Abstract
The miR-33 microRNAs are crucial regulators of cholesterol/lipids and may represent therapeutic targets for the treatment of atherosclerosis. A recent report by Price et al. showed that miR-33−/− mice exhibit obesity, insulin resistance, and increased food intake, suggesting that metabolic regulation by miR-33 is more complex than previously known.
Keywords: miR-33, microRNA, obesity, insulin resistance
Main text
Mammalian metabolism is highly regulated and elaborate control circuits amongst cell, tissues and organs, participate in maintaining whole body metabolic homeostasis. Metabolic diseases such as obesity, insulin resistance/type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), circulating cholesterol/lipid abnormalities, and atherosclerosis have risen dramatically in the developed world, however, the mechanisms contributing to metabolic dysregulation remain unclear and are intensively investigated to find new therapeutic avenues. Most studies to date have focused on the functions of metabolic enzymes, hormones and signaling molecules, and regulatory proteins such as transcription factors and co-factors in metabolic diseases. However, regulatory non-coding RNAs such as microRNAs (miRNAs) [1] have also recently emerged as important modulators of metabolic homeostasis [2].
The miR-33a and miR-33b miRNAs, located in introns in the genes encoding the SREBP-2 and SREBP-1 transcription factors respectively (master regulators of genes involved in cholesterol/lipid biosynthesis and trafficking), inhibit the expression of cholesterol transporters (ABCA1 and ABCG1) in mammals, with effects on cholesterol efflux in peripheral tissues and cells such as macrophages, and on circulating high-density lipoprotein (HDL)-cholesterol, a component of the anti-atherosclerotic reverse cholesterol transport (RCT) pathway [2]. Accordingly, inhibition of miR-33 in mouse atherosclerosis models (LDLr or Apoe null mice fed atherogenic diets) by antisense oligonucleotides (ASOs) or genetic ablation have potent beneficial effects, at least in short-term (4–12 weeks) studies [3]. These findings have elicited considerable interest in evaluating miR-33 as a therapeutic target in cardiovascular disease.
Results from longitudinal studies with ASO treatment [4] or miR-33 knockout (KO) in mice [5, 6] have however raised questions about possible deleterious metabolic consequences of antagonizing miR-33. Early work by Horie et al. showed that miR-33 KO mice developed obesity and elevated circulating TAGs over time (after about one year in chow-fed mice, or after 12 weeks on a high-fat diet) [5]. Their analysis suggested that these metabolic abnormalities might be due to elevated SREBP-1 protein in liver (SREBP-1 was shown to be a target of miR-33), as heterozygous genetic ablation of SREBP-1 ameliorated obesity and elevated blood TAGs in miR-33 KO mice. In accord with this, it was also reported that expression of SREBP-1 lipogenic target genes such as FASN and ACC1 is elevated in the liver of mice treated chronically with miR-33-targeting ASOs [4].
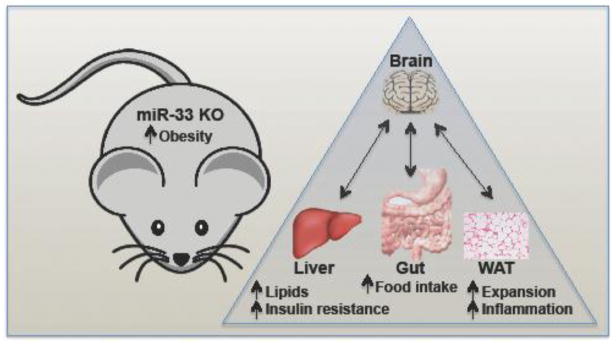
In the current work, Price et al. [6] further characterized the metabolic dysfunction of miR-33 KO mice. Similar to previous studies [4, 5, 7], they found that miR-33 null mice develop obesity and elevated circulating TAGs and liver lipids (Figure 1). However, by contrast with findings in other models [4, 5] there was no evidence of increased SREBP-1 activity or lipogenic target gene expression during fasting/re-feeding experiments of the miR-33 null mice, and no alteration of SREBP-1 proteolytic processing was observed. The reason for these differences is unclear, and the mechanisms contributing to the modest increase in liver lipids have not been fully elucidated. Future studies will need to address whether elevated liver lipids observed in miR-33 null mice may be accounted for by increased lipid uptake, decreased fatty acid degradation or lowered lipid export.
Figure 1. Metabolic dysregulation in miR-33 KO mice.
Previous studies had suggested that the miR-33 microRNA might represent a therapeutic target for the treatment of cardiovascular disease. However, Price et al. found that whole animal genetic ablation of miR-33 results in obesity, with expanded white adipose tissue (WAT) fat storage and inflammation, as well as abnormal liver and circulating lipids, and systemic and liver insulin resistance and impaired glucose tolerance. Pair-feeding studies suggested that increased food intake in the miR-33 KO mice might explain many of the deleterious phenotypes. Future longitudinal studies, especially using more relevant nonhuman primate models, will be needed to further explore the impact of miR-33 antagonism on metabolic homeostasis before launching human intervention studies for the treatment of cardiovascular disease.
In agreement with the previous study by Horie et al. [5], Price et al. also found that loss of miR-33 in the whole animal predisposes to decreased insulin sensitivity in liver, WAT and skeletal muscle, even in mice fed a chow diet [6]. Extending these findings, hyperinsulinemic-euglycemic clamp studies revealed that miR-33 KO mice exhibit decreased glucose uptake in WAT and skeletal muscle, poor suppression of endogenous glucose production in liver, and decreased ability to suppress release of non-esterified fatty acids (NEFA) from WAT. There was additionally an increased infiltration of inflammatory cells in WAT in miR-33 null mice fed the HFD. These results suggest that complete miR-33 loss in mice, especially in the context of a HFD, can have important deleterious metabolic consequences that are similar to obesity-associated insulin resistance, type 2 diabetes and WAT inflammation in humans.
The authors found that the increased body weight and adiposity could not be explained by changes in fuel preference or decreased energy expenditure [6], similar to findings reported by the Horie et al. 2013 study [5], however, they also showed that young mice exhibited increased lipid uptake into adipose and decreased free fatty acid hydrolysis, suggesting an increased propensity to store fat as a contributing factor to the elevated body weight and obesity. What might be the cause of increased adiposity? The most important, and unexpected, finding by Price et al. is that miR-33 KO mice exhibit increased food intake, and pair-feeding studies suggested that the hyperphagia is the primary cause of obesity and metabolic dysfunction, as limiting food intake to match that of wild-type animals rescued metabolic dysfunction. This indicates that miR-33 regulation is somehow involved in central metabolic control of feeding, however altered levels of feeding-regulating hormones such as ghrelin or leptin appeared not to be involved [6]. Brain-specific KO of miR-33 would help to tease out whether the effect is due to peripheral action or a direct function of miR-33 in regulating brain circuits involved in satiety control.
Unlike humans and other primates, rodents lack the miR-33b isoform located in an intron in the SREBF1 gene [2]. Previous work has shown that although miR-33b has partially redundant functions with miR-33a in cholesterol regulation, it appears to exert more prominent regulatory impact on targets involved in fatty acid degradation and regulation of mitochondrial energy homeostasis and glucose control [3]. Importantly, two studies reporting ASO targeting of both miR-33a and miR-33b in nonhuman primates did not reveal deleterious metabolic effects, although these studies were only carried out for 12 weeks [8, 9]. Moreover, whereas the miR-33a KO mice would lack miR-33 from birth, ASOs would be used for therapeutic targeting in adults and would not cause complete ablation of miR-33 in any tissue, except perhaps in liver, and they do not traverse the blood-brain barrier, and should have no direct inhibitory effect on brain miR-33. The relevance of rodent miR-33a KO studies to human ASO-based therapeutic targeting efforts is thus uncertain. Nevertheless, longitudinal studies in nonhuman primates with anti-miR-33a/b ASOs would help to ascertain the safety of this approach for the treatment of cardiovascular disease.
Acknowledgments
A.M.N is supported by grants from the National Institutes of Health (DK114277 and P01GM047467) and a Massachusetts General Hospital Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13(4):239–50. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aryal B, et al. MicroRNAs and lipid metabolism. Curr Opin Lipidol. 2017;28(3):273–280. doi: 10.1097/MOL.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goedeke L, et al. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol Med. 2014;6(9):1133–41. doi: 10.15252/emmm.201404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horie T, et al. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price NL, et al. Genetic Ablation of miR-33 Increases Food Intake, Enhances Adipose Tissue Expansion, and Promotes Obesity and Insulin Resistance. Cell Rep. 2018;22(8):2133–2145. doi: 10.1016/j.celrep.2018.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price NL, et al. Genetic Dissection of the Impact of miR-33a and miR-33b during the Progression of Atherosclerosis. Cell Rep. 2017;21(5):1317–1330. doi: 10.1016/j.celrep.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayner KJ, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–7. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rottiers V, et al. Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci Transl Med. 2013;5(212):212ra162. doi: 10.1126/scitranslmed.3006840. [DOI] [PMC free article] [PubMed] [Google Scholar]