Summary
Inflammation has been involvement in the pathophysiology and treatment response of major depressive disorder (MDD). Plasma cytokine profiles of 171 treatment-naïve, MDD patients (none of the MDD patients received an adequate trial of antidepressants or evidence-based psychotherapy) and 64 healthy controls (HC) were obtained. MDD patients exhibited elevated concentrations of 18 anti- and proinflammatory markers and decreased concentrations of 6 cytokines. Increased inflammasome protein expression was observed in MDD patients, indicative of an activated inflammatory response. The plasma of MDD patients was immunosuppressive on healthy donor peripheral blood mononuclear cells inducing reduced activation of monocytes/dendritic cells and B cells and reduced T cell memory. Comparison between 33 non-responders and 71 responders at baseline and 12 weeks, revealed that after treatment, anti-inflammatory cytokine levels increase in both groups, whereas 5 pro-inflammatory cytokine levels were stabilized in responders, but continued to increase in non-responders. MDD patients exhibit remodeling of their inflammatory landscape.
Keywords: Major depressive disorder, treatment naïve, cytokines, PBMC, inflammasome
ETOC paragraph
Treatment-naïve depressed patients have increased levels of pro- and anti-inflammatory markers, but overall the balance shifts towards immunosuppression of immune cells. Consistent with these findings, absence of response to antidepressant treatments has been associated with defective anti-inflammatory response.
Introduction
Major depressive disorder (MDD) is a widespread and debilitating disorder with a lifetime prevalence rate in the United States of 21% in women and 11% in men (Kessler et al., 2005). It is characterized by disturbances in sleep, appetite, concentration, ability to experience pleasure, and psychomotor alterations [Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)]. It is also associated with a markedly increased risk for suicide and a variety of comorbid medical disorders (stroke, myocardial infarction, diabetes and others) (Otte et al., 2016). Various approaches ranging from functional brain imaging of patients to animal models of depression have been proposed to discover biomarkers to identify at risk populations and/or to predict individual treatment responses. However, because of the current lack of validated biomarkers and heterogeneity in different MDD patient populations, these approaches with some exceptions (Raison et al., 2013; Schmidt et al., 2011; Sen et al., 2008) have provided only limited advances towards these goals. This current limitation is particularly evident in studies of the role of inflammation in depression (Maes et al., 2009). It is now well-established that psychological stress, a prominent risk factor for MDD, induces an inflammatory response, and in multiple meta-analyss, two cytokines, interleukin (IL)-6 and tumor necrosis factor (TNF), are reproducibly elevated in the blood and cerebrospinal fluid (CSF) of depressed patients (Dowlati et al., 2010; Howren et al., 2009; Köhler et al., 2017; Liu et al., 2012; Maes et al., 2009; Miller et al., 2009). Furthermore, elevation of these proinflammatory cytokines in healthy volunteers is associated with the development of depressive symptoms (Miller and Raison, 2015), suggesting that investigating proinflammatory cytokines represents a reasonable strategy to identify biomarkers for depression.
The immune system induces the expression of anti-inflammatory cytokines to diminish inflammation. Thus, it is particularly intriguing that the levels of antiinflammatory cytokines IL-2, IL-4, and IL-10 are also often elevated in depressed patients, raising the question of whether these anti-inflammatory cytokines play a role in the onset or recovery from depression (Dowlati et al., 2010). As discussed below, elevated anti-inflammatory cytokines may represent responses to antidepressant treatments or compensatory mechanisms related to the duration of depressive episodes; this emphasizes the need for a study with a relatively homogeneous population of patients with a minimal number of depressive episodes and free of antidepressant treatment exposure to interpret changes in the inflammatory system.
The role of inflammation in MDD is supported, in part, by the results of a meta-analysis that supported the efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) compared with placebo in the treatment of depression (Köhler et al., 2014). It is, important to note, however, that although NSAIDs have broad anti-inflammatory actions, they do not block the effects of inflammatory cytokines. Indeed, in the SADHART study all patients received NSAIDs, but they continued to exhibit an elevation in inflammatory markers (Glassman et al., 2002). With the development of novel targeted therapeutics in other inflammatory diseases such as psoriasis and rheumatoid arthritis, FDA-approved monoclonal antibodies and other cytokine inhibitors have been used to block individual cytokines in depressed patients, and they have demonstrated significant antidepressant properties in some patient cohorts (Kappelmann et al., 2016). For example, TNF inhibitors such as adalimumab (Loftus et al., 2008; Menter et al., 2010) or etanercept (Tyring et al., 2013; Tyring et al., 2006), IL-12/IL-23 antagonists (Langley et al., 2010), or IL-4Ra antagonists (Simpson et al., 2015) have been shown to be more efficacious than placebo in the treatment of MDD symptoms. Similar effects have been observed in non-randomized and/or non-placebo controlled trials that targeted TNF or IL-6 (Kappelmann et al., 2016), indicating an improvement of depressive symptoms with anti-cytokine treatments. Infliximab, a TNF neutralizing antibody, only benefited a sub-population of treatmentresistant MDD patients with elevated levels of inflammation (CRP>5 mg/l) (Miller and Raison, 2015; Raison et al., 2013). This suggests that anti-cytokine approaches might only provide benefit in depressed patients with prominent inflammation.
Response to antidepressant treatments has been reported to be impaired by proinflammatory cytokines, which may be overcome by co-administering antiinflammatory drugs (Köhler et al., 2014). Although antidepressants are generally thought to shift the balance towards anti-inflammatory response (Kubera et al., 2001; Lanquillon et al., 2000; Maes et al., 1999; Sluzewska et al., 1997), the overall net effect of antidepressants on cytokines remains unclear, as antidepressants have also been reported to promote proinflammatory cytokine production (Warner-Schmidt et al., 2011). Nonetheless, high levels of proinflammatory cytokines are often observed in treatment-resistant depressed patients, suggesting a negative correlation between pro-inflammatory cytokine levels and treatment response (Kubera et al., 2001; Lanquillon et al., 2000). Taken together, the data suggest that changing the balance between pro- and anti-inflammatory cytokines may promote antidepressant actions. However, no prior studies have focused on previously untreated patients and, furthermore, there are limited data on multiple inflammatory and anti-inflammatory cytokines.
The Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study was designed to identify predictors of the response to 3 well-established and effective interventions: escitalopram (10–20 mg/day), duloxetine (30–60 mg/day) and Cognitive Behavior Therapy (CBT:16 sessions) among MDD patients who had never previously received an evidence-based treatment for depression (Dunlop et al., 2012). This unique and relatively homogeneous population of treatment-naïve depressed patients represents an ideal paradigm to identify predictors of treatment response and to identify biomarkers by comparing MDD patients to healthy volunteers. The PReDICT study demonstrated that antidepressants and CBT treatment were similarly efficacious with remission rates in the 44–52% range (Dunlop et al., 2017a). Further, magnetic resonance imaging (MRI) using resting state functional connectivity distinguished between the likelihood of remitting or failing to benefit from CBT or antidepressant treatment in the PReDICT cohort (Dunlop et al., 2017b).
In the current investigation, we measured 27 cytokines, chemokines, and growth factors in the PReDICT cohort of treatment-naïve MDD patients and healthy volunteers. The majority of patients exhibited elevated levels of both proinflammatory and anti-inflammatory cytokines, pointing towards an increased inflammatory response. We also found that independent of treatment type, responders exhibited stabilized levels of proinflammatory cytokines whereas non-responders exhibited continued increases in proinflammatory cytokines. In contrast, anti-inflammatory cytokines, remained elevated in both responders and non-responders.
In addition, we found associated with the increased cytokine production, an increase in the inflammasome protein levels, pointing towards an upregulation of the cytokine production machinery in MDD patients compared to healthy controls. Examination of the potential effect of the plasma content of MDD patients on PBMC activation revealed that plasma of MDD patients exhibit immunosuppressive capacity consistent with previous literature (Maes, 1995). Nevertheless, response to antidepressant treatments was not associated with changes in the inflammasome proteins, nor PBMC activation suggesting that cytokine concentrations might represent better biomarkers to evaluate the effects of intervention in treatment-naïve MDD patients 12 weeks after initiating the treatment.
STAR methods
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-CD4 clone SK3 | BD Horizon | Cat# 566104 Lot7068600 |
| anti-CD11b clone CBRM1/5 | BD Horizon | Cat# 566313 Lot6105692 |
| anti-CD19 clone SJ25C1 | BD Horizon | Cat# 566396 Lot7146702 |
| anti-CD45RO clone UCHL1 | BD Pharmingen | Cat# 555493 RRID:AB_395884 Lot6320585 |
| anti-CD25 clone 2A3 | BD Horizon | Cat# 564467 Lot7054527 |
| anti-CD69 clone FN50 | BD Horizon | Cat# 562617 Lot7158806 |
| anti-CD8 clone RPA-T8 | BD Horizon | Cat# 563823 RRID:AB_2687487 Lot7167933 |
| anti-CD86 clone FUN-1 | BD Horizon | Cat# 562999 Lot7144880 |
| Critical Commercial Assays | ||
| human 27Plex cytokine multiplex assay | Bio-rad | M500KCAF0Y |
| CART/Simple Plex Assay | Protein simple | SPCKA-PS-000786 |
| Biological samples | ||
| Human PBMCs | ALLCELLS | PB001 Lot A5962 |
| Instruments | ||
| MAGPIX | Luminex | Cat# MAGPIX |
| Celesta flow cytometer | BD Bioscience | Cat# 660343 |
| Ella System | Protein Simple | |
| Software | ||
| SPSS 24.0 software | IBM | RRID:SCR_002865 |
| FACS DIVA 8.0 software | BD Bioscience | RRID:SCR_001456 |
| FlowJo 9.9.5 software | FlowJo | RRID:SCR_008520 |
| PRISM 5.0 software | Graph Pad | |
CONTACT FOR REAGENT AND RESOURCE SHARING
For further information and requests for reagents may be directed to, and will be fulfilled by, the Lead Contact and Corresponding author, Charles Nemeroff (cnemeroff@med.miami.edu).
EXPERIMENTAL MODEL AND SUBJECTS DETAILS
Study overview
The rationale, methods and design of the PReDICT study has been published previously (Dunlop et al., 2012) (ClinicalTrials.gov ID NCT00360399). The study was conducted between January 2007 and May 2013 through the Emory University’s Mood and Anxiety Disorders Program. The study was approved by the Emory Institutional Review Board and the Grady Hospital Research Oversight committee.
Patients
Male and female patients were recruited through clinical referrals and advertising. Eligible participants were adult outpatients 18–65 years of age who met the DSM-IV criteria for a primary diagnosis of MDD without psychotic features. The primary diagnosis and absence of exclusionary diagnoses were assessed through a study psychiatrists interview and administration of the Structured Interview for DSM-IV (SCID). Inclusion criteria included a 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) total score ≥15 at the randomization visit. Patients had to have never previously received an evidence-based treatment for a mood disorder, operationalized as lifetime exposure to 4 sessions of an evidence-based psychotherapy or 4 weeks of an antidepressant medication. Of the patients entered into the study, 15 (8.8%) had ever received an antidepressant medication. Of these 7 had received an inadequate trial as defined in the inclusion criterion. The mean period of time they were treated was 10.5 ± 2.4 days and this occurred 8.9± 3.1 years prior to study entry. The only exception was one patient who had been treated for “premenstrual tension” 2 years prior to study entry. These patients did not differ from the others in the study either in terms of inflammatory markers or treatment response.
Exclusion criteria: Lifetime criteria for bipolar disorder or a psychotic disorder, currently met criteria for OCD (past 12 months), substance abuse within 3 months prior to screen or substance dependence within 12 months, significant current suicidality or homicidality, pregnant or breast-feeding women, contraindication to MRI scanning. If present, comorbid current post traumatic stress disorder (PTSD) could be only mild in severity as assessed by the study psychiatrist and could not be the focus of treatment.
Interested patients were seen for a screening visit, which was comprised of a SCID interview, HDRS, medical history, routine laboratory testing (including urine drug screen), electrocardiogram, and physical exam.
Randomization and Treatments
Patients were randomized at a 1:1:1 ratio to receive 12 weeks of treatment with either escitalopram (10–20 mg/day), duloxetine (30–60 mg/day), or 16 sessions of CBT of 50 min, administered according to a standardized protocol (Beck, 1979). Escitalopram and duloxetine were initiated at 10 mg/day and 30 mg/day, respectively, with the option to increase the dose beginning at week three depending on tolerability and response. All patients who had not achieved remission by week six had the dose increased to 20 mg/day of escitalopram or 60 mg/day of duloxetine unless prevented by tolerability concerns.
Clinical outcomes
We defined positive response if HDRS scores at week 12 were < 50% of baseline.
Sample collection
Blood samples were collected from antecubital veins using standard techniques into EDTA tubes at baseline, and at week 12 between 8 am and 4 pm. Within 10 minutes of being obtained, the EDTA tubes were centrifuged at 4°C, the plasma aliquoted into 1 ml samples and frozen at −80°C. Healthy control ( HC) volunteer plasma were also collected in EDTA tubes. We also selected healthy control volunteers who reported no medication (comprising psychiatric or other types of medications) at the time of blood collection.
Cytokine Measurements
Cytokines, chemokines and growth factors were measured in plasma samples isolated as above using a commercially available multiplex analysis human 27Plex cytokine multiplex assay (M500KCAF0Y, Bio-Rad) on a MAGPIX. Samples were run on 9 plates, blind to treatment. 4 samples were run on every plate to control for the inter-plate variability (%CV varied between 2.3–12%, supplementary Figure 1C). Assays were checked for quality control to fit the standard curves. A standard curve was run for each lot, and samples were normalized to the averaged standard curve values.
Inflammasome Protein Measurements
Inflammasome signalling protein levels (ASC, caspase-1 and IL-18) in plasma were analysed as described in (Brand et al., 2016), using the Ella System and analysed by the Simple Plex Explorer software (Protein System). Results correspond to the mean of each sample run in triplicates for each analyte.
Peripheral Blood Mononuclear Cells (PBMC) stimulation
PBMCs from a non-hispanic white female healthy donor (age 50, weight: 142 lbs, height 68 in) unmedicated for a week, were freshly isolated at ALLCELLS. No other information regarding this donor was available. When received in the lab, PBMCs were plated at 4×105 cells/well (86% viability) in 96-well plates, and the next day, PBMCs were stimulated with LPS (100 ng/mL) or not and/or with 10% of plasma of HC subjects or MDD patients for 24 h. All the samples were run in duplicate. The plasma of 27 HC subjects and 40 MDD patients (20 non-responders and 20 responders) were tested. The demographics of the patients were chosen so there was no difference between the 3 groups.
Cells were then stained for flow cytometry using BV480-conjugated anti-CD4, BV786-conjugated anti-CD8, BV421-conjugated anti-CD11b, BB700-conjugated antiCD19, PE-conjugated anti-CD45RO, BB515-conjugated anti-CD25, PE-CF594conjugated anti-CD69, and BV605-conjugated anti-CD86 (BD Bioscience, supplementary material) and analysed on a Celesta, BD bioscience flow cytometer. Data were analysed using Flow Jo.
Data analysis
The data were analyzed using SPSS Version 24. For all analyses, any samples that were under the detection limit were included as the minimal detectable value of the assay. More than 50% of the values for IL-10, IL-5, IL-12, G-CSF and GM-CSF were under the minimal detection range in the HC group. Depending on the analytes, some samples demonstrated aberrant values and were excluded.
Statistical Analyses
Baseline demographic differences between HC and MDD groups were performed using one-way analysis of variance procedures and chi-square analyses for dichotomous variables. The criteria for statistical significance was p<0.05. In analyses comparing variables associated with an inflammatory response and the upregulation of the inflammasome pathway, we employed ANCOVA analyses to account for HC and MDD initial differences in age, gender, percentage of African-American participants and Hispanic participants. The Bonferroni correction was applied to adjust for family-wise Type 1 alpha error rates due to multiple comparisons. Following a statistically significant Bonferroni Test, additional nonparametric Mann-Whitney Tests were performed to insure that distributional characteristics of the data and heterogeneity of variance in a comparison of groups with unequal n’s did not affect the obtained findings.
We tested if antidepressant treatments for 12 weeks altered the inflammatory molecule profile and if there were differences between responders and non-responders. Due to the modest number of subjects who completed treatment in each arm, we pooled all the treatments together as there were no differences in the cytokine levels and response outcome between the 3 treatments (Supplementary Table 5C). We hypothesized that MDD responders would have reduced proinflammatory cytokine levels compared with MDD non-responders after 12-weeks of treatment. To test this hypothesis, a 2 X 2 (Responder by Time) multivariate analyses of variance (MANOVA) with IL-6, IL-1β, TNF, IFNγ, and IL-17A serving as outcome measures. We focused on the Responder X Time interaction term since this would reveal any potential differences in patterns of change of pro-inflammatory cytokines in responders versus non-responders, overtime. Following a statistically significant omnibus multivariate test of significance, individual univariate analyses conducted for each pro-inflammatory cytokine. A similar MANCOVA approach was employed for anti-inflammatory markers.
For analyses comparing cytokine values at baseline of MDD patients in the PReDICT study versus healthy controls, a series of Levine tests were used and demonstrated significant heterogeneity of variance. As a result, Welch’s F-Value for Unequal Variances was applied. We also completed ANCOVAs using baseline differences between groups with regards to age and gender, BMI as covariates. Since the underlying assumptions of ANCOVA may be violated in the presence of both heterogeneity of variance described above coupled with unequal numbers of subjects in MDD patients versus HC subjects, it was judged that a most robust test of group differences could be obtained using a series of Mann-Whitney U tests of ranks that do not require parametric assumptions such as homogeneity of variances, normality and unequal numbers of subjects. Both parametric and non-parametric tests yielded similar results.
To examine changes in cytokines as a function of treatment condition among responders versus non-responders, we conducted a series of Diagnostic Group x responder x Time (Baseline versus 12 weeks) mixed model repeated measures MANOVA for both proinflammatory cytokines and anti-inflammatory cytokines. Given the equivalence of age, gender and body mass index in responders versus non-responders, no covariates were required in the model. Following a statistically significant multivariate effect, post-hoc univariate analyses (ANOVAs) were conducted for proinflammatory and anti-inflammatory cytokines respectively. Multiple comparison corrections were made and stated within each legend.
Results
Patient characteristics
171 patients meeting DSM-IV criteria for MDD and 64 healthy controls were included in the analyses. The sociodemographic and clinical characteristics of the patients are presented in Table 1, Supplemental Tables 1A, 3, and Supplemental Figures 3A, 4A.
Table 1.
Demographic characteristics of HC subjects and MDD patients
ANCOVA, mean ±SD, HSRD: Hamilton Depression Rating Scale, BDI: Beck depression Inventory, Major Dep ep: Major depressive episode, Fam hx MDD: Family history of MDD, BMI: body mass index
| HC n=64 |
MDD n=171 |
p-value | |
|---|---|---|---|
| Participants | 64 | 171 | - |
| Gender (F/M) | 30/34 | 113/58 | p=0.007** |
| Age | 45±11.8 | 39.4±11.8 | p=0.001** |
| BMI | 29.2±7.2 | 28.8±6.18 | p=0.728 |
| Race, n (%) | p=0.562 | ||
| Caucasian | 8 (13%) | 52 (30%) | |
| African-American | 41 (64%) | 41 (24%) | |
| Other | 15 (23%) | 78 (46%) | |
| Ethnicity | |||
| -Hispanic | 15 (23%) | 72 (42%) | p=0.002** |
| -Non-Hispanic | 49 (77%) | 99 (58%) | |
| Depression History | |||
| HSRD-Baseline | - | 20.0±3.73 | |
| HSRD-Week12 | - | 7.78±3.73 | |
| BDI-Baseline | - | 23.3±6.78 | |
| BDI-Week12 | - | 7.23±7.54 | |
| # Major dep ep | - | 5.15±16.7 | |
| Fam hx MDD | - | 57/171 | |
p<0.01
Depressed patients exhibit an inflammatory response
Because the populations of HC subjects and MDD patients were significantly different in demographic characteristics (age, gender, ethnicity, BMI) that could significantly impact the dependent variables (Table 1), we carefully matched 62 HC subjects with 62 MDD patients on these demographic characteristics to avoid any bias in our conclusions (Supplementary Table 1A-B). Two HC participants could not be effectively matched. This matching strategy resulted in equivalent age, gender and ethnicity as well as BMI among the HC and MDD matched groups (Supplementary Table 1A). We then compared these results to statistical analysis performed with the full compliment of 64 HC subjects and 171 MDD patients. Because the results were identical for the cytokine data, we present the data for the total sample below.
Compared to HC subjects, the 171 MDD patients exhibited elevated levels for six of the seven measured proinflammatory cytokines, including IL-12, TNF, IL-6, IFNγ IL9, and IL-17A, with levels that were ~2- to 13-fold those in HC subjects, and elevations were evident in 66–100% of the MDD patients (Table 2, Supplementary Fig 1A). Of the 7 potential anti-inflammatory cytokines measured, among the 171 MDD patients 5 were elevated (IL-5, IL-15, IL-10, IL-2, IL-13) ranging from ~1.6- to 17-fold levels in HC subjects, and 2 were diminished (IL-1RA, IL-4) by ~25%. The effects found with the potential anti-inflammatory cytokines occurred in 79–100% of MDD patients (Table 2, Supplementary Fig 1B).
Table 2:
Levels of cytokines, chemokines and growth factors in HC subjects and MDD patients
Due to differences between the HC subjects and the MDD patients, and to adjust for various covariates (age, gender, race), we performed a 1:1 match HC subjects and MDD patients and excluded any effects of the various covariates on the production of cytokines, chemokines and growth factors except for RANTES/CCL5, FGF and GM-CSF (Supplementary Figure 1). In addition, because there were a number of occasions where there was heterogeneity of variance, questionable distributional normality and an unequal n, a Non-Parametric Mann-Whitney U Test of Ranks test was also used, mean ±SD, CCL: chemokine ligand, FGF: Fibroblast Growth Factor, G-CSF: granulocyte colony-stimulating factor, GM-CSF: granulocyte-macrophage colony-stimulating factor, IFNγ: interferonγ, IL: interleukin, CXCL: chemokine (C-X-C motif) ligand, MCP-1: monocyte chemoattractant protein-1, MIP: macrophage inflammatory protein, PDGF: platelet derived growth factor, RANTES: regulated on activation, normal T cell expressed and secreted, TNF: tumor necrosis factor, VEGF: vascular endothelial growth factor
| HC Mean (pg/mL) n=64 |
MDD Mean (pg/mL) n=171 |
MDD/ HC (%) |
p-value | |
|---|---|---|---|---|
| Proinflammatory cytokines | ||||
| Increased | ||||
| IL-12 | 4.6±6.2 | 60.3±36.0 | 1311 | p<0.001 ** |
| TNF | 27.4±14.7 | 101±47.9 | 369 | p<0.001 ** |
| IL-6 | 11.6±13.0 | 41.2±16.5 | 355 | p=0.001 ** |
| IFNγ | 97.5±30.8 | 279±117 | 286 | p<0.001 ** |
| IL-9 | 24.0±3.5 | 60.7±2.1 | 253 | p<0.001 ** |
| IL-17A | 93.4±65.8 | 182±173 | 195 | p<0.001 ** |
| No change | ||||
| IL-1β | 9.1±6.8 | 9.2±4.9 | 101 | p=0.089 |
| Anti-inflammatory cytokines | ||||
| Increased | ||||
| IL-10 | 13.7±73.2 | 37.9±21.0 | 277 | p<0.001 ** |
| Decreased | ||||
| IL-1RA | 394±280 | 290±170 | 74 | p=0.001 ** |
| Other cytokines | ||||
| Increased | ||||
| IL-5 | 3.6±3.9 | 60.5±21.3 | 1681 | p<0.001 ** |
| IL-15 | 2.1±0.0 | 23.0±7.8 | 1095 | p<0.001 ** |
| IL-2 | 17.6±33.3 | 32.1±12.8 | 182 | p<0.001 ** |
| IL-13 | 18.7±17.5 | 30.0±21.3 | 160 | p<0.001 ** |
| Decreased | ||||
| IL-4 | 8.0±4.1 | 6.0±2.1 | 75 | p<0.001 ** |
| Chemokines | ||||
| Increased | ||||
| MIP1α/CCL3 | 2.0±0.6 | 13.6±1.6 | 680 | p<0.001 ** |
| RANTES/CCL5 | 7310±856 | 12200±2270 | 167 | p<0.001 ** |
| No change | ||||
| Eotaxin/CCL11 | 81.6±7.1 | 71.2±1.7 | 87 | p=0.617 |
| Decreased | ||||
| MIP1α/CCL4 | 61.4±3.06 | 50.7±1.9 | 83 | p<0.001 ** |
| IL-8 | 20.7±9.5 | 16.3±6.0 | 79 | p<0.001 ** |
| MCP-1/CCL2 | 116±9.3 | 69.1±4.8 | 60 | p<0.001 ** |
| IP-10/CXCL10 | 1530±129 | 525±30.8 | 34 | p<0.001 ** |
| Growth factors | ||||
| Increased | ||||
| G-CSF | 10.0±2.5 | 106±3.4 | 1060 | p<0.001 ** |
| PDGF | 574±73.1 | 1290±126 | 225 | p<0.001 ** |
| FGF | 52.0± 2.9 | 104.3 ±3.9 | 201 | p<0.001 ** |
| IL-7 | 31.8±11.4 | 38.7±13.4 | 122 | p<0.001 ** |
| No change | ||||
| GM-CSF | 62.8±1 | 62.2±2.8 | 99 | p=0.008 * |
| VEGF | 81.6±12.4 | 64.4±5.3 | 79 | p=0.320 |
GM-CSF did not survive the Bonferroni correction
p≤0.001
Chemokine level elevations in the 171 MDD patients compared to HC subjects were evident in 2 (MIP1α/CCL3, RANTES/CCL5; 1.7-fold and 6.8-fold of HC levels, respectively) out of 7 measured, and 4 were lower by 66–17% in the 171 MDD patients (IP10/CXCL10, MCP1/CCL2, IL-8, MIP1β/CCL4) (Table 2, Supplementary Fig 2A). The 171 MDD patients demonstrated elevated levels on 4 of the 6 measured growth factors, including G-CSF, PDGF, FGF, IL-7, which were elevated to 1.2–11fold levels in HC subjects (Table 2, Supplementary Figure 2B). It is important to note, however, there were 3 chemokines and growth factors that were differentially regulated between the matched and whole sample analyses. RANTES/CCL5 and FGF were increased in the whole sample analysis in the MDD patients, but did not change or decreased, respectively, in the matched sample analysis in MDD patients compared to HC subjects. Similarly, GM-CSF did not change in the whole sample analysis in MDD patients, but significantly decreased in the matched sample analysis in MDD patients when compared to HC subjects. Although IL-6 concentration was increased in both the matched and whole sample analyses, the fold increase was ~3 fold lower in the 62 matched MDD patients. This suggests that these factors might be sensitive to age, gender, ethnicity and BMI variation.
Taken together, these results indicate that MDD patients commonly demonstrated elevated levels of many inflammatory molecules, along with several that had lower levels; this is in contrast to the notion that only a subset of MDD patients experience inflammatory dysregulation (Raison et al., 2013). In addition, both pro- and anti-inflammatory cytokines were increased in MDD patients, suggesting an overall increased inflammatory response in treatment-naïve MDD subjects.
Depressed patients exhibit an upregulation of the inflammasome pathway
We also examined whether MDD patients exhibited alterations of the cytokineproducing inflammasome pathway, which can be reliably analysed in plasma samples. Inflammasomes are intracellular multiprotein complexes that function as sensors of danger-associated molecular patterns (DAMPS) or pathogen-associated molecular patterns (PAMP), which leads to the activation of proinflammatory caspases and the cleavage and release of proinflammatory cytokines. Inflammasomes generally comprise three proteins: 1) an NLR (nucleotide binding domain, leucine-rich repeat family member); 2) the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and 3) the pro-inflammatory cysteine aspartase, caspase-1 (Guo et al., 2015). The inflammasome regulates caspase-1 activity and, consequently, regulates the activation of the pro-inflammatory cytokines IL-1β and IL-18 (Fantuzzi and Dinarello, 1999). To date, there is only one report showing that caspase-1, NLRP3 mRNA expression, and NLRP3 protein levels are increased in PBMCs of MDD patients compared to HC subjects (Alcocer-Gómez et al., 2014).
Results here demonstrate that IL-18, caspase-1 and ASC-1, were significantly elevated in MDD patients compared to HC subjects (Table 3 and Supplementary Figure 3) suggesting that the upstream pathway responsible for the production of IL18 or IL-1β was elevated in MDD patients. However, IL-1β (Table 2) was not significantly different between the MDD and HC groups. These findings substantiate the idea that MDD patients exhibit elevated inflammatory responses.
Table 3:
Inflammasome protein levels in HC subjects and MDD patients
Non-Parametric Mann-Whitney U Test of Ranks, mean ±SD, All measures survived Bonferroni correction. ASC apoptosis associated speck-like protein containing a caspase recruitment domain, IL: interleukin
| HC Mean (pg/mL) n=24 |
MDD Mean (pg/mL) n=24 |
p-value | |
|---|---|---|---|
| ASC1 | 233.9±85.7 | 443.9±164.7 | p<0.001* |
| Caspase-1 | 0.7± 0.6 | 2.7 ±1.6 | p<0.001* |
| IL-18 | 178.4±82.2 | 258.0±119.7 | p=0.013* |
p<0.05
Plasma of MDD patients displays impaired activation of PBMCs
Because plasma from MDD patients exhibited increased levels of both pro- and anti-inflammatory cytokines, we tested the overall effects of the plasma from MDD patients on freshly isolated PBMCs from a healthy volunteer donor. Both pro- and anti-inflammatory cytokines have been shown to modulate PBMC activation (Sancho et al., 2005; Subauste et al., 1998; Wieland and Shipkova, 2016). Compared with plasma from HC subjects, addition of plasma from MDD patients on donor PBMCs led to a lower population of the CD86+CD11b+ cells (p=0.005) (Table 4 and Supplementary Fig 4B) in control PBMCs, indicating a reduced activation of monocytes/dendritic cells, and a reduced proportion of CD69+CD19+ (p=0.001) cell population (Table 4 and Supplementary Fig 4B), indicative of reduced activated B cells. There was no change in the overall populations of CD4+ (p=0.292), CD8+ (p=0.531), CD19+ (p=0.913) and CD11b+ (p=0.351) cells from the donor PBMCs exposed to the plasma of MDD patients or HC subjects (Supplementary Table 2A). Furthermore, the percent of activated donor PBMCs exposed to plasma from MDD patients or HC subjects were similar after treatment with lipopolysaccharide (LPS) (supplementary Table 2B), suggesting that the reduced activation of PBMCs in the presence of the MDD patient’s plasma was not the results of an incapacity of the PBMCs to become activated. The amount of lymphocyte activation marker CD69 expression on CD8+ cells, Tregs (CD4+CD25+), and B cells was also significantly reduced after LPS treatment confirming there was less activation of the donor PBMCs by LPS in the presence of plasma from MDD patients compared to the plasma of HC subjects (Supplementary Table 2C). We also excluded any potential activating effect of the HC plasma on PBMCs, as PBMCs that did not receive any plasma, have similar PBMC activation level as PBMCs exposed to plasma from HC subjects (Supplementary Table 2D). We excluded any change in cell viability as the counts and frequency data were similar (data not shown). CD4+CD45RO+CD69- (p<0.001) memory cells from the healthy donor were also reduced after exposure to MDD patient’s plasma compared to plasma from HC subjects (Table 4), suggesting that plasma of MDD patients exhibit properties that also inhibit cellular memory formation.
Table 4:
Frequency of activated PBMC cells after stimulation with plasma of HC subjects and of MDD patients
Non-Parametric Mann-Whitney U Test of Ranks, mean ±SD, All statistical significant results survived the Bonferroni correction. CD69 and CD86 are activation markers of the adaptive and the innate immune systems, respectively. CD4+CD25+ are T regulatory cells. CD4+CD45+CD69- are memory cells, and CD4+CD69+CD45RO- are effector T cells.
| HC Mean (%) n=27 |
MDD Mean (%) n=40 |
p-value | |
|---|---|---|---|
| CD4+CD25+CD69+ | 28.9±8.5 | 24.4±7.3 | p=0.242 |
| CD4+CD45RO+CD69- | 34.4±5.0 | 30.1±4.5 | p<0.001* |
| CD4+CD69+CD45RO- | 3.0±4.2 | 2.2±1.6 | p=0.682 |
| CD8+CD69+ | 25.3±8.0 | 20.9±7.7 | p=0.182 |
| CD19+CD69+ | 25.4±5.3 | 21.4±3.7 | p=0.001* |
| CD11b+CD86+ | 0.7±0.3 | 0.5±0.18 | p=0.005* |
*p < 0.05
Taken together, these data suggest that MDD patients experienced a relatively major remodelling of the cytokine landscape, accompanied by an overall immunosuppressive phenotype at the cellular level.
Antidepressant treatment effects on the inflammatory response
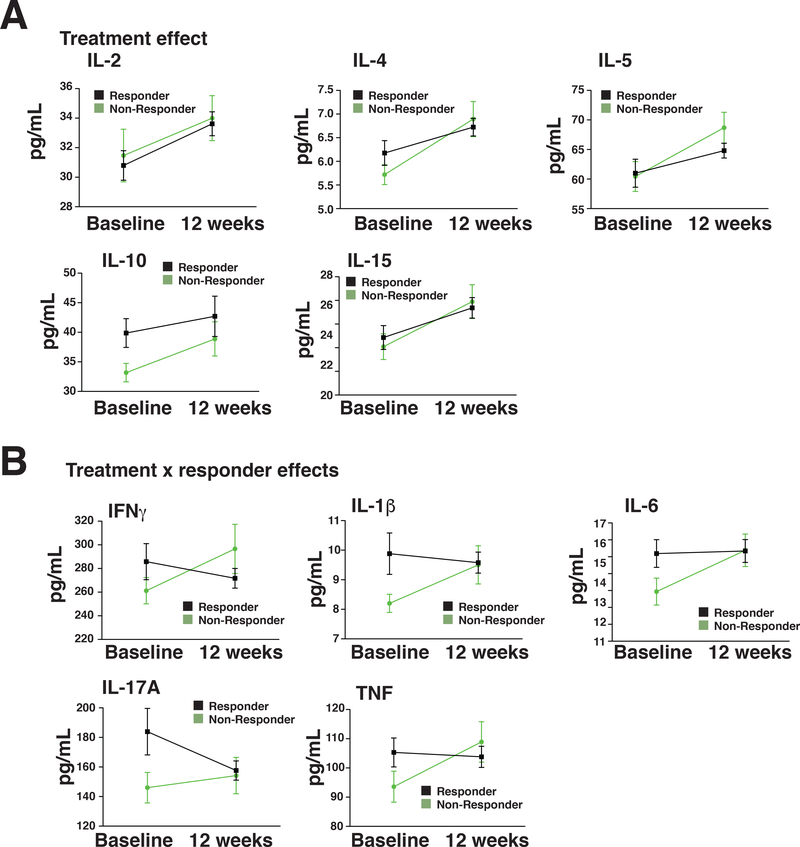
We tested if antidepressant treatments for 12 weeks altered the inflammatory molecule profile and whether there were differences between responders and non-responders. Due to the modest number of subjects who completed treatment in each arm (33 non-responders and 71 responders), we pooled all the treatments together as there were no differences in the cytokine levels and response outcome among the 3 treatments (Supplementary Table 5) or on PBMC counts (Supplementary Table 7). We hypothesized that MDD responders would have reduced proinflammatory cytokine levels compared with MDD non-responders after 12-weeks of treatment. To test this hypothesis, we conducted a 2 X 2 (Responder by Time) multivariate analyses of variance (MANOVA) with IL-6, IL-1β, TNF, IFNγ, and IL-17A serving as outcome measures. No covariates were entered into the model because Responders and Non-Responders did not differ on demographic factors such as age, gender, or BMI (Supplementary Table 3). We focused on the Responder X Time interaction term because this would reveal any potential differences in patterns of change of proinflammatory cytokines in responders versus non-responders, over time. Following a statistically significant multivariate test of significance, individual univariate analyses were conducted for each pro-inflammatory cytokine. As predicted, there was a Responder X Time effect [Wilks’ lambda F(1,99)= 5.84; p=0.017] but there was no main effect for Time [Wilks’ lambda [F(1,99)=0.46; p=0.5] or Responder [Wilks’ lambda F(1,99)=0.74; p=0.392]. Univariate analyses on the interaction terms for each proinflammatory cytokines revealed that each of the five proinflammatory cytokines reached statistical significance (IFNγ, p=0.027; IL-1β, p=0.026; IL-6, p=0.041, IL-17A, p=0.035 and TNF, p=0.017) (Table 5A and Supplementary Fig 5A); furthermore, the pro-inflammatory markers tended to rise in non-responders during treatment while they were relatively lower or stabilized in treatment responders (Figure 1B).
Table 5:
Pro- and anti-inflammatory cytokine levels in responders and non-responders
A: Proinflammatory cytokine levels in responders and non-responders: Repeated measure MANOVA, mean ±SD, *p<0.05, IFNγ: interferonγ, IL: interleukin, TNF: tumor necrosis factor. B: Anti-inflammatory cytokine levels in responders and non-responders Repeated measure MANOVA, mean ±SD
| Baseline Responder Mean (pg/mL) (n = 71) |
Baseline Non-Responder Mean (pg/mL) (n = 33) |
12 week Responder Mean (pg/mL) (n = 71) |
12 week Non- Responder Mean (pg/mL) (n = 33) |
Effect Time F p-value |
Effect Responder F p-value |
Effect Time x Responder F p-value |
|
|---|---|---|---|---|---|---|---|
| A IFNγ | 287.7±142.3 | 248.0±67.5 | 271.6±70.4 | 296.5±119.5 | 1.28 p=0.261 |
0.178 p=0.674 |
5.05 p=0.027* |
| IL1β | 10.2±6.6 | 7.9±1.9 | 9.6±3.0 | 9.5±3.7 | 1.092 p=0.299 |
2.060 p=0.154 |
5.096 p=0.026* |
| IL-6 | 15.5±7.7 | 12.5±5.3 | 15.4±5.6 | 15.4±5.3 | 3.41 p=0.068 |
1.85 p=0.177 |
4.3 p=0.041* |
| IL-17A | 189.9±147.3 | 134.0±56.7 | 157.2±54.0 | 154.9±73.9 | 0.221 p=0.639 |
2.93 p=0.09 |
4.56 p=0.035* |
| TNF | 108.2±45.5 | 91.4±33.1 | 103.7±30.4 | 108.8±39.7 | 2.07 p=0.154 |
0.779 p=0.379 |
5.93 p=0.017* |
| B IL2† | 30.0±8.9 | 30.8±10.5 | 33.1±5.9 | 33.5±6.5 |
5.97 p=0.018* |
0.202 p=0.655 |
0 p=0.99 |
| IL-4 | 6.2±2.4 | 5.5±1.3 | 6.7±1.5 | 6.8±2.1 |
11.6 p=0.001* |
0.884 p=0.35 |
3.05 p=0.084 |
| IL-5 | 61.6±21.9 | 58.1±15.6 | 64.8±10.5 | 68.6±14.9 |
10.5 p=0.002* |
0.004 p=0.951 |
3.09 p=0.083 |
| IL-10 | 40.1±22.3 | 32.5±9.7 | 42.7±28.7 | 38.9±16.6 |
5.05 p=0.027* |
1.74 p=0.19 |
0.913 p=0.342 |
| IL-15†† | 23.4±5.4 | 21.6±5.4 | 25.2±5.7 | 25.1±5.7 |
6.64 p=0.013* |
0.541 p=0.466 |
0.573 p=0.453 |
p<0.05, IL: interleukin
31 responders-21 non-responders
28 responders-21 non-responders
Figure 1: Anti-inflammatory cytokines increase in both responders and non-responders whereas proinflammatory cytokines are stabilized in responders but increased in non-responders.
Anti-inflammatory cytokines (A) and proinflammatory cytokines (B) were measured in the plasma of responders and non-responders, and the means were presented at baseline and 12 weeks after treatment.
We conducted a similar repeated measures MANOVA on anti-inflammatory cytokines (IL-4, IL-5; IL-10). IL-2 and IL-15 were not included because of significant missing data. There was no Responder X Time effect [Wilks’ lambda F(1,102)=2.56; p=0.087], but there was a main effect for Time [Wilks’ lambda [F(1,102)=12.11; p<0.001]. There was no Responder effect [Wilks’ lambda F(1,102)=0.78; p=0.378]. Post-hoc tests on the main effect for time revealed the 3 cytokines (IL-4, p=0.001; IL5, p=0.002; and IL-10, p=0.027) generally thought to be anti-inflammatory cytokines were elevated in response to antidepressant treatments (Table 5B; Figure 1A and supplementary Fig 5B). There were only 31 responders and 21 non-responders who had a detectable level of IL-2 and a similar number of participants who had a detectable level of IL-15. The results of ANCOVA analyses revealed an identical main effect as observed on the MANOVA analyses of other anti-inflammatory markers. For IL-2 and IL-15, there was a statistically significant time effect [F(1,47)= 5.97; p=0.018] and F(1,47)=6.6; p=0.013], respectively. No Responder or Responder X Time Interaction effects were observed. Taken together, these results indicate that depressed patients undergoing 12 weeks of treatment have increases in anti-inflammatory markers independent of treatment response.
The pattern of results suggested by these data indicate that antidepressant treatments in general, promote anti-inflammatory cytokine production and inhibition of some pro-inflammatory cytokines (Supplementary Table 4 and Supplementary Fig 5 and 6).
We conducted exploratory analyses and did not find any difference between responders and non-responders in the activation of the inflammasome pathway (Supplementary Table 6), or in the proportion of activated CD4, CD8, B cells and CD11b cells (Supplementary Fig 7), suggesting that antidepressant treatments might target pathways downstream of cytokines rather than their production.
However, memory T cells (CD4+CD45RO+CD69−) were significantly lower in responders compared to non-responders (Table 6). Associated with the reduced memory T cells, we found no change in the frequency of cells expressing the activation marker CD69 (Table 6), but reduced expression of CD69 at the cell level, on CD8+ cells and B cells in responders compared to non-responders (Table 7). In contrast, CD69 expression on Tregs, though significantly lower in responders compared to non-responders, did not survive the Bonferroni correction, suggesting that lower activation of T and B cells might be associated with lower memory.
Table 6:
Frequency of activated PBMC cells after receiving plasma of responders and non-responders
Repeated measure ANCOVA, after Bonferroni correction, mean ±SD.
| Responder Baseline N=20 |
Non-Responder Baseline N=20 | Responder 12 weeks N=20 | Non-Responder 12 weeks N=20 | Time effect F p-value |
Responder effect F p-value |
Time x responder effect F p-value |
|
|---|---|---|---|---|---|---|---|
| CD4+CD25+CD69+ | 23.6±11.1 | 26.9±12.7 | 24.8±10.5 | 24.8±13.4 | 0.02 p=0.8 |
0.49 p=0.49 |
0.30 p=0.56 |
| CD4+CD45RO+CD69− | 27.1±4.8 | 33.3±3.4 | 26.6±3.9 | 31.9±3.3 | 2.3 p=0.14 |
20 p<0.001* |
0.57 p=0.45 |
| CD4+CD69+CD45RO- | 3.9±2.3 | 4.6±2.6 | 4.1±3.1 | 4.3±2.7 | 0.11 p=0.73 |
0.07 p=0.78 |
0.22 p=0.64 |
| CD8+CD69+ | 19.2±8.7 | 23.2±11.5 | 20.5±8.9 | 22.6±9.7 | 0.035 p=0.85 |
1.5 p=0.21 |
0.21 p=0.65 |
| CD19+CD69+ | 20.5±3.3 | 22.2±3.9 | 20.7±4.3 | 20.7±3.9 | 0.67 p=0.42 |
0.77 p=0.39 |
1.1 p=0.3 |
| CD11b+CD86+ | 0.5±0.1 | 0.5±0.1 | 0.6±0.1 | 0.6±0.2 | 1.9 p=0.18 |
0.23 p=0.63 |
0.33 p=0.57 |
p<0.005
Table 7:
CD69 expression (MFI) in PBMC cells receiving plasma from HC or of depressed patients
Repeated measure ANCOVA
| Responder Baseline N=20 |
Non-Responder Baseline N=20 |
Responder 12 weeks N=20 |
Non-Responder 12 weeks N=20 |
Time effect F p-value |
Responder effect F p-value |
Time x responder effect F p-value |
|
|---|---|---|---|---|---|---|---|
| CD4+CD25+CD69+ | 296±127 | 313±142 | 270±121 | 318±165 | 0.5 p=0.82 |
4.8 p=0.03* |
0.01 p=0.93 |
| CD4+CD45RO+CD69- | 677±107 | 627±116 | 579±80 | 614±139 | 2.5 p=0.12 |
1.7 p=0.20 |
0.6 p=0.44 |
| CD8+CD69+ | 973±255 | 1171±473 | 952±308 | 1043±358 | 0.001 p=0.997 |
7.1 p=0.01* |
0.54 p=0.47 |
| CD19+CD69+ | 1636±174 | 1816±248 | 1619±184 | 1704±217 |
6.3 p=0.014 |
12.6 p=0.001* |
3.5 p=0.065 |
p<0.05
Discussion
In this comprehensive assessment of inflammatory markers in treatment-naïve MDD patients, who had never received an adequate trial of antidepressant medication or evidence-based psychotherapy for the treatment of depression, we observed elevated levels of 18 pro- and anti-inflammatory cytokines, chemokines, and growth factors, together with lower levels of 6 cytokines and chemokines compared to HC subjects. These widespread differences indicate that a major remodelling of the inflammatory landscape occurred in MDD patients. Associated with these changes in cytokines was an upregulation of the inflammasome proteins IL-18, caspase-1 and ASC in MDD patients. There was a decrease of the activation and cellular memory of healthy donor PBMCs exposed to plasma of MDD patients compared to the plasma of HC subjects, suggestive of cellular immunosuppressive properties of the plasma of MDD patients. Overall, these findings demonstrated that treatment-naïve MDD patients exhibited features of a significant remodelling of their cytokine profiles.
Although the idea that depressed patients have increased pro-inflammatory and anti-inflammatory cytokines is now well-accepted (Dowlati et al., 2010), the novelty of the present study is based on the measurement of 27 cytokines in a unique population of treatment-naïve MDD patients. Most studies measuring cytokines in MDD patients have only measured a limited number of cytokines per patient. We found that levels of most cytokines (23/27) were different in MDD patients and HC subjects, which contrasts with a recent study that reported that treatment resistant depression (TRD) patients have only 5 cytokines differentially expressed compared to HC subjects (Kiraly et al., 2017). The discrepancy between our study and others’ findings likely originates from the populations of MDD patients studied, and the fact that most previous studies focused on MDD patients that were previously exposed to multiple trials of antidepressant medications. Our population is unique because they are treatment-naïve and many were diagnosed for the first time, thereby providing a unique group for interrogation the role of the inflammatory response in MDD patients. Surprisingly, and in contrast to recent findings proposing that only a subgroup of MDD patients exhibit increased inflammatory markers (Raison et al., 2013), we found that the majority of MDD patients have elevated inflammatory markers above the mean of the HC subjects. Moreover, this increase in the cytokine levels occurred early in the disease as 5 cytokine (IL-2, IL-10, IL-12, IL15 and IL-17A), 1 chemokine (MIP1a/CCL3) and 2 growth factor (GM-CSF, FGF) levels were elevated in MDD patients within their first episode of the disease compared to MDD patients who experienced several episodes of the disease (data not shown).
Consistent with an increased production of cytokines in MDD patients (Dowlati et al., 2010; Howren et al., 2009; Köhler et al., 2017; Liu et al., 2012; Maes et al., 2009; Miller et al., 2009), the inflammasome pathway was also induced in MDD patients compared to healthy controls. There were differences in IL-18, ASC-1 and caspase-1 levels, but not in IL-1β levels in MDD patients compared to HC subjects. Caspase-1 and ASC-1 might possibly be the limiting factors in the regulation of inflammasome activation, whereas the IL-18 and IL-1β are produced in excess (Marshall et 1999, Puren et al 1998) or encounter other regulatory interactions downstream of ASC-1 and capase-1, such as potassium efflux or cathepsin release (Guo et al 2015). This suggests that the cytokine production machinery is turned on in MDD patients, since the inflammasome is primed by an NF-KB dependent stimulus and most cytokines that are increased in MDD patients do not depend on the inflammasome pathway, but on the NF-KB pathway since NF-KB is considered the main transcription factor controlling cytokine synthesis (Martinon et al., 2002). Importantly, inflammasome activation appears to bridge the gap between immune activation and metabolic danger signals or stress exposure, which are key factors in the pathogenesis of MDD (Dantzer et al., 2008b; Miller et al., 2009). Our study suggests that activation of the inflammasome is associated with the subsequent proteolysis and release of the pro-inflammatory cytokine IL-18 in MDD patients.
To test the overall effect of the cytokine profile remodelling on immune cells, we analysed the activation of healthy donor PBMCs in the presence of plasma of MDD patients or HC subjects. We found that the overall phenotype of plasma from MDD patients was immunosuppressive, as the PBMCs incubated with the plasma of MDD patients were less frequently activated than the PBMC cultured with plasma of HC subjects. This suggests that MDD patients experience an upregulation of the production of cytokines, whereas the overall effect of MDD plasma on immune cells are immunosupressive. It remains to be explored whether these immunosuppressive properties are due to the cytokine milieu favoring an anti-inflammatory response or other molecules present in the plasma providing immunosuppression. In addition, the fact that both pro- and anti-inflammatory cytokines are elevated in MDD patients but that the overall effect on healthy donor PBMCs is immunosuppressive, suggests that the anti-inflammatory response in MDD patients might be failing to terminate the inflammatory responses; this is consistent with the presence of chronic inflammation in MDD patients (Dantzer et al., 2008a).
Although CD69 is considered to be an activation marker of T and B cells, CD69 has ambiguous immunoregulatory functions (Sancho et al., 2005). In humans, its expression is associated with an ongoing immune response and tissue damage. Our data are consistent with a proinflammatory role of CD69 in MDD patients, though the role of CD69 in MDD patients will need to be confirmed in PBMCs isolated directly from MDD patients. Cytokines have been shown to modulate CD69 expression (Sancho et al., 2005), with anti-inflammatory cytokines such as IL-10 that reduces CD69 expression (Mocellin et al., 2003). Reduced levels of CD69 have been shown to be associated with reduced memory cell formation, which might explain why memory cells are reduced in the responders compared to non-responders (Shinoda et al., 2012). Nevertheless, a deeper characterization of the memory immune cells is needed to follow-up this finding.
Because MDD patients have a significant remodelling of their immune response, we also analysed the effect of antidepressant treatments on the immune response, as antidepressants have been shown to modulate both cytokine production (Kenis and Maes, 2002) and immune cells (Miller and Raison, 2016). Because there were no differential effects in pro-inflammatory or anti-inflammatory cytokines in the different types of depression treatments, we were able to model the profiles of MDD responders versus non-responders over the 12-week treatment period. The current results indicated that anti-inflammatory cytokines were increased in both MDD responders and non-responders, whereas only proinflammatory cytokines were stabilized in responders while they continued to increase in non-responders. This suggests that the antidepressant response may be associated with the ability of anti-inflammatory cytokines to block increasing levels of proinflammatory cytokines (e.g. IL-1β, IL-6, and TNF), while non-responders may have a defect in the response to anti-inflammatory cytokines. However, the mechanism of defective anti-inflammatory response remains to be determined.
It is unlikely that the difference in the production of cytokines between responders and non-responders is due to a reduction in the production of cytokines as upstream inflammasome activation is similar between responders and non-responders; thus, it is most likely due to an absence of the response to inflammatory termination signals. Consistent with this conclusion, the percent of CD86+CD11b+ cells, indicative of activated monocytes/dendritic cells cells, which represent activated cells of the innate immune response which in part regulate the inflammasome pathway (Guo et al., 2015), were similar between non-responders and responders. Furthermore, CD69, which is present on adaptive immune cells, was expressed similarly between non-responders and HC but it decreased in responders. Altogether, these findings suggest that the plasma of responders has anti-inflammatory and anti-activation properties that are absent in the non-responder group and are likely mediated by different cell types.
It is interesting to note that antidepressant actions, although likely expected to be different between escitalopram, duloxetine (Frampton and Plosker, 2007), and CBT (Hofmann et al., 2012), have similar effects on the immune response. Better mood outcomes were associated with a healthier immune response. It is possible that a direct central nervous system effect of the antidepressant medication and CBT influences the immune system response, perhaps by modulating HPA axis activity or the production of growth factors (McKay and Zakzanis, 2010). Because escitalopram and duloxetine are present in plasma, it is also possible that the medications directly affect the PBMCs (Greeson et al., 2016). However, because we found no difference between duloxetine, escitalopram and CBT in the activation levels of CD69, or cytokine levels, it is unlikely that the observed effects are due to a direct effect of the medication on PBMC activation, but rather results from downstream effects of the treatments leading to cytokine changes. In addition, it is possible that the absence of effects on the PBMC activation between responders and non-responders at the frequency level are due to the small changes of cytokine concentrations observed between these two groups. The differentiation of T cells, which is known to be regulated by cytokines (Zhu et al., 2009), might also be an important component to measure as T cells even though exhibiting the same level of activation might differentiate towards pro or anti-inflammatory subsets. It is particularly interesting that the regulatory T cells (CD4+CD25+), known to be anti-inflammatory cells, or CD8 cells or B cells expressing CD69 were reduced in responders compared to non-responders. This suggests that T and B cell activation markers might help to discriminate between patient’s response to antidepressant treatments, consistent with a recent study of (Grosse et al., 2016).
Taken together, the results of this study show a broad effect of depression on the immune system, although unknown confounds may contribute to some of the differences reported here and that causality was not directly tested. Interestingly, effective treatments for depression modulate the inflammatory response, although these effects might be the results of epiphenomena e.g. sleeplessness, stress, weight loss associated with depression. Effective treatments seem to target both the innate and adaptive immune system.
Supplementary Material
Highlights.
Treatment-naive depressed patients have elevated levels of inflammatory markers.
Overall, plasma of treatment-naïve depressed patients is immunosuppressive.
Defective anti-inflammatory response occurs in non-responders.
Acknowledgements
This work was supported by the NIH (MH077083, MH080880, MH0866901, AA024933 MH104656, MH110415). Forest Laboratories and Eli Lilly and Company donated the study medications escitalopram and duloxetine.
Footnotes
Declaration of Interests
Dr. Nemeroff discloses the following:
Research/Grants:
National Institutes of Health (NIH), Stanley Medical Research Institute Consulting (last three years):
Xhale, Takeda, Taisho Pharmaceutical Inc., Prismic Pharmaceuticals, Bracket (Clintara), Total Pain Solutions (TPS), Fortress Biotech, Sunovion Pharmaceuticals Inc., Janssen Research & Development LLC, Magstim, Inc., Navitor Pharmaceuticals, Inc., TC MSO, Inc., Intra-Cellular Therapies, Inc.
Stockholder:
Xhale, Celgene, Seattle Genetics, Abbvie, OPKO Health, Inc., Antares, BI Gen Holdings, Inc.
Scientific Advisory Boards:
American Foundation for Suicide Prevention (AFSP), Brain and Behavior Research Foundation (BBRF), Xhale, Anxiety Disorders Association of America (ADAA), Skyland Trail, Bracket (Clintara), Laureate Institute for Brain Research (LIBR), Inc.
Board of Directors:
AFSP, Gratitude America, ADAA
Income sources or equity of $10,000 or more:
American Psychiatric Publishing, Xhale, Bracket (Clintara), CME Outfitters, Takeda,
Magstim, Intra-Cellular Therapies, Inc.
Patents:
Method and devices for transdermal delivery of lithium (US 6,375,990B1)
Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2)
Speakers Bureau:
None
Dr. Craighead receives research support from NIH, is a board member of Hugarheill ehf, an Icelandic company dedicated to prevention of depression, receives book royalties from John Wiley, and is supported by the Mary and John Brock Foundation, and the Fuqua Family Foundation. He is a consultant to the George West Mental Health Foundation, and a member of the Scientific Advisory Boards of AIM for Mental Health and the ADAA.
Dr. Dunlop reports research support from Acadia Pharmaceuticals, Axsome Therapeutics, Janssen Pharmaceuticals, the National Institute of Mental Health
(NIMH), and Takeda Pharmaceutical Company.
Dr. Loewenstein receives research support from the National Institutes of Health and is a consultant with Mitsubishi Tanabe Pharma Development America.
Drs. De Rivero, Keane, and Dietrich are co-founders and managing members of InflamaCORE, LLC and have patents on inflammasome proteins as biomarkers of injury and disease as well as on targeting inflammasome proteins for therapeutic purposes.
Dr Beurel receives research support from the National Institutes of Health, and declares no competing interests.
Dr. Mayberg reports research support from NIMH, NINDS, and the Hope For Depression Research Foundation; and has licensed intellectual property to St Jude Medical Corp (now Abbott Labs).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, SánchezAlcazar JA, Fernández-Rodríguez A, and Cordero MD (2014). NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain, behavior, and immunity 36, 111–117. [DOI] [PubMed] [Google Scholar]
- Beck AT (1979). Cognitive therapy of depression (Guilford press; ). [Google Scholar]
- Brand FJ, Forouzandeh M, Kaur H, Travascio F, and de Rivero Vaccari JP (2016). Acidification changes affect the inflammasome in human nucleus pulposus cells. Journal of Inflammation 13, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, and Kelley KW (2008a). From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, and Kelley KW (2008b). From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, and Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biological psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, Kutner M, Nemeroff CB, Newport DJ, and Owens MJ (2012). Predictors of remission in depression to individual and combined treatments (PReDICT): study protocol for a randomized controlled trial. Trials 13, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Kelley ME, Aponte-Rivera V, Mletzko-Crowe T, Kinkead B, Ritchie JC, Nemeroff CB, Craighead WE, Mayberg HS, and Team PR (2017a). Effects of patient preferences on outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study. American Journal of Psychiatry 174, 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Rajendra JK, Craighead WE, Kelley ME, McGrath CL, Choi KS, Kinkead B, Nemeroff CB, and Mayberg HS (2017b). Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. American Journal of Psychiatry 174, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G, and Dinarello CA (1999). Interleukin-18 and interleukin-1β: two cytokine substrates for ICE (caspase-1). Journal of clinical immunology 19, 1–11. [DOI] [PubMed] [Google Scholar]
- Frampton JE, and Plosker GL (2007). Duloxetine. CNS drugs 21, 581–609. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr., Krishnan KR, van Zyl LT, Swenson JR, Finkel MS , et al. (2002). Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 288, 701–709. [DOI] [PubMed] [Google Scholar]
- Greeson JM, Gettes DR, Spitsin S, Dubé B, Benton TD, Lynch KG, Douglas SD, and Evans DL (2016). The selective serotonin reuptake inhibitor citalopram decreases human immunodeficiency virus receptor and coreceptor expression in immune cells. Biological Psychiatry 80, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, and Bergink V (2016). Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology 233, 1679–1688. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, and Ting JPY (2015). Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Medicine 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, and Fang A (2012). The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive therapy and research 36, 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, and Suls J (2009). Associations of depression with Creactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, and Khandaker GM (2016). Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenis G, and Maes M (2002). Effects of antidepressants on the production of cytokines. International Journal of Neuropsychopharmacology 5, 401–412. [DOI] [PubMed] [Google Scholar]
- Kessler D, Sharp D, and Lewis G (2005). Screening for depression in primary care. Br J Gen Pract 55, 659–660. [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, Patel M, Hodes GE, Russo SJ, and Merad M (2017). Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Translational psychiatry 7, e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler CA, Freitas TH, Maes M, Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, and Herrmann N (2017). Peripheral cytokine and chemokine alterations in depression: a meta‐analysis of 82 studies. Acta Psychiatrica Scandinavica 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, and Krogh J (2014). Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and metaanalysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Kubera M, Maes M, Holan V, Basta-Kaim A, Roman A, and Shani J (2001). Prolonged desipramine treatment increases the production of interleukin-10, an anti-inflammatory cytokine, in C57BL/6 mice subjected to the chronic mild stress model of depression. Journal of Affective Disorders 63, 171–178. [DOI] [PubMed] [Google Scholar]
- Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu M-C, Ortonne J-P, Gordon KB, and Kimball AB (2010). Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. Journal of the American Academy of Dermatology 63, 457–465. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, and Vedder H (2000). Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22, 370–379. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC-M, and Mak A (2012). Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. Journal of Affective Disorders 139, 230–239. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Feagan BG, Colombel J-F, Rubin DT, Wu EQ, Andrew PY, Pollack PF, Chao J, and Mulani P (2008). Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. The American Journal of Gastroenterology 103, 3132–3141. [DOI] [PubMed] [Google Scholar]
- Maes M (1995). Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuro-Psychopharmacology and Biological Psychiatry 19, 11–38. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A-H, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, and Scharpé S (1999). Negative immunoregulatory effects of antidepressants: inhibition of interferon-γ and stimulation of interleukin-10 secretion. Neuropsychopharmacology 20, 370–379. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, and Maj M (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic Brain Disease 24, 27–53. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, and Tschopp J (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Molecular Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- McKay MS, and Zakzanis KK (2010). The impact of treatment on HPA axis activity in unipolar major depression. Journal of Psychiatric Research 44, 183192. [DOI] [PubMed] [Google Scholar]
- Menter A, Augustin M, Signorovitch J, Andrew PY, Wu EQ, Gupta SR, Bao Y, and Mulani P (2010). The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. Journal of the American Academy of Dermatology 62, 812–818. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, and Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, and Raison CL (2015). Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA psychiatry 72, 527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, and Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology 16, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Panelli MC, Wang E, Nagorsen D, and Marincola FM (2003). The dual role of IL-10. Trends in immunology 24, 36–43. [DOI] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, and Schatzberg AF (2016). Major depressive disorder. Nature Reviews Disease Primers 2, 16065. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, and Miller AH (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Gómez M, and Sánchez-Madrid F (2005). CD69 is an immunoregulatory molecule induced following activation. Trends in Immunology 26, 136–140. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, and Duman RS (2011). Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36, 2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, and Sanacora G (2008). Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological psychiatry 64, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Tokoyoda K, Hanazawa A, Hayashizaki K, Zehentmeier S, Hosokawa H, Iwamura C, Koseki H, Tumes DJ, and Radbruch A (2012). Type II membrane protein CD69 regulates the formation of resting Thelper memory. Proceedings of the National Academy of Sciences 109, 7409–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Worm M, Soong W, Blauvelt A, Eckert L, Wu R, Ardeleanu M, Graham N, Pirozzi G, and Sutherland ER (2015). Dupilumab improves patient-reported outcomes (PROs) in a Phase 2 study in adults with moderateto-severe atopic dermatitis. Journal of Allergy and Clinical Immunology 135, AB167. [Google Scholar]
- Sluzewska A, Sobieska M, and Rybakowski JK (1997). Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology 35, 123–127. [DOI] [PubMed] [Google Scholar]
- Subauste CS, de Waal Malefyt R, and Fuh F (1998). Role of CD80 (B7. 1) and CD86 (B7. 2) in the immune response to an intracellular pathogen. The Journal of Immunology 160, 1831–1840. [PubMed] [Google Scholar]
- Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EHZ, Gandra SR, Shi Y, and Kricorian G (2013). Patient‐reported outcomes in moderate‐to‐severe plaque psoriasis with scalp involvement: results from a randomized, doubleblind, placebo‐controlled study of etanercept. Journal of the European Academy of Dermatology and Venereology 27, 125–128. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, and Zitnik R (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. The Lancet 367, 29–35. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, and Greengard P (2011). Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by anti-inflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences 108, 9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland E, and Shipkova M (2016). Lymphocyte surface molecules as immune activation biomarkers. Clinical biochemistry 49, 347–354. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, and Paul WE (2009). Differentiation of effector CD4 T cell populations. Annual review of immunology 28, 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.