Abstract
Olaparib is an oral poly ADP-ribose polymerase inhibitor with activity in germline BRCA1 and BRCA2 (BRCA1/2)-associated breast and ovarian cancers. There is no report about treatment with olaparib in BRCA1/2-mutated intrahepatic cholangiocarcinomas. This study is to observe the efficacy and safety of olaparib monotherapy in the refractory BRCA1/2-mutant intrahepatic cholangiocarcinoma (ICC) patient. The clinical record of a patient with BRCA2-mutated refractory advanced ICC treated with olaparib was analyzed. The patient was administered with olaparib (400 mg orally twice daily) and followed up for 11 months. The clinical tumor response was evaluated after 4 weeks of olaparib treatment, and then every 8 weeks (two treatment cycles). The patient achieved partial response confirmed by the computed tomography and the tumor marker CA19.9, CA50, and CA125 levels decreased significantly as an outcome of the treatment. The quality of life improved significantly. Major adverse events were fatigue, thrombocytopenia, leukopenia, and anemia, which were manageable with medication. The patient is still receiving treatment. Olaparib in the treatment of BRCA2-mutation-associated refractory advanced ICC patent is effective, and the adverse effects are tolerated. Large-scale studies should be conducted to further the adoption of genomic profiling, which may help clinicians identify suitable biomarkers for therapy of ICCs. A possible line of therapy is often extrapolated from case reports or small case series.
Keywords: intrahepatic cholangiocarcinomas, ICC, olaparib, BRCA1/2
Introduction
Hepatobiliary cancers are highly lethal cancers including a spectrum of invasive carcinomas arising in the liver (hepatocellular carcinoma), gall bladder, and bile ducts (intrahepatic and extrahepatic cholangiocarcinoma [CCA]). In 2017, it was estimated that 40,710 people in the USA would be diagnosed with liver cancer and intrahepatic bile duct cancer and 28,920 deaths would occur.1 Intrahepatic cholangiocarcinomas (ICCs) are the second most common malignant tumors of the liver with a poor prognosis. Analyses of Surveillance, Epidemiology, and End Results (SEER) data from 1973 to 2012 showed that incidence of ICC is increasing (annual percentage change, 2.3%) in the USA.2 Most patients have unresectable tumors when they are diagnosed and chemotherapies provided only limited benefit. No molecular targeted drugs or immunotherapy for ICC have been recommended or approved. Here we report one encouraging case using olaparib (Lynparza®, AstraZeneca) to treat advanced ICC with BRCA2 mutation in our hospital.
Case report
A female patient, 64 years old, felt pain in the left upper quadrant of abdomen with no obvious cause in December 2015. CA19.9 level increased to 170.0 μ/mL, and CEA, CA125, and CA50 levels were tested normal on December 18, 2015. ECOG (Eastern Cooperative Oncology Group) performance status score =1. 18-fluoride positron emission tomography-computed tomography scan (18F-PET/CT) showed that there was a mass (about 5 cm in diameter) in the left lobe of the liver and multiple retroperitoneal lymph nodes, which were deemed metastatic on December 20, 2015. The patient underwent a liver biopsy on December 28, 2015. The pathology showed moderately differentiated tubular adenocarcinoma. Immunohistochemically, the tumor cells tested positive for cytokeratin 7 (CK7), cytokeratin 19 (CK19), CDX2, Ki-67 (labeling index =15%), MUC1, and MUC5AC and the tumor cells were negative for cytokeratin 20 (CK20), CEA, MUC2, and P53. Based on the pathologic results presented earlier, the patient was diagnosed with ICCs. She underwent treatment of transcatheter arterial chemoembolization (TACE) for the first time with agents of epirubicin (50 mL), fluorouracil (5-FU 1.0 g), and liquid lipiodol (10 mL) on December 30, 2015. She accepted the accurate cyberknife treatment to liver tumor (DT 42.5 GY/4F) from January 15, 2016 to January 19, 2016 and CA19.9 level decreased to 89.3 μ/mL after radiotherapy.
On May 20, 2016, the patient’s CA19.9 level increased to 132.9 μ/mL. Magnetic resonance imaging (MRI) of upper abdomen showed the remained left hepatic lobe lesions, multiple disseminated metastatic lesions in the right hepatic lobe, and multiple abdominal metastatic lymph nodes on May 25, 2016. She accepted the additional treatment of TACE for three times followed with gemcitabine (800 mg), oxaliplatin (100 mg), and liquid lipiodol (10 mL) on June 24, 2016, September 15, 2016, and October 31, 2016, respectively. CA19.9 level decreased to 77.6 μ/mL after treatments of TACE.
The patient experienced persistent pain in the lower back in January 2017. CA19.9 level increased to 466.6 μ/mL, CA125 level increased to 108.0 μ/mL, and CA50 level increased to 91.4 μ/mL on February 22, 2017. 18F-PET/CT showed that left clavicular area, mediastinum, and right cardiophrenic angle lymph nodes (SUVmax =5.1), multiple low-density shadows in the liver (SUVmax =3.8), and multiple retroperitoneal lymph nodes (SUVmax =4.0), whose FDG metabolism all increased on February 24, 2017. Peripheral blood (method: next generation sequencing) has been tested in March 2017. The gene mutation was negative for epithelial growth factor receptor (EGFR) exons 18–21, KRAS codons 12–13, 61, and 146, NRAS codons 12–13 and 61, KIT exons 9, 11, 13, and 17, PDGFRA exons 12 and 18, ALK, ROS1, C-MET, RET, HER-2, fibroblast growth factor receptor 1–4 (FGFR1–4), or isocitrate dehydrogenase 2 (IDH2). The gene mutation was positive for BRCA2 (c.8009C.A, p>S2670*, heterozygous germline mutation). She received helical tomotherapy (DT50GY/25F) from March 23, 2017 to April 26, 2017, aimed at left supraclavicular, mediastinal, retroperitoneal metastatic lymph nodes and liver metastatic lesions. The lower back pain relieved after radiotherapy. CA19.9 level decreased to 79.3 μ/mL, CA125 level decreased to 43.4 μ/mL, and CA50 level decreased to 55.8 μ/mL after radiotherapy on May 10, 2017.
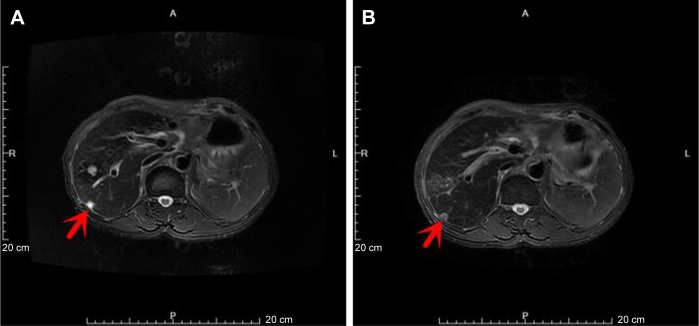
The patient developed upper abdominal pain and lower back pain in June 2017. CT of the chest and abdomen showed multiple nodular annular enhancement lesions in the left and right lobes of the liver, and left supraclavicular swollen lymph nodes, which considered metastasis; bilateral neck, hepatic gastric space, and retroperitoneal multiple lymph nodes were smaller than before (March 15, 2017); uterine rectal fossa nodules were considered enlarged lymph node (1.5×2.3 cm) (Figure 1) on June 2, 2017. MRI of the upper abdomen showed that multiple nodules in the liver may be intrahepatic metastases on June 2, 2017, in which a new lesion (arrowhead in Figure 2) in the right lobe of the liver was found (0.8×0.6 cm). Due to the new pelvic swollen lymph node and the new lesion in the liver, the therapeutic evaluation is progressive disease. CA19.9 level increased to 109.0 μ/mL, CA125 level increased to 74.6 μ/mL, and CA50 level increased to 63.9 μ/mL on June 20, 2017. The patient’s liver biopsy specimens tested negative for programmed death-ligand 1 (PD-L1) and pMMR on June 16, 2017.
Figure 1.
The tumor (arrow) in uterine rectal fossa disappeared.
Notes: (A) Pretreatment of olaparib. (B) Posttreatment of olaparib.
Figure 2.
The new tumor (arrowhead) in the right lobe of the liver is the same as before.
Notes: (A) Pretreatment of olaparib. (B) Posttreatment of olaparib.
The patient started to take olaparib (400 mg bid po) from June 26, 2017. There have been grade 2 fatigue, grade 1 nausea and vomit, grade 4 leukopenia, grade 4 neutropenia, grade 3 anemia, and grade 3 thrombocytopenia during the treatment. On January 23, 2018, white blood cell count decreased to 0.9×109/L, neutrophil count decreased to 0.5×109/L, hemoglobin decreased to 71 g/L, and platelet count decreased to 47×109/L. She stopped taking olaparib for 7 days and got relief after taking positive measures, such as granulocyte-colony-stimulating factor, thrombopoietin, and transfusion. She resumed olaparib at a reduced dosage (200 mg bid po). During the treatment, CA19.9 level decreased to 91.1 μ/mL, CA125 level decreased to 60.1 μ/mL, and CA50 level decreased to 40.3 μ/mL on July 10, 2017. CA19.9, CA125, and CA50 all returned to normal level on August 25, 2017. CT of the chest and abdomen showed that multiple low-density lesions in the liver are the same as before (Figure 2), the left supraclavicular fossa, left hilus pulmonis, hepatic gastric space, and para-aortic lymph nodes are the same as before; the former uterine rectal fossa nodules disappeared (Figure 1) on March 29, 2018. The therapeutic evaluation is partial response (PR). The patient’s upper abdominal pain was relieved during the treatment. The ECOG performance status declined from 2 scores to 1 score. Currently, the patient is still undergoing treatment.
Discussion
ICC originates in the epithelium of the intrahepatic biliary tract. It is a rare disease and is associated with poor clinical outcome and survival. Radical surgical removal with clear margins is the only potentially curative therapy, although most patients are not candidates for surgery due to the presence of advanced disease at diagnosis. So systemic treatment is particularly important.
National Comprehensive Cancer Network guideline for Hepatobiliary Cancers (Version 1.0, 2018 – February 14, 2018)3 recommends clinical trial, fluoropyrimidine-based or gemcitabine-based chemotherapy, or best supportive care for advanced ICCs.4–7 And there is no established second-line systemic therapy following progression after first-line treatment.
With the development of tumor molecular biology and genomics, the strategies for the diagnosis and treatment of tumors have been gradually transformed into a precise target treatment era, which has been centered around the tumor location or pathologic type. There are a number of emerging therapeutic biomarkers and therapeutic concepts that show promise. The EGFR and vascular endothelial growth factor (VEGF) have been studied the most. A phase II trial of 44 patients with advanced CCA suggested that the addition of cetuximab to gemcitabine-based chemotherapy may have activity in unresectable disease, but KRAS status was not associated with longer PFS.8 Prospective randomized phase II studies targeting VEGF have failed to demonstrate a benefit of adding sorafenib to single-agent gemcitabine9 or cediranib to the cisplatin/gemcitabine combination.10 IDH1 mutations are found in 10%–23%11–14 and FGFR fusion rearrangements are found in 8%–14% of patients.15–17 BGJ39818 and ARQ087 (NCT01752920),19 two orally bioavailable, selective pan-FGFR kinase inhibitors, have shown preliminary clinical activity against chemotherapy-refractory CCA with FGFR2 fusions. Unfortunately, there are currently no molecular targeted therapies approved for advanced ICCs. We need to understand more about the molecular pathology of ICCs, which may help us identify suitable targets for therapy. Maybe the addition of comprehensive genomic profiling adds a possible line of therapy for ICCs that have little treatment options under the current standard of care.
In 2014, the American Cancer Research Association proposed an innovative clinical trial design called “Basket Trial” under the guidance of the new oncology genomics.20 The “Basket Trial” is to compare a particular molecular event such as gene mutation, fusion, and amplification to a basket, and put different kinds of tumors with the same molecular event into the same basket for clinical trials and treat them with the same target drug. The essence of “Basket Trial” is a specific drug treating different cancers with same genomics. This study design is noteworthy. Tumor treatment is no longer restricted by the traditional location or pathologic type, but it has been added to the genetic background and molecular profiling of tumor genes.
Hyman et al20 undertook a histology-independent phase II “Basket” study of vemurafenib in BRAF V600 mutation-positive nonmelanoma cancers. A total of 122 patients of different tumor types without standard options were enrolled. Clinical activity, including PR or complete response (CR), and tumor regression were observed in several tumor types, which include non-small-cell lung cancer (eight PRs in the cohort of 20 patients), Erdheim–Chester disease or Langerhans’-cell histiocytosis (one complete and five PRs in the cohort of 18 patients), anaplastic thyroid cancer (one complete and one PR in the cohort of seven patients), and CCA (one PR in the cohort of eight patients).
MyPathway21 is a multicenter, nonrandomized, phase IIa multiple “Basket” study. Two hundred fifty-one patients of 35 different tumor types with advanced refractory solid tumors harboring molecular alterations in HER2, EGFR, BRAF, or the Hedgehog pathway are treated with pertuzumab plus trastuzumab, erlotinib, vemurafenib, or vismodegib, respectively. Fifty-two patients (23%) with 14 different tumor types had objective responses (CR =4; PR =48). Tumor-pathway cohorts with notable objective response rates included HER2-amplified/overexpressing colorectal (38% [14 of 37]; 95% CI, 23%–55%) and BRAF V600-mutated non-small-cell lung cancer (43% [six of 14]; 95% CI, 18%–71%). Two of seven patients (29%; 95% CI, 4%–71%) with HER2-amplified/overexpressing biliary cancer treated with trastuzumab plus pertuzumab had PR, and three had SD >120 days.
Olaparib is an oral poly ADP-ribose polymerase inhibitor with activity in germline BRCA1 and BRCA2 (BRCA1/2)-associated breast and ovarian cancers. A multicenter, phase II study enrolled individuals with a germline BRCA1/2 mutation and recurrent cancer including advanced refractory ovarian cancer, breast cancer, pancreatic cancer, and prostate cancer who had received at least one previous line of therapy.22 A total of 298 patients received treatment and were evaluable. The tumor response rate was 26.2% (78 of 298; 95% CI, 21.3–31.6) overall and 31.1% (60 of 193; 95% CI, 24.6–38.1), 12.9% (eight of 62; 95% CI, 5.7–23.9), 21.7% (five of 23; 95% CI, 7.5–43.7), and 50.0% (four of eight; 95% CI, 15.7–84.3) in ovarian, breast, pancreatic, and prostate cancers, respectively. Responses to olaparib were observed across different tumor types associated with germline BRCA1/2 mutations.
Systematic genomic profiling efforts have shown that many approved and investigational biomarkers are present in various tumor types, although often at low frequencies.23 The importance of “Basket” studies is that they provide potential therapies for patients with refractory tumors, including the refractory state of common malignant tumor multiline therapy and rare malignant tumors without guidelines associated with genomic profiling.
In this case, the patient was initially diagnosed as advanced ICC and was unresectable. The patient accepted local treatment mainly, including TACE and local radiotherapy without systemic chemotherapy. The disease progressed again because of new lesions occurring in the liver and pelvis, which is not suitable for local treatment. Systemic chemotherapy was called for. Considering her weak constitution, the patient is not suitable for systemic chemotherapy. There were no appropriate drugs for systemic chemotherapy because she took 5-FU and gemcitabine during the previous TACE treatment. She was not suitable for PD-L1 therapy because of the negative result of PD-L1 and mismatch repair deficiency. As a result of the BRCA2 mutation, we tried to treat with olaparib consulting the results of a series of “Basket Trials”. The tumor markers CA50, CA19.9, and CA125 all returned to normal level after 2 months of treatment, while CA19.9 never returned to normal level in the past local treatment. Multiple liver lesions were stable during the treatment of olaparib and the pelvic lymph nodes gradually regressed and disappeared after 10 months. There are grade 3–4 hematologic toxicity, grade 1–2 nonhematologic toxicity, and no specific adverse events during the treatment. All toxicities were managed by dose interruption and dose reduction. The ECOG score declined from 2 scores to 1 score. The patient has survived the 11 months of treatment and her quality of life is good.
This is the first case of treating ICC with olaparib after genomic profiling and demonstrated favorable efficacy. The case showed the concept of precision medicine and different types of tumors may receive the same treatment with the same molecular biomarkers.
The US Food and Drug Administration (FDA) granted breakthrough therapy designation of entrectinib (RXDX-101) for the treatment of NTRK fusion-positive locally advanced or metastatic solid tumors with no standard therapy. Pembrolizumab (Keytruda) was granted accelerated approval for treatment of adult and pediatric patients with unresectable or metastatic microsatellite instability–high (MSI-H) or mismatch repair deficient (dMMR) solid tumors progressing following prior treatment and who have no satisfactory alternative treatment options or with MSI-H or dMMR colorectal cancer progressing following treatment with fluoropyrimidine, oxaliplatin, and irinotecan. These are precedents for FDA to differentiate drug indications based on molecular biomarkers rather than tumor origin.
However, anatomical location of tumors is an important reference factor in the treatment and prognosis of diseases and it cannot be completely ignored. We noticed that most “Basket Trials” have reported mixed results. Different tumor types with the same molecular biomarker might differ in their sensitivity to therapy targeted at that biomarker. Therefore, the combination of genetic background and histopathologic types may be more accurate to reflect efficacy of molecular target therapy. This is also our research direction in the future.
Conclusion
CCA is a cancer of poor prognosis with limited treatment options. As precision medicine becomes more common, we will find more and more patients with ICCs who can benefit from targeted therapy and treatment is often extrapolated from case reports or small case series. Olaparib could potentially be a promising therapeutic agent for chemotherapy-refractory BRCA1/2 mutant CCA patients.
Informed consent
Written informed consent has been provided by the patient to have the case details and any accompanying images published.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A, Statistics C. CA Cancer J Clin. 2017;2017(67):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines®) Hepatobiliary Cancers Version 1 [EB/OL] [2018-02-14] Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#hepatobiliary.
- 4.Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15(3):478–483. doi: 10.1093/annonc/mdh096. [DOI] [PubMed] [Google Scholar]
- 5.Ducreux M, van Cutsem E, van Laethem JL, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41(3):398–403. doi: 10.1016/j.ejca.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 7.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borbath I, Ceratti A, Verslype C, et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a phase II study of the Belgian Group of Digestive Oncology. Ann Oncol. 2013;24:2824–2829. doi: 10.1093/annonc/mdt337. [DOI] [PubMed] [Google Scholar]
- 9.Moehler M, Maderer A, Schimanski C, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50(18):3125–3135. doi: 10.1016/j.ejca.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol. 2015;16(8):967–978. doi: 10.1016/S1470-2045(15)00139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32(25):3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss JS, Holtegaard LM, Kerr SE, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44(7):1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9(12):e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intra-hepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19(3):235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45(8):1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59(4):1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 18.Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):JCO2017755009. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzaferro V, Droz M, Busset D, et al. ARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrations[C] Poster-ASCO-2017;4017NO. [Google Scholar]
- 20.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;34(4_suppl):JCO2017753780–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol. 2015;19(1A):68–77. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]