNA as a noninvasive marker suggest that enteric zoster may also be a common problem. The guinea pig model of VZV latency and reactivation may help to determine how VZV establishes latency in the ENS, what provokes its reactivation, the manifestations of enteric zoster, and, ultimately, its contribution to GI disease.
Notes
Acknowledgments. The authors thank Dr. Paul Kinchington for supplying VZV ORF 66.GFP for the guinea pig experiments.
Financial support. This work is funded by National Institutes of Health grant R01DK093094.
Supplement sponsorship. This work is part of a supplement sponsored by the Royal Society of Medicine (Royal Charter number RC000525) funded through unrestricted educational grants from Merck, Sanofi Pasteur MSD, The Research Foundation for Microbial Diseases of Osaka University, Seqirus and GlaxoSmithKline.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Stern ES. The mechanism of herpes zoster and its relation to chickenpox. Brit J Dermatol Syphil 1937; 49:264–71. [Google Scholar]
- 2. Arvin A, Gilden D. Molecular mechanisms of varicella zoster virus pathogenesis. In: Knipe D, Howley P, eds. Fields Virology. 6th ed Philadelphia, PA: Lippincott, Williams, and Wilkins, 2013: pp 2015–57. [Google Scholar]
- 3. Gilden DH, Gesser R, Smith J, et al. . Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 2001; 23:145–7. [DOI] [PubMed] [Google Scholar]
- 4. Richter ER, Dias JK, Gilbert JE II, Atherton SS. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis 2009; 200:1901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilden D, Nagel MA. Varicella zoster virus and giant cell arteritis. Curr Opin Infect Dis 2016; 29:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gershon AA, Chen J, Davis L, et al. . Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc 2012; 123:17–33; discussion 5. [PMC free article] [PubMed] [Google Scholar]
- 7. Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol 2011; 17:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gershon AA, Chen J, Gershon MD. Use of saliva to identify varicella zoster virus infection of the gut. Clin Infect Dis 2015; 61:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jong MD, Weel JF, van Oers MH, Boom R, Wertheim-van Dillen PM. Molecular diagnosis of visceral herpes zoster. Lancet 2001; 357:2101–2. [DOI] [PubMed] [Google Scholar]
- 10. Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 2005; 39:S184–93. [DOI] [PubMed] [Google Scholar]
- 11. Furness JB. The Enteric Nervous System. Malden, MA: Blackwell Publishing, 2006. [Google Scholar]
- 12. Edelman DA, Antaki F, Basson MD, Salwen WA, Gruber SA, Losanoff JE. Ogilvie syndrome and herpes zoster: case report and review of the literature. J Emerg Med 2010; 39:696–700. [DOI] [PubMed] [Google Scholar]
- 13. Pui JC, Furth EE, Minda J, Montone KT. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie’s syndrome). Am J Gastroenterol 2001; 96:1627–30. [DOI] [PubMed] [Google Scholar]
- 14. Giunta R, Marfella MA, Maffei A, Lucivero G. Herpes zoster infection and Ogilvie’s syndrome in non-Hodgkin’s lymphoma with hypogammaglobulinemia. Ann Ital Med Int 2001; 16:50–3. [PubMed] [Google Scholar]
- 15. Nomdedéu JF, Nomdedéu J, Martino R, et al. . Ogilvie’s syndrome from disseminated varicella-zoster infection and infarcted celiac ganglia. J Clin Gastroenterol 1995; 20:157–9. [DOI] [PubMed] [Google Scholar]
- 16. Alpay K, Yandt M. Herpes zoster and Ogilvie’s syndrome. Dermatology 1994; 189:312. [DOI] [PubMed] [Google Scholar]
- 17. Pai NB, Murthy RS, Kumar HT, Gerst PH. Association of acute colonic pseudo-obstruction (Ogilvie’s syndrome) with herpes zoster. Am Surg 1990; 56:691–4. [PubMed] [Google Scholar]
- 18. Caccese WJ, Bronzo RL, Wadler G, McKinley MJ. Ogilvie’s syndrome associated with herpes zoster infection. J Clin Gastroenterol 1985; 7:309–13. [DOI] [PubMed] [Google Scholar]
- 19. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai SY, Yang TY, Lin CL, Tsai YH, Kuo CF, Kao CH. Increased risk of varicella zoster virus infection in inflammatory bowel disease in an Asian population: a nationwide population-based cohort study. Int J Clin Pract 2015; 69:228–34. [DOI] [PubMed] [Google Scholar]
- 21. Milligan KL, Jain AK, Garrett JS, Knutsen AP. Gastric ulcers due to varicella-zoster reactivation. Pediatrics 2012; 130:e1377–81. [DOI] [PubMed] [Google Scholar]
- 22. Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 2004; 119:915–26. [DOI] [PubMed] [Google Scholar]
- 23. Levin MJ. Varicella-zoster virus and virus DNA in the blood and oropharynx of people with latent or active varicella-zoster virus infections. J Clin Virol 2014; 61:487–95. [DOI] [PubMed] [Google Scholar]
- 24. Sen N, Arvin AM. Dissecting the molecular mechanisms of the tropism of varicella-zoster virus for human T cells. J Virol 2016; 90:3284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sen N, Mukherjee G, Sen A, et al. . Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Rep 2014; 8:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ku CC, Padilla JA, Grose C, Butcher EC, Arvin AM. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J Virol 2002; 76:11425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med 2004; 200:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gershon MD, Gershon AA. VZV infection of keratinocytes: production of cell-free infectious virions in vivo. Curr Top Microbiol Immunol 2010; 342:173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Head H,A.W. C. The pathology of herpes zoster and its bearing on sensory localization. Brain 1900; 23:353–523. [DOI] [PubMed] [Google Scholar]
- 30. Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med 1991; 325:1545–50. [DOI] [PubMed] [Google Scholar]
- 31. Gershon A, Takahashi M, Seward JF. Live attenuated varicella vaccine. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 6th ed Philadelphia, PA: WB Saunders, 2013: pp 837–69. [Google Scholar]
- 32. Weinmann S, Chun C, Schmid DS, et al. . Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis 2013; 208:1859–68. [DOI] [PubMed] [Google Scholar]
- 33. Ihara T, Kamiya H, Torigoe S, Sakurai M, Takahashi M. Viremic phase in a leukemic child after live varicella vaccination. Pediatrics 1992; 89:147–9. [PubMed] [Google Scholar]
- 34. Chen JJ, Gershon AA, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J Med Virol 2003; 70(Suppl 1):S71–8. [DOI] [PubMed] [Google Scholar]
- 35. Gershon AA, Chen J, Gershon MD. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis 2008; 197(Suppl 2):S61–5. [DOI] [PubMed] [Google Scholar]
- 36. Chen J, Gershon AA, Cohen JI, Silverstein S, Gershon M. ORF61P determines whether VZV infection of isolated enteric neurons is lytic or latent. In: 31 St Annual Herpesvirus Workshop July 2006; Seattle, WA. Abstract 2.26. [Google Scholar]
- 37. Gan L, Wang M, Chen JJ, Gershon MD, Gershon AA. Infected peripheral blood mononuclear cells transmit latent varicella zoster virus infection to the guinea pig enteric nervous system. J Neurovirol 2014; 20:442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 2018; 74:66–77. [DOI] [PubMed] [Google Scholar]
- 39. Kalamvoki M, Du T, Roizman B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc Natl Acad Sci U S A 2014; 111:E4991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Konno H, Barber GN. The STING controlled cytosolic-DNA activated innate immune pathway and microbial disease. Microbes Infect 2014; 16:998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abe T, Harashima A, Xia T, et al. . STING recognition of cytoplasmic DNA instigates cellular defense. Mol Cell 2013; 50:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalamvoki M, Roizman B. HSV-1 degrades, stabilizes, requires, or is stung by STING depending on ICP0, the US3 protein kinase, and cell derivation. Proc Natl Acad Sci U S A 2014; 111:E611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemos H, Huang L, McGaha T, Mellor AL. STING, nanoparticles, autoimmune disease and cancer: a novel paradigm for immunotherapy?Expert Rev Clin Immunol 2015; 11:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lemos H, Huang L, Chandler PR, et al. . Activation of the STING adaptor attenuates experimental autoimmune encephalitis. J Immunol 2014; 192:5571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pourchet A, Modrek AS, Placantonakis DG, Mohr I, Wilson AC. Modeling HSV-1 latency in human embryonic stem cell-derived neurons. Pathogens 2017; 6: doi: 10.3390/pathogens6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ouwendijk WJD, van Veen S, Mehraban T, Mahalingam R, Verjans G. Simian varicella virus infects enteric neurons and alpha4beta7 integrin-expressing gut-tropic T-cells in nonhuman primates. Viruses 2018; 10: doi: 10.3390/v10040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ouwendijk WJ, Choe A, Nagel MA, et al. . Restricted varicella-zoster virus transcription in human trigeminal ganglia obtained soon after death. J Virol 2012; 86:10203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baker CJ, Gilsdorf JR, South MA, Singleton EB. Gastritis as a complication of varicella. South Med J 1973; 66:539–41. [DOI] [PubMed] [Google Scholar]
- 49. Ussery XT, Annunziato P, Gershon AA, et al. . Congenital varicella-zoster virus infection and Barrett’s esophagus. J Infect Dis 1998; 178:539–43. [DOI] [PubMed] [Google Scholar]
- 50. Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol 2008; 80:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol 2004; 72:174–9. [DOI] [PubMed] [Google Scholar]
- 52. Payne DA, Mehta SK, Tyring SK, Stowe RP, Pierson DL. Incidence of Epstein-Barr virus in astronaut saliva during spaceflight. Aviat Space Environ Med 1999; 70:1211–3. [PubMed] [Google Scholar]
- 53. Leung J, Harpaz R, Baughman AL, et al. . Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis 2010; 51:23–32. [DOI] [PubMed] [Google Scholar]
- 54. Mehta SK, Tyring SK, Gilden DH, et al. . Varicella-zoster virus in the saliva of patients with herpes zoster. J Infect Dis 2008; 197:654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nagel MA, Choe A, Cohrs RJ, et al. . Persistence of varicella zoster virus DNA in saliva after herpes zoster. J Infect Dis 2011; 204:820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Papaevangelou V, Quinlivan M, Lockwood J, et al. . Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin Microbiol Infect 2013; 19:E245–51. [DOI] [PubMed] [Google Scholar]
- 57. Birlea M, Cohrs RJ, Bos N, Mehta SK, Pierson DL, Gilden D. Search for varicella zoster virus DNA in saliva of healthy individuals aged 20-59 years. J Med Virol 2014; 86:360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gershon AA, Chen J, Gershon MD. Use of saliva to identify varicella zoster virus infection of the gut. Clin Infect Dis 2015; 61:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol 2007; 81:9034–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peritz DC, Duncan C, Kurek K, Perez-Atayde AR, Lehmann LE. Visceral varicella zoster virus (VZV) after allogeneic hematopoietic stem cell transplant (HSCT) in pediatric patients with chronic graft-versus-host disease (cGVHD). J Pediatr Hematol Oncol 2008; 30:931–4. [DOI] [PubMed] [Google Scholar]
- 61. Leena M, Ville V, Veli-Jukka A. Visceral varicella zoster virus infection after stem cell transplantation: a possible cause of severe abdominal pain. Scand J Gastroenterol 2006; 41:242–4. [DOI] [PubMed] [Google Scholar]
- 62. Morgan ER, Smalley LA. Varicella in immunocompromised children. Incidence of abdominal pain and organ involvement. Am J Dis Child 1983; 137:883–5. [DOI] [PubMed] [Google Scholar]
- 63. Verdonck LF, Cornelissen JJ, Dekker AW, Rozenberg-Arska M. Acute abdominal pain as a presenting symptom of varicella-zoster virus infection in recipients of bone marrow transplants. Clin Infect Dis 1993; 16:190–1. [DOI] [PubMed] [Google Scholar]
- 64. Magi E. Severe varicella in an immunocompromised adult presenting with abdominal pain. West J Med 2000; 173:376–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rau R, Fitzhugh CD, Baird K, et al. . Triad of severe abdominal pain, inappropriate antidiuretic hormone secretion, and disseminated varicella-zoster virus infection preceding cutaneous manifestations after hematopoietic stem cell transplantation: utility of PCR for early recognition and therapy. Pediatr Infect Dis J 2008; 27:265–8. [DOI] [PubMed] [Google Scholar]