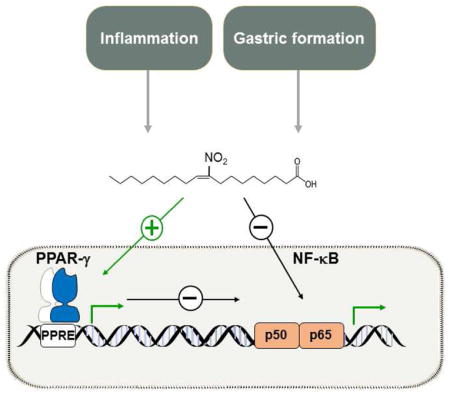
Abstract
The addition of nitrogen dioxide (•NO2) to the double bond of unsaturated fatty acids yields an array of electrophilic nitro-fatty acids (NO2-FA) with unique biochemical and signaling properties. During the last decade, NO2-FA have been shown to exert a protective role in various inflammatory and metabolic disorders. NO2-FA exert their biological effects primarily by regulating two central physiological adaptive responses: the canonical inflammatory signaling and metabolic pathways. In this mini-review, we summarize current knowledge on the regulatory role of NO2-FA in the inflammatory and metabolic response via regulation of nuclear factor kappa B (NF-κB) and peroxisome proliferator-activated receptor γ (PPARγ), master regulators of inflammation and metabolism. Moreover, the engagement of novel signaling and metabolic pathways influenced by NO2-FA, beyond NF-κB and PPAR signaling, is discussed herein.
Keywords: Nitro-fatty acids, Inflammation, Metabolism, NF-κB, PPARγ
Graphical Abstract
1. Introduction
Endogenous nitro-fatty acids (NO2-FA) are generated during inflammation and digestion through non-enzymatic reactions of unsaturated fatty acids with nitrogen dioxide (•NO2) [1]. Conjugated linoleic acid (CLA) is the preferred fatty acid target of nitration reactions due to the highly reactive external flanking carbons in the conjugated diene, yielding electrophilic nitro-conjugated linoleic acid (NO2-CLA), the predominant endogenous isomer detected in vivo [2].
A large body of evidence has accumulated during the last decade supporting a protective role for NO2-FA in numerous experimental settings [3]. These include endotoxin-induced vascular inflammation, endotoxemia and multi-organ injury [4; 5], inflammatory bowel disease (IBD) [6], allergic airway disease [7], renal ischaemia and reperfusion (I/R) injury and diabetic kidney disease [8; 9], pulmonary arterial hypertension (PAH) [10; 11], myocardial I/R injury [12], hypertension [13; 14], and atherosclerosis [15]. Beyond the above experimental models, the safety and pharmacokinetics of NO2-FA have been clinically examined in four successfully completed phase I trials (NCT02127190, NCT02248051, NCT02460146, NCT02313064) [16; 17; 18; 19]. Currently, NO2-FA administration is entering phase II clinical trials for the treatment of focal segmental glomerulosclerosis (FSGS), PAH and obese asthmatics.
The above protective effects on NO2-FA are meditated by their pluripotent cell signaling capabilities, affecting various intracellular pathways. First, NO2-FA modulate nitric oxide (•NO) signaling by yielding low concentrations of •NO (via Nef reaction) that mediate cGMP-dependent cell signaling activities and via a non cGMP-dependent manner in which NO2-FA regulate endothelial and inducible nitric oxide synthase (eNOS and iNOS)-mediated •NO generation and reactions [20; 21; 22]. Second, NO2-FA can potently regulate the expression of key inflammatory, cell proliferation and differentiation-related genes [4; 23; 24; 25; 26; 27]. Third, NO2-FA are endogenous ligands for peroxisome proliferator-activated receptors (PPARs), mainly PPARγ, centrally involved in lipid and glucose homeostasis as well as inflammation [28; 29; 30]. Finally, NO2-FA facilitate reversible adduction by nucleophilic targets (e.g. Cys and His protein residues), leading to the post-translational modifications (PTM) of proteins [31; 32]. Particularly, the electrophilic nature of NO2-FA results in nitroalkylation of nuclear factor kappa B (NF-κB), the master regulator of the immune and inflammatory response, inhibiting its DNA binding activity and repressing inflammatory gene expression, underlying a key role for NO2-FA as endogenous anti-inflammatory signaling mediators [24]. This mini-review focuses on NO2-FA regulation of NF-κB and PPARγ, key regulators of inflammation and metabolism, and the implication for inflammatory and metabolic disorders.
2. NO2-FA promote cellular anti-inflammatory responses via NF-κB inhibition
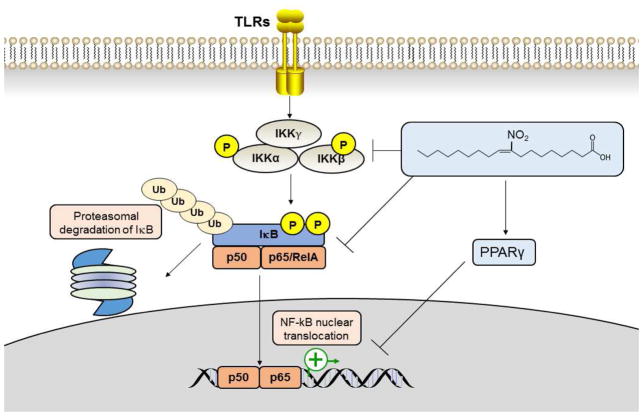
NO2-FA exerts potent anti-inflammatory actions, primarily by antagonizing the activities of NF-κB, and signal transducers and activators of transcription (STATs) [25; 33], while activating PPARγ and the anti-inflammatory nuclear factor E2-related factor 2 (Nrf2) [23; 28; 34]. Yet, a key mechanism behind the role of NO2-FA is their ability to inhibit NF-κB activity by preventing the phosphorylation of inhibitor of kappa B (IκB) and its subsequent degradation as well as by activating PPARγ (Fig. 1). Studies using affinity labeling and mass spectrometry strategies reveal that NO2-FA derivatives inhibit NF-κB via nitroalkylation of Cys residues in the NF-kB p65 subunit in vitro and a similar reaction with p65 in macrophages treated with NO2-FA [24]. Both nitro-oleic acid (NO2-OA) and nitro-linoleic acid (NO2-LA) or novel NO2-FA derivatives inhibit lipopolysaccharide (LPS)-induced macrophage secretion of pro-inflammatory cytokines including interleukin 6 (IL-6), tumor necrosis factor α (TNF-α) and monocyte chemoattractant protein 1 (MCP-1) as well as iNOS expression [24; 35; 36]. NO2-FA down-regulate expression or activity of pro-inflammatory signaling molecules induced by various stimuli (e.g., TNF-α, LPS, IL6, TGF-β) [24; 26].
Fig. 1.
NO2-FA promote cellular anti-inflammatory responses through NF-κB suppression via inhibition of IκB phosphorylation and its subsequent degradation, PTM (e.g. p65 nitroalkylation) or PPARγtransrepression signaling. NO2-FA-induced NF-κB inhibition contributes to prevention of vascular inflammation and endotoxemia, allergic airway disease, myocardial I/R injury and myocardial fibrosis [3].
Further experimental evidence reveals that NO2-FA regulate the NF-κB signaling pathway at multiple levels (Fig 1). These include reduced membrane expression of Toll-like receptor 4 (TLR4) and impaired recruitment of TLR4 and TNF receptor associated factor 6 (TRAF6) to the lipid rafts compartment with subsequent inhibition of of IκB kinase β (IKKβ) phosphorylation as well as phosphorylation and ubiquitination of IκB-α, [4]. In addition, alkylation the NF-κB p65/RelA by NO2-FA, targeting RelA for proteasomal degradation has been demonstrated in triple negative breast cancer (TNBC) cells [37].
The anti-inflammatory properties of NO2-FA via NF-κB suppression resemble that of known NF-κB inhibitors. For instance, TNBC cells treated with NO2-OA or the IKKβ inhibitor 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile (Bay 11-7082) show a similar inhibitory effect on TNFα-induced IKKβ phosphorylation and IκB degradation [37]. Also, the oleanane triterpenoid 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid methyl ester (CDDO-Me, Bardoxolone methyl) contains α,β-unsaturated carbonyl in the A-ring that forms reversible adducts with thiol nucleophiles. CDDO-Me inhibits the NF-κB pathway by interacting with Cys-179 in the IKKβ activation loop [38]. Although CDDO-Me showed beneficial effects of improving renal function in patients with chronic kidney disease and type 2 diabetes [39], a phase III trial of CDDO-Me was terminated early due to safety concerns associated with increased rate of cardiovascular events in these patients [40]. While both NO2-FA and CDDO-Me are equally effective in the activation of Nrf2-dependent signaling, NO2-FA more efficiently inhibits NF-κB than CDDO-Me [36], which may serve as an advantage over bardoxolone. NO2-FA are endogenously-generated NF-κB inhibitors suggesting improved safety as shown in past and ongoing phase II trials [16; 17; 18; 19].
Electrophilic prostaglandin metabolites such as the cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is another class of lipid mediators that inhibit NF-κB with comparable actions to NO2-FA [41]. In spite of the similar reactivity (e.g. Michael addition) [42], differences in the electrophilic potential or accessibility to nucleophilic thiols likely account for the divergent biological outcomes. 15d-PGJ2 inhibits NF-κB transcriptional activity via covalent modification of Cys 62 in p50 [43]. 15d-PGJ2 do not readily dissociate from the sites of adduction, accumulate and promote toxicity at low concentrations [44], while NO2-FA allows rapid Cys adduct formation as well as dissociation, possibly via transalkylation of different thiol sites [45]. For a more comprehensive discussion, see [41].
In addition to inhibiting NF-κB via direct PTM of the p65 subunit or upstream phosphorylation of IKK or IκB, NO2-FA may inhibit NF-κB signaling through the activation of PPAR-mediated transrepression of inflammatory responses, term coined to describe the ability of nuclear receptors to antagonize the expression of pro-inflammatory transcription factors, such as NF-kB [46]. As for PPARγ, this occurs via ligand-dependent and -independent transrepression mechanisms. For example, PPARγ can directly interact with NF-κB subunits p50 and p65 to inhibit pro-inflammatory signaling events [47]. Additionally, the sumoylation of specific lysine residues in the PPARγ ligand binding domain (LBD) does not allow clearance of corepressors which results in protein complexes bound to promoters in the actively repressed state [48]. Specifically, the sumoylation of PPARγ results in the corepressor NCoR complex bound to iNOS promoter preventing transcriptional activation in response to LPS [49]. Transrepression signaling by NO2-FA has been experimentally observed under inflammatory stimuli. Co-immunoprecipitation analysis of PPARγ and NF-κB has indeed been described in endothelial cells exposed to inflammatory insult and treatment with NO2-OA further promotes PPARγ/NF-κB interaction [7].
3. NO2-FA regulate metabolism and inflammation as PPARγ ligands
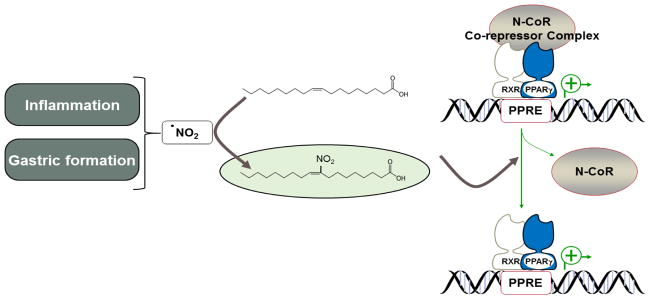
NO2-FA are potent endogenous ligands for PPARs, in particular PPARγ [28; 29; 30]. A comparative analysis of equimolar concentrations of several proposed endogenous PPARγ agonists (15d-PGJ2, linoleic acid, conjugated linoleic acid, 16:0 lysophosphatidic acid, 18:1 lysophosphatidic acid, 1-O-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine and 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine) or exogenous PPARγ agonists thiazolidinediones (TZDs, rosiglitazone) revealed a similar activation by NO2-LA compared to rosiglitazone and exceeded all of the endogenous agonists [28]. A common structural feature of PPARγ binding domain is the relatively large binding pocket resulting in a rather promiscuous affinity for lipids [50]. Yet, structure-function relationships prioritized electrophilic lipids as preferential PPARγ endogenous ligands, including unsaturated ketones and most selectively NO2-FA derivatives [51]. Cys 285 at the PPARγ LBD is critical for covalent binding of NO2-FA to PPARγ via Michael addition, while docking of rosiglitazone does not require reaction with Cys 285. This critical structural difference in the mode of interactions of endogenous NO2-FA and rosiglitazone is reflected in the differential recruitment of coactivators. Both rosiglitazone and NO2-FA share a similar displacement of the NCoR ID2 corepressor from PPARγ. However, unlike rosiglitazone, NO2-FA does not induce coactivator Trap220/Drip2 recruitment upon PPARγ binding and activation [52] (Fig. 2). Further studies applying mutation analysis of key PPARγ LBD residues demonstrated that NO2-LA interaction with PPARγ and its activation by NO2-LA differ from rosiglitazone [29].
Fig. 2.
Endogenous generation of NO2-FA and PPARγ transcriptional activation. Fatty acid nitration occurs primarily during inflammation and gastric formation via non-enzymatic reaction of •NO2 with unsaturated fatty acids. NO2-FA covalently bind to Cys 285 in the LBD of PPARγ via Michael addition, resulting in displacement of N-CoR corepressor complex yet with unique PPARγ agonism that induces different physiologic responses than known PPARγ agonists TZDs.
The discovery of unique PPARγ agonism by NO2-FA, suggests that NO2-FA can induce different physiologic responses than TZDs, giving hope that NO2-FA can be used as a therapeutic to improve insulin sensitivity without causing undesirable TZDs-mediated side-effects such as weight gain, edema and increased adverse cardiovascular events [53; 54; 55]. Unlike rosiglitazone which improves glucose homeostasis, while promoting weight gain, administration of NO2-OA increases insulin sensitivity without undesirable weight gain in leptin deficient mice [52]. Similarly, administration of NO2-OA to mice with high-fat diet (HFD)-induced adiposity, hyperglycemia and PAH improves glucose tolerance without altering body weight [11].
Beyond the controversial aspects on PPARγ dependent and independent outcomes by TZDs and aligned side-effects [56], PPARγ has multiple functions in the immune system and is implicated in major inflammatory diseases [57; 58]. Recent reports demonstrate an essential role of PPAR in the gut responses to bacterial infections by generating endogenous ligands thus improving systemic energy metabolism [59]. Also, PPARγ controls cell development of type-2 immunity (including T lymphocytes and dendritic cells) [60]. Although some reports have demonstrated that the anti-inflammatory properties of NO2-FA are independent of PPARγ activation [13; 24], other studies have shown that PPARγ regulation by NO2-FA exert an anti-inflammatory response [6; 7; 61].
4. Regulation of lipid metabolism by NO2-FA
Regulation of PPARγ activity by NO2-FA has been proven to promote an anti-inflammatory response and to improve glucose homeostasis without inducing obesity [6; 7; 52], yet a putative role of NO2-FA in lipid metabolism is intriguing. Administration of NO2-OA to apolipoprotein E-deficient (apoE−/−) mice reduces atherosclerosis and macrophage foam-cell formation without altering the lipoprotein profile [15]. Still, administration of NO2-FA in pre-clinical models of metabolic disorders suggests an active role in lipid metabolism, with observed improved lipid profiles and liver steatosis, with decreased plasma triglycerides [62; 63] and reduced expression of lipoprotein-associated phospholipase A2 (Lp-PLA2), a biomarker of cardiovascular risk [64]. In macrophages, NO2-FA prevents triglyceride accumulation via down-regulation of diacylglycerol acyltransferase 1 (DGAT1), and induced expression of triglyceride hydrolysis enzymes, hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) [65]. Novel evidence demonstrates an active incorporation of free NO2-FA into monoacyl-, diacyl- and triglycerides [66], suggesting that the aforementioned lipases might be putative targets of NO2-FA nitroalkylation. Electrophilic NO2-FA are preferentially incorporated into monoacyl- and diacylglycerides, while saturated and β-oxidized metabolites are more often incorporated into triglycerides [67]. Thus, NO2-FA may influence fluxes of fatty acid interconversion between di-and triglycerides and subsequent metabolism by yet unidentified mechanisms [67]. These examples certainly pave the way for a deeper appreciation of the putative regulatory role of NO2-FA on fatty acid distribution and gain further molecular insight on the regulation of key enzymatic activities involved in lipid metabolism [68; 69].
Given the intimate link between inflammation and metabolic derangements [70], understanding the mechanisms by which NO2-FA regulates signaling pathways centrally involved in immune regulation and energy metabolism is highly provocative [71]. Whereas a role for NO2-FA in innate immune responses has been well-established in models of acute inflammatory responses and sterile inflammation [4; 35; 72], a role of NO2-FA in metabolic adaptation of immune cells rather than the classical inflammatory responses [73], is only starting to be explored.
5. Conclusions and perspectives
The pharmacokinetics and pharmacologic actions of NO2-FA continue to actively be pursued. Biodistribution of nitrated fatty acid species indicates an active signaling role of NO2-FA in adipose tissue, liver, kidney, lung, the immune and cardiovascular systems [66; 74]. To what extent NO2-FA regulation of NF-κB and PPARγ signaling discussed herein contributes to the beneficial outcomes under disease conditions is largely unknown. While the role of NO2-FA on inflammatory processes continues to be elucidated, with established protective role in chronic and acute inflammatory responses, their role in adaptive immunity remains to be explored with molecular mechanisms that expand beyond PPAR and NF-κB signaling [73]. Given that PTM of key signaling mediators by NO2-FA continues to be uncovered [75; 76], it will be possible to assess these functions and mechanisms in further molecular detail in vivo. These remaining questions are relevant to establish the therapeutic efficacy of nitrated fatty acids and further elucidate their role on the mutual regulation of energy metabolism and immune function.
Highlights.
NO2-FA modulate key signaling pathways via posttranslational modifications.
NO2-FA inhibit NF-κB via direct p65 nitroalkylation and regulate Toll-like receptor signaling.
NO2-FA are PPARγ ligands that differ from thiazolidinediones in receptor affinity and extent of activation.
This mini-review conveys the protective role of NO2-FA in inflammatory responses and regulation of metabolic pathways.
Acknowledgments
Funding: This study was supported by National Institutes of Health Grant R01-HL123333 (L.V.), R01-HL068878 (Y.E.C.), P01-HL103455 (N.K.H.K) and the Michigan-Israel Partnership Research Grant (O.R.).
Abbreviations
- CLA
conjugated linoleic acid
- eNOS
endothelial nitric oxide synthase
- IBD
inflammatory bowel disease
- iNOS
inducible nitric oxide synthase
- NCoR
Nuclear receptor co-repressor
- NF-κB
nuclear factor kappa B
- •NO
nitric oxide
- NO2−
nitrite
- •NO2
nitrogen dioxide
- NO2-FA
nitro-fatty acids
- NO2-OA
nitro-oleic acid
- NO2-LA
nitro-linoleic acid
- NO2-CLA
nitro-conjugated linoleic acid
- PAH
pulmonary arterial hypertension
- PPARγ
peroxisome proliferator activating receptorγ
- PTM
posttranslational modification
- TZDs
thiazolidinediones
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NK, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Cole MP, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J Biol Chem. 2012;287:44071–82. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villacorta L, Gao Z, Schopfer FJ, Freeman BA, Chen YE. Nitro-fatty acids in cardiovascular regulation and diseases: characteristics and molecular mechanisms. Front Biosci (Landmark Ed) 2016;21:873–89. doi: 10.2741/4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98:116–24. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Liu H, Jia Z, Olsen C, Litwin S, Guan G, Yang T. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am J Physiol Renal Physiol. 2010;298:F754–62. doi: 10.1152/ajprenal.00439.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med. 2010;48:499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy AT, Lakshmi SP, Dornadula S, Pinni S, Rampa DR, Reddy RC. The nitrated fatty acid 10-nitro-oleate attenuates allergic airway disease. J Immunol. 2013;191:2053–63. doi: 10.4049/jimmunol.1300730. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Jia Z, Liu S, Downton M, Liu G, Du Y, Yang T. Combined losartan and nitro-oleic acid remarkably improves diabetic nephropathy in mice. Am J Physiol Renal Physiol. 2013;305:F1555–62. doi: 10.1152/ajprenal.00157.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Jia Z, Soodvilai S, Guan G, Wang MH, Dong Z, Symons JD, Yang T. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F942–9. doi: 10.1152/ajprenal.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinke A, Moller A, Pekarova M, Ravekes T, Friedrichs K, Berlin M, Scheu KM, Kubala L, Kolarova H, Ambrozova G, Schermuly RT, Woodcock SR, Freeman BA, Rosenkranz S, Baldus S, Rudolph V, Rudolph TK. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am J Respir Cell Mol Biol. 2014;51:155–62. doi: 10.1165/rcmb.2013-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NK. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res. 2014;101:352–63. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2010;85:155–66. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107:540–8. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2014;111:8167–72. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NK, Hasty AH, Baldus S, Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:938–45. doi: 10.1161/ATVBAHA.109.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. [accessed 24.07.17];A service of the US National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT02127190.
- 17.ClinicalTrials.gov. [accessed 24.07.17];A service of the US National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT02313064.
- 18.ClinicalTrials.gov. [accessed 24.07.17];A service of the US National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT02248051.
- 19.ClinicalTrials.gov. [accessed 24.07.17];A service of the US National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT02460146.
- 20.Khoo NK, Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol. 2010;10:179–84. doi: 10.1016/j.coph.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mata-Perez C, Sanchez-Calvo B, Begara-Morales JC, Carreras A, Padilla MN, Melguizo M, Valderrama R, Corpas FJ, Barroso JB. Nitro-linolenic acid is a nitric oxide donor. Nitric Oxide. 2016;57:57–63. doi: 10.1016/j.niox.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr, Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem. 2005;280:19289–97. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 23.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–6. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–98. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambrozova G, Martiskova H, Koudelka A, Ravekes T, Rudolph TK, Klinke A, Rudolph V, Freeman BA, Woodcock SR, Kubala L, Pekarova M. Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in pro-fibrotic responses. Free Radic Biol Med. 2016;90:252–260. doi: 10.1016/j.freeradbiomed.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambrozova G, Fidlerova T, Verescakova H, Koudelka A, Rudolph TK, Woodcock SR, Freeman BA, Kubala L, Pekarova M. Nitro-oleic acid inhibits vascular endothelial inflammatory responses and the endothelial-mesenchymal transition. Biochim Biophys Acta. 2016;1860:2428–2437. doi: 10.1016/j.bbagen.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kansanen E, Jyrkkanen HK, Volger OL, Leinonen H, Kivela AM, Hakkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem. 2009;284:33233–41. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102:2340–5. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PR, Freeman BA, Chen YE, Xu HE. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol. 2008;15:865–7. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–75. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–63. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–93. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichikawa T, Zhang J, Chen K, Liu Y, Schopfer FJ, Baker PR, Freeman BA, Chen YE, Cui T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–94. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286:14019–27. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villacorta L, Minarrieta L, Salvatore SR, Khoo NK, Rom O, Gao Z, Berman RC, Jobbagy S, Li L, Woodcock SR, Chen YE, Freeman BA, Ferreira AM, Schopfer FJ, Vitturi DA. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol. 2018;15:522–531. doi: 10.1016/j.redox.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoo NKH, Li L, Salvatore SR, Schopfer FJ, Freeman BA. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-kappaB signaling:A medicinal chemistry investigation of structure-function relationships. Sci Rep. 2018;8:2295. doi: 10.1038/s41598-018-20460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodcock CC, Huang Y, Woodcock SR, Salvatore SR, Singh B, Golin-Bisello F, Davidson NE, Neumann CA, Freeman BA, Wendell SG. Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. J Biol Chem. 2018;293:1120–1137. doi: 10.1074/jbc.M117.814368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 39.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG Investigators BS. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 40.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM Investigators BT. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surh YJ, Na HK, Park JM, Lee HN, Kim W, Yoon IS, Kim DD. 15-Deoxy-Delta(1)(2),(1)(4)-prostaglandin J(2), an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling. Biochem Pharmacol. 2011;82:1335–51. doi: 10.1016/j.bcp.2011.07.100. [DOI] [PubMed] [Google Scholar]
- 42.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-Deoxy-Delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J Biol Chem. 2001;276:35530–6. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Li W, Rose ME, Pascoe JL, Miller TM, Ahmad M, Poloyac SM, Hickey RW, Graham SH. Prostaglandin D2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology. 2013;39:35–44. doi: 10.1016/j.neuro.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvatore SR, Vitturi DA, Baker PR, Bonacci G, Koenitzer JR, Woodcock SR, Freeman BA, Schopfer FJ. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. J Lipid Res. 2013;54:1998–2009. doi: 10.1194/jlr.M037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–35. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–21. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey ST, Ghosh S. ‘PPAR’ting ways with inflammation. Nat Immunol. 2005;6:966–7. doi: 10.1038/ni1005-966. [DOI] [PubMed] [Google Scholar]
- 50.Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem. 2010;285:40409–15. doi: 10.1074/jbc.R110.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waku T, Shiraki T, Oyama T, Fujimoto Y, Maebara K, Kamiya N, Jingami H, Morikawa K. Structural insight into PPARgamma activation through covalent modification with endogenous fatty acids. J Mol Biol. 2009;385:188–99. doi: 10.1016/j.jmb.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 52.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–33. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–66. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 55.Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 2009;116:205–18. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–91. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94:1168–78. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 58.Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–81. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- 59.Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Baumler AJ. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nobs SP, Natali S, Pohlmeier L, Okreglicka K, Schneider C, Kurrer M, Sallusto F, Kopf M. PPARgamma in dendritic cells and T cells drives pathogenic type-2 effector responses in lung inflammation. J Exp Med. 2017;214:3015–3035. doi: 10.1084/jem.20162069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonacci G, Schopfer FJ, Batthyany CI, Rudolph TK, Rudolph V, Khoo NK, Kelley EE, Freeman BA. Electrophilic fatty acids regulate matrix metalloproteinase activity and expression. J Biol Chem. 2011;286:16074–81. doi: 10.1074/jbc.M111.225029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Liu H, Jia Z, Guan G, Yang T. Effects of Endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese Zucker rats. PPAR Res. 2010;2010:601562. doi: 10.1155/2010/601562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Sun J, Jia Z, Yang T, Xu L, Zhao B, Yu K, Wang R. Nitrooleic acid attenuates lipid metabolic disorders and liver steatosis in DOCA-salt hypertensive mice. PPAR Res. 2015;2015:480348. doi: 10.1155/2015/480348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang G, Ji Y, Li Z, Han X, Guo N, Song Q, Quan L, Wang T, Han W, Pang D, Ouyang H, Tang X. Nitro-oleic acid downregulates lipoprotein-associated phospholipase A2 expression via the p42/p44 MAPK and NFkappaB pathways. Sci Rep. 2014;4:4905. doi: 10.1038/srep04905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenblat M, Rom O, Volkova N, Aviram M. Nitro-oleic acid reduces J774A.1 macrophage oxidative status and triglyceride mass: involvement of paraoxonase2 and triglyceride metabolizing enzymes. Lipids. 2016;51:941–53. doi: 10.1007/s11745-016-4169-2. [DOI] [PubMed] [Google Scholar]
- 66.Fazzari M, Khoo NK, Woodcock SR, Jorkasky DK, Li L, Schopfer FJ, Freeman BA. Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. J Lipid Res. 2017;58:375–385. doi: 10.1194/jlr.M072058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eichmann TO, Kumari M, Haas JT, Farese RV, Jr, Zimmermann R, Lass A, Zechner R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J Biol Chem. 2012;287:41446–57. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fazzari M, Khoo N, Woodcock SR, Li L, Freeman BA, Schopfer FJ. Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides. Free Radic Biol Med. 2015;87:113–24. doi: 10.1016/j.freeradbiomed.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vitturi DA, Chen CS, Woodcock SR, Salvatore SR, Bonacci G, Koenitzer JR, Stewart NA, Wakabayashi N, Kensler TW, Freeman BA, Schopfer FJ. Modulation of nitro-fatty acid signaling: prostaglandin reductase-1 is a nitroalkene reductase. J Biol Chem. 2013;288:25626–37. doi: 10.1074/jbc.M113.486282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quijano C, Trujillo M, Castro L, Trostchansky A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016;8:28–42. doi: 10.1016/j.redox.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vitturi DA, Minarrieta L, Salvatore SR, Postlethwait EM, Fazzari M, Ferrer-Sueta G, Lancaster JR, Jr, Freeman BA, Schopfer FJ. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat Chem Biol. 2015;11:504–10. doi: 10.1038/nchembio.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salvatore SR, Vitturi DA, Fazzari M, Jorkasky DK, Schopfer FJ. Evaluation of 10-nitro oleic acid bio-elimination in rats and humans. Sci Rep. 2017;7:39900. doi: 10.1038/srep39900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy AT, Lakshmi SP, Muchumarri RR, Reddy RC. Nitrated fatty acids reverse cigarette smoke-induced alveolar macrophage activation and inhibit protease activity via electrophilic S-alkylation. PLoS One. 2016;11:e0153336. doi: 10.1371/journal.pone.0153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lakshmi SP, Reddy AT, Zhang Y, Sciurba FC, Mallampalli RK, Duncan SR, Reddy RC. Down-regulated peroxisome proliferator-activated receptor gamma (PPARgamma) in lung epithelial cells promotes a PPARgamma agonist-reversible proinflammatory phenotype in chronic obstructive pulmonary disease (COPD) J Biol Chem. 2014;289:6383–93. doi: 10.1074/jbc.M113.536805. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]