Abstract
Background
The use of radiofrequency ablation (RFA) procedures to treat chronic knee pain has surged in the past decade, though many questions remain regarding anatomical targets, selection criteria, and evidence for effectiveness.
Methods
A comprehensive literature review was performed on anatomy, selection criteria, technical parameters, results of clinical studies, and complications. Databases searched included MEDLINE and Google Scholar, with all types of clinical and preclinical studies considered.
Results
We identified nine relevant clinical trials, which included 592 patients, evaluating knee RFA for osteoarthritis and persistent postsurgical pain. These included one randomized, placebo-controlled trial, one randomized controlled trial evaluating RFA as add-on therapy, four comparative-effectiveness studies, two randomized trials comparing different techniques and treatment paradigms, and one non-randomized, controlled trial. The results of these studies demonstrate significant benefit for both reduction and functional improvement lasting between 3 and 12 months, with questionable utility for prognostic blocks. There was considerable variation in the described neuroanatomy, neural targets, radiofrequency technique, and selection criteria.
Conclusion
RFA of the knee appears to be a viable and effective treatment option, providing significant benefit to well-selected patients lasting at least 3 months. More research is needed to better identify neural targets, refine selection criteria to include the use of prognostic blocks, optimize treatment parameters, and better elucidate relative effectiveness compared to other treatments.
Keywords: Knee pain, osteoarthritis, radiofrequency, ablation, denervation, genicular nerve
Introduction
Knee pain has a lifetime prevalence rate of ~45%,1 and represents a source of significant disability and reduced quality of life.2,3 Risk factors for the development of knee pain include a history of prior injury or surgery, obesity, and advancing age.4 The most common cause of chronic knee pain is osteoarthritis (OA), which is characterized by the progressive loss of articular cartilage, with other etiologies including rheumatoid arthritis, trauma, crystal arthropathies, and persistent postsurgical pain.5,6
Available treatments for knee pain vary depending on the etiology and diagnosis, but broadly include physical therapy, oral medications, injections, and surgery.7 Injections for knee pain consist of several types, and may be directed to the soft tissues of the knee joint or the intra-articular joint space. Intra-articular injections encompass a wide range of medications to include anti-inflammatory corticosteroids, pro-inflammatory prolotherapy and platelet-rich plasma (PRP) solutions, viscosupplements, and stem cell preparations.8–11 All intra-articular injections require the presence of an intact joint, and are therefore not applicable following total arthroplasty. Knee surgery is similarly heterogeneous and ranges from minimally invasive arthroscopic procedures to open partial or total arthroplasties.12,13 Pain due to severe OA is not reliably responsive to conservative therapies, and chronic pain may persist in over 40% of patients who undergo joint replacement, being characterized as severe in 15% of cases.14–16 Delivery of radiofrequency (RF) energy to the knee’s nerve supply is a relatively new intervention that can be safely done in the presence of an artificial joint, and may offer an alternative to surgery or surgical revision.
Radiofrequency ablation (RFA) entails the discrete delivery of thermal energy produced by an alternating current to neural tissue, thereby degrading its ability to conduct pain signals.17 First described as a treatment for pain in the 1960’s,18 RFA evolved from a therapy primarily employed to alleviate neuropathic pain to one used today predominantly for mechanical joint pain amidst reports of increased pain stemming from deafferentation and neuroma formation. Since the neurosurgeon Norman Shealy adapted its use for the treatment of pain arising from the spinal facet joints in the mid-1970’s,19 the accepted indications for RFA have expanded steadily over the ensuing decades. The advent of cooled radiofrequency ablation (CRFA)20 and the non- ablative pulsed radiofrequency (PRF)21 therapy have further broadened the clinical utility of RF for chronic pain states. Accepted targets for RF treatment now include most neural structures to include major nerves and ganglia.22–24 The use of RF as a treatment for knee pain was first described in a small case series involving the treatment of different types of joint pain by Sluijter et al, who noted complete eradication of pain with intra-articular PRF in a patient with refractory post-traumatic knee pain.25 This area of pain medicine has evolved in the past decade, and though knee RFA has been the subject of numerous publications, high-quality randomized controlled trials (RCTs) remain sparse. The objectives of this publication are to review the relevant neuroanatomy of the knee and the available literature on RFA.
Search strategy
We systematically searched and screened all titles and abstracts from MEDLINE and Google Scholar from inception to February 1, 2018 using the following key words: “knee,” “pain,” “arthritis,” “persistent postsurgical pain,” “chronic postsurgical pain,” “radiofrequency,” “ablation,” “denervation,” and “pulsed radiofrequency.” This initial search yielded 92 trials of varying design, which were then separately reviewed by the authors. For the purposes of this review only randomized and comparative trials were selected, with all other study types being excluded. A total of nine randomized or comparative trials were ultimately identified by independent searches conducted by each author. The literature search was complemented by reviewing the reference lists of the selected publications to search for additional articles missed by our initial electronic search.
Neuroanatomy of the knee
The innervation of the knee joint is complex given that genicular nerves arise from branches of three major nerves: the sciatic, femoral, and obturator, all of which are themselves derived from the lumbar plexus.26,27 The sciatic nerve bifurcates into the tibial and common peroneal nerves in the popliteal fossa. The tibial nerve remains in the posterior compartment of the lower leg and gives off the superomedial (SM) and inferomedial (IM) genicular nerves to the posterior aspect of the knee joint. The common peroneal nerve passes into the anterior compartment of the lower leg, and contributes the superior lateral (SL) genicular nerve to the anterior portion of the knee. These genicular branches of the sciatic nerve reliably course in approximation to the periosteum at the medial and lateral junctions of the distal femoral shaft and epicondyles, and at the medial junction of the proximal tibia and epicondyle. The saphenous nerve is a cutaneous sensory branch of the femoral nerve and gives off suprapatellar and infrapatellar (IP) genicular nerves to the anterior portion of the knee. The contribution of the obturator nerve is more variable, but its posterior branch can provide an articular branch to the posterior knee.
The complexity of the knee joint’s innervation has resulted in a disparity in procedural technique among the available controlled and observational studies. Studies report on a range of procedural targets to include the SM, IM, and SL genicular nerves in combination,28–33 the saphenous nerve,34 the sciatic nerve,35 the IP genicular nerve,36 the IP and SM genicular nerves in combination,37 the femoral, tibial, saphenous nerves, and peripatellar plexus in combination,38 and the intra-articular joint space6,25,39–41 (Figures 1 and 2).
Figure 1.
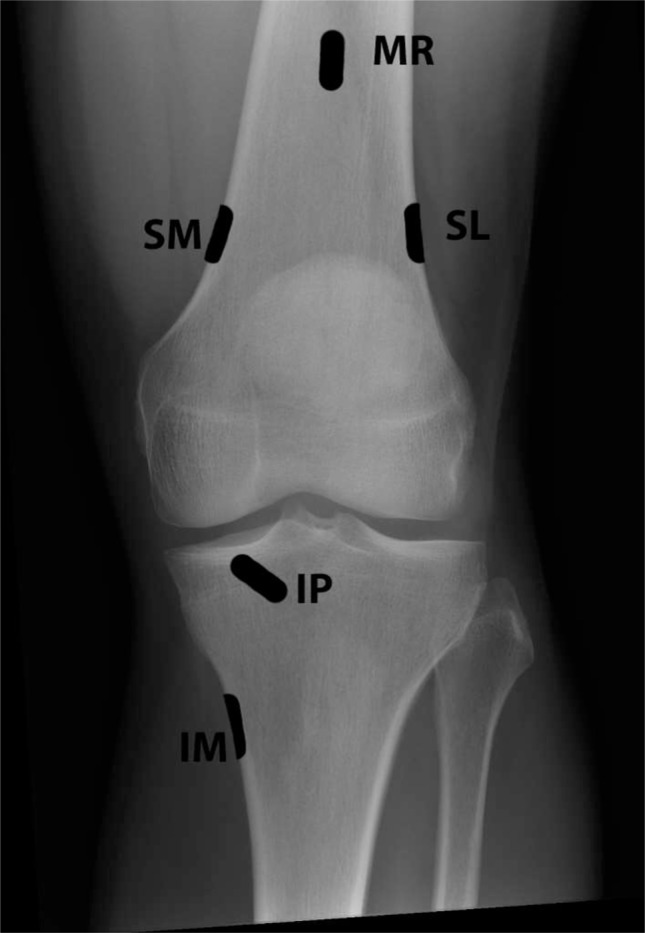
Anterior-posterior radiograph of the knee depicting locations for genicular nerve targeting.
Abbreviations: IM, inferomedial; IP, infrapatellar; MR, medial retinacular; SL, superolateral; SM, superomedial.
Figure 2.
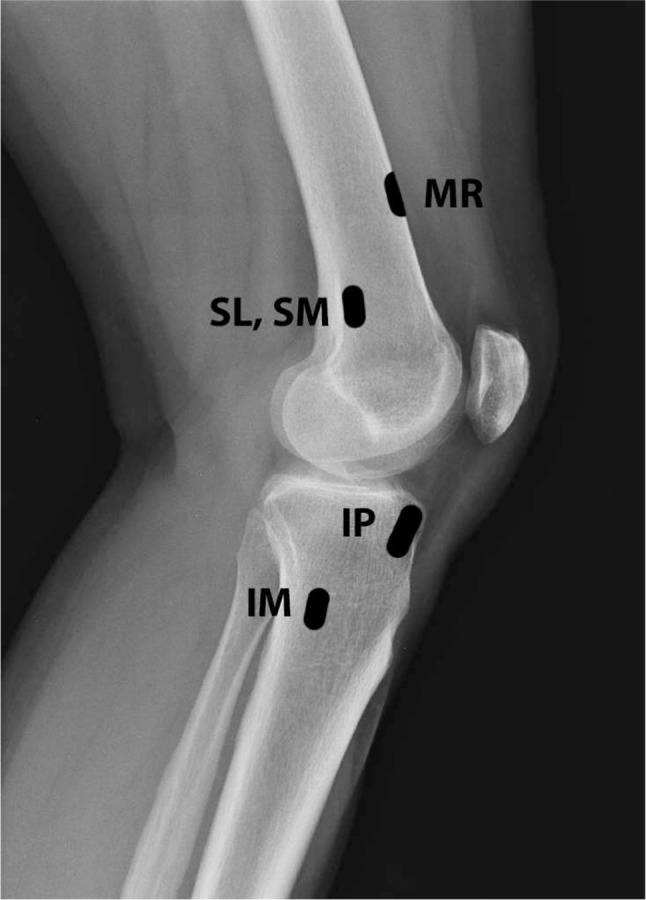
Lateral radiograph of the knee depicting locations for genicular nerve targeting.
Abbreviations: IM, inferomedial; IP, infrapatellar; MR, medial retinacular; SL, superolateral; SM, superomedial.
RCTs
The earliest published RCT is the 2011 study by Choi et al28 This trial was designed to compare genicular nerve RFA to sham RFA. Nerves targeted in this trial were the SM, IM, and SL genicular branches. Inclusion criteria were age between 50 and 80 years, presence of refractory knee pain of at least 3 months duration, and at least grade 2 radiographic evidence of OA on the 5-point Kellgren–Lawrence scale.42 Thirty-eight patients who met the criteria and had a positive response to genicular nerve blocks were randomized to receive either continuous, fluoroscopically guided RFA at 70°C, or sham RFA that consisted of superficial and deep local anesthesia with lidocaine. Primary outcomes were pain level as assessed by visual analog scale (VAS) and the proportion of patients reporting at least 50% pain relief at 12-week follow-up. Overall follow-up was obtained at 1, 4, and 12 weeks post- procedure. The results showed that RFA significantly lowered the VAS at all time periods compared to sham, but both groups showed similar improvement at 1 week, suggesting a temporary improvement with local anesthetic nerve block. Ten of the 19 RFA patients achieved at least 50% pain reduction at 12 weeks, compared to no patients in the control group. Secondary outcomes to include Oxford knee score (OKS) and patient satisfaction were also significantly improved in the RFA group. The limitations of this study are primarily its small size, the large discrepancy between the volume of prognostic blocks and the small electrodes used, the high proportion of positive prognostic blocks (82.5%), and lack of long-term follow-up.
RFA was first compared to intra-articular injections in the 2016 Sarı et al trial.29 Seventy-three patients with at least grade 2 Kellgren–Lawrence OA were randomized to receive either RFA of the SL, SM, and IM genicular nerves at 80°C for 90 seconds or intra-articular injection of bupivacaine, morphine, and betamethasone. Patients were assessed at baseline, 1 and 3 months for pain level via VAS and function via the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index. Results showed statistically superior pain relief with RFA at 1 and 3 months, but superiority in the total WOMAC score with RFA only at 1 month. Limitations of the study include the lack of prognostic blocks, the unrestricted and undocumented use of oral analgesics, and the lack of a true control group.
The SM, IM, and SL genicular nerves were also targeted in the 2017 Qudsi-Sinclair et al trial, but in this trial the effect of RFA was examined only in patients with a history of total knee arthroplasty (TKA).30 Thirty patients with refractory knee pain that persisted at least 6 months following TKA were enrolled in the study, with 28 completing follow-up to 12 months. Patients were randomized to receive either continuous RFA at 80°C or sham RFA that consisted of genicular nerve blocks with local anesthetic and corticosteroid. Both procedures were performed under fluoroscopic guidance. Outcome measures were pain level assessed via a numeric rating scale (NRS), and function assessed via both the OKS and Knee Society Score. Outcomes with respect to function were modest and similar between groups, with most improvements occurring between months 1 and 6, and declining toward baseline by 12 months. Pain also decreased in both groups, but the reduction following RFA peaked at 3 months and persisted at 12 months, while the control group experienced their lowest NRS on day 1 and then steadily increased toward baseline at 12 months. This trial is limited by a small size and the lack of prognostic blocks pre-RFA, which may have led to the inclusion of nonresponders in the RFA group.
The Shen et al trial compared RFA with PRP and sodium hyaluronate (HA) to PRP and HA alone.43 Inclusion criteria were refractory pain of at least 3 months duration due to OA and pain level of at least 6 on a 0–10 VAS. Both groups received intra-articular injections of PRP and HA weekly for 5 weeks, but the treatment group also received RFA at 70°C, although the timing of the RFA was not described. The precise nerves were also neither named, nor was it specified whether image guidance was used. Twenty-seven patients were randomized to each group and follow-up was obtained at the completion of intra-articular injections and 3 months. Outcome measures included pain intensity as measured on a VAS, life quality as measured on the 36-item Short-Form Health Survey, and function via the American Knee Society Score. Both groups showed improvement in pain and function, although the gains in the RFA group were statistically superior at all time periods. The RFA group also demonstrated significant improvement in quality of life at 3 months, while the control group did not.
The 2018 trial by Davis et al is the largest study and was also the first to employ CRFA.31 Similar to the Qudsi-Sinclair and Choi trials, the SM, SL, and IM genicular nerves were targeted. Inclusion criteria were the presence of at least grade 2 Kellgren–Lawrence radiographic OA, refractory knee pain of at least 6 month duration, pain of at least 6 of 10 on a NRS, OKS score of at least 35, and at least 50% improvement with genicular nerve blocks. One hundred and fifty-one patients met the inclusion criteria and were randomized to receive either CRFA or intra-articular steroid (IAS) injection. CRFA was performed under fluoroscopic guidance with 17-gage introducers at 60°C for 150 seconds. The primary outcome was the percentage of patients achieving at least 50% pain reduction at 6 month follow-up as measured by a NRS. Secondary outcome measures included function measured on OKS, patient’s overall perception of the treatment, and analgesic usage. Pain relief with CRFA was superior to that obtained with IAS at all time periods, and at 6 month follow- up 74% of the CRFA group had at least 50% relief compared to just 16% of the IAS group. Function and global perception were also superior in the CRFA cohort, although there was no statistical difference between the groups in terms of oral opioid use. The longer duration of relief noted in this study provides evidence for the theoretical benefit of CRFA, namely the creation of larger lesions to reduce the technical failure rate (ie, missed nerves).
The most recent RCT by El-Hakeim et al compared RFA to non-interventional therapy.32 Sixty patients with at least grade 3 Kellgren–Lawrence OA were randomized to receive either RFA of the SM, SL, and IM branches or conventional treatment with oral acetaminophen and diclofenac. RFA was accomplished with three 90 seconds cycles at 90°C per site, which is a substantially longer duration of RFA than that employed by any other RCT. Patients were evaluated at baseline, 2 weeks, 3 months, and 6 months. Results showed statistically superior pain relief with RFA at all follow-up intervals. Function as assessed by the WOMAC index was improved in both groups at 6 months, but was superior with RFA. Lastly, patient satisfaction as measured on a Likert scale was significantly higher at 3 and 6 month follow-up in the RFA group. However, the study is limited by the lack of pre-RFA prognostic blocks and the lack of patient blinding.
Randomized, non-comparative studies
The 2017 randomized trial by McCormick et al also employed CRFA, but the study was designed to determine the predictive value of pre-RFA nerve blocks, not to compare RFA to other modalities.33 Fifty-four patients with chronic knee pain due to OA all received CRFA, but 29 did so after prognostic blocks and 25 did not. Notably, only three of 32 (9.3%) patients had a negative block, defined as <50% pain relief. Some patients had the procedure done bilaterally; so a total of 36 knees had CRFA following prognostic blocks and 35 knees proceeded directly to CRFA. Inclusion criteria were age between 30 and 80 years, >6 months of refractory knee pain, NRS pain score of at least four and at least grade 2 radiographic OA. Follow-up was conducted at 1, 3, and 6 months, but the primary outcome measure was attainment of at least 50% pain relief at the 6-month mark. Results showed significant improvements in both groups at 6 months, with 58.6% of the nerve block group and 64% of the non-nerve block group achieving at least 50% relief at 6 months. There were no significant differences between groups in terms of pain and function at any of the time periods. These findings raise questions regarding the utility of single, uncontrolled blocks before knee RF ablation, and suggest the need for more research to better refine selection criteria.
The intra-articular delivery of RF energy is advocated by some as an alternative to ablating the genicular nerves, though studies evaluating intra-articular RFA for other conditions have yielded equivocal results.44,45 A 2017 trial by Gulec et al randomized 100 patients with chronic knee pain for at least 3 months to receive either intra-articular bipolar or monopolar PRF, without a control group.39 Two RF cannulae were placed into the anterior knee joint from either side of the patellar ligament under fluoroscopic guidance. PRF in all cases was delivered at 42°C for 10 minutes, but patients and the provider were blinded as to whether it consisted of bipolar energy coursing between the cannulae, or a monopolar system set up between one of the cannulae and a dispersive (grounding) pad. The primary outcome measure was the percent of patients with at least 50% pain reduction at 3-month follow-up as measured by VAS while walking on a flat ground. The results showed superior improvements in the bipolar group, with 84% achieving a positive primary outcome, compared to only 50% in the monopolar group. Limitations of this trial include the lack of a control group and no long-term follow-up.
Comparative, non-randomized trials
The 2010 Ikeuchi et al trial compared RFA to nerve blocks, but was not randomized.37 Nineteen patients who met the selection criteria during one time frame received RFA, while another nineteen patients treated within a later time frame received sham RFA; one RFA and two control patients did not complete the study. Inclusion criteria included radiographic evidence of moderate to severe OA, age >65 years, and knee pain for longer than 3 months. Patients in the RFA group received two non-image guided, continuous RFA treatments at 70°C at a 2-week interval. Two lesion sites were targeted, the SM and IP genicular nerves. Patients in the control group also received two procedures 2 weeks apart, but they consisted only of topical and deep anesthesia with lidocaine; RFA was not performed. Other complementary treatments were withheld for 12 weeks, and assessment was obtained at 4 weeks, 8 weeks, 12 weeks, and 6 months. Outcome measures included the WOMAC functional assessment, VAS pain scores, and a 4-point patient global assessment. Outcomes were overall mixed. The total WOMAC score remained lower in the RFA group throughout the 6 month follow-up, but the difference was not statistically significant. VAS scores were significantly lower in the RFA group at 4-, 8-, and 12-week follow-ups, but the effect tapered off by 6 months. Limitations of the this study include the lack of randomization, lack of prognostic nerve blocks, and the targeting of only two nerve branches (Table 1).
Table 1.
Randomized and comparative studies
| Trial | Study type | Trial size, diagnosis | RF method | Anatomic targets | Prognostic block | Follow-up | Results | Comment |
|---|---|---|---|---|---|---|---|---|
| Choi et al28 | RCT | 38, OA | RFA (70°C, 90 seconds) + LA vs sham | SM, SL, IM | Yes | 12 weeks | RFA > sham for pain and function at 4 and 12 weeks | High volume (2 mL) used for prognostic blocks |
| Sarı et al29 | RCT | 73, OA | RFA (80°C, 90 seconds) vs IA steroid, LA, and morphine | SM, SL, IM | No | 3 months | RFA superior for pain and function | No prognostic blocks |
| Shen et al43 | RCT | 54, OA | RFA (70°C, 120 seconds) + PRP + HA vs PRP + HA | Not specified | No | 3 months | RFA superior for pain and function | Nerves targeted not described, unclear if imaging guidance was used |
| Qudsi-Sinclair et al30 | RCT | 28, post- TKA | RFA (80°C, 90 seconds) vs LA + steroid | SM, SL, IM | No | 12 months | RFA > nerve block for pain at 12 months; functional relief modest and not different between groups | Significant short-term relief with nerve blocks. Functional improvement modest and not statistically different between groups |
| Davis et al31 | RCT | 151, OA | Cooled RFA (60°C, 150 seconds) vs IA steroid | SM, SL, IM | Yes | 6 months | RFA > IA injections for pain and function | No difference in opioid usage between groups |
| El-Hakeim et al32 | RCT | 60, OA | RFA (90°C, 270 seconds) vs PO acetaminophen and NSAIDs | SM, SL, IM | No | 6 months | RFA superior for pain and function | Patients not blinded |
| McCormick et al33 | RCT | 53, OA | Cooled RFA (60°C, 150 seconds) either after or without prognostic nerve block | SM, SL, IM | Yes/no | 6 months | Both groups improved in pain and function, no difference between groups | 29 of 32 patients in the nerve block group received RFA |
| Gulec et al39 | RCT | 100, OA | Bipolar vs monopolar IA PRF (42°C, 10 minutes) | IA | No | 12 weeks | Bipolar > monopolar RFA for pain relief at 12 weeks | No control group |
| Ikeuchi et al37 | CS-P | 35, OA | RFA (70°C, 90 seconds) + LA vs LA | SM, IP | No | 6 months | RFA > LA for pain at 12 weeks | Not image guided, non-randomized |
Abbreviations: CS-P, case series, prospective; HA, hyaluronic acid; IA, intra-articular; IM, inferomedial; IP, infrapatellar; LA, local anesthetic; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis; PO, per os; PRF, pulsed radiofrequency; PRP, platelet-rich plasma; RCT, randomized controlled trial; RF, radiofrequency; RFA, radiofrequency ablation; SL, superolateral; SM, superomedial; TKA, total knee arthroplasty.
RFA predictors of outcome
Predictors of success or failure with RFA relate to both patient characteristics and procedural technique. This subject has been reported extensively as it relates to other pain interventions, notably lumbar facet and sacroiliac joint (SIJ) RFA. Patient factors predictive of failure with SIJ RFA include age >65 years, higher baseline pain level, and opioid use.46 Patient factors predictive of poor success with lumbar or cervical RFA include advanced age, certain pain referral patterns and physical exam signs, opioid use, prior history of depression, previous back surgery, longer duration of pain, and ongoing legal action.47–49 In individuals with chronic neck pain due to whiplash, low levels of pain catastrophizing and functional disability were found to be predictive of success with cervical RFA.50 In a study evaluating prognostic factors before PRF of the occipital nerves, treatment failure was associated with extension of pain beyond the confines of the targeted nerve distribution, while a lower diagnostic block volume, multiple treatment cycles, and a traumatic precipitating event predicted success.51
Procedural factors that contribute to success with RFA include the type of RF energy applied, the duration and temperature of treatment, and the gage of the RF probe itself. An ex vivo trial compared several types of RF and found that CRFA created larger lesions than either continuous or PRF.52 Within RFA and PRFA groups, larger probes and longer duration of treatment were associated with larger lesions. Results from studies on SIJ RFA have been mixed, with one reporting superior relief with CRFA when compared to RFA,53 one showing a trend toward superiority with CRFA,54 and another demonstrating no difference.55 Orientation of the RF probe parallel to the targeted nerve has also been demonstrated in preclinical studies to create larger lesions,56 but results from uncontrolled trials are mixed.55,57
The predictive value of prognostic genicular nerve blocks prior to RFA is similarly questionable. Among the eight RCTs discussed, only three employed positive response to nerve block as a criterion for the performance of RFA. The Choi et al trial, which is the only RCT to compare RFA to sham, required a positive response to prognostic nerve blocks for patients to proceed to the RF portion of the study.28 However, 2 mL lidocaine per site was used to perform the nerve blocks, and a positive response was defined as at least 50% pain relief lasting for 24 hours. Higher volume local anesthetic nerve blocks have been shown to decrease the specificity of prognostic blocks as local anesthetics may spread farther than a subsequent RF lesion,51 and pain relief lasting 24 hours is inconsistent with the pharmacodynamics of lidocaine.
The McCormick et al trial specifically addressed the prognostic value of nerve blocks by comparing RFA outcomes performed after positive genicular nerve blocks to results obtained without the use of prior injections.33 Blocks were performed with 1 mL of lidocaine, with a positive response defined as ≥50% pain reduction persisting for at least 1 hour following injection. Given the lack of statistical difference between the groups, the authors concluded that their method of genicular nerve block has little prognostic value. Methods postulated by the authors to increase the prognostic utility of genicular nerve blocks include the use of a higher threshold for defining a positive response to nerve blocks (90% relief), or using double comparative nerve blocks. However, studies evaluating the effect of using higher cutoffs to select patients for SIJ and lumbar and cervical RFA have mostly found no improvement in outcomes.46,58,59 The use of comparative medial branch blocks with local anesthetics of differing durations of action is also of unclear benefit, having been shown to have marginal sensitivity60 and little impact on lumbar facet RFA outcomes61,62 (Table 2).
Table 2.
Factors associated with radiofrequency ablation treatment outcomes for knee pain and other conditions
| Predictors of success | Predictors of failure |
|---|---|
| Medial compartment osteoarthritis and concordant pain | Greater disease burden (eg, longer duration of symptoms, greater disability) |
| Large and/or multiple lesions | Previous surgery |
| Controlled prognostic blocks | Opioid use |
| Psychopathology | |
| Diffuse pain symptomatology (fibromyalgianess) |
Complications
No major adverse events were reported in any randomized or observational trial. All patients in the RF limb of the Ikeuchi trial noted 2–6 weeks of hypoesthesia in the IP region, but these symptoms self-resolved.37 A review in 2016 discussed the potential adverse effects of knee RFA given the proximity of genicular arteries to the genicular nerves.63 Multiple genicular arteries arise from the popliteal artery, and they course along the epicondyles of the distal femur and proximal tibia in the region where the SL, SM, and IM genicular nerves are normally targeted64 (Figure 3). Injuries to the genicular arterial system are described in the surgical literature, from both open and arthroscopic procedures, and sequelae include pseudoaneurysm formation, hemarthrosis, arteriovenous fistula formation, and patellar osteonecrosis.65–68 The authors are also aware of several unpublished reports of skin burns, which may arise because of the close proximity of the target nerves to the skin. Other potential complications include those generic to other interventional procedures, namely infection, bleeding, or bruising. Intra-articular approaches also confer the risk of joint sepsis or chondrolysis, but these have not been described following RFA.
Figure 3.
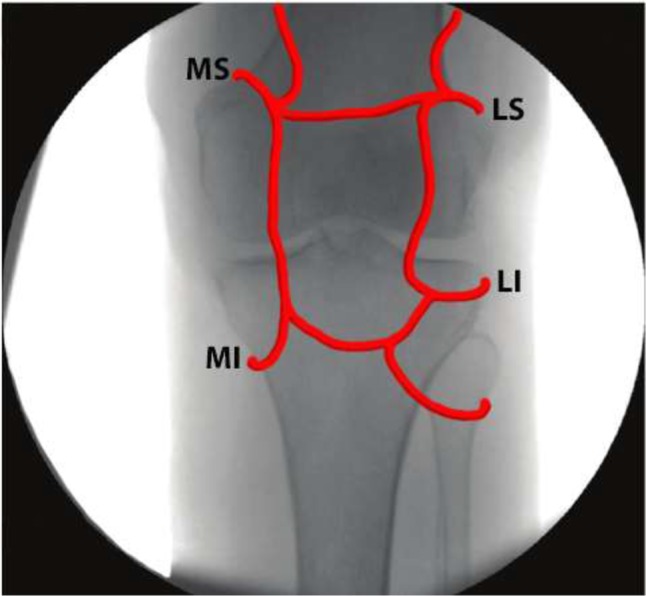
Anterior-posterior radiograph of the knee with overlay of the genicular arteries.
Abbreviations: LI, lateral inferior; LS, lateral superior; MI, medial inferior; MS, medial superior.
Discussion
Chronic knee pain is a recent addition to the growing list of indications for RF, having first been described only a decade ago. Among the eight RCTs found in the literature, six compared knee RFA to sham28,30 or other accepted treatments,29,31,32,43 but all demonstrated superior pain relief and function with RFA.28–33,37,39,43 Fundamental differences between studies make the aggregate body of literature difficult to interpret, as the type of RFA and the anatomic targets vary considerably. However, the preponderance of evidence suggests that RF denervation can be a safe and effective treatment for chronic knee pain, with the duration of benefit similar to that targeting other joints, ranging between 3 and 12 months.
The use of PRF for knee pain is not well supported, and was featured in only one RCT that compared two types of PRF, without a control group.39 PRF is generally acknowledged as an effective treatment for neuropathic pain based on dozens of preclinical studies and numerous clinical trials.69–72 Considering that knee arthritis is a non-neuropathic pain condition, the conceptual basis for the use of PRF in this condition (ie, neuromodulation without significant injury to the neural architecture) is lacking. RCTs comparing PRF to RFA for lumbar facet joint pain have shown greater and more sustained pain relief with continuous RFA, raising further questions regarding its utility for knee pain.73,74 Although pain was improved compared to baseline in the PRF groups of both studies, these differences were small and may be attributable to a placebo response. In any case, these results are not easily extrapolated to PRF of the knee given that the targets were neural structures (ie, the medial branch nerves), and not the intra-articular space.
The utility of prognostic blocks prior to RFA is also unclear. Employment of a higher pain relief threshold and the use of comparative local anesthetic blocks may increase the specificity of prognostic blocks, but at the inevitable cost of decreased sensitivity (ie, increased false-negative results).60,62 The primary downside for a prognostic procedure associated with a high false-negative rate is the denial to patients of a safe and effective RFA procedure.
It should be acknowledged that comparisons to and from the spine pain literature regarding RFA are based primarily on the similarity of the intervention, not on similarities in pathology or anatomy. Therefore, in addition to neuroanatomical studies and large multicenter studies that further elucidate the benefit of knee RFA in comparison to sham ablation (ie, placebo-controlled studies), different types of ablation (eg, cooled vs conventional, techniques targeting different genicular nerves and anatomical locations), and alternative treatments (ie, comparative-effectiveness studies), other areas ripe for future research include modification of facet and SIJ RFA trials that have shed light on patient selection criteria, and the effects of prognostic nerve blocks on RFA outcomes.
Acknowledgments
The assistance offered by the Center for Rehabilitation Sciences Research, Bethesda, MD, USA is gratefully acknowledged.
Footnotes
Disclosure
SPC has served as a consultant to Halyard, Boston Scientific, and Abbott within the past 3 years. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the US Department of Defense. The authors report no other conflicts of interest in this work.
References
- 1.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannoni A, Briganti MP, di Bari M, et al. Epidemiological profile of symptomatic osteoarthritis in older adults: a population based study in Dicomano, Italy. Ann Rheum Dis. 2003;62(6):576–578. doi: 10.1136/ard.62.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehabil. 2006;85(11 Suppl):S2–S14. doi: 10.1097/01.phm.0000245568.69434.1a. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69(3):483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 6.Karaman H, Tüfek A, Kavak GÖ, et al. Intra-articularly applied pulsed radiofrequency can reduce chronic knee pain in patients with osteoarthritis. J Chin Med Assoc. 2011;74(8):336–340. doi: 10.1016/j.jcma.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Onishi K, Utturkar A, Chang E, et al. Osteoarthritis: a critical review. Crit Rev Phys Rehabil Med. 2012;24(3–4):251–264. doi: 10.1615/CritRevPhysRehabilMed.2013007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcalindon TE, Lavalley MP, Harvey WF, et al. Effect of intra- articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–1975. doi: 10.1001/jama.2017.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan F, Trebinjac S, Murrell WD, Maffulli N. The effectiveness of prolotherapy in treating knee osteoarthritis in adults: a systematic review. Br Med Bull. 2017;122(1):91–108. doi: 10.1093/bmb/ldx006. [DOI] [PubMed] [Google Scholar]
- 10.Louis ML, Magalon J, Jouve E, et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: a randomized double blind noninferiority trial compared with visco-supplementation. Arthroscopy. 2018;34(5):1530–1540. doi: 10.1016/j.arthro.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Goncars V, Kalnberzs K, Jakobsons E, et al. Treatment of knee osteoarthritis with bone marrow-derived mononuclear cell injection: 12-month follow-up. Cartilage. 2018 Jan 1; doi: 10.1177/1947603517746721. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith NA, Parsons N, Wright D, et al. A pilot randomized trial of meniscal allograft transplantation versus personalized physiotherapy for patients with a symptomatic meniscal deficient knee compartment. Bone Joint J. 2018;100-B(1):56–63. doi: 10.1302/0301-620X.100B1.BJJ-2017-0918.R1. [DOI] [PubMed] [Google Scholar]
- 13.Arendt-Nielsen L, Simonsen O, Laursen M, et al. Pain and sensitization after total knee replacement or non-surgical treatment in patients with knee osteoarthritis: identifying potential predictors of outcome at 12 months. Eur J Pain. 2018;22(6):1088–1102. doi: 10.1002/ejp.1193. [DOI] [PubMed] [Google Scholar]
- 14.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27(7):1745–1752. [PubMed] [Google Scholar]
- 15.Baker PN, van der Meulen JH, Lewsey J, Gregg PJ, National Joint Registry for England and Wales The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89(7):893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 16.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Cosman ER, Cosman ER. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6(6):405–424. doi: 10.1111/j.1526-4637.2005.00076.x. [DOI] [PubMed] [Google Scholar]
- 18.Sweet WH, Mark VH, Hamlin H. Radiofrequency lesions in the central nervous system of man and cat: including case reports of eight bulbar pain-tract interruptions. J Neurosurg. 1960;17:213–225. doi: 10.3171/jns.1960.17.2.0213. [DOI] [PubMed] [Google Scholar]
- 19.Shealy CN. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurg. 1975;43(4):448–451. doi: 10.3171/jns.1975.43.4.0448. [DOI] [PubMed] [Google Scholar]
- 20.Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 1996;3(7):556–563. doi: 10.1016/s1076-6332(96)80219-4. [DOI] [PubMed] [Google Scholar]
- 21.Munglani R. The longer term effect of pulsed radiofrequency for neuropathic pain. Pain. 1999;80(1–2):437–439. doi: 10.1016/s0304-3959(98)00183-3. [DOI] [PubMed] [Google Scholar]
- 22.Ho KWD, Przkora R, Kumar S. Sphenopalatine ganglion: block, radiofrequency ablation and neurostimulation – a systematic review. J Headache Pain. 2017;18(1):118. doi: 10.1186/s10194-017-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxena AK, Lakshman K, Sharma T, et al. Modulation of serum BDNF levels in postherpetic neuralgia following pulsed radiofrequency of intercostal nerve and pregabalin. Pain Manag. 2016;6(3):217–227. doi: 10.2217/pmt.16.3. [DOI] [PubMed] [Google Scholar]
- 24.Lindquist J, Bäckryd E. Pulsed radiofrequency in clinical practice – a retrospective analysis of 238 patients with chronic non-cancer pain treated at an academic tertiary pain centre. Scand J Pain. 2016;12:68–73. doi: 10.1016/j.sjpain.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Sluijter ME, Teixeira A, Serra V, Balogh S, Schianchi P. Intra-articular application of pulsed radiofrequency for arthrogenic pain – report of six cases. Pain Pract. 2008;8(1):57–61. doi: 10.1111/j.1533-2500.2007.00172.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirasawa Y, Okajima S, Ohta M, Tokioka T. Nerve distribution to the human knee joint: anatomical and immunohistochemical study. Int Orthop. 2000;24(1):1–4. doi: 10.1007/s002640050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10(6):329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 28.Choi WJ, Hwang SJ, Song JG, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;152(3):481–487. doi: 10.1016/j.pain.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Sarı S, Aydın ON, Turan Y, et al. Which one is more effective for the clinical treatment of chronic pain in knee osteoarthritis: radiofrequency neurotomy of the genicular nerves or intra-articular injection? Int J Rheum Dis. 2016 Aug 12; doi: 10.1111/1756-185X.12925. Epub. [DOI] [PubMed] [Google Scholar]
- 30.Qudsi-Sinclair S, Borrás-Rubio E, Abellan-Guillén JF, Padilla del Rey ML, Ruiz-Merino G. A comparison of genicular nerve treatment using either radiofrequency or analgesic block with corticosteroid for pain after a total knee arthroplasty: a double-blind, randomized clinical study. Pain Pract. 2017;17(5):578–588. doi: 10.1111/papr.12481. [DOI] [PubMed] [Google Scholar]
- 31.Davis T, Loudermilk E, Depalma M, et al. Prospective, multicenter, randomized, crossover clinical trial comparing the safety and effectiveness of cooled radiofrequency ablation with corticosteroid injection in the management of knee pain from osteoarthritis. Reg Anesth Pain Med. 2018;43(1):84–91. doi: 10.1097/AAP.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Hakeim EH, Elawamy A, Kamel EZ, et al. Fluoroscopic guided radiofrequency of genicular nerves for pain alleviation in chronic knee osteoarthritis: a single-blind randomized controlled trial. Pain Physician. 2018;21(2):169–177. [PubMed] [Google Scholar]
- 33.McCormick ZL, Reddy R, Korn M, et al. A prospective randomized trial of prognostic genicular nerve blocks to determine the predictive value for the outcome of cooled radiofrequency ablation for chronic knee pain due to osteoarthritis. Pain Med. 2017 Dec 28; doi: 10.1093/pm/pnx286. Epub. [DOI] [PubMed] [Google Scholar]
- 34.Akbas M, Luleci N, Dere K, Luleci E, Ozdemir U, Toman H. Efficacy of pulsed radiofrequency treatment on the saphenous nerve in patients with chronic knee pain. J Back Musculoskelet Rehabil. 2011;24(2):77–82. doi: 10.3233/BMR-2011-0277. [DOI] [PubMed] [Google Scholar]
- 35.Djibilian Fucci R, Pascual-Ramírez J, Martínez-Marcos A, Mantecón JM. Ultrasound-guided sciatic nerve pulsed radiofrequency for chronic knee pain treatment: a novel approach. J Anesth. 2013;27(6):935–938. doi: 10.1007/s00540-013-1624-6. [DOI] [PubMed] [Google Scholar]
- 36.Clendenen S, Greengrass R, Whalen J, O’Connor MI. Infrapatellar saphenous neuralgia after TKA can be improved with ultrasound-guided local treatments. Clin Orthop Relat Res. 2015;473(1):119–125. doi: 10.1007/s11999-014-3812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeuchi M, Ushida T, Izumi M, Tani T. Percutaneous radiofrequency treatment for refractory anteromedial pain of osteoarthritic knees. Pain Med. 2011;12(4):546–551. doi: 10.1111/j.1526-4637.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- 38.Vas L, Pai R, Khandagale N, Pattnaik M. Pulsed radiofrequency of the composite nerve supply to the knee joint as a new technique for relieving osteoarthritic pain: a preliminary report. Pain Physician. 2014;17(6):493–506. [PubMed] [Google Scholar]
- 39.Gulec E, Ozbek H, Pektas S, Isik G. Bipolar versus unipolar intraarticular pulsed radiofrequency thermocoagulation in chronic knee pain treatment: a prospective randomized trial. Pain Physician. 2017;20(3):197–206. [PubMed] [Google Scholar]
- 40.Masala S, Fiori R, Raguso M, Morini M, Calabria E, Simonetti G. Pulse-dose radiofrequency for knee osteoarthrithis. Cardiovasc Intervent Radiol. 2014;37(2):482–487. doi: 10.1007/s00270-013-0694-z. [DOI] [PubMed] [Google Scholar]
- 41.Eyigor C, Eyigor S, Akdeniz S, Uyar M. Effects of intra-articular application of pulsed radiofrequency on pain, functioning and quality of life in patients with advanced knee osteoarthritis. J Back Musculoskelet Rehabil. 2015;28(1):129–134. doi: 10.3233/BMR-140500. [DOI] [PubMed] [Google Scholar]
- 42.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen WS, Xu XQ, Zhai NN, et al. Radiofrequency thermocoagulation in relieving refractory pain of knee osteoarthritis. Am J Ther. 2017;24(6):e693–e700. doi: 10.1097/MJT.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 44.Ferrante FM, King LF, Roche EA, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137–142. doi: 10.1053/rapm.2001.21739. [DOI] [PubMed] [Google Scholar]
- 45.Lim JW, Cho YW, Lee DG, Chang MC. Comparison of intraarticular pulsed radiofrequency and intraarticular corticosteroid injection for management of cervical facet joint pain. Pain Physician. 2017;20(6):E961–E967. [PubMed] [Google Scholar]
- 46.Cohen SP, Strassels SA, Kurihara C, et al. Outcome predictors for sacroiliac joint (lateral branch) radiofrequency denervation. Reg Anesth Pain Med. 2009;34(3):206–214. doi: 10.1097/AAP.0b013e3181958f4b. [DOI] [PubMed] [Google Scholar]
- 47.Christensen TJ, Deberard MS, Wheeler AJ. Outcomes and prognostic variables of radiofrequency zygapophyseal joint neurotomy in Utah workers’ compensation patients. J Pain Res. 2017;10:1207–1215. doi: 10.2147/JPR.S132853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen SP, Hurley RW, Christo PJ, et al. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23(1):45–52. doi: 10.1097/01.ajp.0000210941.04182.ea. [DOI] [PubMed] [Google Scholar]
- 49.Cohen SP, Bajwa ZH, Kraemer JJ, et al. Factors predicting success and failure for cervical facet radiofrequency denervation: a multi-center analysis. Reg Anesth Pain Med. 2007;32(6):495–503. doi: 10.1016/j.rapm.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Smith AD, Jull GA, Schneider GM, et al. Low pain catastrophization and disability predict successful outcome to radiofrequency neurotomy in individuals with chronic whiplash. Pain Pract. 2016;16(3):311–319. doi: 10.1111/papr.12282. [DOI] [PubMed] [Google Scholar]
- 51.Huang JH, Galvagno SM, Hameed M, et al. Occipital nerve pulsed radiofrequency treatment: a multi-center study evaluating predictors of outcome. Pain Med. 2012;13(4):489–497. doi: 10.1111/j.1526-4637.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 52.Cedeno DL, Vallejo A, Kelley CA, Tilley DM, Kumar N. Comparisons of lesion volumes and shapes produced by a radiofrequency system with a cooled, a protruding, or a monopolar probe. Pain Physician. 2017;20(6):E915–E922. [PubMed] [Google Scholar]
- 53.Tinnirello A, Barbieri S, Todeschini M, Marchesini M. Conventional (Simplicity III) and cooled (SInergy) radiofrequency for sacroiliac joint denervation: one-year retrospective study comparing two devices. Pain Med. 2017;18(9):1731–1744. doi: 10.1093/pm/pnw333. [DOI] [PubMed] [Google Scholar]
- 54.Cohen SP, Hurley RW, Buckenmaier CC, et al. Randomized placebo- controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–288. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng J, Pope JE, Dalton JE, Cheng O, Bensitel A. Comparative outcomes of cooled versus traditional radiofrequency ablation of the lateral branches for sacroiliac joint pain. Clin J Pain. 2013;29(2):132–137. doi: 10.1097/AJP.0b013e3182490a17. [DOI] [PubMed] [Google Scholar]
- 56.Bogduk N, Macintosh J, Marsland A. Technical limitations to the efficacy of radiofrequency neurotomy for spinal pain. Neurosurgery. 1987;20(4):529–535. doi: 10.1227/00006123-198704000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Loh JT, Nicol AL, Elashoff D, Ferrante FM. Efficacy of needle-placement technique in radiofrequency ablation for treatment of lumbar facet arthropathy. J Pain Res. 2015;8:687–694. doi: 10.2147/JPR.S84913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen SP, Strassels SA, Kurihara C, et al. Establishing an optimal “cutoff ” threshold for diagnostic lumbar facet blocks: a prospective correlational study. Clin J Pain. 2013;29(5):382–391. doi: 10.1097/AJP.0b013e31825f53bf. [DOI] [PubMed] [Google Scholar]
- 59.Cohen SP, Stojanovic MP, Crooks M, et al. Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: a multicenter analysis. Spine J. 2008;8(3):498–504. doi: 10.1016/j.spinee.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 60.Lord SM, Barnsley L, Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11(3):208–273. doi: 10.1097/00002508-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Holz SC, Sehgal N. What is the correlation between facet joint radiofrequency outcome and response to comparative medial branch blocks? Pain Physician. 2016;19(3):163–172. [PubMed] [Google Scholar]
- 62.Cohen SP, Williams KA, Kurihara C, et al. Multicenter, randomized, comparative cost-effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology. 2010;113(2):395–405. doi: 10.1097/ALN.0b013e3181e33ae5. [DOI] [PubMed] [Google Scholar]
- 63.Kim SY, Le PU, Kosharskyy B, et al. Is genicular nerve radiofrequency ablation safe? A literature review and anatomical study. Pain Physician. 2016;19(5):E697–E705. [PubMed] [Google Scholar]
- 64.Callaghan J, Rosenberg A, Rubash H. Surgical anatomy of the knee. In: Wsielewski R, editor. The Adult Knee. Lippincott Williams & Wilkins; Philadelphia: 2003. pp. 77–78. [Google Scholar]
- 65.Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following total knee replacement. Knee. 2002;9(4):349–351. doi: 10.1016/s0968-0160(02)00061-3. [DOI] [PubMed] [Google Scholar]
- 66.Saboo SS, Juan YH, Belkin M, et al. Multi-detector CT angiography in case of concomitant pseudoaneurysm and arteriovenous fistula of the lateral superior geniculate artery. Postgrad Med J. 2014;90(1060):118–119. doi: 10.1136/postgradmedj-2013-132274. [DOI] [PubMed] [Google Scholar]
- 67.Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty – a case report. Acta Orthop. 2005;76(1):138–140. doi: 10.1080/00016470510030463. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim M, Booth RE, Clark TW. Embolization of traumatic pseudoaneurysms after total knee arthroplasty. J Arthroplasty. 2004;19(1):123–128. doi: 10.1016/j.arth.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Cohen SP, Peterlin BL, Fulton L, et al. Randomized, double-blind, comparative-effectiveness study comparing pulsed radiofrequency to steroid injections for occipital neuralgia or migraine with occipital nerve tenderness. Pain. 2015;156(12):2585–2594. doi: 10.1097/j.pain.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Zundert J, Patijn J, Kessels A, et al. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain. 2007;127(1–2):173–182. doi: 10.1016/j.pain.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Hagiwara S, Iwasaka H, Takeshima N, Noguchi T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain. 2009;13(3):249–252. doi: 10.1016/j.ejpain.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Wu B, Ni J, Zhang C, et al. Changes in spinal cord met-enkephalin levels and mechanical threshold values of pain after pulsed radio frequency in a spared nerve injury rat model. Neurol Res. 2012;34(4):408–414. doi: 10.1179/1743132812Y.0000000026. [DOI] [PubMed] [Google Scholar]
- 73.Tekin I, Mirzai H, Ok G, Erbuyun K, Vatansever D. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clin J Pain. 2007;23(6):524–529. doi: 10.1097/AJP.0b013e318074c99c. [DOI] [PubMed] [Google Scholar]
- 74.Kroll HR, Kim D, Danic MJ, et al. A randomized, double-blind, prospective study comparing the efficacy of continuous versus pulsed radiofrequency in the treatment of lumbar facet syndrome. J Clin Anesth. 2008;20(7):534–537. doi: 10.1016/j.jclinane.2008.05.021. [DOI] [PubMed] [Google Scholar]


