Abstract
Diabetes significantly increases the risk of stroke and post-stroke mortality. Recurrent hypoglycemia (RH) is common among diabetes patients owing to glucose-lowering therapies. Earlier we showed that RH in a rat model of insulin-dependent diabetes exacerbates cerebral ischemic damage. Impaired mitochondrial function has been implicated as a central player in the development of cerebral ischemic damage. Hypoglycemia is also known to affect mitochondrial functioning. The present study tested the hypothesis that prior exposure of insulin-treated diabetic (ITD) rats to RH exacerbates brain damage via enhanced post-ischemic mitochondrial dysfunction. In a rat model of streptozotocin-induced diabetes, we evaluated post-ischemic mitochondrial function in RH-exposed ITD rats. Rats were exposed to five episodes of moderate hypoglycemia prior to the induction of cerebral ischemia. We also evaluated the impact of RH, both alone and in combination with cerebral ischemia, on cognitive function using the Barnes circular platform maze test. We observed that RH exposure to ITD rats leads to increased cerebral ischemic damage, and decreased mitochondrial complex I activity. Exposure of ITD rats to RH impaired spatial learning and memory. Our results demonstrate that RH exposure to ITD rats potentially increases post-ischemic damage via enhanced post-ischemic mitochondrial dysfunction.
Keywords: Ischemic damage, mitochondria, hippocampus, Barnes maze test, open field, type 1 diabetes
Introduction
The prevalence of diabetes is rapidly increasing worldwide. The International Diabetes Federation and the CDC estimates that 415 and 29 million people suffer with diabetes globally and in the USA, respectively (International Diabetes Federation, 2015; Center for Disease Control and Prevention, 2016). Diabetes is a chronic disease that requires continuous medical care to prevent acute complications and reduce the risk of long-term complications (American Diabetes Association, 2011). Diabetes significantly increases the risk of stroke/cerebral ischemia, and also increases mortality following stroke/cerebral ischemia (Beckman et al., 2008).
Earlier studies reported that management of the disease with intensive glycemic control can limit, delay or even prevent the chronic complications of diabetes (DCCT research group, 1993; Nathan, 2014). However, intensive glycemic control could increase the risk of hypoglycemia in both type 1 (T1D) and type 2 (T2D) diabetics, consequently increasing the risk of hypoglycemic brain injury (Lincoln et al., 1996; Davis et al., 1998; EDIC group, 1999; Cryer, 2001; Donnelly et al., 2005). Diabetics frequently experience episodes of symptomatic and asymptomatic hypoglycemia (Janssen et al., 2000; Pedersen-Bjergaard et al., 2004; Donnelly, et al., 2005; UK Hypoglycaemia Study Group, 2007; Shafiee et al., 2012). Recurrent exposure to hypoglycemia may lead to an impaired awareness of hypoglycemia (Geddes et al., 2008; Yeoh et al., 2015). Diabetics with impaired hypoglycemia awareness experience much higher incidences of both asymptomatic and severe hypoglycemia than those with normal awareness (Schopman et al., 2011; Shafiee, et al., 2012; Gehlaut et al., 2015). Studies involving continuous glucose monitoring systems detect a high frequency of previously unrecognized hypoglycemic episodes in individuals with both T1D and T2D (Boland et al., 2001; McNally et al., 2007; Weber et al., 2007; Tamborlane et al., 2008).
The presence of oxidative stress during hypoglycemia is well documented (Patockova et al., 2003; Singh et al., 2004; Suh et al., 2007; Amador-Alvarado et al., 2014). Using in vivo and in vitro models, earlier studies demonstrated that hypoglycemia leads to an increase in mitochondrial reactive oxygen species (ROS) production and a decrease in mitochondrial membrane potential (MMP)(Kauppinen et al., 1986; McGowan et al., 2006; Isaev et al., 2008; Dave et al., 2011). The production of ROS and oxidative stress is involved in recurrent hypoglycemia (RH)-induced dendritic damage in the hippocampus (Won et al., 2012). Repetitive moderate hypoglycemia in the developing brain causes selective impairment of synaptic plasticity in the absence of hippocampal neuronal death, and without complete disruption of basal synaptic transmission (Yamada et al., 2004). We earlier demonstrated that ROS generation is increased in isolated mitochondria from the hippocampus of insulin-treated diabetic (ITD) rats exposed to RH compared to the control groups (Dave, et al., 2011). Also, we observed that the most sensitive populations of neurons, the cornusammonis (CA) 1 hippocampal neurons survived least in the ITD + RH group compared to the control groups (Dave, et al., 2011). Increased ROS production is also observed during and following an acute ischemic stroke (Cuzzocrea et al., 2001). Thus, oxidative stress is an important mediator of tissue injury in both hypoglycemia and acute ischemic stroke.
Mitochondria have long been known to play a critical role in the pathogenesis of cerebral ischemia/reperfusion injury via ROS generation, mitochondrial failure or dysfunction, and mitochondrial-mediated apoptosis (Fiskum et al., 1999; Chan, 2001). Impaired function of respiratory chain complexes and ATP synthase after ischemia are potential major causes of enhanced mitochondrial ROS production (Moro et al., 2005; Niatsetskaya et al., 2012). The excess production of ROS during and after ischemia causes mitochondrial dysfunction as well as induces the release of cytochrome c from mitochondria through the opening of the mitochondrial permeability transition pore (MPTP), which in turn initiates the apoptotic cascade (Zoratti et al., 1995; Borutaite et al., 1999; Kowaltowski et al., 2000; Nicholls et al., 2000; Iijima, 2006). Impaired mitochondrial energy metabolism is the most immediate result of mitochondrial dysfunction in cerebral ischemia (Canevari et al., 1997; Zaidan et al., 1997).
The present study tested the hypothesis that exposure to RH in diabetic conditions exacerbates cerebral ischemia-induced CA1 hippocampal neuronal death via aggravated post-ischemic mitochondrial dysfunction.
Experimental Procedures
Animals
All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by an institutional animal care and use committee. Efforts were made to minimize the number of animals used and their suffering.
Induction of diabetes
Male Wistar rats were made diabetic by intraperitoneal injection (i.p) of the β cell toxin streptozotocin (STZ; (58 mg / kg body weight; Sigma Aldrich, St Louis, MO) (Dave, et al., 2011). After STZ injection, blood glucose levels were measured (using portable glucose meter: Freestyle Freedom, Abbott Diabetes Care Inc., Alameda, CA, USA; detection range 20–550 mg/dL) in non-fasting rats by tail pricking twice a week. Blood glucose values of >550 mg/dL were assigned a value of 550 mg/dL for statistical analysis.
Insulin treatment of diabetic rats
Two to three weeks after the induction of diabetes, insulin pellets (LinShin Canada, Inc, Ontario, Canada) were implanted subcutaneously (s.c.) (Dave, et al., 2011). Blood glucose levels were monitored in the following week and if the level was >220mg/dL an additional insulin pellet (or part of the pellet) was implanted to keep the glucose level in the target range (≤220 mg/dL). This group of animals was considered ITD rats.
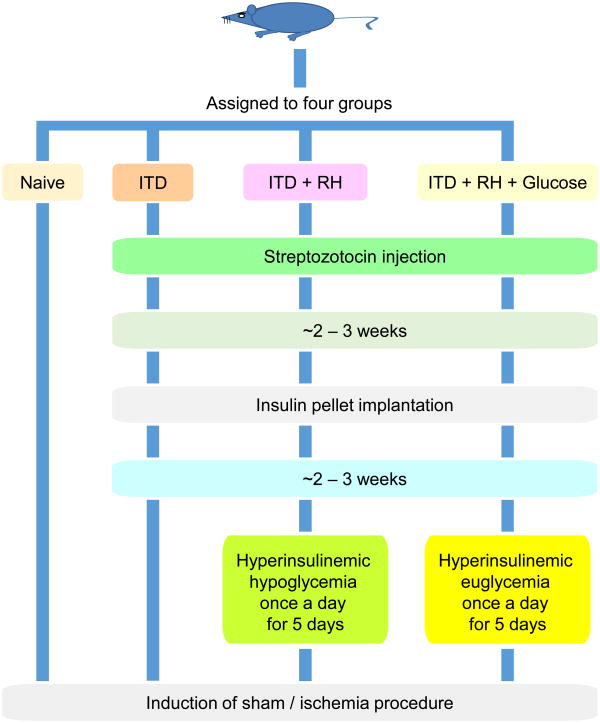
Rats belonging to the ITD group were randomized into three groups: 1) ITD (n = 19), 2) ITD + RH (n = 29), and 3) ITD + RH + glucose (n = 26). Rats belonging to ITD + RH or ITD + RH + glucose groups were exposed to a total of five episodes of RH (hyperinsulinemic hypoglycemia) or RH + glucose (hyperinsulinemic euglycemia) over 5 consecutive days (1 episode / day), respectively.
Exposure of ITD rats to RH
RH was induced 2-3 weeks after insulin pellet implantation, with the aim of maintaining blood glucose levels in the moderate hypoglycemia range for 3 hours, by s.c. injection of Novolog (insulin aspart, Novo Nordisk, AIS, Denmark) (Dave, et al., 2011). Duration, frequency and total number of hypoglycemia episodes was decided based on earlier studies (McNay et al., 2006; Herzog et al., 2008; Won, et al., 2012; McNay, 2015). Glucose levels were measured at baseline and every hour during hypoglycemia. Additional insulin or 50% dextrose solution was administered by s.c. injection if the target glucose level was not achieved within the first hour of insulin injection. After 3 hours of hypoglycemia the rats were infused s.c. with 50% dextrose solution to raise the blood glucose to pre-hypoglycemia levels. Blood glucose levels were also measured 30 minutes post-dextrose injection (recovery) confirming termination of the hypoglycemia episode.
Animals belonging to the ITD + RH + glucose group were treated with insulin similar to the ITD + RH group. However, their glucose levels were maintained close to baseline by s.c. injection of 50% dextrose. Similar to the ITD + RH group, their blood glucose levels were measured at baseline, at every hour for three hours following insulin + glucose injection, and at 30 minutes post-recovery.
Rats belonging to four experimental groups were included in the study: 1) Naïve (n = 17), 2) ITD (n = 19), 3) RH (n = 29), and 4) RH + glucose (n = 26). A schematic diagram detailing the experimental groups is presented in Figure 1.
Figure 1. Schematic diagram of experimental groups.
Induction of ischemia
After the last exposure to hypoglycemia, on the next day the rats were subjected to sham or global cerebral ischemia surgery (Figure 1) (Dave et al., 2001). Rats were first anesthetized with 4% isoflurane in a mixture of 70% nitrous oxide and 30% oxygen. Both femoral arteries were cannulated for blood pressure measurements, controlled hemorrhage, and for arterial sampling of blood gases and glucose. The femoral vein was cannulated for rocuronium infusion. Temperature probes were inserted into the rectum and the left temporalis muscle, and separate thermostat-regulated heating lamps were used to maintain rectal and temporalis muscle temperature between 36.5 °C to 37.5 °C throughout the experiment. The rats were endotracheally intubated and mechanically ventilated during the surgery procedure. During ventilation, the rats were immobilized with rocuronium (10 mg/kg). Blood glucose levels were measured before the induction of ischemia. Arterial blood gases (ABL80, Radiometer, Westlake, OH) were measured intermittently throughout the experiment. The goal was to maintain blood gases in the physiological range. Both common carotid arteries were exposed by a midline ventral incision and were gently dissected free of surrounding nerve fibers. Ligatures of polyethylene (PE-10) tubing, contained within double-lumen silastic tubing, were passed around each carotid artery. To induce cerebral ischemia, blood was gradually withdrawn from the femoral artery into a heparinized syringe to reduce systemic blood pressure to 50 mmHg, then the carotid ligatures were tightened bilaterally. Loosening the carotid ligatures and slowly reinfusing the shed blood into the femoral artery terminated the ischemic insult. The carotid arteries were inspected to verify that recirculation was reestablished. The duration of ischemia was 8 minutes. Arterial and venous catheters were then removed with appropriate caution. After 45 – 60 minutes, the animals were transferred to an individual cage. Sham animals were treated the same except for induction of cerebral ischemia.
Behavior assessments
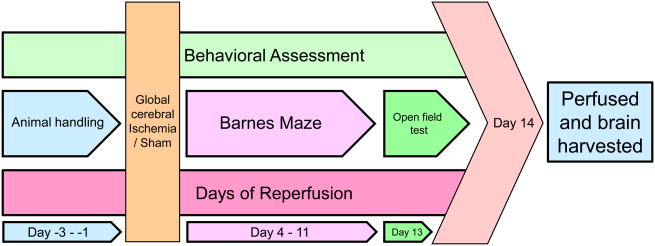
All rats used for behavior assessment were handled for three days (15 min per session, last session on the day before surgical procedure) prior to sham ischemia surgery. Rats were subjected to behavioral assessment starting on day 4 post-surgery. The details of the behavioral test schedule appear in Figure 2. Behavioral assessments included the Barnes circular platform maze and the open field test to evaluate spatial learning and memory and locomotor activity, respectively (Cohan et al., 2015).
Figure 2. Behavioral test schedule.
Barnes circular platform maze test
Rats from each experimental group were subjected to the Barnes maze test on day 4 post-ischemia for a total of 8 trials, one trial per day up to day 11 post-ischemia. The Barnes maze test was performed as described earlier (Cohan, et al., 2015). In brief, the apparatus consisted of a circular platform with 18 escape holes located along the circumference. Only one of the escape holes was connected to the escape box. To start the test, rats were placed in the center of the platform and covered with a black box for 15 seconds. Rats were given 4 minutes to find the escape box following removal of the black box. Bright lights (noxious stimuli) were used to increase the incentive in finding the escape box. For each trial, the Barnes maze platform and escape box were thoroughly cleaned with 70% ethanol solution and dried with paper towels. Each day the maze was rotated 90 degrees in order to disperse residual odorant cues on the maze. All trials were recorded using an EQ 610 Polestar II Everfocus camera. The distance traveled until the rat first located the escape tunnel (known as a primary measurement, a potentially more accurate measure of learning (Harrison et al., 2006)and the latency to the rat located the escape box (primary measure) were quantified using Ethovision 8.5 video tracking software (Noldus, Leesburg, VA, USA). The primary search strategy (random or systematic) used by each rat was also evaluated (details are in (Cohan, et al., 2015)).
Open field test
The rats from each experimental group were subjected to an open field test on day 13 post-ischemia using an open field chamber (17” L × 17” W × 12” H; Med Associates Inc., St. Albans, VT) (Prut et al., 2003). To start the trial, rats were placed into the center of the open field and allowed to explore the apparatus for 30 minutes. For each trial, the open field box was thoroughly cleaned with 70% ethanol solution and afterward by dry paper towels. The analysis included the distance traveled by the rat over the arena in 30 minutes.
Histological studies
The number of normal neurons was counted as described earlier (Dave et al., 2005). In brief, on day 14 post-ischemia/sham surgery, rats were perfused using FAM (a mixture of 40% formaldehyde, glacial acetic acid, and methanol, 1:1:8 by volume) under isoflurane anesthesia for histological analysis. Coronal sections were made from paraffin-embedded brains and stained with hematoxylin and eosin. In sections containing hippocampus at the level of -3.6, -3.8, and -4.0 mm from bregma, the number of surviving normal neurons within the entire CA1 region of hippocampus was counted using MCID Elite 6.0 software (InterFocus Imaging Ltd, Cambridge, UK) attached to a Nikon microscope (Nikon Microphot-SA; Nikon Corporation, Tokyo, Japan), a Sony 3CCD color video camera (Sony Corporation, Tokyo, Japan), and an LEP motorized stage (Ludl Electronic Products Ltd, Hawthorne, NY). The counts at the three bregma levels were averaged.
Isolation of Mitochondria and determination of mitochondrial function
Mitochondria were isolated twenty-four hours after sham/ischemia surgery. In brief, rats were decapitated under isoflurane anesthesia. The hippocampus was removed immediately and mitochondria were isolated as described earlier (Lee et al., 1993; Dave, et al., 2011). The entire procedure was completed within 1 to 1.5 hours. All procedures were carried out at 4°C or on ice. Polarographic measurement of substrate oxidation rates, and spectrophotometric measurements of the specific activities of complexes I - IV were performed as described earlier (Dave, et al., 2001; Dave, et al., 2011).
Statistical analysis
All statistical analyses were done using either Graph Pad prism software version 5 or SAS version 9.4 (SAS Institute Inc., Cary, NC). Multiple comparison analysis among the groups for open field test, mitochondrial respiration rate and mitochondrial respiratory chain complex activity were done by one way analysis of variance (ANOVA) followed by Tukey's post-hoc test. Rats that did not respond on the Barnes maze, animals with abnormal ischemic injury (absence, unilateral or severe), and significant outlier data points as identified by the Grubbs' test were excluded from analysis. Linear mixed-effect models for repeated measurements were used to assess the effects of treatment on spatial learning and memory with repeated Barnes maze tests over time. The Chi-square test was used to compare the search strategy results among the groups. All data are expressed as mean ± SEM. A p value of less than 0.05 was considered statistically significant.
Results
Blood glucose levels
To confirm the induction of diabetes, and control of blood glucose levels in ITD groups, the blood glucose levels were measured for rats belonging to each group before (after STZ injection)and after insulin pellet implantation (at the time of ischemia/sham surgery). No significant differences in blood glucose level (Figure S1) were observed in all six diabetic groups (i.e., ITD + sham: n=8, ITD + ischemia: n=11, ITD + RH + sham: n=14, ITD + RH + ischemia: n=15, ITD + RH + glucose + sham: n=12, and ITD + RH + glucose + ischemia: n=14).
To confirm hypoglycemia in rats, the glucose levels were measured at baseline (before insulin injection), at 1, 2, and 3 hr post-insulin injection, and after half an hour of dextrose infusion (recovery) in rats belonging to the ITD + RH + sham (n=14), ITD + RH + ischemia (n=15), ITD + RH + glucose + sham (n=12), and ITD + RH + glucose + ischemia (n=14) groups (Figure S2). Our results confirm that we were able to maintain glucose levels in the moderate hypoglycemia range in both ITD + RH groups (sham and ischemia). Our results also indicated that we were able to maintain glucose levels close to euglycemia in the ITD + RH + glucose groups (sham and ischemia).
Exposure to RH in ITD rats exacerbates post-ischemic neuronal damage in the CA1 hippocampus
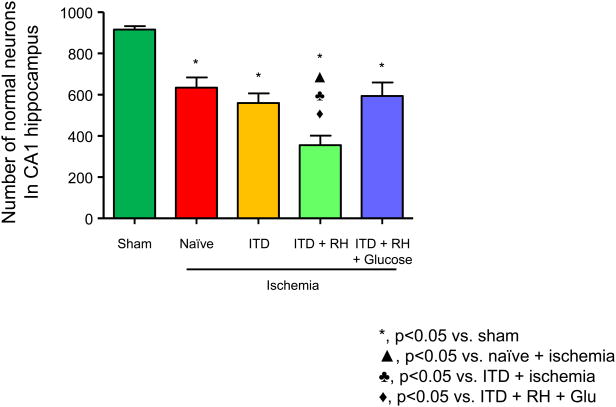
The goal of this experiment was to confirm our earlier results which demonstrated that prior exposure to RH exacerbates cerebral ischemic damage in ITD rats. The groups included were (1) naïve + sham (n=9), (2) naïve + ischemia (n=8), (3) ITD + sham (n=8), (4) ITD + ischemia (n=5), (5) ITD + RH + sham (n=14), (6) ITD + RH + ischemia (n=8), (7) ITD + RH + glucose + sham (n=12), and (8) ITD + RH + glucose + ischemia (n=7). The number of normal neurons in the CA1 region of hippocampus was evaluated for each experimental group on day 14 post-ischemia. Neither RH nor RH + glucose treatment resulted in any cell death as the number of normal neurons in sham-operated rats belonging to all experimental groups was not different. Due to this reason, all sham groups were pooled into one group, and further comparisons are made against this common sham group. We found a significant decrease in normal surviving neurons in the naïve (31%, p<0.001), ITD (39%, p<0.001), ITD + RH (61%, p<0.001), and ITD + RH + glucose (35%, p<0.001) groups after ischemia when compared with the sham group (Figure 3). When further analysis was done between ischemic groups, we found a significant reduction in the number of normal neurons in the ITD + RH + ischemia group compared to three ischemia groups; i.e., naïve (44 %, p<0.001), ITD (37 %, p<0.05) and ITD + RH + glucose (40 %, p<0.01) (Figure 3). These results confirms our earlier findings that the reduction in normal surviving neurons was more severe in the ITD + RH + ischemia group compared to the naïve, ITD, and ITD + RH + glucose ischemic groups. Thus, our results demonstrate that RH in a rat model of ITD exacerbates cerebral ischemic damage.
Figure 3. The effect of RH exposure on neuronal survival in CA1 hippocampus of ITD rats subjected to cerebral ischemia.
Normal neurons were counted post-ischemia/sham surgery in rats belonging to (1) sham (n = 41), (2) naïve + ischemia (n = 8), (3) ITD + ischemia (n = 5), (4) ITD + RH + ischemia (n = 7), and (5) ITD + RH + glucose + ischemia (n = 8). The results are presented as mean ± SEM. *, p<0.05 vs sham; ▲, p<0.05 vs naïve + ischemia; ♣, p<0.05 vs ITD + ischemia; ◆, p<0.05 vs ITD + RH + glucose.
Prior RH exposure induces no significant changes in locomotor activity in ITD animals subjected to cerebral ischemia
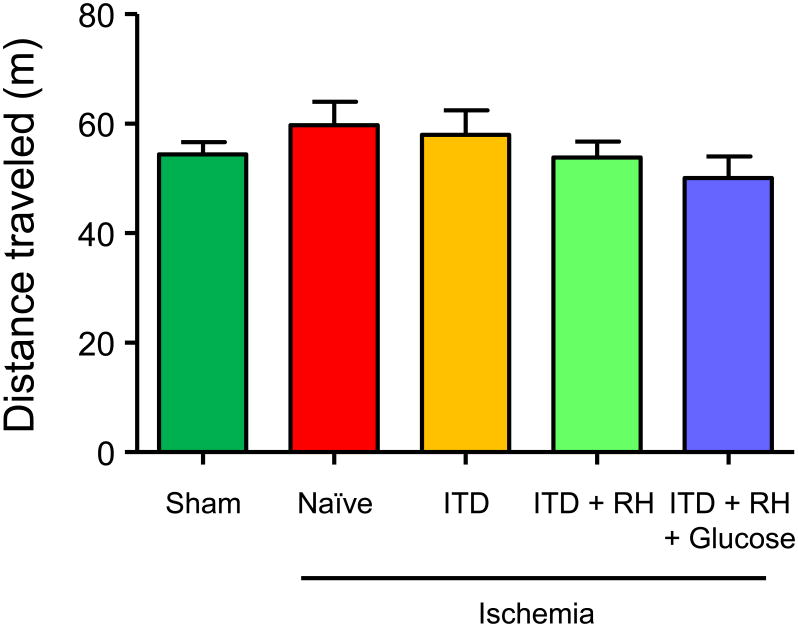
We observed a significant reduction in number of normal neurons in RH-exposed diabetic rats subjected to cerebral ischemia. Before determining the effect of RH on cognitive outcomes, first we confirmed absence of any motor deficits in our experimental groups specifically in RH treated group as motor deficits may affect results of cognitive test used in our study. We measured distance traveled by animals during the test period of 30 minutes in an open field chamber. Groups used for this experiment were same as the one used for histology study (sham: n = 41, naïve + ischemia: n = 8, ITD + ischemia: n = 5, ITD + RH + ischemia: n = 7, ITD + RH + glucose + ischemia: n = 8). The mean distance traveled by animals belonging to the groups subjected to cerebral ischemia was not significantly different from that of their respective sham groups (Figure 4). Also, no significant difference in mean distance traveled was noticed among all experimental groups (Figure 4). Overall, our results suggest that prior exposure of ITD rats to RH does not have any effect on locomotor activity in either control (sham) or ischemia groups.
Figure 4. The effect of RH exposure on distance traveled during an open field test in ITD rats subjected to cerebral ischemia.
Distance traveled by rats belonging to (1) sham (n=41), (2) naïve + ischemia (n=8), (3) ITD + ischemia (n=5), (4) ITD + RH + ischemia (n=7), and (5) ITD + RH + glucose + ischemia (n=8). The results are presented as mean ± SEM.
RH exposure alone impairs spatial learning and memory in ITD rats
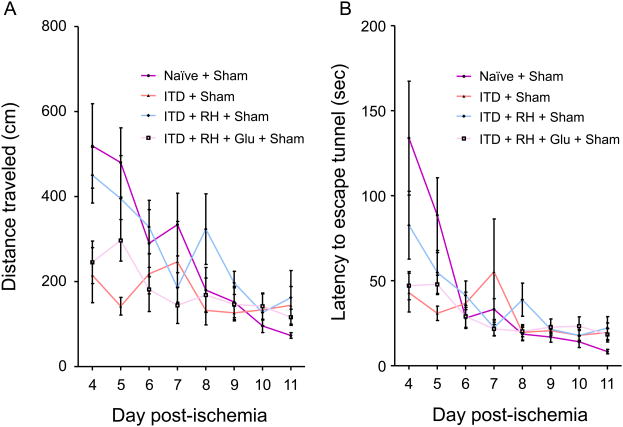
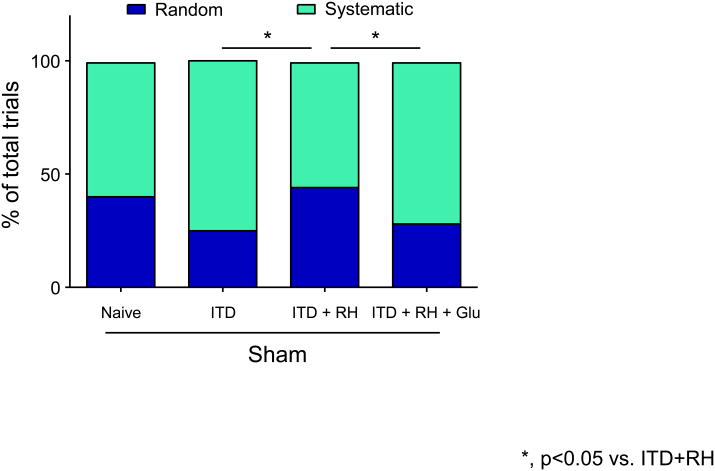
To determine the effect of RH exposure to ITD rats on spatial learning and memory, we evaluated rats belonging to naïve + sham (n = 9), ITD + sham (n = 7), ITD + RH + sham (n = 14), and ITD + RH + glucose + sham (n = 12) using the Barnes maze. The average distance traveled (cm), latency (sec), and strategy (random or systematic) used to locate the escape box over the eight day period of trial was measured in animals belonging to each experimental group (same as histology study). We observed significant group (difference among groups, p<0.005), day (groups improved over time, p<0.001) and day × group (treatment by time interaction, p<0.05) effects among the four experimental groups for total distance traveled (Figure 5A). The rate of learning (calculated from distance traveled) in the ITD + RH + sham group was significantly lower when compared to other two diabetic groups (ITDp<0.005 and ITD + RH + glucose + sham p<0.005) (Figure 5A). However, no significant difference was observed in the rate of learning (distance traveled) between ITD + RH + sham and naïve + sham groups (Figure 5A). Also, no significant differences were observed for latency between ITD + RH + sham and three control groups (Figure 5B). ITD + RH + sham animals used random verses systematic strategy more frequently (45% of the time) compared to the ITD + sham (25% of the time, p<0.05) and ITD + RH + glucose + sham (28% of the time, p<0.05) groups (two diabetic groups) (Figure 6). However, no significant difference was observed in the frequency of random strategy use between ITD + RH + sham (45% of the time) and naïve + sham (40% of the time) groups(Figure 6). Our results indicate that RH exposure to ITD leads to impaired spatial learning and memory.
Figure 5. The effect of RH exposure on (A) distance traveled and (B) latency during the Barnes maze test in ITD rats subjected to sham surgery.
The groups include (1) naïve + sham (n=9), (2) ITD + sham (n=7), (3) ITD + RH + sham (n=14), (4) ITD + RH + glucose + sham (n=12). The results are presented as mean ± SEM. A significant group (difference among groups, p<0.005), day (groups improved over time, p<0.001) and day × group (treatment by time interaction, p<0.05) effects among the four experimental groups for total distance traveled was observed. The rate of learning (calculated from distance traveled) in the ITD + RH + sham group was significantly lower when compared to other two diabetic groups (ITD p<0.005 and ITD + RH + glucose + sham p<0.005).
Figure 6. The search strategy used during the Barnes maze test.
The groups include (1) naïve + sham (n=9), (2) ITD + sham (n=7), (3) ITD + RH + sham (n=14), (4) ITD + RH + glucose + sham (n=12). The results are presented as mean ± SEM. Post hoc analysis revealed that the percentage use of random search strategy was significantly more by ITD + RH + sham rats compared to ITD + sham (Chi-Square, p <0.05) and ITD + RH + glucose + sham rats (Chi-Square, p <0.05).
RH exposure does not affect spatial learning and memory in ITD rats subjected to cerebral ischemia
To examine whether increased CA1 hippocampal neuronal injury observed in RH-exposed ITD rats following cerebral ischemia correlated with spatial learning and memory deficit, we evaluated rats belonging to four experimental groups (naïve, ITD, ITD + RH, ITD + RH + glucose) with sham or ischemia surgeries using Barnes maze testing (naïve + sham: n = 9, naïve + ischemia: n = 8, ITD + sham: n = 7, ITD + ischemia: n = 5, ITD + RH + sham: n = 14, ITD + RH + ischemia: n = 8, ITD + RH + glucose + sham: n = 12, ITD + RH + glucose + ischemia: n = 7). We observed day (groups improved over time) effect (p<0.01 for distance and p<0.005 for latency),but did not observe group, and day × group effects among the four ischemia groups (naïve, ITD, ITD + RH, and ITD + RH + glucose) for these two parameters(Figure S3). The rate of learning (distance traveled and latency) in ITD + RH + ischemia group was not significantly different from naïve, ITD, and ITD + RH + glucose animals subjected to ischemia. The rates of learning (distance traveled) in ITD + ischemia (p<0.05) and ITD + RH + glucose + ischemia (p<0.05) groups were significantly impaired when compared to the respective sham groups (Figure S3B and S3D). We also analyzed the strategy used to find the escape box during the Barnes maze test (Figure S4). We did not observe any significant difference in frequency of random strategy use among the four ischemia groups. When we compared ischemia groups with their respective sham groups, we observed that ITD + ischemia animals used random strategy (48% of the time) more frequently compared to ITD + sham animals (25% of the time, p<0.05) (Figure S4). However, we did not observe any differences in use of random strategy among the other three sham groups when compared with their respective ischemia groups. These results indicate that prior RH exposure has no significant additive effect on spatial learning and memory in ITD rats subjected to cerebral ischemia.
Measurement of mitochondrial respiration rate in RH-exposed ITD rats subjected to cerebral ischemia
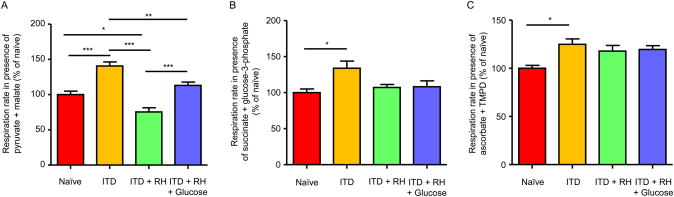
Hypoglycemia leads to an increase in mitochondrial ROS production and decrease in MMP (Kauppinen, et al., 1986; McGowan, et al., 2006; Dave, et al., 2011). This pre-existing mitochondrial dysfunction may further exacerbate cerebral ischemia-induced mitochondrial dysfunction. Thus, we next determined hippocampal mitochondrial function in our experimental conditions at twenty-four hours post sham/ischemia surgery. The oxygen consumption rate was measured in the presence of different pairs of substrates: pyruvate and malate, succinate and glycerol-3-phosphate, and ascorbate and N, N, N′, N′-tetramethyl-p-phenylenediamine (TMPD). We observed that the rate of oxygen consumption in the presence of pyruvate and malate was lower in the ITD + RH (n=7) ischemic group by 25% (p<0.05), 46% (p<0.001), and 33% (p<0.001) compared to the naïve (n=6), ITD (n=6), and ITD + RH + glucose (n=7) ischemic groups, respectively (Figure 7A). No statistically significant differences were observed between ITD + RH and the other three control groups when oxygen consumption was measured in the presence of succinate + glycerol-3-phosphate (Figure 7B) and ascorbate + TMPD (Figure 7C) as substrates. Our results demonstrate that prior exposure to RH in ITD rats following cerebral ischemia leads to decreased rate of oxidation in presence of pyruvate and malate as substrates.
Figure 7. Mitochondrial respiration rates in RH-exposed ITD rats subjected to cerebral ischemia.
Substrate oxidation rates in isolated mitochondria from hippocampus of (1) naïve + ischemia (n = 6), (2) ITD + ischemia (n = 6), (3) ITD + RH + ischemia (n = 7), and (4) ITD + RH + glucose + ischemia (n = 7) was measured in presence of different substrates (A) pyruvate + malate, (B) succinate + glycerol-3-phosphate and (C) ascorbate + tetramethyl-p-phenylenediamine (TMPD). The results are presented as mean ± SEM. *, p<0.05 ITD + RH + ischemia vs naïve; ***, p<0.001 ITD + RH + ischemia vs ITD and ITD + RH + ischemia vsITD + RH + glucose + ischemia.
Measurement of mitochondrial respiratory chain complex activity in RH-exposed ITD rats subjected to cerebral ischemia
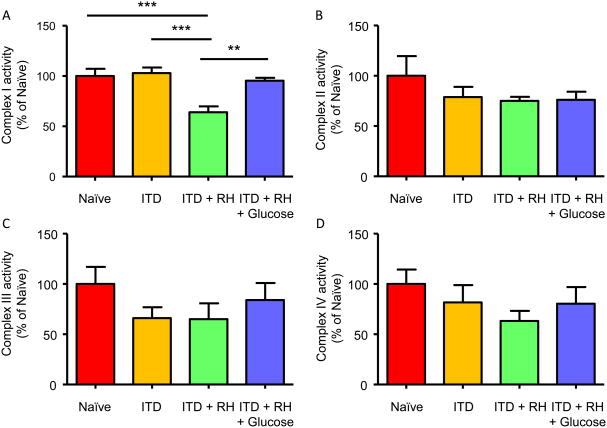
Because we observed a decreased rate of respiration in presence of pyruvate and malate as substrates, next we measured activities of complexes I, II, III, and IV of the mitochondrial electron transport chain (ETC). Similar to the decreased rate of substrate oxidation in ITD + RH + ischemia group, complex I activity was also lower in the ITD + RH + ischemia group (n=7) by 36% (p<0.001), 38% (p<0.001), and 33% (p<0.01) compared to the naïve (n=6), ITD (n=6), and ITD + RH + glucose (n=7) ischemia groups, respectively (Figure 8A). No significant difference in complex II, III and IV activity was observed in the ITD + RH + ischemia group when compared to thenaïve, ITD and ITD + RH + glucose ischemia groups, respectively (Figure 8B-D). Our results demonstrate that prior exposure to RH in ITD rats results in pronounced impairment in mitochondrial complex I activity following cerebral ischemia.
Figure 8. Mitochondrial complex (I, II, III and IV) activities in RH-exposed ITD rats subjected to cerebral ischemia.
(A) Complex I, (B) Complex II, (C) Complex III, and (D) Complex IV activities were measured in mitochondria isolated from hippocampus of (1) naïve + ischemia (n = 6), (2) ITD + ischemia (n = 6), (3) ITD + RH + ischemia (n = 7), and (4) ITD + RH + glucose + ischemia (n = 7). The results are presented as mean ± SEM. **, p<0.01 ITD + RH + ischemia vsITD + RH + glucose + ischemia; ***, p<0.001 ITD + RH + ischemia vs naïve + ischemia and ITD + ischemia.
Discussion
Stroke is one of the leading causes of death and physical disability worldwide and diabetes is a recognized risk factor for ischemic stroke (Kissela et al., 2006). Diabetes increases the risk of stroke and also enhances brain damage caused by cerebral ischemia (Unachukwu et al., 2012; Jing et al., 2013; Jing et al., 2014; Liu et al., 2016). Insulin-induced hypoglycemia is the most frequent side effect of insulin-therapy in diabetes (McCrimmon et al., 1994; Cryer, 2004). Although studies demonstrate that diabetics frequently experience mild/moderate hypoglycemia, the effect of mild/moderate hypoglycemia on brain is not well explored. In the present, as well as in an earlier study, we demonstrated that prior exposure of ITD rats to RH increases cerebral ischemic damage. We also observed that the rate of learning in RH-exposed ITD rats was significantly lower when compared to other two diabetic groups and pronounced ischemia-induced impairment in mitochondrial complex I activity was observed in ITD rats exposed to RH.
It is evident that oxidative stress and mitochondrial dysfunction play an important role in tissue damage associated with cerebral ischemia. Mitochondria undergo morphological and functional changes which contribute to oxidative stress and cell death following cerebral ischemia (Canevari, et al., 1997; Zaidan, et al., 1997; Murakami et al., 1998; Anderson et al., 1999; Sims et al., 2002). Increased ROS production disrupts antioxidant defense and directly impairs mitochondrial homeostasis and energy production. Deficits of mitochondrial energy metabolism is the most immediate cause of mitochondrial damage and dysfunction in cerebral ischemia (Canevari, et al., 1997; Zaidan, et al., 1997). Mitochondrial dysfunction is mainly manifested as decreased mitochondrial ATP synthesis, increased ROS production, dysregulation of intracellular lipid homeostasis, loss of the MMP, and induction of the MPTP (Piantadosi et al., 1996; Feng et al., 1998; Iijima et al., 2003). Further, mitochondrial oxidative stress prevents metabolic recovery and promotes apoptosis (Myers et al., 1995; Liu et al., 1998). Hence, the sequence of post-ischemic events that damage mitochondria and lead to mitochondrial dysfunction, in turn, participate in cerebral ischemic damage. Thus we tested the hypothesis that increased extent of mitochondrial dysfunction plays a role in exacerbating cerebral ischemic damage in RH-exposed ITD rats.
In the present study, we observed that prior exposure of ITD rats to RH leads to more robust decrease in the rate of oxidation in the presence of pyruvate and malate as substrate. We also observed lower complex I activity in the ITD + RH group compared to the naïve, ITD and ITD + RH + glucose groups. Mitochondrial Complex I is the first and largest protein complex in the mitochondrial ETC, which has an essential role in maintaining mitochondrial function and integrity (Giachin et al., 2016). Prior studies observed that tumor necrosis factor-α (TNF-α) adversely affects mitochondria. Using an in vitro model, Higuchi et al demonstrated that mitochondrial ETC complex I is inhibited following TNF-α treatment during the early phase leading to apoptosis via the mitochondrial apoptotic pathway (Higuchi et al., 1998). Recently, Doll et al., observed rapid and profound mitochondrial dysfunction when HT22 hippocampal cells and mouse primary cortical neurons were exposed to TNF-α for a short duration (90 min) (Doll et al., 2015). TNF-α induces significant mitochondrial dysfunction and activation of mitochondrial apoptotic responses (Baregamian et al., 2009; Doll, et al., 2015). The increased level of TNF-α is associated with worsened clinical outcomes after stroke and exacerbation of infarct size in pre-clinical models (Nawashiro et al., 1997; Ormstad et al., 2011). Moreover, intracerebroventricular administration of exogenous TNF-α significantly expands infarct volume (Barone et al., 1997). In an earlier study we observed a robust increase in TNF-α level in RH- exposed ITD rats compared to naïve and ITD rats at 24 h post-cerebral ischemia (Shukla et al., 2015). It is possible that increased post-ischemic TNF-α levels in hippocampus of RH- exposed ITD rats may be responsible for observed lower complex I activity.
Studies have reported that mitochondria are the major source of cellular ROS involved in TNF-α-induced cell death/apoptosis (Schulze-Osthoff et al., 1993; Goossens et al., 1995; Shoji et al., 1995; Goossens et al., 1999; Corda et al., 2001). Treatment of cells with TNF-α alters mitochondrial membrane permeability, inhibits respiratory chain complex I, induces mitochondrial swelling and clustering, and leads to cytochrome c release. Release of cytochrome c activates caspases leading to cell death (Schulze-Osthoff, et al., 1993; Goossens, et al., 1995; Pastorino et al., 1996; Higuchi, et al., 1998; Rath et al., 1999). Mitochondrial respiratory chain complex I inhibitor (i. e., rotenone)-induced apoptosis has been widely studied (Barrientos et al., 1999; Isenberg et al., 2000; Chauvin et al., 2001). Complex I inhibition results in enhanced ROS production and depletion of ATP in cells, membrane depolarization and mitochondrial permeability transition pore opening, all of which contribute to apoptosis (Barrientos, et al., 1999). Free radical overload results in damage of protein, DNA and membrane phospholipids (Guo et al., 2013). Our results indicate that severe inhibition of complex I activity may be, in part, responsible for exacerbating cerebral ischemic damage in ITD rats exposed to RH. However, our results warrant confirmatory studies to establish the role of complex I inhibition on increased cerebral ischemic damage in our experimental conditions.
Mitochondrial respiratory chain complex I catalyzes NADH oxidation and regenerates NAD+. The role of oxidative stress and altered NAD+ metabolism has been established in ischemic brain injury. We observed that the complex I activity was significantly reduced in ITD + RH rats following cerebral ischemia. It has been found that ischemia/reperfusion can induce significant decreases in NAD+ levels in the brain (Endres et al., 1997), and NAD+ administration can significantly decrease ischemic brain injury in an animal model of cerebral ischemia (Ying et al., 2007). It is plausible that the reduced ability of complex I to oxidize NADH to NAD+ in the ITD + RH group contributed to the exacerbation of cerebral ischemic damage.
We further investigated whether RH exposure, by itself or with cerebral ischemia, has any effect on memory and cognitive impairment. We observed that RH exposure in ITD rats (sham surgery group) leads to significant spatial learning and memory impairment compared to other two sham-operated diabetic groups (ITD and ITD + RH + glucose). Our findings are corroborated by an earlier study where diabetic rats exposed to recurrent moderate hypoglycemia showed significant cognitive impairment compared to non-diabetic rats (Won, et al., 2012). Similarly, recently McNeilly and colleagues also reported that exposure of streptozotocin-diabetic mice to moderate RH results in impaired cognitive function when assessed using novel object recognition and spontaneous alteration tests (McNeilly et al., 2016). Considering very few studies investigated the effect of brain with respect to its effect on cognition, the mechanism behind is not well understood. Recurrent moderate hypoglycemia is observed to impair hippocampal synaptic plasticity, suggesting its potential link to observed memory and cognitive deficits as hippocampal long-term potentiation is involved in memory and learning (Yamada, et al., 2004). Uncontrolled blood glucose levels (hyper- or hypoglycemia)also affect brain structure and function, potentiating cognitive impairment (Cardoso et al., 2013). Severe hypoglycemia induces hippocampal neuronal death and impairs learning and memory in rodents (Cardoso, et al., 2013) and in patients with diabetes (Rovet et al., 1997; Hershey et al., 2005). Recurrent moderate hypoglycemia causes oxidative injury in hippocampal dendrites (Won, et al., 2012). Repetitive episodes of moderate hypoglycemia induce synaptic injury in the hippocampus, and consequently may contribute to the development of cognitive impairment (Won, et al., 2012; Choi et al., 2013). An earlier study observed that exposure of diabetic rats to RH potentiated an increase in lipid peroxidation and a decrease in aconitase activity (both markers of oxidative damage) in hippocampal mitochondria (Cardoso, et al., 2013). Conversely, RH improved cognitive ability and preserved basic brain functions in diabetic and non-diabetic rats tested in a euglycemic state (McNay et al., 2004; McNay, et al., 2006). However, under hypoglycemia the animals performed worse compared to control animals. Severe hypoglycemia is observed to induce brain damage and related deficits in spatial learning and memory (Puente et al., 2010). Our results thus confirm previous reports that prior exposure to hypoglycemia results in impaired cognitive function.
Post-stroke cognitive decline is considered a major contributor to post-stroke disability (Brainin et al., 2015; Levine et al., 2015). An earlier prospective clinical study observed that stroke survivors not only suffer from acute cognitive deficits but also experience accelerated and persistent cognitive decline (Levine, et al., 2015). Similarly, cognitive impairments are also observed in survivors of cardiac arrest (Moulaert et al., 2009; Perez et al., 2016). Post-stroke cognitive impairments are also evident in animal models of cerebral ischemia (Okada et al., 1995; Hodges et al., 1997; Kiprianova et al., 1999; Hattori et al., 2000). However, we did not observe an effect of cerebral ischemia on cognitive function evaluated by means of a Barnes circular platform maze. It should be noted that since we anticipated increased ischemic damage in the RH-exposed group, we chose a relatively short duration of global cerebral ischemia (8 min). It is possible that we did not observe a sufficient impact of cerebral ischemia on cognitive function from such a mild ischemic insult. An earlier study that used 30 min (relatively short ischemia duration) of transient middle cerebral artery occlusion in mice also did not observe any impact on cognitive function when that was evaluated using a Morris water maze (Doll et al., 2015). We also did not observe an adverse effect of ischemia on cognitive function in RH-exposed rats compared to sham controls. It is plausible that the effect of ischemia on cognitive function in RH-exposed rats is masked by the effect of RH itself on cognitive function. Further studies using relatively sensitive test to evaluate cognitive deficits may help evaluate post-ischemia cognitive deficits in our experimental conditions.
Inclusion of only male rats is a limitation of our studies. Earlier studies established that response to hypoglycemia is different in normal and diabetic women compared to the respective male population. Hormonal changes in response to hypoglycemia (epinephrine, norepinephrine, and growth hormone) and endogenous glucose production response are lower in T1D women than in T1D men, and in healthy women compared with healthy men (Davis et al., 2000). Another study demonstrated that women are less susceptible to the blunting of neuroendocrine counterregulatory responses following repeated exposure to hypoglycemia, compared to men (Davis et al., 2000). Differential hormonal responses to hypoglycemia in men and women diabetic subjects warrants studies to determine the effect of prior hypoglycemia exposure on ischemic outcomes in female diabetic animals.
Overall, our findings suggest that prior RH exposure in ITD rats increases ischemic damage potentially via inhibition of mitochondrial complex I. The mechanism by which RH exposure inhibits post-ischemic mitochondrial complex I inhibition remains to be defined. Understanding the mechanism by which RH exposure increases cerebral ischemic injury may ultimately help lower cerebral ischemic damage in diabetics.
Supplementary Material
Acknowledgments
We would like to thank Dr. Brant Watson for critical reading of this manuscript.
Funding: The present study is supported by NIH grant NS073779 and the Evelyn F. McKnight Brain Institute. The funding agency had no role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Abbreviations
- CA
cornus ammonis
- ETC
electron transport chain
- ITD
insulin-treated diabetic
- MMP
mitochondrial membrane potential
- MPTP
mitochondrial permeability transition pores
- RH
recurrent hypoglycemia
- ROS
reactive oxygen species
- STZ
streptozotocin
- s.c.
subcutaneous
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TNF-α
tumor necrosis factorα
Footnotes
Compliance with Ethical Standards: Ethical approval: All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by an institutional animal care and use committee.
Conflict of Interest: The authors declare that they have no competing interests.
References
- Amador-Alvarado L, Montiel T, Massieu L. Differential production of reactive oxygen species in distinct brain regions of hypoglycemic mice. Metab Brain Dis. 2014;29:711–719. doi: 10.1007/s11011-014-9508-5. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MF, Sims NR. Mitochondrial respiratory function and cell death in focal cerebral ischemia. J Neurochem. 1999;73:1189–1199. doi: 10.1046/j.1471-4159.1999.0731189.x. [DOI] [PubMed] [Google Scholar]
- Baregamian N, Song J, Bailey CE, Papaconstantinou J, Evers BM, Chung DH. Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid Med Cell Longev. 2009;2:297–306. doi: 10.4161/oxim.2.5.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- Beckman J, Libby P, Creager M. Diabetes mellitus, the metabolic syndrome, and atherosclerotic vascular disease. 2008 [Google Scholar]
- Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Morkuniene R, Brown GC. Release of cytochrome c from heart mitochondria is induced by high Ca2+ and peroxynitrite and is responsible for Ca(2+)-induced inhibition of substrate oxidation. Biochim Biophys Acta. 1999;1453:41–48. doi: 10.1016/s0925-4439(98)00082-9. [DOI] [PubMed] [Google Scholar]
- Brainin M, Tuomilehto J, Heiss WD, Bornstein NM, Bath PM, Teuschl Y, Richard E, Guekht A, Quinn T Post Stroke Cognition Study, G. Post-stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol. 2015;22:229–238. e213–226. doi: 10.1111/ene.12626. [DOI] [PubMed] [Google Scholar]
- Canevari L, Kuroda S, Bates TE, Clark JB, Siesjo BK. Activity of mitochondrial respiratory chain enzymes after transient focal ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:1166–1169. doi: 10.1097/00004647-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Cardoso S, Correia SC, Santos RX, Carvalho C, Candeias E, Duarte AI, Placido AI, Santos MS, Moreira PI. Hyperglycemia, hypoglycemia and dementia: role of mitochondria and uncoupling proteins. Curr Mol Med. 2013;13:586–601. doi: 10.2174/1566524011313040010. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. 2016 http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (Retrieved on September 25th, 2016)
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chauvin C, De Oliveira F, Ronot X, Mousseau M, Leverve X, Fontaine E. Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. J Biol Chem. 2001;276:41394–41398. doi: 10.1074/jbc.M106417200. [DOI] [PubMed] [Google Scholar]
- Choi BY, Kim JH, Kim HJ, Yoo JH, Song HK, Sohn M, Won SJ, Suh SW. Pyruvate administration reduces recurrent/moderate hypoglycemia-induced cortical neuron death in diabetic rats. PLoS One. 2013;8:e81523. doi: 10.1371/journal.pone.0081523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan CH, Neumann JT, Dave KR, Alekseyenko A, Binkert M, Stransky K, Lin HW, Barnes CA, Wright CB, Perez-Pinzon MA. Effect of cardiac arrest on cognitive impairment and hippocampal plasticity in middle-aged rats. PLoS One. 2015;10:e0124918. doi: 10.1371/journal.pone.0124918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab. 2001;281:E1115–1121. doi: 10.1152/ajpendo.2001.281.6.E1115. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- Dave KR, Lange-Asschenfeldt C, Raval AP, Prado R, Busto R, Saul I, Perez-Pinzon MA. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005;82:665–673. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Perez-Pinzon MA. Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab. 2001;21:1401–1410. doi: 10.1097/00004647-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, Saul I, Bhattacharya SK, Pileggi A. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke. 2011;42:1404–1411. doi: 10.1161/STROKEAHA.110.594937. [DOI] [PubMed] [Google Scholar]
- Davis EA, Jones TW. Hypoglycemia in children with diabetes: incidence, counterregulation and cognitive dysfunction. J Pediatr Endocrinol Metab. 1998;11:177–182. doi: 10.1515/jpem.1998.11.s1.177. [DOI] [PubMed] [Google Scholar]
- Davis SN, Fowler S, Costa F. Hypoglycemic counterregulatory responses differ between men and women with type 1 diabetes. Diabetes. 2000;49:65–72. doi: 10.2337/diabetes.49.1.65. [DOI] [PubMed] [Google Scholar]
- Davis SN, Shavers C, Costa F. Gender-related differences in counterregulatory responses to antecedent hypoglycemia in normal humans. J Clin Endocrinol Metab. 2000;85:2148–2157. doi: 10.1210/jcem.85.6.6641. [DOI] [PubMed] [Google Scholar]
- DCCT research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Doll DN, Engler-Chiurazzi EB, Lewis SE, Hu H, Kerr AE, Ren X, Simpkins JW. Lipopolysaccharide exacerbates infarct size and results in worsened post-stroke behavioral outcomes. Behav Brain Funct. 2015;11:32. doi: 10.1186/s12993-015-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll DN, Rellick SL, Barr TL, Ren X, Simpkins JW. Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J Neurochem. 2015;132:443–451. doi: 10.1111/jnc.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, Band MM, Reekie G, Leese GP. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- EDIC group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Feng ZC, Sick TJ, Rosenthal M. Oxygen sensitivity of mitochondrial redox status and evoked potential recovery early during reperfusion in post-ischemic rat brain. Resuscitation. 1998;37:33–41. doi: 10.1016/s0300-9572(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2008;25:501–504. doi: 10.1111/j.1464-5491.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in Type 2 Diabetes--More Common Than You Think: A Continuous Glucose Monitoring Study. J Diabetes Sci Technol. 2015;9:999–1005. doi: 10.1177/1932296815581052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachin G, Bouverot R, Acajjaoui S, Pantalone S, Soler-Lopez M. Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front Mol Biosci. 2016;3:43. doi: 10.3389/fmolb.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens V, Stange G, Moens K, Pipeleers D, Grooten J. Regulation of tumor necrosis factor-induced, mitochondria- and reactive oxygen species-dependent cell death by the electron flux through the electron transport chain complex I. Antioxid Redox Signal. 1999;1:285–295. doi: 10.1089/ars.1999.1.3-285. [DOI] [PubMed] [Google Scholar]
- Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology. 2008;149:1499–1504. doi: 10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Proske RJ, Yeh ET. Inhibition of mitochondrial respiratory chain complex I by TNF results in cytochrome c release, membrane permeability transition, and apoptosis. Oncogene. 1998;17:2515–2524. doi: 10.1038/sj.onc.1202485. [DOI] [PubMed] [Google Scholar]
- Hodges H, Nelson A, Virley D, Kershaw TR, Sinden JD. Cognitive deficits induced by global cerebral ischaemia: prospects for transplant therapy. Pharmacol Biochem Behav. 1997;56:763–780. doi: 10.1016/s0091-3057(96)00424-8. [DOI] [PubMed] [Google Scholar]
- Iijima T. Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res. 2006;55:234–243. doi: 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Iijima T, Mishima T, Tohyama M, Akagawa K, Iwao Y. Mitochondrial membrane potential and intracellular ATP content after transient experimental ischemia in the cultured hippocampal neuron. Neurochem Int. 2003;43:263–269. doi: 10.1016/s0197-0186(02)00228-0. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. IDF diabetes atlas. 2015 [Google Scholar]
- Isaev NK, Stelmashook EV, Dirnagl U, Plotnikov EY, Kuvshinova EA, Zorov DB. Mitochondrial free radical production induced by glucose deprivation in cerebellar granule neurons. Biochemistry (Mosc) 2008;73:149–155. doi: 10.1134/s0006297908020053. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Klaunig JE. Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol Sci. 2000;53:340–351. doi: 10.1093/toxsci/53.2.340. [DOI] [PubMed] [Google Scholar]
- Janssen MM, Snoek FJ, de Jongh RT, Casteleijn S, Deville W, Heine RJ. Biological and behavioural determinants of the frequency of mild, biochemical hypoglycaemia in patients with Type 1 diabetes on multiple insulin injection therapy. Diabetes Metab Res Rev. 2000;16:157–163. doi: 10.1002/1520-7560(0000)9999:9999<::aid-dmrr104>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Jing L, Mai L, Zhang JZ, Wang JG, Chang Y, Dong JD, Guo FY, Li PA. Diabetes inhibits cerebral ischemia-induced astrocyte activation - an observation in the cingulate cortex. Int J Biol Sci. 2013;9:980–988. doi: 10.7150/ijbs.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Wang JG, Zhang JZ, Cao CX, Chang Y, Dong JD, Guo FY, Li PA. Upregulation of ICAM-1 in diabetic rats after transient forebrain ischemia and reperfusion injury. J Inflamm (Lond) 2014;11:35. doi: 10.1186/s12950-014-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kiprianova I, Sandkuhler J, Schwab S, Hoyer S, Spranger M. Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol. 1999;159:511–519. doi: 10.1006/exnr.1999.7109. [DOI] [PubMed] [Google Scholar]
- Kissela B, Air E. Diabetes: impact on stroke risk and poststroke recovery. Semin Neurol. 2006;26:100–107. doi: 10.1055/s-2006-933313. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE, Fiskum G. Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ. 2000;7:903–910. doi: 10.1038/sj.cdd.4400722. [DOI] [PubMed] [Google Scholar]
- Lee C, Sciamanna M, Peterson P. Intact rat brain mitochondria from a single animal: preparation and properties. Methods Toxicol. 1993;2:41–50. [Google Scholar]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of Cognitive Decline After Incident Stroke. JAMA. 2015;314:41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln NB, Faleiro RM, Kelly C, Kirk BA, Jeffcoate WJ. Effect of long-term glycemic control on cognitive function. Diabetes Care. 1996;19:656–658. doi: 10.2337/diacare.19.6.656. [DOI] [PubMed] [Google Scholar]
- Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, Li PA. Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int J Biol Sci. 2016;12:1032–1040. doi: 10.7150/ijbs.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Frier BM. Hypoglycaemia, the most feared complication of insulin therapy. Diabete Metab. 1994;20:503–512. [PubMed] [Google Scholar]
- McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. 2006;399:111–114. doi: 10.1016/j.neulet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- McNally PG, Dean JD, Morris AD, Wilkinson PD, Compion G, Heller SR. Using continuous glucose monitoring to measure the frequency of low glucose values when using biphasic insulin aspart 30 compared with biphasic human insulin 30: a double-blind crossover study in individuals with type 2 diabetes. Diabetes Care. 2007;30:1044–1048. doi: 10.2337/dc06-1328. [DOI] [PubMed] [Google Scholar]
- McNay E. Recurrent Hypoglycemia Increases Anxiety and Amygdala Norepinephrine Release During Subsequent Hypoglycemia. Front Endocrinol (Lausanne) 2015;6:175. doi: 10.3389/fendo.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–1095. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- McNeilly AD, Gallagher JR, Dinkova-Kostova AT, Hayes JD, Sharkey J, Ashford ML, McCrimmon RJ. Nrf2-Mediated Neuroprotection Against Recurrent Hypoglycemia Is Insufficient to Prevent Cognitive Impairment in a Rodent Model of Type 1 Diabetes. Diabetes. 2016;65:3151–3160. doi: 10.2337/db15-1653. [DOI] [PubMed] [Google Scholar]
- Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39:1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80:297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Fiskum G, Liu Y, Simmens SJ, Bredesen DE, Murphy AN. Bcl-2 protects neural cells from cyanide/aglycemia-induced lipid oxidation, mitochondrial injury, and loss of viability. J Neurochem. 1995;65:2432–2440. doi: 10.1046/j.1471-4159.1995.65062432.x. [DOI] [PubMed] [Google Scholar]
- Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, Ten VS. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J Neurosci. 2012;32:3235–3244. doi: 10.1523/JNEUROSCI.6303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Okada M, Tamura A, Urae A, Nakagomi T, Kirino T, Mine K, Fujiwara M. Long-term spatial cognitive impairment following middle cerebral artery occlusion in rats. A behavioral study. J Cereb Blood Flow Metab. 1995;15:505–512. doi: 10.1038/jcbfm.1995.62. [DOI] [PubMed] [Google Scholar]
- Ormstad H, Aass HC, Amthor KF, Lund-Sorensen N, Sandvik L. Serum cytokine and glucose levels as predictors of poststroke fatigue in acute ischemic stroke patients. J Neurol. 2011;258:670–676. doi: 10.1007/s00415-011-5962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–29798. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- Patockova J, Marhol P, Tumova E, Krsiak M, Rokyta R, Stipek S, Crkovska J, Andel M. Oxidative stress in the brain tissue of laboratory mice with acute post insulin hypoglycemia. Physiol Res. 2003;52:131–135. [PubMed] [Google Scholar]
- Pedersen-Bjergaard U, Pramming S, Heller SR, Wallace TM, Rasmussen AK, Jorgensen HV, Matthews DR, Hougaard P, Thorsteinsson B. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20:479–486. doi: 10.1002/dmrr.482. [DOI] [PubMed] [Google Scholar]
- Perez CA, Samudra N, Aiyagari V. Cognitive and Functional Consequence of Cardiac Arrest. Curr Neurol Neurosci Rep. 2016;16:70. doi: 10.1007/s11910-016-0669-y. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Puente EC, Silverstein J, Bree AJ, Musikantow DR, Wozniak DF, Maloney S, Daphna-Iken D, Fisher SJ. Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia. Diabetes. 2010;59:1055–1062. doi: 10.2337/db09-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- Rovet J, Alvarez M. Attentional functioning in children and adolescents with IDDM. Diabetes Care. 1997;20:803–810. doi: 10.2337/diacare.20.5.803. [DOI] [PubMed] [Google Scholar]
- Schopman JE, Geddes J, Frier BM. Frequency of symptomatic and asymptomatic hypoglycaemia in Type 1 diabetes: effect of impaired awareness of hypoglycaemia. Diabet Med. 2011;28:352–355. doi: 10.1111/j.1464-5491.2010.03203.x. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee G, Mohajeri-Tehrani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11:17. doi: 10.1186/2251-6581-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y, Uedono Y, Ishikura H, Takeyama N, Tanaka T. DNA damage induced by tumour necrosis factor-alpha in L929 cells is mediated by mitochondrial oxygen radical formation. Immunology. 1995;84:543–548. [PMC free article] [PubMed] [Google Scholar]
- Shukla V, Rehni A, Dave K. Dysregulated cytokine released by activated microglia in the hippocampus of diabetes associated recurrent hypoglycemic rat brain exacerbate ischemic damage. Society for Neuroscience; 45th Annual Meeting; Chicago. 2015; p. 419. [Google Scholar]
- Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- Singh P, Jain A, Kaur G. Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem. 2004;260:153–159. doi: 10.1023/b:mcbi.0000026067.08356.13. [DOI] [PubMed] [Google Scholar]
- Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- Unachukwu C, Ofori S. Diabetes Mellitus And Cardiovascular Risk. The Internet Journal of Endocrinology. 2012;7:1–10. [Google Scholar]
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with Type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes. 2007;115:491–494. doi: 10.1055/s-2007-984452. [DOI] [PubMed] [Google Scholar]
- Won SJ, Yoo BH, Kauppinen TM, Choi BY, Kim JH, Jang BG, Lee MW, Sohn M, Liu J, Swanson RA, Suh SW. Recurrent/moderate hypoglycemia induces hippocampal dendritic injury, microglial activation, and cognitive impairment in diabetic rats. J Neuroinflammation. 2012;9:182. doi: 10.1186/1742-2094-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, Herrera DG. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res. 2004;55:372–379. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]
- Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA. Interventions That Restore Awareness of Hypoglycemia in Adults With Type 1 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2015;38:1592–1609. doi: 10.2337/dc15-0102. [DOI] [PubMed] [Google Scholar]
- Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, Zhang P, Lu H. Intranasal administration with NAD + profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- Zaidan E, Sims NR. Reduced activity of the pyruvate dehydrogenase complex but not cytochrome c oxidase is associated with neuronal loss in the striatum following short-term forebrain ischemia. Brain Res. 1997;772:23–28. doi: 10.1016/s0006-8993(97)00833-0. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.