Abstract
Salt inducible kinases represent a subfamily of AMPK family kinases. Initially named because SIK1 (the founding member of this kinase family) expression is regulated by dietary salt intake in the adrenal gland, it is now apparent that a major biological role of these kinases is to control gene expression in response extracellular cues that increase intracellular levels of cAMP. Here, we will review four physiologically-relevant examples of how cAMP signaling impinges upon SIK cellular function. By focusing on examples of cAMP-mediated SIK regulation in gut myeloid cells, bone, liver, and skin, we will highlight recent advances in G protein coupled receptor signal transduction. New knowledge regarding the role of salt inducible kinases in GPCR signaling has led to therapeutic applications of novel small molecule SIK inhibitors.
Keywords: kinase, second messengers, inflammation, osteoporosis, glucose metabolism, skin pigmentation
Background on SIKs, and their regulation by cAMP signaling
The AMP-activated protein kinase (AMPK) family contains 14 members, which play broad roles in cellular metabolism (1). Activation of AMPK family members occurs via phosphorylation of the activation loop kinase domain by liver kinase B1 (LKB1) (2). Within the AMPK family, the subfamily of salt inducible kinases (SIKs) contains three kinases (SIK1–3) whose physiologic roles will be the subject of this review. SIK1, the founding member of this subfamily, was identified and named as a kinase whose expression is induced in the adrenal gland of rats fed a high salt diet (3). Subsequent homology searches led to the identification of SIK2 and SIK3. All three SIK family members share a characteristic structure, including an N-terminal kinase domain bearing a LKB1 phosphorylation site, a sucrose non-fermenting-1 (SNF-1) homology domain, and a long C-terminal extension containing multiple potential protein kinase A phosphorylation sites (4).
All three SIK family kinases are expressed broadly. SIK1 mRNA expression is regulated by multiple stimuli, including high dietary salt intake, ACTH signaling (5), glucagon signaling (6), excitable cell depolarization (7), and circadian rhythms (8). In contrast, SIK2 and SIK3 expression is constitutive in tissues in which these kinases are expressed. In humans, SIK2 and SIK3 are expressed ubiquitously, with highest SIK2 levels in adipose tissue and highest SIK3 expression in brain. Interestingly, SIK2 and SIK3 are closely linked on human chromosome 11 and mouse chromosome 9.
Initial studies to define the substrate specificity of SIKs were performed in vitro using recombinant SIK1 kinase domain fragments. Through this approach, a canonical substrate phosphorylation motif of LxB(S/T)xS*xxxL (B=basic amino acid, X=any amino acid) was defined (4). In subsequent years, multiple physiologically-important SIK substrates have been identified. Currently, the best studied SIK substrates are class IIa histone deacetylases (HDAC4, 5, 7, and 9) (9) and cAMP regulated transcriptional coactivators (CRTC1-3) (10). Phosphorylation by SIKs plays a crucial role in regulating subcellular localization and biologic activity of class IIa HDACs and CRTC proteins. When phosphorylated, these SIK substrates are retained in the cytoplasm due to association with cytoplasmic 14–3–3 chaperones. When de-phosphorylated, these SIK substrates are able to translocate into the nucleus, where they regulate gene expression. In the nucleus, class IIa HDACs function as potent inhibitors of MEF2-driven gene expression (9) and can activate forkhead family transcription factors (11, 12), while CRTC factors potentiate the activity of CREB and related bZIP-family transcription factors (10). Beyond class IIa HDACs and CRTC proteins, additional tissue-specific SIK substrates have been suggested (13–15) and will be discussed below.
A key role of SIKs is to control dynamic changes in phosphorylation and subcellular localization of class IIa HDACs and CRTC factors. Therefore, upstream control of SIK activity provides an opportunity to integrate diverse extracellular cues into changes in MEF2- and CREB-driven gene expression. In general, SIK cellular activity is tonically in the “on” state, due to constitutive LKB1-mediated phosphorylation (2, 16, 17). SIK-mediated phosphorylation of class IIa HDAC and CRTC proteins leads to their cytoplasmic retention and latent inactivation (9, 10, 18). Signals that increase intracellular cAMP levels lead to protein kinase A (PKA)-mediated SIK family member phosphorylation (19, 20). PKA-mediated phosphorylation does not alter SIK intrinsic kinase activity (21, 22). However, mutation of PKA phosphoacceptor sites leads to SIK variants whose cellular activity cannot be inhibited by cAMP-inducing signals (23). PKA-mediated SIK phosphorylation promotes interaction between SIK and 14–3–3 proteins (18, 24). This PKA-inducible SIK/14–3–3 association leads to conformational changes and/or shifts in SIK cytoplasmic distribution which block the ability of these kinases to access and phosphorylate their substrates. As discussed below, reducing CRTC phosphorylation via small molecule SIK inhibitors appears to be sufficient to stimulate CREB-dependent gene expression, even in the absence of boosting cellular cAMP levels. Therefore, the relative importance of PKA-dependent CREB versus SIK phosphorylation in stimulating CREB/CRTC-mediated transcriptional output remains to be determined.
Recent work demonstrated that, of the three SIK isoforms, SIK2 is unique in that it bears 4 separate PKA phosphorylation sites (SIK1 and SIK3 each have two PKA sites) that, when phosphorylated, serve as 14–3–3 docking sites (24). Therefore, the cellular activity of all SIK family members can be inhibited by upstream cAMP-inducing signals, with SIK2 perhaps best poised to be blocked by PKA-activating agents. While the role of PKA-mediated SIK1 and SIK2 phosphorylation in vivo remains to be explored, a SIK3 mutant allele lacking these PKA phosphorylation sites was identified during a screen for randomly mutagenized mice with disrupted sleep patterns (25). Of the three SIK isoforms, SIK3 expression is highest in brain. Interestingly, brain phosphoproteomic analysis of these SIK3 gain of function mice versus littermate controls revealed increased phosphorylation of synaptic regulatory proteins, indicating a novel role for SIK3 in sleep-related neurotransmission (26). Although cAMP-activated PKA is a well-accepted mechanism to reduce cellular SIK activity, less is known about the upstream signals that stimulate basal SIK function. Since LKB1 is the best-known SIK activator (2), it is possible that signals that induce LKB1 function (17) may also increase SIK activity.
To highlight the physiologic significance of these signaling events, selected examples of G protein coupled receptor (GPCR)-linked cAMP/PKA/SIK signaling pathways will now be discussed. Although each example reviewed participates very different cellular physiology ranging from cytokine production to bone remodeling to skin pigmentation, the general theme that SIK inhibition is a key downstream step in cAMP signaling events clearly emerges. Moreover, in each instance, key aspects of hormonal signaling action are mimicked using small molecule SIK inhibitors, hinting at possible new therapeutic strategies.
The role of SIKs downstream of prostaglandins in gut myeloid cells
Crohn’s disease (CD) and ulcerative colitis (UC) are the most common forms of inflammatory bowel disease (IBD), a chronic disorder arising in part from impaired anti-inflammatory immune mechanisms that result in an imbalance between pro- and anti-inflammatory cytokines (27). Multiple lines of evidence from human and mouse genetics have highlighted a central role for the anti-inflammatory cytokine IL-10 in inflammatory bowel disease. Impaired IL-10 production by gut resident myeloid cells drives intestinal inflammation; therefore, boosting IL-10 levels could yield therapeutic anti-inflammatory effects in the appropriate setting.
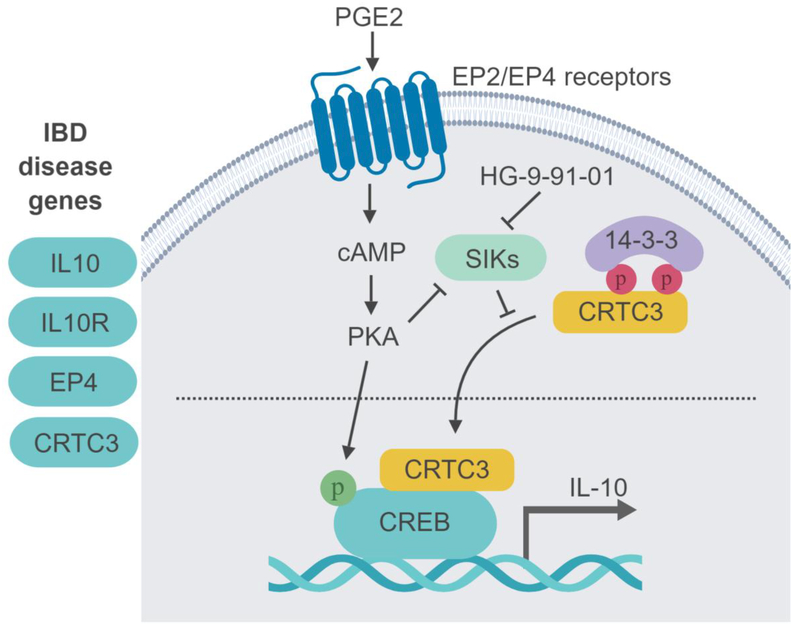
Relative levels of NF-κB- and CREB-target expression programs represent key regulatory nodes governing IL-10 production by myeloid cells (28). Disruption of signaling pathways activating NF-κB downstream of microbial recognition with small molecule inhibitors of PKC or GSK-3β upregulates IL-10 production by stimulated macrophages (29, 30). CREB activation by EP2/EP4 prostanoid receptor agonists such as PGE2 promotes IL-10 production by myeloid cells (31). Recently, SIKs have been identified as key components of the CREB activation cascade downstream of PGE2 (32, 33). In quiescent myeloid cells, SIKs phosphorylate the CREB transcriptional co-activator CRTC3, resulting in its sequestration by cytoplasmic 14–3–3 proteins. and activates CREB, enabling CRTC3 to enhance transcription of IL-10.
Several of these IL-10 pathway regulators are encoded by genes that are also associated with increased risk for IBD, strengthening the link between defective IL-10 signaling and intestinal inflammation (Figure 1). SNPs near the genetic loci encoding the EP4 prostanoid receptor and CRTC3 both confer increased risk for IBD (34). Further, an intronic SNP in SIK2 itself confers susceptibility to primary sclerosing cholangitis, an inflammatory liver disease that is strongly associated with IBD (35). The central role of PKC, GSK3β and SIKs in IL-10 production suggesting that kinase inhibitors are likely to modulate IL-10 production by DCs and macrophages.
Figure 1:
Human disease genetics implicates EP4-SIK2-CRTC3-CREB pathway in the regulation of IL-10 levels and IBD susceptibility. PGE2 signaling, through PKA, leads to SIK inhibition and therefore reduced phosphorylation of the SIK substrate CRTC3. Un-phosphorylated CRTC3 translocates to the nucleus where it drives IL-10 expression. Small molecule SIK inhibitors such as HG-9-91-01 show PGE2-like effects and boost IL-10 production.
To identify compounds that enhance anti-inflammatory IL-10 and suppress proinflammatory IL12 production, over 150 kinase inhibitors were recently screened in bone marrow-derived dendritic cells (BMDCs) (36). SIK inhibition was identified as a common feature of several kinase inhibitors identified that enhanced IL-10 production. HG-9-91-01 analogs with altered selectivities for SIK1-3 were used to determine the relationship between SIK isoform inhibition and IL-10 induction. The strong positive correlation between the potencies of SIK2 inhibition and IL-10 up-regulation suggested that SIK2 is the key family member regulating IL-10 induction. Consistent with this observation, expression of a HG-9-91-01-resistant SIK2 ‘gatekeeper’ mutant is reported to suppress IL-10 potentiation by HG-9-91-01 in myeloid cells (23).
Next, selective SIK inhibitors for in vivo use were developed (37). Molecular modeling was used to guide structure-activity relationship (SAR) optimization of HG-9-91-01. Analysis of derivatives synthesized based on modeling predictions identified the analog YKL-05–099. This compound showed biochemical and cell-based activities consistent with SIK inhibition, and improved PK properties suitable for use in vivo. Kinome-wide profiling of YKL-05–099 revealed that this compound inhibits several protein kinases in addition to SIK1, SIK2, and SIK3, including Brk, Lck, Eph-A4, and p38a. YKL-05–099 modulates cytokine responses in vivo. Mice treated with YKL-05–099 displayed reduced SIK substrate phosphorylation, increased IL-10, and reduced TNFa levels in serum and colonic epithelium. Importantly, daily dosing with YKL-05–099 for one week did not yield metabolic defects observed with chronic SIK2 inhibition by genetic deletion. The improved pan-SIK inhibitor YKL-05–099 was also utilized in mechanistic studies of SIK signaling events in bone and skin, as discussed below.
Notably, the effect of small molecule SIK inhibitors on cytokine production is distinct from that of activation by PGE2 (36). While both enhance IL-10 production, SIK inhibitors suppress proinflammatory cytokines including IL-12 and TNFa to a greater extent than PGE2, suggesting that SIK inhibition converts gut-derived myeloid cells to an anti-inflammatory phenotype marked by enhancing IL-10 production and reducing production of inflammatory cytokines. These observations support the pursuit of SIK inhibitors as potential therapies to regionally modulate cytokines. Moreover, SIK inhibitors represent a unique class of small molecules for the treatment of IBD because of their dual effect enhancing IL-10 and dampening IL-12 production. Current therapies such as anti-TNF agents target only one side of this equilibrium, are broadly immunosuppressive, and can cause severe side effects. There is an urgent need to discover new therapies for these disorders that restore cytokine balance within local compartments of the gut. While protein-based therapies are used to effectively treat a wide variety of complex immunemediated disorders, they have several limitations, notably an inability to regulate intracellular proteins identified as targets by genetic and functional studies. Small molecules constitute a complementary approach to protein-based immunomodulatory drug development by enabling modulation of intracellular networks that regulate cytokine signaling (38). The recent successful example of JAK inhibitors for rheumatoid arthritis highlights how small molecule kinase inhibitors can be used to target redundancies within cytokine signaling networks for chronic diseases.
The role of SIKs downstream of parathyroid hormone action in bone
Parathyroid hormone (PTH) is a peptide hormone that plays a crucial role in maintaining blood calcium levels (39). In response to hypocalcemia, PTH is released and acts to maintain normocalcemia through effects in bone and kidney. Since 99% of total body calcium is stored in bone, skeletal PTH action is key in maintaining calcium homeostasis. Chronic hyperparathyroidism is a disease in which overproduction of PTH from the parathyroid glands causes release of calcium from bone and increases risk of fractures (40). However, PTH also stimulates bone formation and concomitant slow entry of calcium into bone, perhaps in an attempt to protect from excessive bone loss in response to intermittent hypocalcemia. This property of PTH to alter bone formation is the basis for its pharmacologic use as treatment for osteoporosis in the form of intermittent (once daily) injection of PTH analogs, teriparatide or abaloparatide (41,42).
In bone, PTH targets multiple cell types to promote bone resorption and to stimulate bone formation. Osteocytes, terminally-differentiated osteoblasts embedded within mineralized matrix, are the most abundant cell type in bone, and the cells expressing the highest numbers of PTH receptors (43). In response to PTH, osteocytes regulate the expression of paracrine-acting factors, such as sclerostin and RANKL, that in turn modulate the activity of osteoblasts and osteoclasts on bone surfaces. Sclerostin is a secreted WNT inhibitor which blocks osteoblast activity (44); reducing its expression is one mechanism used by PTH to stimulate bone formation (45). RANKL (TNFSF11) is the major osteoclastogenic cytokine made by osteocytes and osteoblasts (46); increasing its expression represents the major pathway through which PTH stimulates bone resorption (47).
Recently, a key role for SIKs has been described in PTH signaling in osteocytes (48). MEF2 family transcription factors control sclerostin expression (49, 50), and class IIa HDACs block MEF2C action in osteocytes (51). CREB and related bZIP factors drive RANKL gene expression (52, 53). PTH signaling in a conditionally-immortalized osteocyte cell line (54) leads to rapid SIK2 phosphorylation at multiple PKA sites. Subsequent to this, PTH signaling leads to reduced phosphorylation of HDAC4/5 and CRTC2 and the nuclear translocation of these key SIK substrates. HDAC4/5 are required for PTH to suppress sclerostin expression, both in vitro and in vivo. In contrast, CRTC2 is required for PTH-regulated RANKL up-regulation in vitro (48).
Since SIK2 suppression leads to regulation of key PTH target genes in osteocytes, the effect of the small molecule SIK inhibitor, YKL-05–099 (55) (discussed above in the setting of its initial development as a compound that induces IL-10 in myeloid lineage cells in the gut), on gene expression in osteocytes was tested. As predicted, this compound led to PTH-like changes, including reduced HDAC4/5 phosphorylation, CRTC2 nuclear translocation, increased RANKL, and reduced sclerostin expression. Remarkably, RNA-seq profiling revealed substantial transcriptomic overlap of PTH and small molecule SIK inhibitors. Furthermore, 2 week in vivo treatment with YKL-05–099 led to increased bone formation and increased bone mass in young, eugonadal mice (48). A working model for the central role of SIK inhibition in skeletal PTH action is shown in Figure 2.
Figure 2:
PTH signaling in osteocytes inhibits SIK2 activity via protein kinase A-mediated phosphorylation. By reducing SIK activity, PTH reduces phosphorylation levels of SIK substrates including class IIa HDACs and CRTC family members. Upon dephosphorylation, these factors translocate to the nucleus. Class IIa HDACs block MEF2C-driven SOST expression. CRTC2 stimulates RANKL expression driven by CREB and other bZIP family transcription factors. Small molecule SIK inhibitors such as YKL-05–099 mimic the actions of PTH, both in vitro and in vivo. As schematized here, SIK inhibition represents an intracellular mechanism to ensure that PTH signaling stimulates both bone formation and bone resorption.
While these PTH-like effects of YKL-05–099 were predicted, it was surprising that the SIK inhibitor also reduced osteoclast numbers and activity. Indeed, these inhibitory effects on osteoclast numbers and activity occurred despite clear increases in bone RANKL gene expression after in vivo SIK inhibitor treatment, similar to the effects of SIK inhibitors on cultured osteocytes in vitro. Therefore, while the possibility remains that the effects of YKL-05–099 on bone are different in vitro and in vivo, these results suggest that SIKs may directly regulate osteoclast function. Future studies are needed to clarify the mechanism through which SIK inhibitors boost bone formation and reduce bone resorption, including investigation as to whether there might be a distinct cellautonomous role for SIKs in osteoclasts (56). Since YKL-05–099 can potentially inhibit multiple kinases in addition to SIK2, future studies using genetic approaches and more specific small molecule inhibitors will be necessary to address this important question. Furthermore, future studies are needed to address the questions of functional redundancy between SIK isoforms in bone, as discussed below in the setting of hepatic glucagon action. Nonetheless, these observations indicate that SIK inhibition is a key signaling mechanism used by PTH to accomplish its physiologic effects in osteocytes. Future work is needed to determine if the actions of PTH its other cellular targets in bone and kidney utilize an analogous mechanism.
The role of SIKs downstream of hepatic glucagon action
Hepatic gluconeogenesis is controlled by pancreatic hormones, insulin and glucagon, which regulate transcription of the rate-controlling enzymes glucose-6-phosphatase (G6Pase, encoded by the G6pc gene) and phosphoenolpyruvate carboxykinase (PEPCK, encoded by the Pck1 gene) in hepatocytes. In the fed state, insulin inhibits hepatic glucose production whereas glucagon upregulates gluconeogenesis during prolonged fasting. Dysregulation of these processes contributes to the development of type 2 diabetes (57). Over recent years, salt inducible kinases (SIKs) have emerged as major downstream effectors of glucagon action in the regulation of hepatic gluconeogenesis.
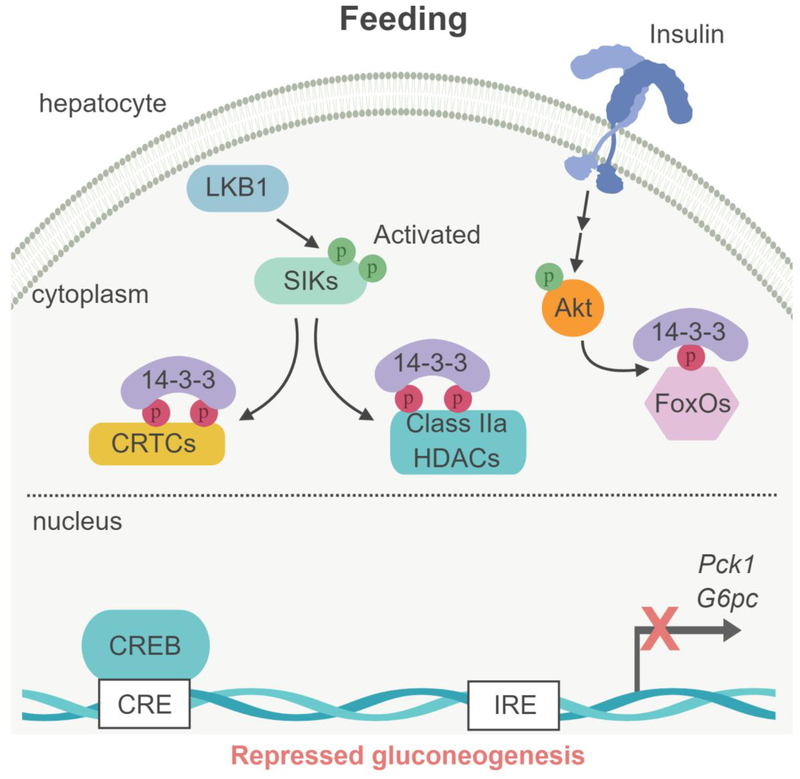
In the fed state, hepatic insulin signaling reduces the expression of G6pc and Pck1 through Aktmediated phosphorylation of forkhead box protein-O (FoxO) transcription factors (58). FoxO phosphorylation by Akt results in binding to 14–3–3 proteins, sequestering FoxO in the cytoplasm and thereby inhibiting gluconeogenic gene expression (Figure 3). Under fasting conditions, glucagon upregulates gluconeogenic gene expression through the protein kinase A (PKA)-mediated phosphorylation and activation of the transcription factor cAMP response element (CRE)-binding protein (CREB), which binds to the G6pc and Pck1 gene promoters (59, 60). PKA-mediated CREB phosphorylation (on Ser133) induces recruitment of the histone acetyltransferases p300 and CREB binding protein (CBP) (61). In concert, the activation of the cAMP-PKA pathway also triggers the dephosphorylation and nuclear translocation of the CREB regulated transcriptional coactivators (CRTCs; CRTC1, 2 and 3) (6), which then bind to CREB to stimulate gluconeogenic gene transcription (Figure 3).
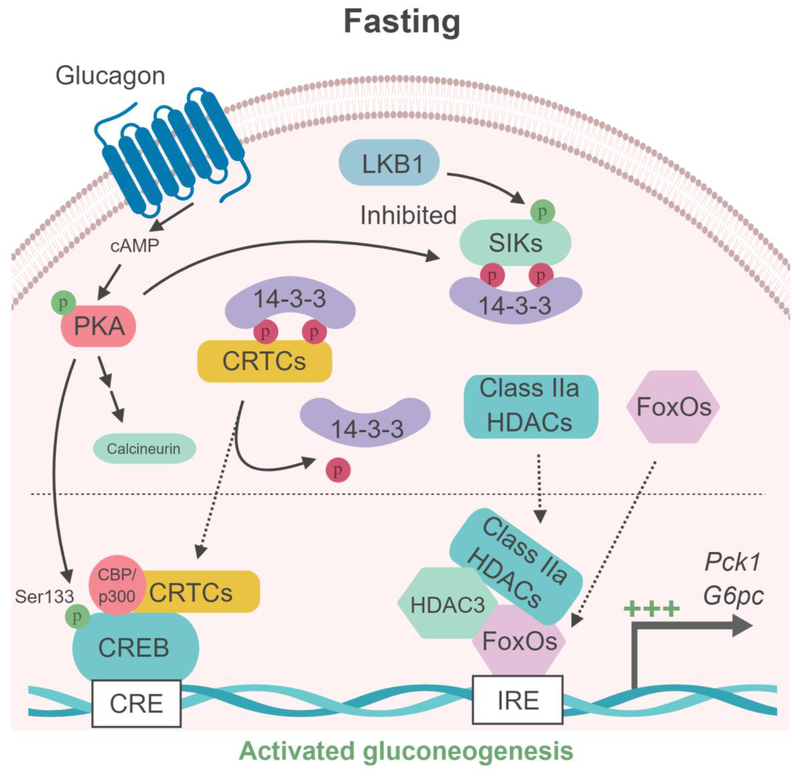
Figure 3. Model of SIK-dependent gluconeogenesis regulation in liver.
(Left box) Under feeding conditions, Akt-dependent phosphorylation of the FoxOs transcription factor results in binding to 14–3–3 proteins, sequestering FoxOs into the cytoplasm. In parallel, the LKB1-dependent SIKs activation induces the phosphorylation of the cofactors CRTCs and class IIa HDAC and their binding to the 14–3–3 proteins and retention into the cytoplasm, leading to the repression of gluconeogenic gene expression (G6pc and Pck1). (Right box) During fasting, glucagon/cAMP-activated PKA phosphorylates the transcription factor CREB on Ser133, promoting the recruitment of the coactivator proteins CBP/p300. In parallel, PKA inhibits SIKs through phosphorylation-dependent interactions with 14–3–3 proteins, resulting in loss of CRTCs and HDACs phosphorylation. Glucagon-PKA pathway also activates the phosphatase calcineurin, which contributes to CRTCs dephosphorylation. Dephosphoryled CRTCs and class IIa HDACs then translocate to the nucleus where CRTCs cooperate with CREB and CBP/p300 while class IIa HDACs recruit HDAC3 causing deacetylation of FoxOs to gluconeogenic gene promoters to activate their transcription. CRE : cAMP responsive element; IRE : insulin responsive element.
CRTC coactivators play a key role in the regulation of hepatic gluconeogenesis (6). During feeding, CRTC2 and CRTC3 are sequestered in the cytoplasm through phosphorylationdependent interactions with 14–3–3 proteins. Hepatic CRTCs are regulated through phosphorylation at multiple sites by salt-inducible kinases (SIK1, 2 and 3) (6, 18, 62). During fasting the activity of all three SIK isoforms is modulated by multiple phosphorylation events outside of the T-loop/kinase domain by the cAMP/PKA pathway. Indeed, as noted earlier, PKA-mediated phosphorylation of the SIKs inhibits their catalytic activity by inducing 14–3–3 protein associations (24), leading to CRTC dephosphorylation and translocation to the nucleus, where they induce the transcription of gluconeogenic genes. The dephosphorylation of CRTCs seems to be in part mediated by the cAMP-PKA dependent activation of the phosphatase, calcineurin (63) (Figure 3).
Similar to CRTCs, under fed conditions the localization of class IIa histone deacetylases (HDACs; HDAC4, 5, 7, and 9) to the nucleus is inhibited through SIKs-mediated phosphorylation and subsequent 14–3–3 protein interactions leading to cytoplasmic sequestration. In response to glucagon, PKA-dependent inactivation of SIKs promotes dephosphorylation of class IIa HDACs and their translocation to the nucleus, where they recruit HDAC3 to G6pc and Pck1 promoters and regulate FoxO acetylation, resulting in transcriptional activation of gluconeogenic genes (11) (Figure 3).
AMPK can also phosphorylate CRTCs and class IIA HDACs (6, 11). However, although AMPK was considered as a key regulator of hepatic gluconeogenesis, liver-specific AMPK deficient mice are normoglycaemic and their hepatic gluconeogenesis is unchanged (64–66). In addition, the use of a small molecule (A-769662) that specifically activates AMPK without modifying the cellular AMP/ATP ratio, had no effect on hepatic glucose production measured in isolated hepatocytes or in vivo (65, 67). In contrast, liver-specific ablation of upstream kinase LKB1 causes increased glucose production in hepatocytes in vitro and fasting hyperglycemia in vivo (64, 65, 68). Although the SIK2 isoform was also proposed to be critical in controlling the gluconeogenic gene program (6, 69), liver-specific ablation of SIK2 alone has no effect on glycemia and hepatic gluconeogenesis, and insulin does not modulate SIK2 phosphorylation/activity (23). Similarly, liver-specific deletion of SIK1 has no impact on hepatic gluconeogenesis (70). Unexpectedly, global-SIK3 deficient mice are hypoglycemic (71). Since SIK3 is deleted in all tissues in these mice, it is difficult to appreciate the role of hepatic SIK3 in this mouse model, especially since most SIK3 knockout mice die shortly after birth due to abnormal chondrocyte hypertrophy (72). Finally, pan-SIK inhibitors recapitulated the entire phenotype observed in LKB1-deficient hepatocytes, including dephosphorylation of CRTC2/3 and HDAC4/5, associated to an increase in gluconeogenic gene expression and glucose production (23, 65). These observations suggest that SIK isoforms play a redundant role in the control of hepatic gluconeogenesis. Furthermore, de-repression of gluconeogenesis observed in response to LKB1 deletion or SIKs inhibition indicates that the LKB1/SIK pathway functions as a key gluconeogenic gatekeeper for hepatic glucose production by keeping the gluconeogenic program repressed, and this can be released by fasting/glucagon signals (23).
With regard of their critical role in the suppression of hepatic glucose production, SIKs may serve as therapeutic targets to treat hyperglycemia in diabetic patients. Indeed, SIK inhibition is sufficient to induce gluconeogenesis (23). Thus, dysregulation of SIK activity may be involved in the impairment of glycemic control in diabetes. Consistent with this hypothesis, SIK1 and SIK2 activities are decreased concomitantly with CRTC2 dephosphorylation/activation in livers of diabetic models (69, 73, 74). In contrast, overexpression of SIKs in liver is sufficient to normalize blood glucose levels in diabetic mice (6). Therefore, therapeutic strategies that specifically activate hepatic SIK activity may curb excessive hepatic glucose output and thus improve glycemic control in patients with type 2 diabetes.
The role of SIKs downstream of MSH effects in melanocytes
Mammalian pigmentation is typically described as either constitutive or adaptive. Constitutive pigmentation is genetically determined—and highly polymorphic in many species—whereas adaptive pigmentation responds to environmental signals, best characterized for UV irradiation/sunlight. Numerous signaling events have been demonstrated to impact control of melanin synthesis (75). However a central and rate-limiting pathway regulating pigmentation is triggered by the GPCR melanocortin 1 receptor (MC1R) and its ligand α-MSH. Non-functional variants of MC1R are present in individuals with the redhair-lightskin phenotype (76, 77). The recognition that redhaired individuals have poor or non-existent tanning responses, led to the observation that UV produces strong (>30-fold) p53-mediated induction of POMC/α-MSH peptide within epidermal keratinocytes (78, 79). UV’s direct effect on melanocytes produced only weak (~50%) upregulation of POMC/α-MSH. MC1R activity triggers cAMP surges via adenylate cyclase, and was shown to upregulate expression of the melanocyte master transcription factor MITF, via a conserved CRE motif in its (melanocyte-specific) promoter. The red/blond pigment within hair of non-functional MC1R (“redhair”) variants is called pheomelanin and is produced via cysteine- or glutathione-mediated reduction of oxidized tyrosine metabolites. The “switch” between pheomelanin and brown/black eumelanin species occurs when activity of CREB and expression of MITF are strongly induced downstream of cAMP, thereby stimulating tyrosinase expression (the tyrosine oxidizing enzyme), consuming and depleting intracellular thiols, and resulting in an alternative thiol-free pathway of melanin biosynthesis. Thus the control over red/blond vs brown/black pigmentation is traceable to the magnitude of MC1R/cAMP signals.
The SIK-CRTC pathway has been demonstrated to potently modulate melanin synthesis within the melanocyte lineage. A landmark study by Horike et al (80) studied this signaling response within the B16 murine melanoma cell line. The investigators observed that all three family isoforms of both SIK and CRTC were expressed in the melanocyte lineage. They found that overexpression of the CRTCs induced cellular hyperpigmentation as well as induction of CREB activity, MITF, and tyrosinase, all of which were suppressed by simultaneous overexpression of SIK2. Importantly, the authors crossed mice exhibiting red hair (due to overexpression of a natural antagonist of Mc1r) together with germline SIK2−/− mice and observed dose-dependent “rescue” of dark fur pigmentation (80). Collectively these data established a key role for the SIK-CRTCCREB/MITF pathway in control of pigmentation.
A more recent study (81) asked whether the eumelanin repressive activity of SIK could be targeted using skin-permeable small molecule antagonists. This concept was an extension of a prior study (79) in which topical forskolin (adenylate cyclase agonist) was shown to induce dark pigmentation in redhaired mice lacking functional Mc1r. The darkening effect of forskolin in redhaired mice suggested that cAMP induction was sufficient to stimulate the CREB/MITF axis, and had previously been assumed to reflect direct phosphorylation/activation of CREB by PKA. This “sunless tanning” was further shown to have produced chemically indistinguishable eumelanin as compared to genetically black mice and to protect from UV-induced DNA damage, sunburn cell formation, and skin carcinogenesis. While topical forskolin produced impressive murine skin melanization, it was subsequently observed to be impermeable to human skin, which is approximately 5-times a thick as mouse skin. The study of Mujahid et al examined a series of small molecule SIK inhibitors, iteratively modifying their chemical features including lipophilicity and size, aiming to enhance human skin penetration. A number of chemical species were ultimately identified that retained cell-based SIK inhibitory activity, induction of MITF and hyperpigmentation, while also being capable of triggering significant darkening of discarded human skin specimens. Melanization was also accompanied by trafficking of melanin-containing melanosomes from melanocytes into epidermal keratinocytes, where they are maintained in an “umbrella” like configuration over the superficial (“sun-exposed”) side of the keratinocyte nucleus—a pattern of intracellular trafficking phenomenon that is known to occur after UV-induced tanning/pigmentation. Topical SIK inhibitor was also seen to potently darken the skin of redhaired mice lacking wildtype Mc1r. Collectively, these studies demonstrated that either forskolin/cAMP or SIK inhibition could strongly induce dark eumelanin pigmentation in the redhair/light-skin genetic background, and that small molecule SIK inhibitors can be designed that are capable of penetrating human skin. Important remaining questions include a better understanding of the precise role of PKA in either suppressing SIK2, stimulating CREB, or both. Do these activities vary by genetic background among humans with different pigmentation features? By what pathway is SIK activity regulated constitutively in human skin? Is SIK activity diminished within skin of darkly pigmented humans? If so, via what mechanism(s)?
The therapeutic potential of small molecule SIK inhibitors
Kinases have been intensively studies as drug targets, with 38 kinase inhibitors currently FDA-approved. While most of these agents are used for oncologic indications, success stories of kinase inhibitors for non-oncologic indications exist (82). As discussed above, recent evidence indicates that beneficial effects of SIK inhibition might include increased bone mass (by mimicking PTH action) (48), modulation of inflammatory cytokine production (by mimicking PGE2 action) (32, 36, 83), and increased skin pigmentation (by mimicking MSH action) (81). Moreover, liver-specific SIK activation may be of benefit for treatment of type 2 diabetes. Finally, recent evidence indicates that catecholamine- and subsequent PKA-induced SIK inhibition participates in adaptive thermogenesis in brown adipose tissue (84).
In addition to these physiology-based mechanisms, it is clear that SIK inhibition may be of benefit in certain malignancies. For example, SIK2 is over-expressed in certain high grade serous ovarian cancers, in which it functions as a centrosome kinase in cell cycle progression (14). Moreover, SIK2 may promote omental ovarian cancer metastasis by activating the PI3K pathway (15). The small molecule SIK inhibitor ARN-3236 shows promising efficacy in ovarian cancer xenograft models (85). Other malignancies in which SIK inhibition may be of benefit include triple negative breast cancer (86), prostate cancer (87), and certain subtypes of acute myelogenous leukemia (88, 89). AML is a particularly interesting example in which a “rational” therapeutic role for SIK inhibitors may emerge: specific mixed lineage leukemia (MLL)-fusion AML subtypes harbor high MEF2 activity and are sensitive to genetic and pharmacologic manipulations that target LKB1/SIK3 (89).
Along these lines, a major focus for future efforts will be to identify and optimize potency and specificity of small molecule SIK inhibitors, especially if these agents are to be used for non oncologic indications. Compounds such as HG-9-91-01 (32), MRT-67307 (32), and YKL-05-099 (55) represent invaluable starting points in these efforts. Moreover, recent unbiased profiling of 243 clinical kinase inhibitors revealed that SIK2 is a relatively common target amongst inhibitors designed to block other kinases (90). Efforts for structure-based drug design are currently limited by the lack of SIK crystal structures.
Concluding remarks and future perspectives
As discussed above, recent evidence points towards a central role for SIK inhibition in the physiologic intracellular actions of several cAMP-linked signals. Despite these advances, major outstanding questions remain. First, do important intracellular SIK substrates exist beyond class IIa HDACs and CRTC proteins? Second, kinase inhibition alone is insufficient to explain the rapid reductions in SIK substrate phosphorylation seen after cAMP signaling or treatment of cells with small molecule SIK inhibitors. Therefore, future studies are needed to identify the role of phosphatases in SIK-mediated class IIa HDAC and CRTC regulated nuclear translocation. Third, as discussed above, discordant phenotypes are observed comparing the effects of small molecule SIK inhibitors and in vivo deletion of individual SIK isoforms. For this reason, in vivo mouse genetics studies are needed to address the potential for functional redundancy amonst the members of this family. Finally, it is possible that HG-9-91-01, MRT-67307, ARN-3236, and YKL-05–099 represent relatively “early” efforts in the development of small molecule SIK inhibitors for therapeutic use. Future development of more potent and specific agents will only be boosted by enhanced knowledge of the role of these kinases in physiology and pathophysiology.
Outstanding questions:
Do cell type-specific SIK substrates exist to account for unique transcriptional outputs downstream of a shared cAMP-regulated signaling mechanism?
Do cAMP-regulated phosphatases participate in rapid signal-dependent reductions in phosphorylation of SIK substrates?
Can cellular SIK activity be increased by signals that stimulate LKB1 kinase activity?
By what pathway is SIK activity regulated constitutively in human skin? Is SIK activity diminished within skin of darkly pigmented humans?
What are the functional similarities and differences between the three distinct members of the SIK family of kinases?
What is the safety profile associated with long term pharmacologic SIK inhibition?
Trends
Salt inducible kinases (SIK) control the phosphorylation and subcellular localization of two key classes of transcriptional regulatory factors: class IIa histone deacetylases (HDACs) and cAMP-regulated transcriptional coactivators (CRTCs)
SIK activity is inhibited by upstream signals that increase intracellular cAMP levels
cAMP-regulated SIK inhibition is a key component in the cellular effects of multiple hormones and paracrine-acting factors
Small molecule SIK inhibitors represent a novel therapeutic strategy to mimic cAMP inducing signals in the settings of inflammatory bowel disease, osteoporosis, and skin pigmentation
Outstanding questions:
Do cell type-specific SIK substrates exist to account for unique transcriptional outputs downstream of a shared cAMP-regulated signaling mechanism?
Do cAMP-regulated phosphatases participate in rapid signal-dependent reductions in phosphorylation of SIK substrates?
Can cellular SIK activity be increased by signals that stimulate LKB1 kinase activity?
By what pathway is SIK activity regulated constitutively in human skin? Is SIK activity diminished within skin of darkly pigmented humans?
What are the functional similarities and differences between the three distinct members of the SIK family of kinases?
What is the safety profile associated with long term pharmacologic SIK inhibition?
Acknowledgements
MNW acknowledges support from the American Society of Bone and Mineral Research, the Harrington Discovery Institute, and NIH (K08 AR067285, R03 AR072150, R03 AR072903). MF acknowledges support by grants from Inserm, CNRS, Université Paris Descartes, the Société francophone du diabète (SFD) and the Fondation pour la Recherche Médicale (FRM). DEF acknowledges support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, Melanoma Research Alliance, and NIH (2R01 AR043369–22; 1R01CA222871–01; 1R01AR072304–01; 5P01 CA163222–05). RJX acknowledges support from the Helmsley Charitable Trust and NIH (P30 DK043351, U19 AI109725, R01 AI110498). HMK acknowledges support from NIH (P01 DK011794, P30 AR066261).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bright NJ, Thornton C, and Carling D. The regulation and function of mammalian AMPK related kinases. Acta Physiol (Oxf). 2009;196(1):15–26. [DOI] [PubMed] [Google Scholar]
- 2.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. The EMBO journal. 2004;23(4):833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Takemori H, Halder SK, Nonaka Y, and Okamoto M. Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS letters. 1999;453(1–2):135–9. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto M, Takemori H, and Katoh Y. Salt-inducible kinase in steroidogenesis and adipogenesis. Trends Endocrinol Metab. 2004;15(1):21–6. [DOI] [PubMed] [Google Scholar]
- 5.Lin X, Takemori H, Katoh Y, Doi J, Horike N, Makino A, et al. Salt-inducible kinase is involved in the ACTH/cAMP-dependent protein kinase signaling in Y1 mouse adrenocortical tumor cells. Molecular endocrinology. 2001;15(8):1264–76. [DOI] [PubMed] [Google Scholar]
- 6.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–11. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Feldman JD, Machado HB, Vician LJ, and Herschman HR. Expression of depolarization-induced immediate early gene proteins in PC12 cells. J Neurosci Res. 2003;72(6):670–8. [DOI] [PubMed] [Google Scholar]
- 8.Jagannath A, Butler R, Godinho SIH, Couch Y, Brown LA, Vasudevan SR, et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell. 2013;154(5):1100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberland M, Montgomery RL, and Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature reviews Genetics. 2009;10(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altarejos JY, and Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nature reviews Molecular cell biology. 2011;12(3):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145(4):607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, et al. A hormone dependent module regulating energy balance. Cell. 2011;145(4):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamaki J, Fu A, Reeks C, Baird S, Depatie C, Al Azzabi M, et al. Role of the SIK2-p35-PJA2 complex in pancreatic beta-cell functional compensation. Nature cell biology. 2014;16(3):234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed AA, Lu Z, Jennings NB, Etemadmoghadam D, Capalbo L, Jacamo RO, et al. SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer cell. 2010;18(2):109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda F, Mannion D, Liu S, Zheng Y, Mangala LS, Redondo C, et al. Salt-Inducible Kinase 2 Couples Ovarian Cancer Cell Metabolism with Survival at the Adipocyte-Rich Metastatic Niche. Cancer cell. 2016;30(2):273–89. [DOI] [PubMed] [Google Scholar]
- 16.Alessi DR, Sakamoto K, and Bayascas JR. LKB1-dependent signaling pathways. Annual review of biochemistry. 2006;75:137–63. [DOI] [PubMed] [Google Scholar]
- 17.Kullmann L, and Krahn MP. Controlling the master-upstream regulation of the tumor suppressor LKB1. Oncogene. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Sonntag T, Moresco JJ, Vaughan JM, Matsumura S, Yates JR 3rd, and Montminy M Analysis of a cAMP regulated coactivator family reveals an alternative phosphorylation motif for AMPK family members. PloS one. 2017;12(2):e0173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, et al. TORCs: transducers of regulated CREB activity. Molecular cell. 2003;12(2):413–23. [DOI] [PubMed] [Google Scholar]
- 20.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119(1):61–74. [DOI] [PubMed] [Google Scholar]
- 21.Berggreen C, Henriksson E, Jones HA, Morrice N, and Goransson O. cAMP-elevation mediated by beta-adrenergic stimulation inhibits salt-inducible kinase (SIK) 3 activity in adipocytes. Cell Signal. 2012;24(9):1863–71. [DOI] [PubMed] [Google Scholar]
- 22.Henriksson E, Jones HA, Patel K, Peggie M, Morrice N, Sakamoto K, et al. The AMPK related kinase SIK2 is regulated by cAMP via phosphorylation at Ser358 in adipocytes. The Biochemical journal. 2012;444(3):503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel K, Foretz M, Marion A, Campbell DG, Gourlay R, Boudaba N, et al. The LKB1-salt inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nat Commun. 2014;5:4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonntag T, Vaughan JM, and Montminy M. 14–3–3 proteins mediate inhibitory effects of cAMP on salt-inducible kinases (SIKs). The FEBS journal. 2018;285(3):467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funato H, Miyoshi C, Fujiyama T, Kanda T, Sato M, Wang Z, et al. Forward genetics analysis of sleep in randomly mutagenized mice. Nature. 2016;539(7629):378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Ma J, Miyoshi C, Li Y, Sato M, Ogawa Y, et al. Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature. 2018;558(7710):435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khor B, Gardet A, and Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saraiva M, and O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Rehani K, Jope RS, and Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6(8):777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto T, Hasegawa H, Onishi S, Ishizaki J, Suemori K, and Yasukawa M. Protein kinase C inhibitor generates stable human tolerogenic dendritic cells. J Immunol. 2013;191(5):2247–57. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez M, Domingo E, Municio C, Alvarez Y, Hugo E, Fernandez N, et al. Polarization of the innate immune response by prostaglandin E2: a puzzle of receptors and signals. Mol Pharmacol. 2014;85(1):187–97. [DOI] [PubMed] [Google Scholar]
- 32.Clark K, MacKenzie KF, Petkevicius K, Kristariyanto Y, Zhang J, Choi HG, et al. Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(42):16986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKenzie KF, Clark K, Naqvi S, McGuire VA, Noehren G, Kristariyanto Y, et al. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J Immunol. 2013;190(2):565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45(6):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundberg TB, Choi HG, Song JH, Russell CN, Hussain MM, Graham DB, et al. Small molecule screening identifies inhibition of salt-inducible kinases as a therapeutic strategy to enhance immunoregulatory functions of dendritic cells. Proc Natl Acad Sci U S A. 2014;111(34):12468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundberg TB, Liang Y, Wu H, Choi HG, Kim ND, Sim T, et al. Development of Chemical Probes for Investigation of Salt-Inducible Kinase Function in Vivo. ACS Chem Biol. 2016;11(8):2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundberg TB, Xavier RJ, Schreiber SL, and Shamji AF. Small-molecule control of cytokine function: new opportunities for treating immune disorders. Curr Opin Chem Biol. 2014;23:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potts JT, and Gardella TJ. Progress, paradox, and potential: parathyroid hormone research over five decades. Annals of the New York Academy of Sciences. 2007;1117:196–208. [DOI] [PubMed] [Google Scholar]
- 40.Silva BC, Costa AG, Cusano NE, Kousteni S, and Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. Journal of endocrinological investigation. 2011;34(10):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England journal of medicine. 2001;344(19):1434–41. [DOI] [PubMed] [Google Scholar]
- 42.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. Jama. 2016;316(7):722–33. [DOI] [PubMed] [Google Scholar]
- 43.Bonewald LF. The amazing osteocyte. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(2):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron R, and Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine. 2013;19(2):179–92. [DOI] [PubMed] [Google Scholar]
- 45.Bellido T, Saini V, and Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54(2):250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wada T, Nakashima T, Hiroshi N, and Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25. [DOI] [PubMed] [Google Scholar]
- 47.Xiong J, and O’Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(3):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wein MN, Liang Y, Goransson O, Sundberg TB, Wang J, Williams EA, et al. SIKs control osteocyte responses to parathyroid hormone. Nature communications. 2016;7:13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(12):1957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collette NM, Genetos DC, Economides AN, Xie L, Shahnazari M, Yao W, et al. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(35):14092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, et al. HDAC5 controls MEF2C driven sclerostin expression in osteocytes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(3):400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. [DOI] [PubMed] [Google Scholar]
- 53.Fu Q, Jilka RL, Manolagas SC, and O’Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. The Journal of biological chemistry. 2002;277(50):48868–75. [DOI] [PubMed] [Google Scholar]
- 54.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, et al. The Wnt Inhibitor Sclerostin Is Up-regulated by Mechanical Unloading in Osteocytes in Vitro. The Journal of biological chemistry. 2015;290(27):16744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundberg TB, Liang Y, Wu H, Choi HG, Kim ND, Sim T, et al. Development of Chemical Probes for Investigation of Salt-Inducible Kinase Function in Vivo. ACS chemical biology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lombardi MS, Gillieron C, Berkelaar M, and Gabay C. Salt-inducible kinases (SIK) inhibition reduces RANKL-induced osteoclastogenesis. PloS one. 2017;12(10):e0185426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biddinger SB, and Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annual review of physiology. 2006;68:123–58. [DOI] [PubMed] [Google Scholar]
- 58.Haeusler RA, Kaestner KH, and Accili D. FoxOs function synergistically to promote glucose production. The Journal of biological chemistry. 2010;285(46):35245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quinn PG, and Granner DK. Cyclic AMP-dependent protein kinase regulates transcription of the phosphoenolpyruvate carboxykinase gene but not binding of nuclear factors to the cyclic AMP regulatory element. Molecular and cellular biology. 1990;10(7):3357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–83. [DOI] [PubMed] [Google Scholar]
- 61.Mayr B, and Montminy M. Transcriptional regulation by the phosphorylation dependent factor CREB. Nature reviews Molecular cell biology. 2001;2(8):599–609. [DOI] [PubMed] [Google Scholar]
- 62.Uebi T, Tamura M, Horike N, Hashimoto YK, and Takemori H. Phosphorylation of the CREB-specific coactivator TORC2 at Ser(307) regulates its intracellular localization in COS-7 cells and in the mouse liver. American journal of physiology Endocrinology and metabolism. 2010;299(3):E413–25. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, et al. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485(7396):128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boudaba N, Marion A, Huet C, Pierre R, Viollet B, and Foretz M. AMPK Re-Activation Suppresses Hepatic Steatosis but its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine. 2018;28:194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. The Journal of clinical investigation. 2010;120(7):2355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hasenour CM, Ridley DE, Hughey CC, James FD, Donahue EP, Shearer J, et al. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) effect on glucose production, but not energy metabolism, is independent of hepatic AMPK in vivo. The Journal of biological chemistry. 2014;289(9):5950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449(7160):366–9. [DOI] [PubMed] [Google Scholar]
- 70.Nixon M, Stewart-Fitzgibbon R, Fu J, Akhmedov D, Rajendran K, Mendoza-Rodriguez MG, et al. Skeletal muscle salt inducible kinase 1 promotes insulin resistance in obesity. Molecular metabolism. 2016;5(1):34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itoh Y, Sanosaka M, Fuchino H, Yahara Y, Kumagai A, Takemoto D, et al. Salt-inducible Kinase 3 Signaling Is Important for the Gluconeogenic Programs in Mouse Hepatocytes. The Journal of biological chemistry. 2015;290(29):17879–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sasagawa S, Takemori H, Uebi T, Ikegami D, Hiramatsu K, Ikegawa S, et al. SIK3 is essential for chondrocyte hypertrophy during skeletal development in mice. Development. 2012;139(6):1153–63. [DOI] [PubMed] [Google Scholar]
- 73.Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, and Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. The Journal of clinical investigation. 2010;120(12):4316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horike N, Takemori H, Katoh Y, Doi J, Min L, Asano T, et al. Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. The Journal of biological chemistry. 2003;278(20):18440–7. [DOI] [PubMed] [Google Scholar]
- 75.Lin JY, and Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843–50. [DOI] [PubMed] [Google Scholar]
- 76.Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72(6):827–34. [DOI] [PubMed] [Google Scholar]
- 77.Rana BK, Hewett-Emmett D, Jin L, Chang BH, Sambuughin N, Lin M, et al. High polymorphism at the human melanocortin 1 receptor locus. Genetics. 1999;151(4):1547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–64. [DOI] [PubMed] [Google Scholar]
- 79.D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443(7109):340–4. [DOI] [PubMed] [Google Scholar]
- 80.Horike N, Kumagai A, Shimono Y, Onishi T, Itoh Y, Sasaki T, et al. Downregulation of SIK2 expression promotes the melanogenic program in mice. Pigment cell & melanoma research. 2010;23(6):809–19. [DOI] [PubMed] [Google Scholar]
- 81.Mujahid N, Liang Y, Murakami R, Choi HG, Dobry AS, Wang J, et al. A UV-Independent Topical Small-Molecule Approach for Melanin Production in Human Skin. Cell reports. 2017;19(11):2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferguson FM, and Gray NS. Kinase inhibitors: the road ahead. Nature reviews Drug discovery. 2018;17(5):353–77. [DOI] [PubMed] [Google Scholar]
- 83.Lombardi MS, Gillieron C, Dietrich D, and Gabay C. SIK inhibition in human myeloid cells modulates TLR and IL-1R signaling and induces an anti-inflammatory phenotype. Journal of leukocyte biology. 2016;99(5):711–21. [DOI] [PubMed] [Google Scholar]
- 84.Paulo E, Wu D, Wang Y, Zhang Y, Wu Y, Swaney DL, et al. Sympathetic inputs regulate adaptive thermogenesis in brown adipose tissue through cAMP-Salt inducible kinase axis. Scientific reports. 2018;8(1):11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou J, Alfraidi A, Zhang S, Santiago-O’Farrill JM, Yerramreddy Reddy VK, Alsaadi A, et al. A Novel Compound ARN-3236 Inhibits Salt-Inducible Kinase 2 and Sensitizes Ovarian Cancer Cell Lines and Xenografts to Paclitaxel. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maxfield KE, Macion J, Vankayalapati H, and Whitehurst AW. SIK2 Restricts Autophagic Flux To Support Triple-Negative Breast Cancer Survival. Mol Cell Biol. 2016;36(24):3048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bon H, Wadhwa K, Schreiner A, Osborne M, Carroll T, Ramos-Montoya A, et al. Salt inducible kinase 2 regulates mitotic progression and transcription in prostate cancer. Molecular cancer research : MCR. 2015;13(4):620–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown FC, Still E, Koche RP, Yim CY, Takao S, Cifani P, et al. MEF2C Phosphorylation Is Required for Chemotherapy Resistance in Acute Myeloid Leukemia. Cancer Discov. 2018;8(4):478–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarumoto Y, Lu B, Somerville TDD, Huang YH, Milazzo JP, Wu XS, et al. LKB1, Salt-Inducible Kinases, and MEF2C Are Linked Dependencies in Acute Myeloid Leukemia. Molecular cell. 2018;69(6):1017–27 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, et al. The target landscape of clinical kinase drugs. Science. 2017;358(6367). [DOI] [PMC free article] [PubMed] [Google Scholar]