Abstract
Nitro-oleic acid (NO2-OA) and related nitroalkenes are electrophilic fatty acid derivatives that are present in normal tissues at nanomolar concentrations and can increase significantly during inflammation. These substances can suppress multiple intracellular signaling pathways contributing to inflammation by reversible Michael addition reactions with nucleophilic residues such as cysteine and histidine leading to post-translational modification of proteins. NO2-OA also can influence inflammation and pain by acting on transient receptor potential (TRP) channels in primary sensory neurons. TRPV1, TRPA1 and TRPC can respond to electrophilic fatty acids because they have ankyrin-like repeats in their N terminus that are rich in cysteine residues that react with electrophiles and other thiol modifying species. NO2-OA acts on TRP channels to initially depolarize and induce firing in sensory neurons followed by desensitization and suppression of firing. In vivo experiments revealed that pretreatment with NO2-OA reduces nociceptive behavior evoked by local administration of a TRPA1 agonist (AITC) to the rat hind paw. These results raise the possibility that NO2-OA might be useful clinically to reduce neurogenic inflammation and certain types of painful sensations by desensitizing TRPA1 expressing nociceptive afferents.
Keywords: Transient Receptor Potential Vanilloid 1 (TRPV1), Transient Receptor Potential Ankyrin 1 (TRPA1), Transient Receptor Potential Canonical (TRPC), Nitro-oleic acid, afferent nerves, Electrophilic fatty acids, Dorsal root ganglia (DRG), Capsaicin, Allyl isothiocyanate (AITC), Urinary bladder
1. Introduction
Nitro-oleic acid (NO2-OA) and related nitroalkenes are electrophilic fatty acid derivatives formed by nitric oxide- or nitrite-mediated redox reactions. These species are present in normal tissues at nanomolar concentrations and can increase significantly during injury or inflammation [1–3]. Fatty acid nitroalkenes induce a variety of pharmacological effects including: (1) activation of peroxisome proliferator-activated receptor γ (PPARγ) [2], (2) activation of the Keap1-Nrf2 pathway [4,5], (3) upregulation of heme oxygenase 1 (HO-1) expression [6], (4) inhibition of NF-κB-dependent gene expression [7,8], (5) inhibition of platelet or neutrophil function [9,10] and (6) inhibition of proinflammatory cytokine secretion by macrophages [7]. These actions can all be ascribed to the post-translational modification of functionally significant proteins by the reversible Michael addition reactions that target nucleophilic residues such as cysteine and histidine [1,7,11–15].
In addition to actions on intracellular signaling pathways, NO2-OA also activates transient receptor potential (TRP) channels in primary sensory neurons [16–18]. These channels are members of a large superfamily of six transmembrane domain cation channels which produce membrane depolarization and firing of sensory neurons when activated. In mammals, this superfamily is divided into 6 subfamilies (TRPA, TRPC, TRPM, TRPML, TRPP, TRPV), with each sub-family consisting of a varying number of members (see partial list in Table 1) [19–21]. TRP channels respond to chemical and mechanical stimuli and detect changes in temperature, pH and osmotic pressure [20,22].
Table 1.
Partial list of known TRP channels in vertebrates, their activators and known distribution. This list is updated from tables previously published by Venkatachalam and Montell (2011) [21] and is not comprehensive due to space constraints.
| Subfamily | Members | Possible Activators | Tissue Expression |
|---|---|---|---|
| TRPA | TRPA1 | Cold temperature, icilin, isothiocyanates, allicin, DAG, PUFAs, bradykinin, cinnamaldehyde, acrolein, cannabinoids | DRG, hair cells, ovary, spleen, testis |
| TRPC | TRPC1 | Ca2+ store depletion, conformational coupling, mechanical stretch | Heart, brain, testis, ovary, liver, spleen |
| TRPC2 | DAG | Vomeronasal organ, testis | |
| TRPC3 | Ca2+ store depletion, conformational coupling, DAG | Brain | |
| TRPC4 | Ca2+ store depletion | Brain, endothelia, testis, adrenal grand, retina | |
| TRPC5 | Ca2+ store depletion, sphingosine-1-phosphate | Brain | |
| TRPC6 | Conformational coupling, DAG, PIP3 | Lung, brain, placenta, ovary | |
| TRPC7 | Ca2+ store depletion, DAG | Eye, heart, lung | |
| TRPV | TRPV1 | Heat, vanilloids, anandamide, camphor, piperine, allicin, ethanol, pH, PIP2, phosphorylation, nitrosylation, resiniferanoids | TG, DRG, neurons, urinary bladder, testis |
| TRPV2 | Heat, hypoosmotic solution | DRG, spinal cord, brain, spleen, intestine | |
| TRPV3 | Warm temperature, PUFAs, menthol, monoterpenes from oregano, cloves, thyme | DRG, spinal cord, brain, keratinocytes, TG | |
| TRPV4 | Warm temperature, hypoosmotic solution, Epoxyeicosatrienoic acid | DRG, kidney, lung, spleen, testis, heart, liver | |
| TRPV5 | Low extracellular Ca2+ | Kidney, intestine, pancreas, placenta | |
| TRPV6 | Ca2+ store depletion | Small intestine, pancreas, placenta |
Abbreviations: DRG: dorsal root ganglion, TG, Trigeminal ganglion, DAG: diacylglycerol, PUFAs: poly unsaturated fatty acids, PIP2: Phosphatidylinositol 4,5-bisphosphate, PIP3: Phosphatidylinositol (3,4,5)-trisphosphate, NAD: Nicotinamide adenine dinucleotide, EGF: Epidermal growth factor.
The sensitivity of TRP channels to natural products facilitated the isolation and chemical characterization of these channels. For example, TRPV1 is activated by vanilloids (Table 1) such as capsaicin, the pungent ingredient in hot pepper, which induces a burning sensation when applied to the skin or ingested with food. It was later discovered that TRPV1 expressed in nociceptive sensory neurons is responsible the burning sensation induced by high temperatures [20]. TRPM8, which is activated by the cooling substance menthol, responds to moderate cold temperatures [23], while TRPA1 responds to extreme cold temperatures which induce pain [24]. The role of various TRP channels in sensory mechanisms including nociception has raised the possibility that drugs might be developed to selectively target these channels to treat pain and other pathological conditions.
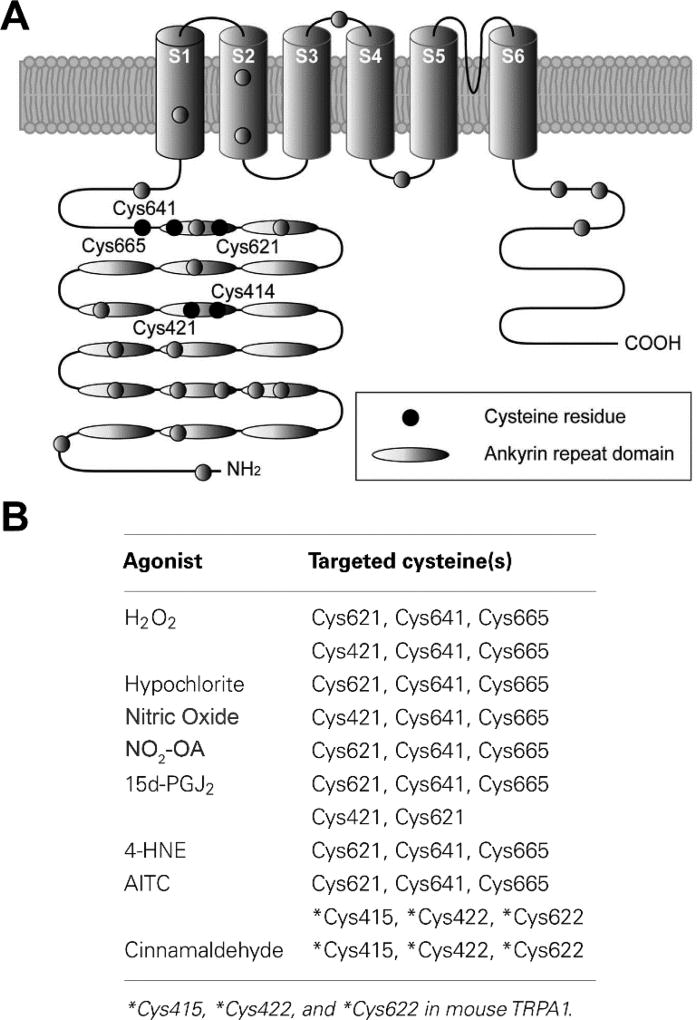
Several members of the TRP family, including TRPV1, TRPA1 and TRPC, have ankyrin-like repeats in the N terminus that are rich in cysteine residues (Fig. 1A) [23,25] that react with electrophiles and other thiol modifying species (Fig. 1B) [23–29] and therefore respond to electrophilic fatty acids. This paper will review the effects of NO2-OA on TRP channels in sensory neurons and the possible role of electrophilic fatty acids in the modulation of nociceptive afferent pathways.
Figure 1.
(A) Structure of the TRPA1 channel highlighting the N-terminus enriched with ankyrin repeats (indicated by ovals) and nucleophilic cysteine residues (filled circles) identified as crucial sites for covalent modification of TRPA1. (B) Table of cysteine residues on TRPA1 channels that have been shown to be covalently modified through redox chemistry with the listed agonists. This figure and table are modified from Takahashi and Mori [47].
2. Effect of 9-NO2-OA on human TRPA1 channels and TRPA1 channels in mouse vagal and trigeminal sensory neurons
HEK293 cells stably transfected with human TRPA1 respond to low concentrations (EC50, 1 uM) of 9-NO2-OA in calcium imaging assays, while cells transfected with human TRPV1 do not respond [16]. The potency of 9-NO2-OA is 10-fold greater than that of a TRPA1 agonist (allyl isothiocyanate, AITC). Human TRPA1 in which three cysteines (Cys619,639,663) are mutated to serine and Lys708 is mutated to glutamine do not respond to 9-NO2-OA [17], indicating that interaction with these nucleophilic residues is necessary for activation of the channel. The effects of 9-NO2-OA are not mimicked by oleic acid in high concentrations (5 mM) and are not blocked by the nitric oxide (NO) scavenger cPTIO, indicating that the parent compound is the active agent [17].
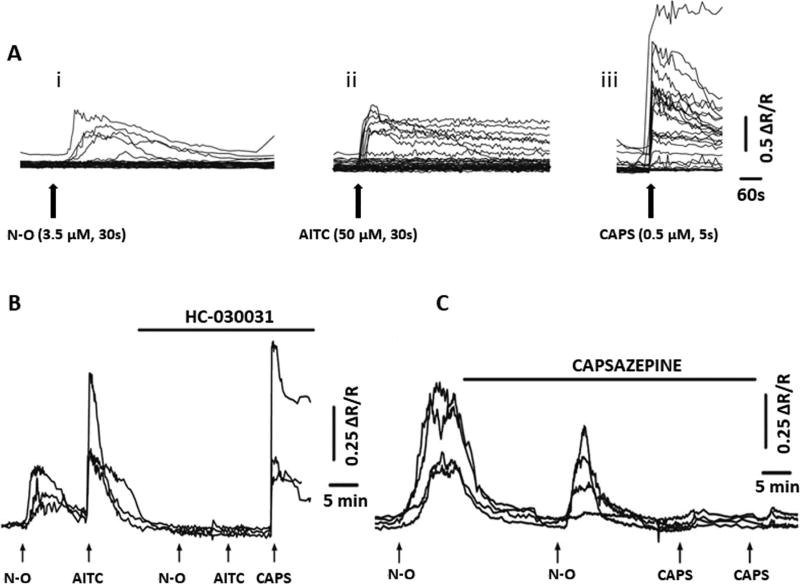
Calcium imaging and patch clamp recording in dissociated sensory neurons revealed that 9-NO2-OA (1–10 µM) activates a subset of mouse vagal and trigeminal nociceptive neurons that respond to agonists for TRPA1 (AITC) and TRPV1 (capsaicin, CAPS) [17]. The responses are eliminated in neurons obtained from TRPA1 (−/− mice) and are suppressed by a selective TRPA1 antagonist (HC-030031), indicating that the effect of 9-NO2-OA on these neurons is mediated primarily by activation of TRPA1 channels. Extracellular recordings from mouse capsaicin-sensitive bronchopulmonary afferents revealed that 9-NO2-OA evokes a robust discharge of action potentials in these afferents, a response inhibited by a TRPA1 antagonist. It was concluded that 9-NO2-OA induces firing in visceral nociceptive afferent nerves in the mouse by directly activating TRPA1 but not TRPV1 channels.
3. Effect of NO2-OA on TRPA1 and TRPV1 channels in rat lumbosacral dorsal root ganglion neurons
NO2-OA also activates TRP channels in sensory neurons dissociated from dorsal root ganglia (DRG) of rats [16]. NO2-OA (3.5–35 µM) elicits Ca2+ transients in 20–40% of DRG neurons (Fig. 2A), the majority of which (60–80%) are likely to be nociceptive because they also respond to TRPA1 (AITC, 1–50 µM) and TRPV1 (CAPS, 0.5 µM) agonists (Fig. 2A, B). The NO2-OA–evoked Ca2+ transients are reduced by a TRPA1 antagonist HC-030031 (5–50 µM) (Fig. 2B) or a TRPV1 antagonist (capsazepine, 10 µM) (Fig. 2C).
Figure 2.
(A) Intracellular Ca2+ transients (Ca2+i) induced by NO2-OA (N-O, 3.5 µM, 30-s duration) (i), allyl isothiocyanate (AITC, 50 µM, 30-s duration) (ii), and capsaicin (CAPS, 0.5 µM, 5-s duration) (iii) in rat DRG neurons. Each line represents the response from one DRG neuron. The fluorescence signal measured at 340 nm, divided by the fluorescence signal measured at 380 nm is proportional to [Ca2+i] and is represented by the ratio (R). The records are shown as percentage increase of R (ΔR/R) above resting levels of [Ca2+i]. (B and C) inhibition of agonist induced Ca2+i transients in DRG neurons by a TRPA1 (HC-030031) or a TRPV1 (capsazepine) antagonist. B, examples from several cells showing responses to NO2-OA (N-O, 3.5 µM; 30-s duration) and AITC (10 µM; 30-s duration) in the absence and in the presence of HC-030031 (50 µM). HC-030031 completely blocked NO2-OA and AITC responses but did not affect the CAPS response. C, examples from several cells showing responses to NO2-OA (3.5 µM; 30-s duration) in the absence and in the presence of capsazepine (10 µM). Capsazepine reduced NO2-OA responses and completely blocked CAPS responses (0.5 µM; 5-s duration). Reproduced with permission from Sculptoreanu et al., 2010 [16].
NO2-OA also depolarizes and induces inward currents in 62% of DRG neurons. The NO2-OA currents are: (1) elicited by concentrations ≥5 nM, (2) blocked by dithiothreitol (DTT, 10 mM) a nucleophile that quenches free NO2-OA and removes any nitroalkene adducts already present through β-elimination, (3) not mimicked by oleic acid in high concentrations (5 mM), (4) not blocked by the nitric oxide (NO) scavenger cPTIO and (5) reduced by TRPV1 (diarylpiperazine, DPA, 5 µM) or TRPA1 (HC-030031, 5 µM) antagonists. These observations suggest that the effects of NO2-OA, like those of various pungent compounds and AITC [24,25,27,28], are mediated by an electrophilic interaction with cysteine residues of TRP channel proteins. However, concentrations of NO2-OA ≥5 nM, also reduce action potential (AP) overshoot, increase AP duration, inhibit firing induced by depolarizing current pulses and suppress Na+ currents [16]. Thus, endogenous NO2-OA generated at sites of inflammation may initially activate multiple TRP channels on nociceptive afferent nerves, evoke afferent nerve activity and release of afferent neurotransmitters but later suppress afferent firing.
4. Effect of NO2-OA on TRPC channels in guinea pig lumbosacral dorsal root ganglion neurons
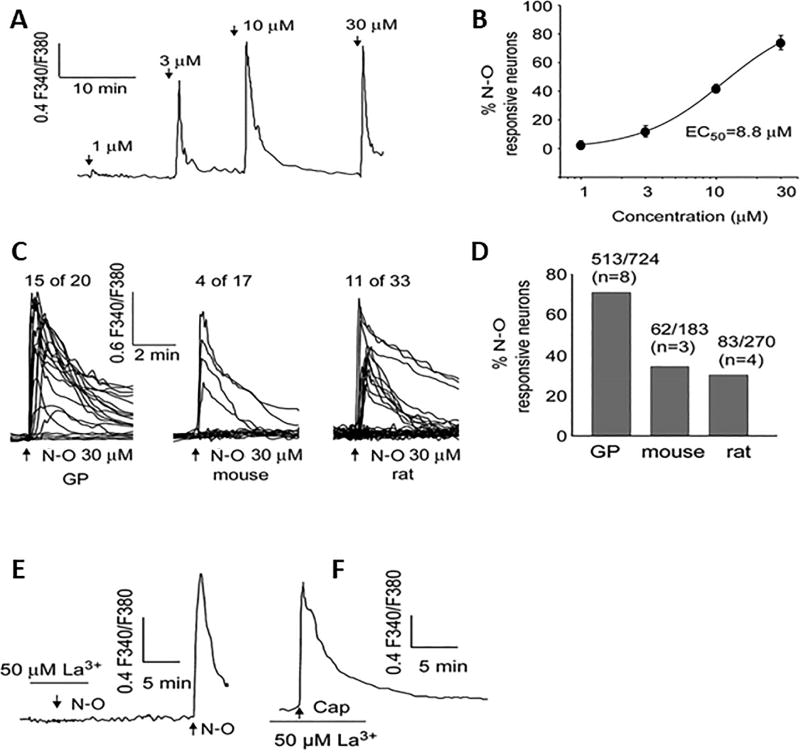
Calcium imaging and patch clamp recording revealed that another TRP channel mediates the effects of NO2-OA on sensory neurons in the guinea pig [30]. NO2-OA increases intracellular Ca2+ (EC50, 8.8 µM, Fig. 3A,B) in 60–80% of cultured neurons from lumbosacral DRGs, a considerably higher percentage than the percentage of neurons in rat or mouse responding to NO2-OA (Fig. 3C,D). 1-oleoyl-2acetyl-sn-glycerol (OAG), a TRPC 3/6/7 agonist, elicits responses in 36% of NO2-OA sensitive neurons while capsaicin or AITC elicit responses in only 16% and 10%, respectively, of these neurons [30]. A TRPV1 antagonist (DPA, 5 µM,) in combination with a TRPA1 antagonist (HC-030031, 30 µM) does not change the amplitude of the Ca2+ transients or the percentage of neurons responding to NO2-OA; however, DTT (50 mM) or La3+ (50 µM), which is known to block TRPC channels, completely abolishes the NO2-OA responses (Fig. 3E,F). Because nitrated fatty acids may induce some of their biological effects by the release of NO [31], the potential role of NO2-OA as an NO donor was also examined. The NO donor SNAP does not elicit detectable increases in intracellular calcium and pretreatment with the NO scavenger, cPTIO, does not prevent the NO2-OA induced calcium transients [30], suggesting that the effects of NO2-OA are not due to NO mediated nitrosylation of TRP channels, which has also been shown to occur when stimulated directly with NO donors [32].
Figure 3.
Comparison of NO2-OA (N-O) evoked Cai2+ transients in guinea-pig (GP), mouse and rat DRG neurons. Cai2+ increase is expressed as the ratio of fluorescence at 340 and 380 nm (F340/F380). NO2-OA at concentrations of 1, 3, 10 and 30 µM applied for 30 s evoked a concentration-dependent increase in both the amplitude (recording from one neuron) (A) and percentage of responsive neurons (summary data from 85 neurons from 6 coverslips) (B). The curve in (B) was fitted with a Hill equation with EC50 = 8.8 µM and Hill coefficient = 1.2. (C and D) NO2-OA (30 µM) applied for (30 s) evoked an increase in a higher percentage of guinea-pig neurons than in rat and mouse neurons. Scales are the same for all the traces in (C); the number of cells and animals (in parentheses) are indicated above each bar in (D). (E and F) analysis of the role of TRPA1 and TRPV1 channels in the NO2-OA evoked Cai2+ increase in guinea-pig DRG neurons. (E) NO2-OA (30 µM) did not evoke an increase in Cai2+ in the presence of 50 µM La3+ but did evoke a normal response after washout of La3+. (F) La3+ did not block the capsaicin response. Reproduced with permission from Zhang et al., 2014 [30].
Patch clamp recording revealed that NO2-OA also induces a transient inward current associated with a membrane depolarization followed by a prolonged outward current and hyperpolarization in 80% of neurons [30]. The reversal potentials of inward and outward currents are approximately −20 mV and −60 mV, respectively. Inward current is reduced in zero extracellular Na+, but is unchanged by niflumic acid (100 µM), a Cl− channel blocker. Outward current is abolished by either zero extracellular Ca2+ or a combination of two Ca2+ activated K+ channel blockers (iberiotoxin, 100 nM and apamin, 1 µM). BTP2 (1 or 10 µM), a broad spectrum TRPC antagonist, or La3+ (50 µM) completely abolish NO2-OA currents. Thus, outward currents are likely induced indirectly by influx of calcium through TRPC channels. RT-PCR revealed the expression of mRNA for the seven subtypes of TRPCs in guinea pig DRGs [30]. These results suggest that NO2-OA activates TRPC channels in addition to TRPV1 and TRPA1 channels already known to be targets in rat and mouse sensory neurons.
5. Effects of NO2-OA on visceral afferent nerves in the urinary bladder of rats and guinea pigs
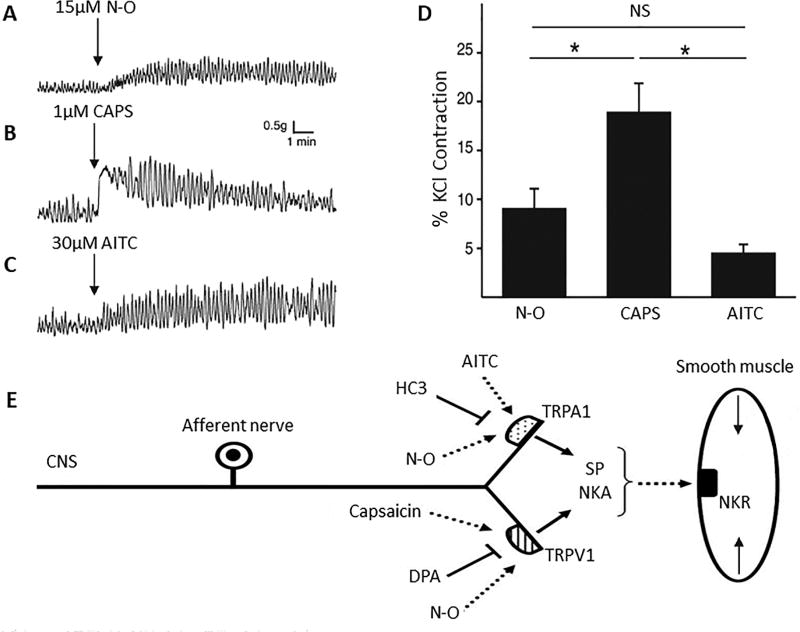
Nociceptive afferent nerves expressing TRP channels and neuropeptides are widely distributed in all organs and have a role in various pathological conditions [33]. Stimulation of TRP channels in afferent nerves releases neuropeptides which can induce inflammation and pain as well as modulate organ motility, blood flow, capillary permeability and glandular secretion by acting on nearby smooth muscle, nerves, epithelial cells or mast cells [18,33–36]. The effects of NO2-OA on visceral afferent nerves were examined indirectly by measuring the spontaneous contractile activity of bladder muscle strips in vitro (Fig. 4A) [18,37]. Application of capsaicin or AITC to rat bladder strips elicits increases in basal tone and contractile activity (Fig. 4 B & C) which are blocked, respectively, by TRPV1 (DPA) and TRPA1 (HC-3) receptor antagonists (Fig. 4E) or by a combination of antagonists for neurokinin receptor subtypes 1, 2 and 3, indicating that the agonists act on afferent nerves to release substance P (SP) or neurokinin A which then stimulate receptors on smooth muscle (Fig. 4E) [18]. NO2-OA (1–33 µM) mimics the effect of TRP agonists producing a concentration-dependent increase in the amplitude of phasic bladder contractions and baseline muscle tone (Fig. 4A and D) which are suppressed by a combination of the TRPV1 and TRPA1 antagonists or by the combination of the three neurokinin receptor antagonists [18]. The amplitude of phasic contractions prior to administration of NO2-OA is reduced 12–25% by the antagonists indicating that tonic activation of TRP channels and neuropeptide release occurs under basal conditions. It is not known if this activation of TRP channels is mediated by endogenous electrophilic nitro-fatty acids.
Figure 4.
Examples of agonist-induced phasic contractions in rat bladder strips showing that NO2-OA (N-O) mimics the effects induced by activation of TRPV1 or TRPA1 receptors. D. Arrows indicate time of application of NO2-OA (15µM, A), CAPS (1µM, B) and AITC (30µM, C). (D) Summary data showing the amplitude of agonist-induced increases in phasic contraction amplitude normalized to KCl-evoked contraction). * p < 0.05, NS p > 0.05 by one-way ANOVA, n = 9 to 13. E, Schematic of NO2-OA mechanism of action. NO2-OA activates TRPV1 and TRPA1 channels on capsaicin-sensitive afferent nerve terminals to trigger the release of neurokinins that act on postjunctional receptors to induce smooth muscle contractions. SP – substance P, NKA – neurokinin A. NKR – neurokinin receptor. Reproduced with permission from Artim et al., 2011 [18].
Given that NO2-OA can activate TRPC channels in guinea pig DRGs, experiments were conducted to determine if activation of TRPC channels also modulates the activity in the guinea pig bladder [37]. Unexpectedly, NO2-OA decreased the amplitude of phasic bladder contractions, the opposite effect of what occurred in rat bladder. This effect was reversed in the presence of La3+ but not by specific TRPV1/TRPA1 antagonists, suggesting that NO2-OA acts primarily on TRPC channels and not TRPV1 or TRPA1 channels in the guinea pig bladder. NO2-OA-mediated inhibition is dependent on the release of calcitonin gene-related peptide (CGRP) from bladder afferent nerve terminals, as it is prevented following application of olcegepant, a selective CGRP antagonist [37].
The difference in the effect of NO2-OA in rat and guinea pig bladders might be due to multiple factors. Studies on dissociated DRG cells suggest that a lower percentage of guinea pig neurons respond to capsaicin or AITC than rat neurons (TRPA1: 5% vs 41%, TRPV1: 21% vs 70%) [30], suggesting that any excitatory effects of NO2-OA on TRPV1 or TRPA1 channels in guinea pig bladder may be masked by a dominant CGRP signal mediated by TRPC channel activation. It is also possible that differences in the affinity/efficacy of NO2-OA at each TRP channel varies between species due to differences in amino acid sequences of the receptors. Regardless, it appears that electrophilic fatty acids may play an important role in bladder function and hence may be an attractive target for treatment of bladder pathology.
6. NO2-OA desensitization of TRPA1 and TRPV1 channels in rat sensory neurons
Sensitization of TRPV1 and TRPA1 channels in sensory neurons is involved in the development of hyperalgesia (hypersensitivity to noxious stimuli) in inflammatory pain models [38,39]; while desensitization is an important mechanism for down-regulation of channel activity and reducing nociceptor function. Capsaicin activates and subsequently desensitizes TRPV1 channels (homologous desensitization) and also reduces the effect of AITC on TRPA1 channels (heterologous desensitization) [40,41]. AITC also elicits homologous and heterologous (TRPV1) desensitization [40,41]. Experiments on rat dissociated dorsal root ganglion cells using Ca2+ imaging and patch clamp techniques [42] and on sensory nerves in the rat hindpaw using pain behavioral testing in vivo [42] revealed that NO2-OA produces heterologous desensitization of responses to TRP agonists. A 5–10 min exposure to NO2-OA reduces by 40–60% the magnitude of the calcium signals and currents as well as the percentage of cells responding to AITC or capsaicin. However, deltamethrin, a phosphatase inhibitor, which reduces the AITC induced heterologous desensitization of capsaicin or NO2-OA does not suppress the NO2-OA induced desensitization of AITC or capsaicin, indicating that heterologous desensitization induced by NO2-OA and AITC occur by different mechanisms.
Subcutaneous injection of a small volume (35 µL) of AITC (10 mM) or NO2-OA (2.5 mM) into a rat hind paw induces nociceptive behaviors (licking or repeated withdrawals of the injected paw) [42]. Homologous desensitization occurs when AITC is applied at 10 minute intervals, but does not occur when NO2-OA is applied at 30 minute intervals. Pretreatment with NO2-OA 30 minutes before AITC reduces by 50% the AITC-evoked nociceptive behaviors but does not alter capsaicin induced nociceptive responses [42]. These results raise the possibility that NO2-OA might be useful clinically to reduce neurogenic inflammation and certain types of painful sensations by desensitizing TRPA1 expressing nociceptive afferents.
7. Conclusions
Nitrated fatty acids, which are generated at sites of inflammation, may target multiple TRP channels in nociceptive sensory neurons producing an initial excitation followed by desensitization of these channels and a reduction in afferent activity. Thus endogenous nitrated fatty acids may have a role in modulating the activity of nociceptive afferents and, in turn, influence the development and/or resolution of inflammatory responses and pain. The demonstration that oral administration of nitrated fatty acids or their precursors can produce significant blood, tissue and urine levels of these substances [3,43–45] raises the possibility that nitro-lipid therapies may be developed to target pathophysiological mechanisms and disorders which involve TRP channels [46] and that passage of these substances from the urine into the bladder wall might be effective in treating certain types lower urinary tract dysfunctions.
Highlights.
Nociceptive sensory neurons express multiple TRP channels (TRPV1, TRPA1, TRPC)
TRP channels have N terminals rich in cysteine residues that react with electrophiles
NO2-OA, an electrophilic fatty acid, activates TRP channels in sensory neurons
NO2-OA also elicits a secondary desensitization of TRP channels in sensory neurons
TRP channel desensitization by NO2-OA may contribute to suppression of inflammation
Acknowledgments
This work was supported by grants from to JMB from the NIH NIDDK (DK106115 and DK114492) and to WCD from the NIH NIDDK (DK093424 and DK111382). The authors would like to thank Ms. Gisel Garcia and Ms. Stephanie Daugherty for their help in preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Authors have no conflict of interest to disclose.
References
- 1.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–63. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–75. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NK, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Cole MP, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J Biol Chem. 2012;287:44071–82. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–6. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem. 2011;286:14019–27. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, Iles KE, Freeman BA, Agarwal A. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci U S A. 2006;103:4299–304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–98. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98:116–24. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O'Donnell VB. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ Res. 2002;91:375–81. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 10.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O'Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–40. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 11.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–93. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–9. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PR, Freeman BA, Chen YE, Xu HE. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol. 2008;15:865–7. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–40. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischemia and reperfusion. Cardiovasc Res. 2009;85:155–66. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sculptoreanu A, Kullmann FA, Artim DE, Bazley FA, Schopfer F, Woodcock S, Freeman BA, de Groat WC. Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats. J Pharmacol Exp Ther. 2010;333:883–95. doi: 10.1124/jpet.109.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–9. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artim DE, Bazely F, Daugherty SL, Sculptoreanu A, Koronowski KB, Schopfer FJ, Woodcock SR, Freeman BA, de Groat WC. Nitro-oleic acid targets transient receptor potential (TRP) channels in capsaicin sensitive afferent nerves of rat urinary bladder. Exp Neurol. 2011;232:90–9. doi: 10.1016/j.expneurol.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–50. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 20.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 21.Venkatachalam K, Montell C. TRP Channels. Annual review of biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 23.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–7. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–57. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 25.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–5. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 26.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–52. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–8. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar H, Llorente I, Jara-Oseguera A, Garcia-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–61. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–24. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Beckel JM, Daugherty SL, Wang T, Woodcock SR, Freeman BA, de Groat WC. Activation of TRPC channels contributes to OA-NO2-induced responses in guinea-pig dorsal root ganglion neurons. J Physiol. 2014;592:4297–312. doi: 10.1113/jphysiol.2014.271783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr, Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem. 2005;280:19289–97. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 33.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–73. doi: 10.1002/nau.20511. [DOI] [PubMed] [Google Scholar]
- 35.Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul Pept. 2001;101:1–18. doi: 10.1016/s0167-0115(01)00285-3. [DOI] [PubMed] [Google Scholar]
- 36.Maggi CA, Giuliani S, Meli A. Effect of ruthenium red on responses mediated by activation of capsaicin-sensitive nerves of the rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:541–6. doi: 10.1007/BF00260609. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Sharaf N, Facchine G, Russo T, Moonert P, Freeman BA, Woodcock SR, Groat WCd, Daugherty SL. Nitro-oleic acid (OA-NO2), an electrophilic fatty acid derivative and TRP channel agonists activate different afferent neuromodulatory mechanisms in guinea pig urinary bladder. Society for Neuroscience Annual Meeting Program No. 736.21. 2014 [Google Scholar]
- 38.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–7. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 39.da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148:431–7. doi: 10.1016/j.pain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–78. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–9. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Koronowski KB, Li L, Freeman BA, Woodcock S, de Groat WC. Nitro-oleic acid desensitizes TRPA1 and TRPV1 agonist responses in adult rat DRG neurons. Exp Neurol. 2014;251:12–21. doi: 10.1016/j.expneurol.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delmastro-Greenwood M, Hughan KS, Vitturi DA, Salvatore SR, Grimes G, Potti G, Shiva S, Schopfer FJ, Gladwin MT, Freeman BA, Gelhaus Wendell S. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic Biol Med. 2015;89:333–41. doi: 10.1016/j.freeradbiomed.2015.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazzari M, Khoo NK, Woodcock SR, Jorkasky DK, Li L, Schopfer FJ, Freeman BA. Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. J Lipid Res. 2017;58:375–385. doi: 10.1194/jlr.M072058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughan KS, Wendell SG, Delmastro-Greenwood M, Helbling N, Corey C, Bellavia L, Potti G, Grimes G, Goodpaster B, Kim-Shapiro DB, Shiva S, Freeman BA, Gladwin MT. Conjugated Linoleic Acid Modulates Clinical Responses to Oral Nitrite and Nitrate. Hypertension. 2017;70:634–44. doi: 10.1161/HYPERTENSIONAHA.117.09016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi N, Mori Y. TRP Channels as Sensors and Signal Integrators of Redox Status Changes. Frontiers in Pharmacology. 2011;2:58. doi: 10.3389/fphar.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]