Abstract
Background
The aim was to evaluate the significance of arteriosclerosis obliterans-related biomarkers in patients with type 2 diabetes mellitus (T2DM), and to compare the effects of sarpogrelate, eicosapentaenoic acid (EPA) and pitavastatin on these markers.
Patients and methods
Seventy-two arteriosclerosis obliterans patients with T2DM were classified into two groups, pitavastatin with either sarpogrelate (PS) or EPA (PE). We observed no differences in all biomarkers between the PS and PE groups before treatments.
Results
The levels of body mass index, hemoglobin A1c, soluble E-selectin, soluble vascular cell adhesion molecule 1, plasminogen activator inhibitor-1 and platelet-derived microparticle in the PE group decreased significantly after treatment. The ankle branchial pressure index and adiponectin levels significantly increased in the PE group after treatment compared with the PS group.
Conclusion
These results suggest that combination therapy using pitavastatin and EPA possesses an antiatherosclerotic effect and may be beneficial for prevention of vascular complications in patients with T2DM.
Keywords: T2DM, pitavastatin, sarpogrelate, eicosapentaenoic acid, EPA, adiponectin, platelet-derived microparticle, PDMP
Introduction
Atherosclerosis is a progressive inflammatory process and is the major cause of death in patients with type 2 diabetes mellitus (T2DM).1,2 In particular, endothelial cells, monocytes and platelets play important roles in the development of atherosclerosis.2 Low-density lipoprotein (LDL) could be used to predict the onset and death of Japanese atherosclerosis-associated diseases.3 In addition, a large-scale clinical trial in Japan in which patients were examined for hypercholesterolemia and subsequently intervened in a lifestyle improvement program has reported that coronary diseases can be significantly controlled.4,5
The underlying pathology of arteriosclerosis obliterans (ASO) is basically atherosclerosis with often observed complete thrombi occlusions.6 The ASO-related biomarkers such as C-reactive protein (CRP), interleukin (IL)-6, soluble E-selectin (sE-selectin), soluble vascular cell adhesion molecule (sVCAM)-1 and plasminogen activator inhibitor (PAI)-1 are markedly elevated in patients with T2DM.7–11 In addition to these biomarkers, platelet-derived microparticle (PDMP) is significant in atherosclerosis in T2DM patients, because these patients typically display hypercoagulability and platelet hyperaggregability with increased levels of platelet activation markers.8,12–14 The PDMPs are generated by platelet activation and play roles in normal hemostatic responses to vascular injury.15–18 In addition, it is thought that PDMPs contribute to thrombin generation and thrombus formation by generating tissue factor.14,19,20 Therefore, PDMPs may ultimately cause vascular complications in T2DM with the participation of the blood coagulation system.
Another important biomarker in T2DM-related atherosclerosis is adiponectin.21–23 Adiponectin is an adipokine with anti-inflammatory, antiatherogenic and insulin-sensitizing properties.23 Adiponectin possibly suppresses the attachment of monocytes to endothelial cells,24 and stimulates nitric oxide production in vascular endothelial cells, which ameliorates endothelial function.21,24 In addition, adiponectin inhibits tissue factor expression and enhances tissue factor pathway inhibitor expression in human endothelial cells.25 These observations suggest that hypoadiponectinemia may be associated with an increased incidence of vascular disease in T2DM.
Therefore, it is thought that the novel therapeutic strategy of ASO-related biomarkers for lipid abnormality is important for atherosclerosis in T2DM patients. In particular, the countermeasure for platelet activation and endothelial dysfunction is necessary. We previously reported that the combination therapy of pitavastatin and eicosapentaenoic acid (EPA) may be beneficial for the prevention of vascular complication in hyperlipidemic patients with T2DM.26 Pitavastatin has various pleiotropic effects, resulting in the improvement of endothelial function and preventing the progression of aortic atherosclerosis.27,28
Studies have reported other effects of EPA on serum lipids, including a unique preventive effect against atherosclerotic disease and coronary events.29–31 However, the influence of other antiplatelet drugs besides EPA on pitavastatin treatment for ASO in T2DM patients is poorly understood. In our present study, we compared the effect of sarpogrelate32,33 or EPA on pitavastatin treatment for ASO in T2DM, focusing on the antiatherosclerotic properties of these combination therapies (SARpogrelate-EPA-PITavastatin-ASO [SAREPITASO] study). All three of these drugs have a common feature that raises the level of adiponectin.26,34–36
Patients and methods
Study design
The SAREPITASO study is a randomized, non-blinded, parallel-group study. We enrolled patients who satisfied all inclusion criteria. All study participants provided signed informed consent, and this study was approved by the ethics committee of Kansai Medical University, Hirakata, Osaka, Japan (no. 110614).
Study population
The study group included 84 T2DM patients and 50 non-diabetic controls. Between August 2012 and March 2016, we selected participants from T2DM patients admitted to our hospital for treatment. Entry criteria required that participants had no history of inflammatory, coronary artery or cerebrovascular disease, or no clinically detectable renal (serum creatinine ≥2.0 mg/dL), hepatic (elevated serum transaminase levels), infectious (fever or elevated white blood cell count) or malignant disease (based on ultrasound or computed tomography examination) within the 3 months before enrollment. Other antithrombotic agents were prohibited to avoid potential influence of data interpretation. The concomitant use of these drugs was fixed for a certain period before study entry.
Study protocol
Pitavastatin of 2 mg with either EPA 1,800 mg or sarpogrelate 300 mg was administered daily for 12 months (Figure 1). No other pharmacologic regimen changes occurred during treatment in any of the patients. Clinical and biochemical data obtained before and after pitavastatin with either EPA (PE group) or sarpogrelate (PS group) administration were compared. There were 19 patients using an angiotensin II receptor blocker (ARB) and 14 patients using a Ca antagonist for hypertension in T2DM. Of these patients, 52 were treated with sulfonylureas, 56 with α-glucosidase inhibitors, 47 with DPP4 inhibitor and 41 with insulin. The doses of drugs were not adjusted during the study. Hyperlipidemia was defined according to the Guidelines for Diagnosis and Treatment of Hyperlipidemias in Adults published by the Japan Atherosclerosis Society.37 The American Diabetes Association criteria were used to define T2DM.38 Table 1 shows the clinical characteristics of the T2DM patients and the control subjects.
Figure 1.
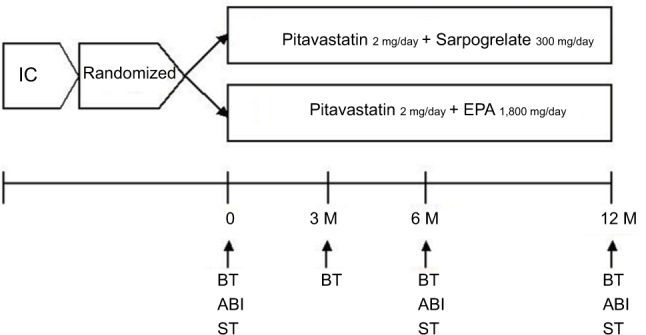
Schema of study protocol.
Abbreviations: ABI, ankle branchial pressure index; BT, blood test; EPA, eicosapentaenoic acid; IC, informed consent; M, months; ST, special test.
Table 1.
Demographic and clinical characteristics of the T2DM patients and nondiabetic controls
| Controls (n=50) | T2DM (n=84) | P-value | |
|---|---|---|---|
| Men/women (no.) | 29/21 | 48/36 | NS |
| Age (years) | 62±13 | 74±10 | <0.05 |
| ABI (ratio) | 1.13±0.25 | 0.93±0.23 | <0.05 |
| BMI (kg/m2) | 22.1±4.1 | 24.5±3.9 | <0.05 |
| FBG (mg/dL) | 108±37 | 129±42 | <0.01 |
| HbA1c (%) | 5.3±1.9 | 7.2±1.2 | <0.001 |
| TC (mg/dL) | 221±47 | 212±41 | NS |
| HDL-C (mg/dL) | 53±16 | 52±14 | NS |
| LDL-C (mg/dL) | 129±39 | 126±37 | NS |
| Risk factor, n (%) | |||
| Current smoking complication, n (%) | 5 (10.0) | 11 (13.1) | NS |
| Angina pectoris | 7 (14.0) | 15 (17.9) | NS |
| Heart failure | 2 (4.0) | 8 (9.5) | NS |
| Cerebral infarction | 4 (8.0) | 9 (10.7) | NS |
| Hypertension medication, n (%) | 11 (22.0) | 24 (28.6) | NS |
| ARBs | 10 (20.0) | 19 (22.6) | NS |
| Ca antagonists | 5 (10.0) | 14 (16.7) | NS |
| Aspirin | 7 (14.0) | 13 (15.5) | NS |
| Sulfonylureas | 0 (0.0) | 52 (61.9) | <0.01 |
| α-GI | 0 (0.0) | 56 (66.7) | <0.001 |
| DPP4-I | 0 (0.0) | 47 (55.9) | <0.01 |
| Insulin therapy | 0 (0.0) | 41 (48.8) | <0.01 |
Notes: Data are shown as mean±SD. P-value, T2DM patients vs nondiabetic controls.
Abbreviations: α-GI, α-glucosidase inhibitor; ABI, ankle brachial pressure index; ARB, angiotensin II receptor blocker; BMI, body mass index; DPP4-I, DPP4 inhibitor; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NS, not significant; T2DM, type 2 diabetes mellitus; TC, total cholesterol.
Endpoints
The primary endpoint was improvement of ankle brachial pressure index (ABI) within 12 months. The secondary endpoints were changes in levels of 1) body mass index (BMI); 2) total cholesterol, high-density lipoprotein cholesterol and LDL cholesterol; 3) glycated hemoglobin (HbA1c); 4) CRP, IL-6 and monocyte-chemoattractant protein (MCP)-1; 5) sE-selectin, sVCAM-1 and PAI-1; 6) PDMP and 7) adiponectin.
Assessment of PDMP
The PDMP levels were measured twice and the mean values were recorded. Furthermore, some basic studies were carried out before this assessment using clinical specimens. An enzyme-linked immunosorbent assay (ELISA) kit was used for PDMP measurements (JIMRO Co. Ltd., Tokyo, Japan).16
Measurements of cytokine, soluble factors and adiponectin
Fasting blood samples from patients and controls were collected into tubes either with or without sodium citrate and allowed to clot at room temperature for a minimum of 1 hour. Citrated plasma or serum samples were isolated by centrifugation at 1,000×g for 20 minutes at 4°C and stored at −30°C until analysis. Plasma concentrations of IL-6, MCP-1, sE-selectin, sVCAM-1 and PAI-1 were measured using monoclonal antibody-based ELISA kits (Thermo Fisher Scientific, Waltham, MA, USA), and plasma adiponectin was measured with adiponectin ELISA kits (Otsuka Pharmaceuticals Co. Ltd., Tokyo, Japan). The recombinant products and standard solutions provided with each kit were used as positive controls in each assay, and all procedures were performed according to the manufacturers’ instructions.
Statistical analysis
Data were expressed as mean±SD and were analyzed using multivariate regression analysis, as appropriate. Between-group comparisons were performed using the Newman–Keuls test and the Scheffe’s test. The correlation between uric acid concentration and continuous variables was assessed using multivariate linear regression analysis. The significance of differences among variables was determined by analysis of variance. P-values <0.05 were considered statistically significant. All analyses were performed using the StatFlex program (version 6).
Results
Omitted patients
There were no cardiovascular events, cases of cerebral infarction or cases of liver dysfunction. Five T2DM patients showed abnormal laboratory data (renal failure and infections) and three T2DM patients changed hospital. Therefore, we collected and analyzed data from 50 nondiabetic and 76 T2DM patients.
Clinical characteristics of T2DM patients
Age, BMI, fasting blood glucose and HbA1c were higher in T2DM patients than in nondiabetic controls (Table 1). However, ABI in T2DM patients was significantly lower than in nondiabetic controls. The values of total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol were similar in both groups before treatment (Table 1).
Univariate and multivariate regression analyses
We investigated the associations between 16 variables and ABI concentration in T2DM patients (Table 2). Univariate analysis showed that BMI, HbA1c, LDL, CRP, MCP-1, sE-selectin, sVCAM-1, PDMP, PAI-1 and adiponectin were factors significantly associated with ABI, whereas HbA1c, CRP, PDMP and PAI-1 were significantly correlated with ABI in multivariate analysis.
Table 2.
Multiple regression analysis of ABI in T2DM patients
| Analysis | Univariate
|
Multivariate
|
||
|---|---|---|---|---|
| β | P-value | β | P-value | |
| Age (years) | 0.2247 | 0.08956 | ||
| Sex (men) | −0.1128 | 0.31257 | ||
| BMI (kg/m2) | −0.3395 | 0.00343* | −0.2139 | 0.07914 |
| HbA1c (%) | −0.4216 | 0.00287* | −0.2969 | 0.04972* |
| UA (mg/dL) | 0.0219 | 0.56475 | ||
| TC (mg/dL) | −0.1869 | 0.11239 | ||
| HDL (mg/dL) | 0.2418 | 0.09774 | ||
| LDL (mg/dL) | −0.3125 | 0.00963* | −0.2537 | 0.05649 |
| CRP (mg/dL) | −0.4129 | 0.00166* | −0.3218 | 0.01024* |
| IL-6 (pg/mL) | 0.1433 | 0.13207 | ||
| MCP-1 (pg/mL) | −0.3127 | 0.01236 | −0.2027 | 0.08961 |
| sE-selectin (ng/mL) | −0.2686 | 0.04423* | −0.1366 | 0.09897 |
| sVCAM-1 (ng/mL) | −0.2762 | 0.04124* | −0.1966 | 0.09567 |
| PDMP (U/mL) | −0.5029 | 0.00012* | −0.3984 | 0.01356* |
| PAI-1 (ng/mL) | −0.5244 | 0.01029* | −0.4313 | 0.01129* |
| Adiponectin (μg/mL) | 0.3235 | 0.02867* | 0.2047 | 0.09833 |
Note:
P<0.05.
Abbreviations: ABI, ankle brachial pressure index; BMI, body mass index; CRP, C-reactive protein; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; IL-6, interleukin-6; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; PAI-1, plasminogen activator inhibitor-1; PDMP, platelet-derived microparticle; sE-selectin, soluble E-selectin; sVCAM-1, soluble vascular cell adhesion molecule 1; T2DM, type 2 diabetes mellitus; TC, total cholesterol; UA, uric acid.
Comparison of changes in all markers of PS or PE groups after treatment
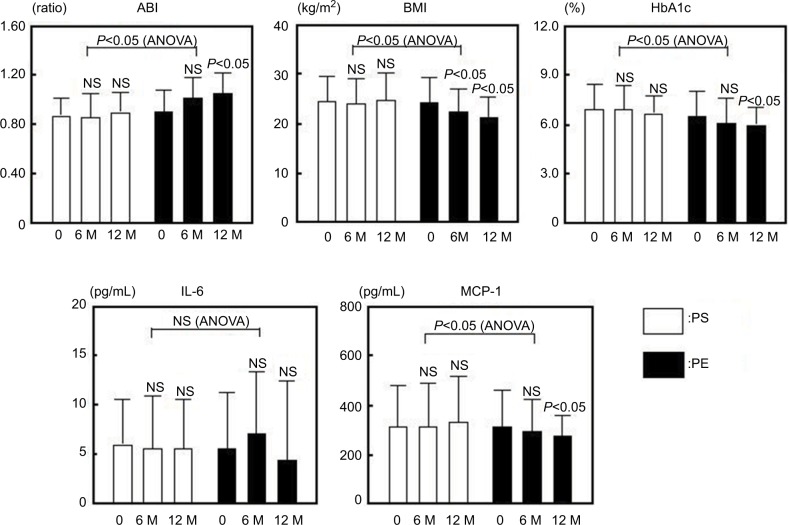
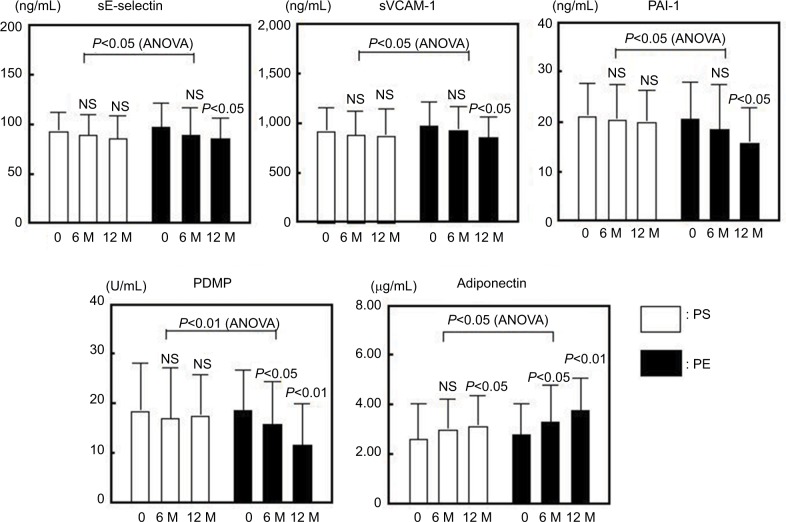
There were no significant differences in the clinical characteristics between the PS and PE groups before treatment (Table 3). The levels of ABI, BMI, HbA1c and MCP-1 improved significantly after treatment in the PE group, although the PS group did not exhibit a significant change (Figure 2). The sE-selectin, sVCAM-1, PDMP and PAI-1 levels decreased significantly in the PE group after treatment, although the PS group did not exhibit significant changes (Figure 3). However, both groups displayed a significant increase in adiponectin levels after treatment, whereas the PE group exhibited greater significant changes (Figure 3).
Table 3.
Demographic and clinical characteristics of the PS and PE groups
| PS (n=38) | PE (n=38) | P-value | |
|---|---|---|---|
| Men/women (no.) | 23/15 | 24/14 | NS |
| Age (years) | 72±10 | 76±9 | NS |
| ABI (ratio) | 0.94±0.19 | 0.93±0.21 | NS |
| BMI (kg/m2) | 24.8±5.5 | 24.3±3.1 | NS |
| FBG (mg/dL) | 123±39 | 127±44 | NS |
| HbA1c (%) | 7.3±1.3 | 7.1±0.9 | NS |
| TC (mg/dL) | 203±36 | 216±45 | NS |
| HDL-C (mg/dL) | 54±12 | 50±16 | NS |
| LDL-C (mg/dL) | 124±34 | 127±39 | NS |
| Risk factor, n (%) | |||
| Current smoking complication, n (%) | 4(10.5) | 6 (15.8) | NS |
| Angina pectoris | 8 (21.1) | 7 (18.4) | NS |
| Heart failure | 4 (10.5) | 3 (7.9) | NS |
| Cerebral infarction | 4 (10.5) | 4 (10.5) | NS |
| Hypertension medication, n (%) | 10 (26.3) | 13 (34.2) | NS |
| ARBs | 8 (21.1) | 8 (21.1) | NS |
| Ca antagonists | 6(15.8) | 5 (13.2) | NS |
| Aspirin | 6 (15.8) | 5 (13.2) | NS |
| Sulfonylureas | 25 (65.8) | 26 (68.4) | NS |
| α-GI | 24 (63.2) | 23 (60.5) | NS |
| DPP4-I | 28 (73.7) | 27 (71.1) | NS |
| Insulin therapy | 19 (50.0) | 22 (57.9) | NS |
Notes: Data are shown as mean±SD. P-value, PE vs PS.
Abbreviations: α-GI, α-glucosidase inhibitor; ABI, ankle brachial pressure index; ARB, angiotensin II receptor blocker; BMI, body mass index; DPP4-I, DPP4 inhibitor; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NS, not significant; PE, pitavastatin with eicosapentaenoic acid; PS, pitavastatin with sarpogrelate; TC, total cholesterol.
Figure 2.
Changes in ABI, BMI, HbA1c, IL-6 and MCP-1 in response to treatment with PS or PE in T2DM patients.
Notes: Bars show the mean±SD. 0: before; M: months (after). P-values are for comparison with each baseline parameter (0 vs 6 and 12 months). ANOVA (PS vs PE).
Abbreviations: ABI, ankle brachial pressure index; ANOVA, analysis of variance; BMI, body mass index; HbA1c, hemoglobin A1c; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; NS, nonsignificant; PE, pitavastatin with eicosapentaenoic acid; PS, pitavastatin with sarpogrelate; T2DM, type 2 diabetes mellitus.
Figure 3.
Changes in sE-selectin, sVCAM-1, PAI-1, PDMP and adiponectin in response to treatment with PS or PE of T2DM patients.
Notes: Bars show the mean±SD. 0: before M: months (after). P-values are for comparison with each baseline parameter (0 vs 6 and 12 months). ANOVA (PS vs PE).
Abbreviations: ANOVA, analysis of variance; NS, nonsignificant; PAI-1, plasminogen activator inhibitor-1; PDMP, platelet-derived microparticle; sE-selectin, soluble E-selectin; sVCAM-1, soluble vascular cell adhesion molecule 1; PE, pitavastatin with eicosapentaenoic acid; PS, pitavastatin with sarpogrelate; T2DM, type 2 diabetes mellitus.
Discussion
Various factors are involved in diabetic vascular changes, one of which is platelet activation.8,12,39,40 Some platelet activation markers are elevated in patients with T2DM.8,13 The marker PDMP, which is derived from activated platelets, plays an important role in the process of coagulation, so that an increase of PDMP causes hypercoagulability.18 We previously reported that PDMPs were significantly increased in diabetic patients with high LDL levels compared with similar patients who had low LDL levels.13 Because PDMP enhanced the expression of adhesion molecules on monocytes and endothelial cells,41 it seems possible that PDMP may participate in the development or progression of atherosclerosis in patients with diabetes. The potential incapability of intensive antiplatelet drugs (cilostazol and ticlopidine) was previously reported.42,43 In addition, these drugs can inhibit the elevation of PDMP.44,45 However, the use of these drugs for primary prevention of atherothrombosis is problematic. For this reason, we evaluated EPA and sarpogrelate. We classified patients with diabetes into two groups according to combination therapy of either PE or PS. Interestingly, T2DM patients with PE exhibited a significant decrease of PDMP after treatment. These results correspond with previous reports on the microvascular complications of diabetes.26,35
In patients with T2DM, a significant decrease in plasma adiponectin which prevents vascular complications has been found.21,24,46 In particular, we evaluated the efficacy of PS and PE therapy for hypoadiponectinemia and found that not only PE but also PS improves hypoadiponectinemia in T2DM. In addition, PE exhibited a better effect for adiponectin compared with PS. This result suggests that PE may exert its antiatherosclerotic effect via modification of adiponectin levels, thus contributing to the adiponectin-dependent PDMP-reducing action. Although the exact mechanism by which PE treatment leads to an increase in circulating adiponectin levels remains unclear, we previously reported about the role of the peroxisome proliferator-activated receptor gamma which can be activated by EPA.36,47
Hypoadiponectinemia is associated with endothelial dysfunction21 and appears to cause platelet activation, since the levels of nitric oxide, which regulates platelet activation, are decreased in patients with hypoadiponectinemia. It has been reported that adiponectin stimulates the production of nitric oxide in vascular endothelial cells,24,48 resulting in platelet activation at low concentrations of nitric oxide. Thus, the increase of adiponectin by EPA may exert a platelet-inhibitory effect via nitric oxide. Our results suggest that EPA possesses multifunctional antithrombotic properties, and that its action is dependent on adiponectin. In particular, the significant reduction of ABI in the PE group might be important, because ABI has diagnostic significance for peripheral artery disease (PAD); the exacerbation of PAD is related to fatty acid and the abnormality of fatty acid is a death risk factor of events of the lower limbs.49,50 Furthermore, statin can control the outcome of lower limbs in PAD.51 Therefore, PE treatment is considered a useful strategy for the progression of vascular events in patients with T2DM.
Conclusion
PE could increase ABI and circulating adiponectin levels in T2DM. In addition, PE could improve PDMP and endothelial activation markers such as sVCAM-1, sE-selectin and PAI-1 in T2DM. Therefore, PE may offer promise as a primary preventive therapy for atherothrombosis in T2DM, even though we were unable to recognize any specific clinical characteristics or occurrence of microvascular complications in this group of patients.
Acknowledgments
We thank Kenneth Tiezen, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Steiner G. Atherosclerosis, the major complication of diabetes. Adv Exp Med Biol. 1985;189:277–297. doi: 10.1007/978-1-4757-1850-8_15. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Yokokawa H, Yasumura S, Tanno K, et al. Serum low-density lipoprotein to high-density lipoprotein ratio as a predictor of future acute myocardial infarction among men in a 2.7-year cohort study of a Japanese northern rural population. J Atheroscler Thromb. 2011;18(2):89–98. doi: 10.5551/jat.5215. [DOI] [PubMed] [Google Scholar]
- 4.Ito H, Ouchi Y, Ohashi Y, et al. A comparison of low versus standard dose pravastatin therapy for the prevention of cardiovascular events in the elderly: the pravastatin anti-atherosclerosis trial in the elderly (PATE) J Atheroscler Thromb. 2001;8(2):33–44. doi: 10.5551/jat1994.8.33. [DOI] [PubMed] [Google Scholar]
- 5.Saito I, Iso H, Kokubo Y, Inoue M, Tsugane S. Metabolic syndrome and all-cause and cardiovascular disease mortality: Japan Public Health Center-based Prospective (JPHC) Study. Circ J. 2009;73(5):878–884. doi: 10.1253/circj.cj-08-1025. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz SM, Deblois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77(3):445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 7.Steiner M, Reinhardt KM, Krammer B, Ernst B, Blann AD. Increased levels of soluble adhesion molecules in type 2 (non-insulin dependent) diabetes mellitus are independent of glycaemic control. Thromb Haemost. 1994;72(6):979–984. [PubMed] [Google Scholar]
- 8.Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121(3):437–443. doi: 10.1046/j.1365-2249.2000.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99(16):2221–2222. [PubMed] [Google Scholar]
- 10.Festa A, Williams K, Tracy RP, Wagenknecht LE, Haffner SM. Progression of plasminogen activator inhibitor-1 and fibrinogen levels in relation to incident type 2 diabetes. Circulation. 2006;113(14):1753–1759. doi: 10.1161/CIRCULATIONAHA.106.616177. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa S, Kayaba K, Gotoh T, Nakamura Y, Kajii E. Metabolic syndrome and C-reactive protein in the general population: JMS Cohort Study. Circ J. 2007;71(1):26–31. doi: 10.1253/circj.71.26. [DOI] [PubMed] [Google Scholar]
- 12.García Frade LJ, de La Calle H, Alava I, Navarro JL, Creighton LJ, Gaffney PJ. Diabetes mellitus as a hypercoagulable state: its relationship with fibrin fragments and vascular damage. Thromb Res. 1987;47(5):533–540. doi: 10.1016/0049-3848(87)90358-6. [DOI] [PubMed] [Google Scholar]
- 13.Nomura S, Suzuki M, Katsura K, et al. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis. 1995;116(2):235–240. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 14.Nomura S, Imamura A, Okuno M, et al. Platelet-derived microparticles in patients with arteriosclerosis obliterans: enhancement of high shear-induced microparticle generation by cytokines. Thromb Res. 2000;98(4):257–268. doi: 10.1016/s0049-3848(00)00186-9. [DOI] [PubMed] [Google Scholar]
- 15.Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123(1):8–23. doi: 10.1016/j.thromres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Nomura S, Shouzu A, Taomoto K, et al. Assessment of an ELISA kit for platelet-derived microparticles by joint research at many institutes in Japan. J Atheroscler Thromb. 2009;16(6):878–887. doi: 10.5551/jat.2642. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S, Shimizu M. Clinical significance of procoagulant microparticles. J Intensive Care. 2015;3(1):2–11. doi: 10.1186/s40560-014-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura S. Microparticle and atherothrombotic diseases. J Atheroscler Thromb. 2016;23(1):1–9. doi: 10.5551/jat.32326. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–3464. [PubMed] [Google Scholar]
- 20.Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–434. [PubMed] [Google Scholar]
- 21.Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. 2013;227(2):216–221. doi: 10.1016/j.atherosclerosis.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Carbone F, Mach F, Montecucco F. The role of adipocytokines in atherogenesis and atheroprogression. Curr Drug Targets. 2015;16(4):295–320. doi: 10.2174/1389450115666141109213439. [DOI] [PubMed] [Google Scholar]
- 23.Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectine, lipids and atherosclerosis. Curr Opin Lipidol. 2017;28(4):347–354. doi: 10.1097/MOL.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 24.Margaritis M, Antonopoulos AS, Digby J, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127(22):2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 25.Chen YJ, Zhang LQ, Wang GP, et al. Adiponectin inhibits tissue factor expression and enhances tissue factor pathway inhibitor expression in human endothelial cells. Thromb Haemost. 2008;100(2):291–300. [PubMed] [Google Scholar]
- 26.Nomura S, Inami N, Shouzu A, et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. 2009;20(1):16–22. doi: 10.1080/09537100802409921. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T, Rani P, Fukatsu A, et al. A new HMG-CoA reductase inhibitor, pitavastatin remarkably retards the progression of high cholesterol induced atherosclerosis in rabbits. Atherosclerosis. 2004;176(2):255–263. doi: 10.1016/j.atherosclerosis.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Nomura S. Pitavastatin in the management of hypercholesterolemia. Clin Med Ther. 2009;1:1477–1488. [Google Scholar]
- 29.Koba S, Sasaki J. Treatment of hyperlipidemia from Japanese evidence. J Atheroscler Thromb. 2006;13(6):267–280. doi: 10.5551/jat.13.267. [DOI] [PubMed] [Google Scholar]
- 30.Nomura S, Kanazawa S, Fukuhara S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with type 2 diabetes mellitus. J Diabetes Complications. 2003;17(3):153–159. doi: 10.1016/s1056-8727(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama M, Origasa H, Matsuzaki M. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blind endopoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Rhee SY, Oh S, et al. Evaluation of the effectiveness of sarpogrelate on the surrogate markers for macrovascular complications in patients with type 2 diabetes. Endocr J. 2012;59(8):709–716. doi: 10.1507/endocrj.ej12-0047. [DOI] [PubMed] [Google Scholar]
- 33.Lee DH, Chun EJ, Hur JH, et al. Effect of sarpogrelate, a selective 5-HT2A receptor antagonist, on characteristics of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2017;257:47–54. doi: 10.1016/j.atherosclerosis.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Nomura S, Shouzu A, Omoto S, Nishikawa M, Iwasaka T. 5-HT2A receptor antagonist increases circulating adiponectin in patients with type 2 diabetes. Blood Coagul Fibrinolysis. 2005;16(6):423–428. doi: 10.1097/01.mbc.0000176197.48134.08. [DOI] [PubMed] [Google Scholar]
- 35.Nomura S, Shouzu A, Omoto S, et al. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thromb Res. 2008;122(1):39–45. doi: 10.1016/j.thromres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Nomura S, Shouzu A, Omoto S, et al. Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J Atheroscler Thromb. 2009;16(2):83–90. doi: 10.5551/jat.e091. [DOI] [PubMed] [Google Scholar]
- 37.Hata Y, Mabuchi H, Saito Y, et al. Investigating Committee of Guideline for Diagnosis and Treatment of Hyperlipidemias Japan Atherosclerosis Society. Guideline for Diagnosis and Treatment of Hyperlipidemias in Adults. Doumyakukouka. 1997;25:1–34. [Google Scholar]
- 38.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 39.Packham MA, Mustard JF. The role of platelets in the development and complications of atherosclerosis. Semin Hematol. 1986;23(1):8–19. [PubMed] [Google Scholar]
- 40.Tschoepe D, Roesen P, Esser J, et al. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemost. 1991;17(04):433–438. doi: 10.1055/s-2007-1002650. [DOI] [PubMed] [Google Scholar]
- 41.Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158(2):277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- 42.Uchiyama S, Demaerschalk BM, Goto S, et al. Stroke prevention by cilostazol in patients with atherothrombosis: meta-analysis of placebo-controlled randomized trials. J Stroke Cerebrovasc Dis. 2009;18(6):482–490. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33(2):143–153. doi: 10.1007/s11239-011-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nomura S, Shouzu A, Omoto S, et al. Effect of cilostazol on soluble adhesion molecules and platelet-derived microparticles in patients with diabetes. Thromb Haemost. 1998;80(3):388–392. [PubMed] [Google Scholar]
- 45.Nomura S, Inami N, Iwasaka T, Liu Y. Platelet activation markers, microparticles and soluble adhesion molecules are elevated in patients with arteriosclerosis obliterans: therapeutic effects by cilostazol and potentiation by dipyridamole. Platelets. 2004;15(3):167–172. doi: 10.1080/09537100410001682779. [DOI] [PubMed] [Google Scholar]
- 46.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 47.Yilmaz MI, Sonmez A, Caglar K, et al. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist increases plasma adiponectin levels in type 2 diabetic patients with proteinuria. Endocrine. 2004;25(3):207–214. doi: 10.1385/ENDO:25:3:207. [DOI] [PubMed] [Google Scholar]
- 48.Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S, Iwasaka T. Effect of valsartan on monocyte/endothelial cell activation markers and adiponectin in hypertensive patients with type 2 diabetes mellitus. Thromb Res. 2006;117(4):385–392. doi: 10.1016/j.thromres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Gautam M, Izawa A, Shiba Y, et al. Importance of fatty acid compositions in patients with peripheral arterial disease. PLoS One. 2014;9(9):e107003. doi: 10.1371/journal.pone.0107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hishikari K, Kimura S, Yamakami Y, et al. The prognostic value of the serum eicosapentaenoic acid to arachidonic acid ratio in relation to clinical outcomes after endovascular therapy in patients with peripheral artery disease caused by femoropopliteal artery lesions. Atherosclerosis. 2015;239(2):583–588. doi: 10.1016/j.atherosclerosis.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Kumbhani DJ, Steg PG, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35(41):2864–2872. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]