Abstract
Objectives
To describe a step-by-step guide for using the first transperineal targeted prostate biopsy platform available in the United States.
Patients and Methods
A total of 32 men with elevated prostate specific antigen (PSA) diagnosed with a region of interest (ROI) on multiparametric magnetic resonance imaging (mpMRI) between February 2017 and January 2018. The procedure was accomplished transperineal accompanied by a stepper, combined with advanced mpMRI/TRUS fusion software to perform targeted prostate biopsy. The detection of overall and clinically significant prostate cancer was assessed as well as rate of complications.
Results
The median age was 68.0 years and median PSA was 8.0 ng/mL. Two (6%) patients were active surveillance candidates and 16 (50%) had a prior negative prostate biopsy. The detection rate for overall and clinically significant prostate cancer (csPCa) was 81% and 59%, respectively. Two (100%) candidates for active surveillance and 8 (50%) patients with a prior negative prostate biopsy had csPCa confirmed on targeted biopsy. There were no perioperative complications.
Conclusion
These results demonstrate the promising potential of the first transperineal targeted prostate biopsy platform in the United States as an alternative diagnostic method for prostate cancer.
Keywords: transperineal, MRI, targeted, prostate biopsy, UroNav
Introduction
Prostate biopsy with multiple samples using a standardized template under transrectal ultrasound (TRUS) is the current standard diagnostic approach in patients with a clinical suspicion of prostate cancer (PCa). [1, 2] Unfortunately, many biopsies are unnecessary and/or do not detect clinical significant prostate cancer (csPCa). Moreover, the majority of prostate biopsies are performed with a transrectal approach which carries risks of infection and sepsis.
Many other solid organ cancers utilize a biopsy strategy that is targeted with imaging to more accurately diagnose cancer. With the introduction of mpMRI we are able to detect clinically suspicious lesions which can aid in targeted sampling. Prior level one evidence comparing mpMRI targeted biopsy using either a transrectal or transperineal approach versus standard TRUS noted a higher detection rate of overall and csPCa. [3] Until recently, there was no commercially available mpMRI/TRUS targeted transperineal platform in the United States. We report the first case series of multiparametric magnetic resonance imaging/transrectal ultrasound (mpMRI/TRUS) fusion targeted transperineal prostate biopsy using the UroNav mpMRI/ultrasound fusion device (Invivo®, Gainesville, FL, USA).
Patient Selection
All patients undergoing transperineal targeted prostate biopsy at out institution from February 2017 and January 2018 were included. All patients underwent mpMRI on a 3.0-T MRI (MAGNETOM Verio or Skyra, Siemens Healthineers, Erlangen, Germany) with triplanar T2-weighted, fat-saturated T2-weighted, diffusion weighted (including b 1800 images), dynamic contrast-enhanced, and large field-of-view T2-weighted images. Images were acquired with a 16-channel diaper coil (PROCURE™, ScanMed, Omaha, NE, USA) on the Verio and an 18-channel body coil (Body 18, Siemens Healthineers, Erlangen, Germany) on the Skyra. Two highly experienced genitourinary radiologists (GH and EW) with 6 and 13 years of experience interpreting prostate mpMRI performed independent review of all studies in this series with consensus reads on challenging cases. Prior to biopsy, an mpMRI was interpreted by the radiologist and ROI locations were recorded (DynaCAD, Invivo, Gainesville, FL, USA). Indications to perform a targeted biopsy included patients diagnosed with an mpMRI prostate imaging reporting and data system, version 2 (PI-RADSv2) score ≥3. [4–6]
Room Layout
The UroNav cart and the BK ultrasound machine, 8848 ultrasound probe (BK Medical®, Peabody, MA, USA), and the field generator were placed to the patient’s left. The field generator was placed approximately 30 centimeters over the anticipated site of the transperineal stepper with ultrasound probe and was secured via a pole to the surgical bed with cables connected to the UroNav cart. The ultrasound machine and UroNav cart were connected using a High-Definition Multimedia Interface (HDMI) to Digital Visual Interface (DVI) cable.
Patient Positioning
All procedures were performed in the operating theater under sedation via anesthesia preference (total intravenous anesthesia or laryngeal mask airway) with patients placed in the dorsal lithotomy position as shown in Figure 1. A total of 60 milliliters of ultrasound gel was injected into the rectum. A total of 10 milliliters of 1% lidocaine plain was injected into the perineum. An endocavity balloon (CIVCO®, Peabody, MA, USA) was placed over the ultrasound probe which was prepped in an inverted fashion using 5 centimeters of ultrasound gel nearest the probe handle then rotated to orthotopic position. This was connected to a 60-milliliter syringe of sterile water which was instilled to evacuate air bubbles. This allowed improved image visualization and removed any echogenic artifacts. An exaggerated hip flexion was used in order to move the pubic bone away from the trajectory line needed to access the anterior prostate. The scrotum was secured superiorly using a 3D drape. The perineum was prepped with chlorhexidine and draped with surgical towels. All patients received enemas the morning of the procedure and antibiotics were given intraoperatively.
Figure 1.
Patient placed in the dorsal lithotomy position. UroNav mpMRI/ultrasound fusion device (Invivo®, Gainesville, FL, USA), transducer type 8848 ultrasound probe (BK Medical®, Peabody, MA, USA) and transperineal stepper with brachytherapy-like grid (CIVCO®, Peabody, MA, USA).
Transperineal Stepper
A transperineal stepper with brachytherapy-like grid (CIVCO®, Peabody, MA, USA) was connected to the UroNav pole cart and allowed for transperineal access. The probe was placed in the carriage and connected to trackers which allowed for tracking of the ultrasound probe throughout the procedure. The stepper was draped and the sterile grid inserted. The field generator, secured to a mobile arm with flexible locking joints, was tilted slightly towards the physician throughout the procedure which allowed the electromagnetic field to encompass the stepper where the grid and trackers were placed to reduce interference.
Biopsy
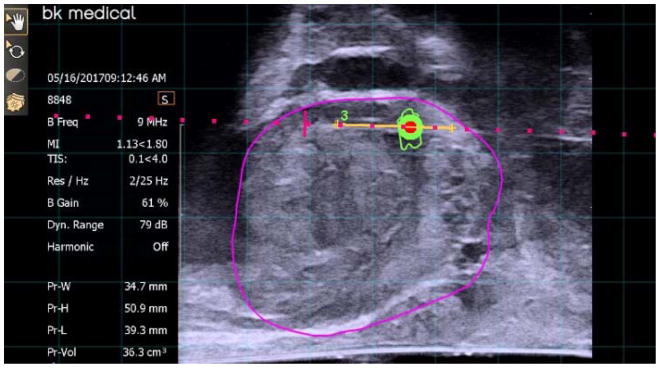
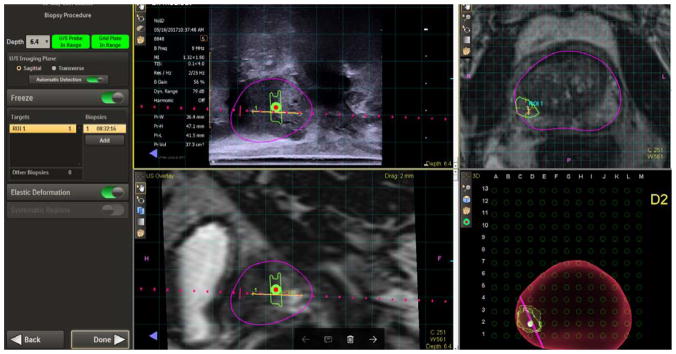
The stepper with BK 8848 TRUS was aligned and inserted into the rectum, respectively. A TRUS prostatic sweep was performed under real time in the sagittal plane. The preoperative MRI and TRUS images were then fused and contoured where necessary via the UroNav review and segmentation screen. Both the prostate volume using TRUS and MRI was obtained and recorded, respectively. For the actual biopsy procedure, prostate images were projected on the UroNav cart screen (left upper quadrant: TRUS sagittal plane and right lower quadrant: 3D volume generated image of prostate on grid-like screen), and biopsies were obtained using selected grid holes with corresponding letters and numbers (i.e. E1) (Figures 2 and 3). A core biopsy needle was used to obtain the samples under fusion guidance. Targeted biopsy was performed on a maximum of two ROIs, and three to six cores were obtained from each lesion. Systematic biopsies were performed in biopsy naïve patients. The steps in performing an mpMRI/ultrasound fusion transperineal biopsy are outlined in Video S1. After all samples were obtained the perineum was cleaned, covered with bacitracin, gauze and Tegaderm (3M). No pain or antibiotics prescriptions were prescribed. Patients were seen within 2 weeks to discuss pathology results.
Figure 2.
Lesion sampled transperineal in the sagittal plane.
Figure 3.
The steps in performing an mpMRI/ultrasound fusion transperineal biopsy.
Outcomes
Out of 32 consecutive patients, 16 (50%) had a prior negative prostate biopsy. Two (6%) patients were active surveillance candidates. Median age was 68.0 years, median PSA was 8.0 ng/mL with a median PSA density of 0.18, and 29 (91%) patients had a normal digital rectal examination. The majority of patients had PI-RADS 4 lesions with 21 (54%) lesions were located peripherally and 16 (41%) were located anteriorly with a median maximum ROI diameter of 12 millimeters (IQR: 8.0, 16.0) (Table 1).
Table 1.
Characteristics of Patients and Tumor Characteristics
| No. (%) | |
|---|---|
|
| |
| Total patients | 26 (100) |
|
| |
| Patients with PCa | 21 (80.7) |
|
| |
| Pretreatment PSA level, ng/mL | |
|
| |
| Median, IQR | 8.5 (6.5, 10.6) |
|
| |
| PSA Density, median, IQR | 0.20 (0.12, 0.32) |
|
| |
| Age, years | |
|
| |
| Median, IQR | 67.1 (60.4, 70.3) |
|
| |
| Race/ethnicity | |
| White | 20 (76.9) |
| Black | 4 (15.4) |
| Hispanic | 2 (7.7) |
|
| |
| Prior Negative Prostate Biopsy | 13 (50.0) |
|
| |
| 2002 AJCC T category | |
| T1c | 24 (92.3) |
|
| |
| Prostate volume (MRI, cc), median, IQR | 43 (36.3, 59.3) |
|
| |
| Number of ROI, total | 33 |
|
| |
| Max ROI Diameter (MRI, mm), median, IQR | 12 (8.0, 16.0) |
|
| |
| Lesion Location | |
| Anterior | 12 (36.4) |
| Central | 0 (0) |
| Transition | 2 (6.1) |
| Peripheral | 19 (57.6) |
|
| |
| MRI Grade* | |
| 3 | 9 (27.3) |
| 4 | 18 (54.5) |
| 5 | 6 (18.2) |
|
| |
| Number of ROI with PCa | 23 (69.7) |
|
| |
| Number of ROI with csPCa** | 17 (51.5) |
|
| |
| Maximum CCL (mm), median, IQR | 6.0 (3.3, 8.8) |
|
| |
| Gleason Grade Group | |
| 1 | 7 (30.4) |
| 2 | 12 (52.2) |
| 3 | 2 (8.7) |
| ≥4 | 2 (8.7) |
|
| |
| Complications | 0 |
Note: Percentages may not sum to 100% due to rounding.
Abbreviations: AJCC, American Joint Committee on Cancer; T: tumor; PSA, prostate specific antigen; IQR, interquartile range; CCL, cancer core length
PIRADS v2 classification
Defined according to START criteria
The detection rate for overall and csPCa was 81.3% and 59.4%, respectively. The detection of Gleason grade groups 1, 2, 3, 4 and 5 were 9 (34.6%), 12 (46.2%), 3 (11.5%), 1 (3.8%) and 1 (3.8%), respectively. The overall and csPCa detection by targeted biopsy for PI-RADS scores 3, 4, and 5 were: 3 (33.3%) and 2 (22.2%), 16 (76.2%) and 11 (52.4%), and 9 (100%) and 8 (88.9%), respectively. Both of the two patients who were candidates for active surveillance were found to have csPCa. Eight (50%) patients with a prior negative prostate biopsy had csPCa confirmed on targeted biopsy. A total of 10 patients underwent simultaneous targeted and systematic biopsy of which only 2 (20%) patients were found to have csPCa not identified by targeted biopsy. There were no perioperative complications. While promising, further application to a larger series of patients should be investigated and we are currently enrolling patients in a trial comparing this device to the standard transrectal diagnostic approach (ClinicalTrials.gov Identifier: NCT03044197).
Supplementary Material
Acknowledgments
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award Mentored Career Development (KL2) Award (KL2TR001441) from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) and The Herzog Foundation (SBW).
Abbreviations and Acronyms
- csPCa
clinically significant prostate cancer
- mpMRI
multiparametric magnetic resonance imaging
- PCa
prostate cancer
- PI-RADS™
Prostate Imaging Reporting and Data System
- PI-RADSv2
PI-RADS, version 2
- PSA
prostate specific antigen
- ROI
region of interest
- TRUS
transrectal ultrasound
Footnotes
Conflict of Interest: None
References
- 1.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. Journal of the National Comprehensive Cancer Network : JNCCN. 2016 Jan;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014 Jan;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Porpiglia F, Manfredi M, Mele F, et al. Diagnostic Pathway with Multiparametric Magnetic Resonance Imaging Versus Standard Pathway: Results from a Randomized Prospective Study in Biopsy-naive Patients with Suspected Prostate Cancer. Eur Urol. 2016 Aug 27; doi: 10.1016/j.eururo.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Vargas HA, Hotker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. European radiology. 2016 Jun;26:1606–12. doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015 Jan 27;313:390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013 Oct;64:544–52. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.