Abstract
Purpose
An Artificial Placenta (AP) utilizing extracorporeal life support (ECLS) could avoid the harm of mechanical ventilation (MV) while allowing the lungs to develop.
Methods
AP lambs (n=5) were delivered at 118 days gestational age (GA; term=145 days) and placed on venovenous ECLS (VV-ECLS) with jugular drainage and umbilical vein reinfusion. Lungs remained fluid-filled. After 10 days, lambs were ventilated. MV control lambs were delivered at 118 (“early MV”; n=5) or 128 days (“late MV”; n=5), and ventilated. Compliance and oxygenation index (OI) were calculated. After sacrifice, lungs were procured and H&E-stained slides scored for lung injury. Slides were also immunostained for PDGFR-α and α-actin; alveolar development was quantified by the area fraction of alveolar septal tips staining double-positive for both markers.
Results
Compliance of AP lambs was 2.79±0.81 Cdyn compared to 0.83±0.19 and 3.04±0.99 for early and late MV, respectively. OI in AP lambs was lower than early MV lambs (6.20±2.10 vs. 36.8±16.8) and lung injury lower as well (1.8±1.6 vs. 6.0±1.2). Double-positive area fractions were higher in AP lambs (0.012±0.003) than early (0.003±0.0005) and late (0.004±0.002) MV controls.
Conclusions
Lung development continues and lungs are protected from injury during AP support relative to mechanical ventilation.
Keywords: Artificial Placenta, extracorporeal life support, prematurity, lung development
1. Introduction
One in ten infants born in the United States each year is premature.[1] About 5% of these are deemed extremely low gestational age newborns (ELGANs), defined as neonates born at ≤28 weeks estimated gestational age (EGA).[2] Morbidity and mortality are unacceptably high in this population, with survival well below 50% for infants born before 24 weeks. Morbidities in these patients arise from the immaturity of multiple organ systems, perhaps most notably the lungs.[3–6] Fetal lungs transition from the canalicular to saccular phase of development at approximately 22-24 weeks EGA; this currently marks the cusp of viability for preterm infants.[7] The current standard of care for respiratory support of these ELGANs is mechanical ventilation. However, this technology is often inadequate, or even harmful to the neonate, leading to pulmonary injury and arrested lung development.[8–10]
A solution to this problem is the development of an Artificial Placenta (AP). This technology consists of four components: 1) extracorporeal life support (ECLS), 2) maintenance of fluid-filled lungs, 3) avoidance of mechanical ventilation, and 4) preservation of fetal circulation. Given that pulmonary morbidity decreases with increasing gestational age[3], the goal of the AP is to allow premature lungs to grow and develop to the point where they can provide adequate gas exchange to support the neonate, with or without the safe assistance of mechanical ventilation. Prior experimentation in our laboratory has shown that the AP can support otherwise moribund premature lambs for over seven days.[11] We more recently have sought to determine if lung development continues during AP support, and if so, if this continued development allows for transition from AP support to mechanical ventilatory support. We hypothesized that, in an ovine model of prematurity, AP support would allow for continued lung development, and that as a result, safe transition to mechanical ventilation is possible.
2. Methods
The sheep in this experiment were treated in compliance with the Guide for Care and Use of Laboratory Animals (US National Institutes of Health publication No. 85-23, National Academy Press, Washington D.C., revised 1996) and all methods were approved by the University of Michigan Institutional Animal Care and Use Committee (protocol 00007211).
Premature lambs were placed in three experimental groups, all with n=5: AP, early MV, and late MV. Of note, the AP lambs in this study represent a cohort within a larger group of experimental animals (n=12) in whom the goal was to transition from mechanical ventilation following AP support. The other animals within this group were not transitioned to mechanical ventilation following support and were used for alternative experimentals, the results of which are not reported in the present study.
2.1. AP Lambs
AP lambs of EGA 118±3 days were delivered via midline laparotomy and transverse hysterotomy. This gestational age was selected as previous experimentation has determined that fetal sheep lung development at this stage is analogous to that of a 24-week human fetus.[11] While the lamb was supported by the native placenta, the right jugular vein was exposed and cannulated with a 10-14Fr drainage cannula (Terumo: Ann Arbor, MI). A 10-12Fr reinfusion cannula was placed in the umbilical vein, and the circuit was completed using ¼” tubing (Tygon: Lima, OH), a roller pump (MC3: Ann Arbor, MI), and oxygenator/heat exchanger (either Capiox Baby Rx, Terumo: Ann Arbor MI, or Medos HiLite, Xenios: Heilbronn, Germany; Figure 1). The umbilical cord was then divided and venovenous (VV) ECLS was initiated. A 5Fr triple lumen venous line was placed in the second umbilical vein and used for IV fluid and medication administration, and a 5Fr umbilical arterial line (both lines from Covidien-Medtronic: Minneapolis, MN) was placed in the umbilical artery for hemodynamic monitoring and arterial blood gas (ABG) sampling. The lambs were then intubated, and the endotracheal tube (ETT) was either filled with amniotic fluid and capped (n=1), connected to pressure line primed with Ringer’s Lactate (LR) at 4-6 mmHg (n=3), or filled with perfluorodecalin (Origen: Austin, TX) to a visible meniscus in the ETT (n=1), as these strategies would maintain fluid-filled lungs and positive intra-pulmonary pressure akin to that seen in the intra-uterine environment.[12] ECLS was managed according to goal ABG parameters: pH 7.30-7.45, pCO2 35-50, pO2 25-35, and SpO2 60-75. AP support was continued for 10 days. Lambs were given total parenteral nutrition (TPN), empiric piperacillin-tazobactam, and solumedrol 0.63 mg/kg every 6 hours (due to low baseline cortisol levels in premature lambs). Heparin sulfate was administered by IV infusion, starting at 100U/hr, and titrated to a goal activated clotting time (ACT) of 200-250, checked every 2-3 hours. Prostaglandin E1 (0.2 mcg/kg/min) was administered continuously to prevent ductus arteriosus closure. Buprenorphine (0.3 mg) and diazepam (2.5 mg) were given sparingly as needed for pain and/or agitation, and lambs were not paralyzed. Echocardiography was performed daily to assess for patency of the ductus arteriosus.
Figure 1.

Schematic of the Artificial Placenta. Blood is drained from the right jugular vein by a collapsible-tubing roller pump (M-pump, MC3: Ann Arbor, MI) and propelled to an oxygenator/heat exchanger (Medos HiLite, Xenios: Heilbronn, Germany), then returned via an umbilical vein. The second umbilical vein is accessed for IV fluid and medication administration, and an umbilical arterial line is placed for hemodynamic monitoring and blood gas sampling. The lamb is intubated and the lungs remain filled with amniotic fluid by clamping the endotracheal tube. Ao – aorta; DV – Ductus venosus; IJV – internal jugular vein; IVC – inferior vena cava; RA – right atrium; SVC – superior vena cava
After 10 days, the lambs’ lungs were suctioned of fluid, exogenous surfactant (Survanta – Abbvie: North Chicago, IL) was administered to maximize initial compliance and oxygenation and to represent standard therapy [11], prostaglandin was discontinued, and mechanical ventilation (MV) was initiated using a Maquet Servo-I pediatric ventilator (Maquet Holding B.V. and Co., Rastatt, Germany). Initial ventilator settings included (pressures in cmH2O): respiratory rate=60, positive end-expiratory pressure (PEEP)=5, inspiratory pressure (IP)=15, and fraction of inspired oxygen (FiO2)=40%, and were adjusted based on arterial blood gas values. Concurrent AP and MV support were continued for at least a 24 hours of lung recruitment, during which time surfactant was administered every 8 hours for a total of three doses. At this point, AP support was withdrawn, and the lamb was supported by MV alone. Pressure-controlled MV was continued until development of non-correctible complications of prematurity arose, at which point the lamb was sacrificed.
2.2. MV Lambs
Early MV (delivered at 118±3 days EGA) or late MV (delivered at 128±2 days) lambs were delivered in the same method as AP lambs. They were immediately intubated, the lungs were suctioned of fluid, surfactant was administered (for the reasons previously described), and pressure-controlled mechanical ventilation was initiated at the same initial settings as detailed in AP lambs. Goal ABG values were pH 7.25-7.35, pCO2 40-60 mmHg, and SpO2 > 85. If peak airway pressures exceeded 25 cmH2O, permissive hypercapnia was practiced with administration of IV alkaline therapy. Lambs were given TPN and solumedrol at regular intervals. Support was continued until lung failure, or until 48 hours, at which point the animal was sacrificed.
2.3. Measures of Lung Function
During MV support in all experimental groups, ventilator data were recorded every half hour, including tidal volume, peak inspiratory pressure (PIP), PEEP, mean airway pressure (MAP), and FiO2. ABGs were collected every 0.5-2 hours. Calculated measures of lung function were dynamic compliance (Cdyn = ΔV/ΔP) and oxygenation index (OI = (FiO2 * MAP) / paO2). Compliance was calculated every 30 minutes starting 30 minutes after initiation of support, and OI with every ABG. OI was only calculated following full withdrawal of AP support.
2.4. Necropsy and Lung Preparation
Following sacrifice, lungs were procured and the pulmonary vasculature flushed with phosphate-buffered saline plus EDTA, followed by 10% formalin. The airways were then inflated with 10% formalin to a pressure of 30 cmH2O. Five micron sections were taken from the left lower lobe for histopathologic evaluation and immunofluorescence.
2.5. Histopathology and Lung Injury
Lung slides were stained with hematoxylin and eosin (H&E) and evaluated by a single pathologist who was blinded to experimental group, who assigned each slide an injury severity score.[13] Variables scored were alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, edema, atelectasis, and necrosis. Each variable was scored using a 0- to 4-point scale: No injury scored 0, and a point was added for every 25% of the field identified as injured, up to a score of 4 for 100%.
2.6. Immunofluorescence
Since alveolarization in sheep begins around 120 days gestation [14, 15], we chose to use alveolar secondary septal formation as a marker of lung development. The tips of these secondary septal crests are defined by the presence of platelet-derived growth factor receptor-α (PDGFR-α) and α-smooth muscle actin double-positive myofibroblasts.[16–18] Lung sections were labeled with antibodies for anti-PDGFR-α (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-α-smooth muscle actin (Cy3 conjugate, Sigma Aldrich, St Louis, MO). The anti PDGFR-a was conjugated to AlexaFluor 488 N-hydroxy succinimidyl ester (Fisher Scientific: Hampton, NH). Nuclei were visualized with Hoechst 33342 (Sigma-Aldrich). The tips of alveolar secondary crests were identified by the co-localization of PDGFR-α and α-actin. Alveolarization was quantified using a morphometric technique. An 18×13 point grid was laid over lung micrograph images using the public domain NIH Image program (developed at the U.S. National Institutes of Health, available at http://rsb.info.nih.gov/nih-image). The ratio of points falling on double-positive alveolar tips compared to the total number of reference points (area fraction) was calculated, with a larger area fraction signifying more advanced lung development. For each sample, five fields at 40x magnification were used to calculate area fraction, and these values were averaged.
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad: La Jolla, CA) and SPSS v. 24.0 (IBM Corp, Armonk, NY). Comparisons were made using ANOVA, with Tukey’s used for individual comparisons in cases of statistical difference. A p-value<0.05 was considered significant.
3. Results
3.1. Support, Hemodynamics, and Organ Function
Total survival for AP lambs was 294±41 hours, compared to 7.0±1.2 hours for early MV lambs and 40±16 hours for late MV lambs (p<0.0001). No early MV lambs survived longer than 8 hours. Four late MV lambs were electively sacrificed (three at 48 hours, one at 41 hours); the fifth died after 12 hours due to tension pneumothorax. AP lambs spent 230±11 hours on pure AP support, followed by 37±36 hours of concurrent ECLS and mechanical ventilation (recruitment period), and 11±10 hours of pure MV support. One lamb was unable to be weaned off ECLS completely, and so OI was not calculated for this lamb. The longest total survival was 341 hours, and longest period of pure MV support after discontinuation of AP support was 26 hours. The support durations, complications, and outcomes for each AP-supported lamb are displayed in Table 1, as is the status of the ductus arteriosus on day 9 of support (prior to initiation of mechanical ventilation). Ductal patency could not be assessed in lamb #5 as the intra-pulmonary perfluorocarbons obscured visualization with echocardiography. Lambs gained 0.43±0.77 kg during support, for an average growth of 44±43 g/day. There were no signs of hemorrhagic complications in any lambs during AP support except for one case of umbilical vein erosion following AP discontinuation, and there was no evidence of intracranial hemorrhage at the time of autopsy in any lamb.
Table 1.
Support durations, complications, and outcomes of Artificial Placenta-supported Lambs.
| Airwaya | Ductus (Day 9) | Support Times (hours)
|
Complications during AP Support | Outcome | |||
|---|---|---|---|---|---|---|---|
| AP | AP+MV | MV alone | |||||
| 1 | LR | Patent | 231 | 91 | 7 | Heat exchanger malfunction causing hypothermic arrest → CPR with ROSCb | Fatal hemorrhage due to perforated umbilical vein |
| 2 | LR | Patent | 222 | 17 | 13 | None | Agitation on ventilator → dyssynchrony and cardiac arrest |
| 3 | LR | Closed | 240 | 25 | 26 | Hypernatremia | Mucus plugging in ETT → acute respiratory failure |
| 4 | TO | Patent | 242 | 15 | 0 | Renal failure of unclear etiology | Hyperkalemia → cardiac arrest |
| 5 | PFC | Unable to assess | 217 | 115 | 9 | Anemia | Intestinal perforation/NEC |
Airway management strategies: LR – ETT filled with lactated Ringer’s and attached to pressure-transducing line; TO – ETT filled with amniotic fluid, then clamped, resulting in tracheal occlusion; PFC – ETT filled with perfluorodecalin and attached to pressure-transducing line.
ROSC – Return of spontaneous circulation.
Hemodynamic, gas exchange, and organ function data during AP support are displayed in Figure 2. Heart rate and mean arterial pressure remained stable throughout support, as did arterial pCO2, pO2, and SaO2, and lambs required slight increases in circuit flow over the course support to maintain adequate oxygen delivery (Figure 2A-C). Lactate values were stable at approximately 2 mmol/L over the course of AP support (Figure 2D). Lambs urinated spontaneously, and though blood urea nitrogen (BUN) tended to increase, creatinine decreased. Total bilirubin (Tbil) remained stable during AP support, and though alanine aminotransferase (ALT) increase slightly after 7-8 days, this remained within physiologic limits (Figure 2E-F). During support, all AP lambs moved all limbs spontaneously and in response to external stimuli.
Figure 2.

Hemodynamic, Gas Exchange, and Laboratory Data during Artificial Placenta Support. Data pictured only include values obtained prior to initiation of mechanical ventilation for all lambs (217 hours; n=5), due to variation in timing of ventilation initiation, amount of ventilator support, and duration of support. A) Heart rate (HR) and Mean arterial pressure (MAP). B) Circuit flow (weight-normalized). C) Umbilical artery blood gas values: pCO2, pO2, and SaO2. D) Arterial lactate. E) Measures of renal function: blood urea nitrogen (BUN) and creatinine. F) Measures of liver function: alanine aminotransferase (ALT) and total serum bilirubin (Tbil).
3.2. Lung Function
Lung compliance and OI data are pictured in Figure 3. Over the course of MV support, AP lambs demonstrated significantly higher average and maximum compliance than early MV lambs (2.8±0.7 vs. 0.8±0.2 Cdyn; p=0.002 and 4.3±1.3 vs. 1.0±0.4 Cdyn; p=0.002, respectively). Average and maximum compliance were similar between AP lambs and late MV controls (2.8±0.7 vs. 3.0±1.0 Cdyn; p=0.86 and 4.3±1.3 vs. 4.3±1.4 Cdyn; p=1.0, respectively). Similarly, average OI was significantly lower for AP lambs compared to early MV lambs (5.8±1.9 vs. 30.4±6.8; p=0.03); minimum OI appeared lower than early MV lambs but did not reach significance (3.1±0.4 vs. 10.7±7.6; p=0.08). No significant differences were observed in average or minimum OI between AP and late MV lambs (5.8±1.9 vs. 14.0±18.8; p=0.59 and 3.1±0.4 vs. 2.1±1.3; p=0.94, respectively).
Figure 3.
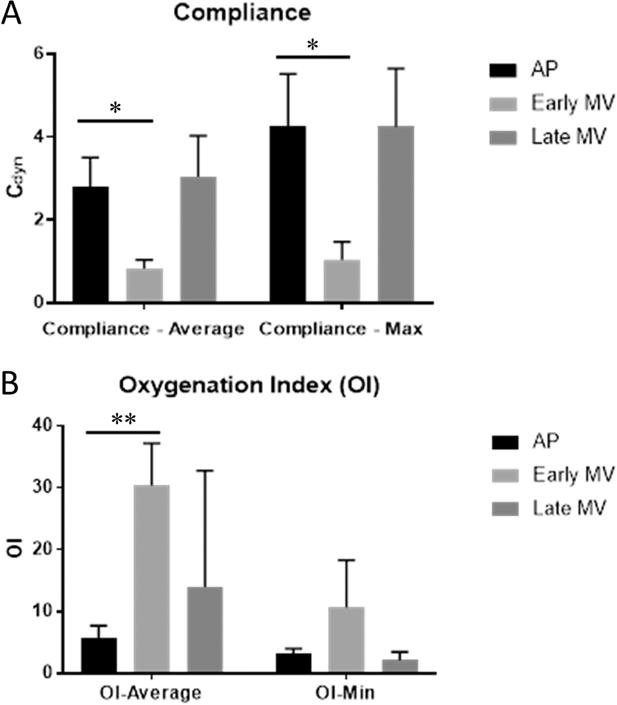
Lung Function Data. A) Lung compliance was calculated as ΔV/ΔP with units of Cdyn (mL/cmH2O). After 10 days of AP support and transition to mechanical ventilation, both average and maximum compliance over the course of ventilatory support were significantly higher than in early MV lambs, and were similar to late MV lambs which were delivered at 10 days greater gestational age. B) Oxygenation Index (OI) was calculated as (mean airway pressure (MAP) * FiO2) / PaO2. After 10 days of AP support and transition to mechanical ventilation, average OI was significantly higher than in early MV lambs. (Mean ± SD; *p<0.01, **p<0.001, ANOVA, Tukey)
3.3. Lung Injury
Lung histopathology from each lamb was analyzed by a pathologist blinded to experimental group and scored in seven categories; representative images are pictured in Figure 4A-C. No alveolar or interstitial inflammation was observed in any sample from any experimental group, so these data are not depicted graphically. AP lungs demonstrated significantly less alveolar and interstitial hemorrhage than early MV lungs (0.4±0.5 vs. 1.6±0.5; p=0.02 and 0.2±0.4 vs. 2.0±0.0; p=0.003) as well as significantly less necrosis (0.0±0.0 vs. 1.0±0.0; p=0.001). AP lungs also manifested less necrosis than late MV lungs (0.0±0.0 vs. 0.6±0.5; p=0.03; Figure 4D). Categorical lung injury scores were summed to calculate a total injury score (Figure 4E). AP lungs again demonstrated significantly less total injury than early MV lungs (1.8±1.6 vs. 6.0±1.2; p=0.007), and also approached significantly lower total injury than late MV lungs (4.6±2.3; p=0.07).
Figure 4.
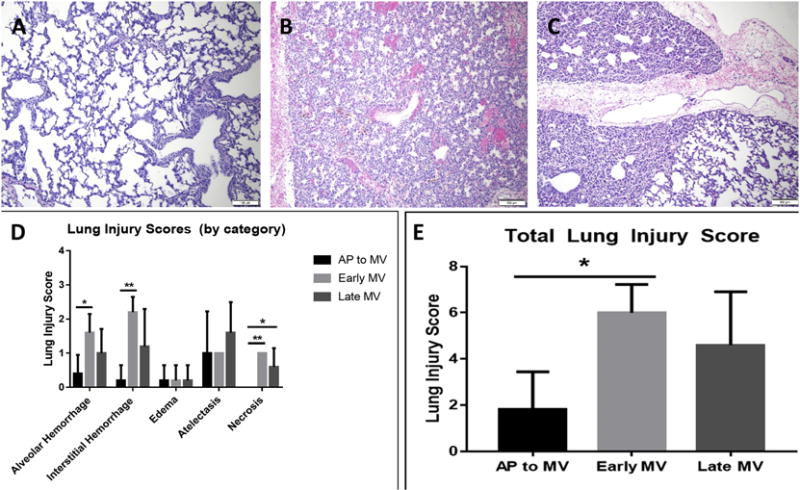
Lung Injury Scoring. Representative slides of H&E staining from the left lower lobe of lungs from AP lambs (A), early MV lambs (B), and late MV lambs (C) are shown (10x magnification). Lungs from AP lambs were better inflated and contained less hemorrhage than early MV lambs. D) Lung slides were scored 0-4 for injury by a pathologist blinded to experimental group in multiple categories. Significant differences were seen between lungs from AP lambs and early MV lambs in alveolar and interstitial hemorrhage as well as necrosis. E) Total injury scores were significantly lower in AP lungs than early MV lungs, and also appeared lower, though not significantly, than late MV lungs. (Mean ± SD; *p<0.01, **p<0.001, ANOVA, Tukey)
3.4. Immunofluorescence
Representative sections of immunofluorescent staining for AP, early MV, and late MV lungs are displayed in Figure 5A-C. Red represents α-actin and green PDGFR-α, while yellow staining at the tips of alveolar crests represents co-localization, signifying positive tips. Two early MV lungs were too injured to allow for adequate morphometric analysis, so for this group, n=3. The area fraction of positive alveolar tips was significantly higher in AP lungs than both early MV and late MV lungs (0.012±0.003 vs. 0.003±0.0005; p=0.001 and 0.004±0.002; p=0.001; Figure 5D).
Figure 5.
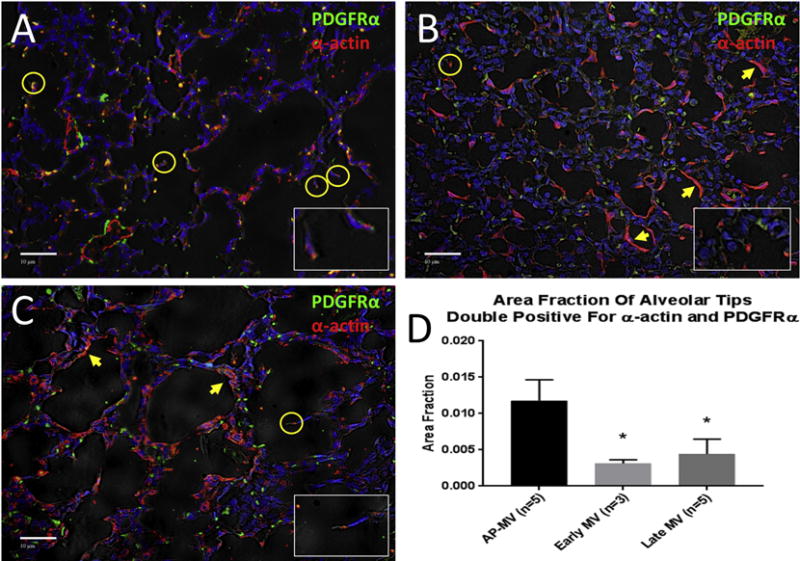
Immunofluorescent staining for PDGFR-α (green) and α-actin (red) at 20x magnification. Co-localization of signals at the tips of alveolar crests represent double-positive tips, signifying active alveolarization (circled, enlarged in inset images). Double-positive tips were more evident in lungs from AP lambs (A) than early MV (B) or late MV (C) lungs. Early and late MV lungs also displayed layering of α-actin along the alveolar wall without the presence of PDGFR-α (arrows), signifying a maladaptive responsive to positive pressure ventilation. D) The ratio of double-positive tips falling on standardized grid points to total grid points represents the area fraction. Area fractions of double-positive tips in AP lungs was significantly higher than that in both early and late MV lungs. (Mean ± SD; *p<0.005 vs. AP, ANOVA, Tukey)
4. Discussion
Using a fetal lamb model with lung development analogous to that of a human fetus at 24 weeks EGA, we report support of five lambs with the AP for 10 days with stable hemodynamics, gas exchange, and organ function. These five lambs represent a cohort within a larger group of lambs (n=12) that were supported with the AP during this period of experimentation with slightly varying experimental goals within our overall Artificial Placenta project. The goals in this cohort of five were to establish the potential of weaning from AP support to mechanical ventilation, and to address our hypothesis that lung development continues during AP support.
All five lambs in this study were transitioned to mechanical ventilation following AP support; four of the five were able to be weaned completely off the AP and were supported by mechanical ventilation alone. Lambs of similar EGA placed directly on MV support at birth survived no longer than 8 hours, again demonstrating that the AP confers a dramatic survival benefit.[11] After 10 days of AP support and transition to MV, lambs demonstrated significantly higher pulmonary compliance and lower oxygenation index than their early MV counterparts that were delivered at the same gestational age. Meanwhile, compliance and OI in AP-supported lambs were very similar to that demonstrated by late MV control lambs, which spent 10 more days developing in utero. The lungs of AP lambs also demonstrated significantly less injury than those of early MV lambs on histopathologic evaluation, and even appeared less injured that those of late MV lambs. And when examining molecular markers of lung development, AP lambs had lungs that exhibited a higher level of active development than both early and late MV lambs, as evidenced by higher area fractions of alveolar tips double-positive for both α-actin and PDGFR-α. These findings lead us to conclude that AP-support prevents lung injury relative to mechanical ventilation, and allows lung development to proceed.
We previously reported the survival advantage of the AP over mechanical ventilation using our current AP system.[11] The present study now demonstrates that lung development continues, and injury is prevented, during AP support compared to MV. There are likely several reasons for these findings. First, AP support allows time for continued lung development. Mortality, pulmonary morbidity, and necessity of mechanical ventilator support decrease with increasing gestational age in premature neonates.[3] Since the design of the AP is to emulate the intrauterine environment, we would hypothesize that mortality and morbidity secondary to pulmonary complications would decrease with duration of AP support and increasing post-conceptional age. Second, our means of maintaining fluid-filled lungs – tracheal occlusion, crystalloid under pressure, and perfluorocarbon instillation – all produce some degree of mechanotransduction. Fetal breathing movements in utero produce intermittent stretch that has been implicated in lung development, even to the level of inducing cellular proliferation.[19, 20] This growth is accelerated with tracheal occlusion, as demonstrated both in lambs supported with the AP and fetuses with CDH subject to prenatal tracheal ligation/occlusion.[21, 22] Lung growth has also been accelerated by postnatal partial liquid ventilation with perfluorocarbons in fetuses and neonates with congenital diaphragmatic hernia (CDH).[23, 24] It is also possible that medications administered during AP support, especially corticosteroids, could impact lung development over the course of support. These steroids are administered in part due to inherent hypocortisolism in premature animals, and to treat inflammation and hypotension that had been observed in prior experiments as a result of interface between blood and foreign material. A potential added benefit could be augmentation of lung development during support; further study would be needed to elucidate the individual contribution of corticosteroids to this development.
Another likely explanation for the AP’s ability to prevent lung injury associated with mechanical ventilation is that it eases the transition from fetal to neonatal physiology. Even in less premature neonates, the abrupt transition to neonatal circulation and alveolar gas exchange often requires, at least temporarily, potentially harmful positive pressure ventilation. Conversely, the artificial placenta allows for gentle lung recruitment and a slow weaning of extracorporeal support, a strategy we employed in our experiments, with lower ventilator pressures used early and then gradually increased. It is possible that the differences in initial ventilator settings between AP and MV lambs in our study affected lung functional data. However, both compliance and oxygenation index are dynamic values that are dependent not only on intrinsic lung structure and function, but also on the necessary ventilator settings to provide adequate support. Therefore, the promising compliance and OI values seen after 10 days of AP support not only suggest ongoing lung development during this period, but also suggest the clinical applicability of this technology and of the weaning process.
With the goal of clinical implementation, we must consider the advantages and disadvantages of our AP system versus others in development. Usuda and Partridge both describe pumpless AV-ECLS systems using the umbilical vessels, with lambs submerged in different versions of an amniotic fluid bath. While Usuda et al noted survival of 1 week using this method [25], Partridge et al. reported prolonged support of eight extremely premature lambs for up to four weeks, with normal lung development and function following support.[26] This model has the advantage of being highly analogous to the true intra-uterine environment, with circuit flow driven by the animal’s cardiac output, and preservation of normal fetal breathing movements which are important to lung development. In our opinion, the major disadvantage is limited clinical utility. The submerged system limits access to the neonate, complicating nursing care and the ability to intervene if complications arise. Support with this rendition of the AP also requires a carefully timed EXIT procedure, which carries significant maternal risk in this setting. Furthermore, apart from gestational age, this approach eliminates any risk stratification for the premature infant, potentially subjecting neonates to complications of ECLS who would otherwise have a favorable prognosis without requiring AP support.
In contrast, our AP system is dry, allowing ease of access to the neonate by nursing and physician staff while preserving fetal circulation and fluid-filled lungs. Though our system requires an external pump to drive extracorporeal flow, the jugular outflow accommodates a much larger cannula than an umbilical artery. Since circuit flow is primarily dependent on drainage, this likely is more translatable into the much smaller human micro-preemies that represent the target patient population. This also avoids the potential arterial vasospasm which has been observed in prior studies of umbilical arterial outflow (unpublished data). More importantly, our model could be implemented in the setting of a spontaneous, preterm vaginal delivery. The infant could undergo a trial of mechanical ventilation first, and in the case of impending respiratory failure, be rescued by the artificial placenta. Though we delivered and cannulated lambs via EXIT procedure in the experiments described in this manuscript, we have successfully preceded AP support with a trial of mechanical ventilation in other experiments. In these studies of this “AP rescue” concept, gas exchange and hemodynamics normalized rapidly, fetal circulation was preserved, and minimal lung injury was apparent.[27]
Admittedly, the major drawbacks of our system are the need for systemic anti-coagulation (a hurdle for any AP application) and prolonged intubation. We are addressing these disadvantages by investigating the use of non-thrombogenic surfaces within the extracorporeal circuit to obviate the need for heparin, and by searching for ways to accelerate lung development to minimize time spent intubated. In moving toward clinical translation, another challenge will be adjusting the size of the AP system, including cannulas, for use in a 22-24 week human premature neonate. Neonates at this size will obviously not tolerate 10-14Fr cannulas as the lambs in this experiment, but also will not require the same circuit flows and oxygen delivery. Future directions in our lab include adopting a smaller animal model to address these concerns of size.
We should note that, while our animal model was selected primarily for study of lung development, it also represents a good model for the complexity of prematurity and its associated complications and critical care. None of the AP-supported lambs in our study died of lung failure, but all succumbed to complications of prematurity. These included renal failure, agitation with resulting ventilator dyssynchrony, and NEC resulting in intestinal perforation. With our data supporting that lung development during AP support by itself should allow weaning from ECLS and respiratory support, the next steps in our experimentation will include fine-tuning our critical care of these animals to prevent and/or treat these complications of prematurity so that long-term survival can be achieved.
A limitation of our experimental cohort was that our AP experimental group included multiple strategies for maintaining fluid-filled lungs. This was a result of evolving experimentation. While different, all three strategies employ the same principle of mechanotransduction. Future experiments will aim to clarify which method – tracheal occlusion, crystalloid instillation, or perfluorocarbon – is best. Our study also is affected by some variation in EGA of the lambs used, a limitation inherent to work with large animal fetal models. An additional limitation was potential selection bias. Initially, only lambs that were hemodynamically stable after seven days of AP support were selected to continue support and transition to mechanical ventilation. However, lung development and function were unknown at that time-point, and now nearly all AP-supported animals in our lab remain hemodynamically stable after seven days.
5. Conclusion
After 10 days of AP support, extremely premature lambs can be transitioned to mechanical ventilation. Based on lung function, histopathology, and immunofluorescence data, lung injury is prevented by AP support relative to mechanical ventilation, and lung development continues during support.
Acknowledgments
The Authors would like to thank Cindy Cooke for her editorial assistance.
Statement of Financial Support
The research presented in this manuscript was funded by the National Institutes of Health NIH 1R01HD073475-01A1, as well as Coller Surgical Society Resident Research Fellowship.
Footnotes
Level of Evidence: n/a (basic/translational science)
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.National Prematurity Awareness Month. Centers for Disease Control and Prevention. 2015 [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England) 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. Jama. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon M, O’Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ, et al. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics. 2009;124:637–648. doi: 10.1542/peds.2008-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manktelow BN, Seaton SE, Field DJ, Draper ES. Population-based estimates of in-unit survival for very preterm infants. Pediatrics. 2013;131:e425–432. doi: 10.1542/peds.2012-2189. [DOI] [PubMed] [Google Scholar]
- 7.Smith LJ, McKay KO, van Asperen PP, Selvadurai H, Fitzgerald DA. Normal development of the lung and premature birth. Paediatric respiratory reviews. 2010;11:135–142. doi: 10.1016/j.prrv.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Chambers HM, van Velzen D. Ventilator-related pathology in the extremely immature lung. Pathology. 1989;21:79–83. doi: 10.3109/00313028909059539. [DOI] [PubMed] [Google Scholar]
- 9.Attar MA, Donn SM. Mechanisms of ventilator-induced lung injury in premature infants. Seminars in neonatology : SN. 2002;7:353–360. doi: 10.1053/siny.2002.0129. [DOI] [PubMed] [Google Scholar]
- 10.Philip AG. Bronchopulmonary dysplasia: then and now. Neonatology. 2012;102:1–8. doi: 10.1159/000336030. [DOI] [PubMed] [Google Scholar]
- 11.Bryner B, Gray B, Perkins E, Davis R, Hoffman H, Barks J, et al. An extracorporeal artificial placenta supports extremely premature lambs for 1 week. Journal of pediatric surgery. 2015;50:44–49. doi: 10.1016/j.jpedsurg.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fewell JE, Johnson P. Upper airway dynamics during breathing and during apnoea in fetal lambs. The Journal of physiology. 1983;339:495–504. doi: 10.1113/jphysiol.1983.sp014729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mrozek JD, Smith KM, Bing DR, Meyers PA, Simonton SC, Connett JE, et al. Exogenous surfactant and partial liquid ventilation: physiologic and pathologic effects. American journal of respiratory and critical care medicine. 1997;156:1058–1065. doi: 10.1164/ajrccm.156.4.9610104. [DOI] [PubMed] [Google Scholar]
- 14.Docimo SG, Crone RK, Davies P, Reid L, Retik AB, Mandell J. Pulmonary development in the fetal lamb: morphometric study of the alveolar phase. The Anatomical record. 1991;229:495–498. doi: 10.1002/ar.1092290409. [DOI] [PubMed] [Google Scholar]
- 15.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. American journal of respiratory and critical care medicine. 1999;159:945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 16.Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD, et al. Characterization of the platelet-derived growth factor receptor-alpha-positive cell lineage during murine late lung development. American journal of physiology Lung cellular and molecular physiology. 2015;309:L942–958. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 17.Branchfield K, Li R, Lungova V, Verheyden JM, McCulley D, Sun X. A three-dimensional study of alveologenesis in mouse lung. Developmental biology. 2016;409:429–441. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popova AP, Bentley JK, Cui TX, Richardson MN, Linn MJ, Lei J, et al. Reduced platelet-derived growth factor receptor expression is a primary feature of human bronchopulmonary dysplasia. American journal of physiology Lung cellular and molecular physiology. 2014;307:L231–239. doi: 10.1152/ajplung.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copland I, Post M. Lung development and fetal lung growth. Paediatric respiratory reviews. 2004;5(Suppl A):S259–264. doi: 10.1016/s1526-0542(04)90049-8. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Xu J, Tanswell AK, Post M. Stretch-induced growth-promoting activities stimulate fetal rat lung epithelial cell proliferation. Experimental lung research. 1993;19:505–517. doi: 10.3109/01902149309064360. [DOI] [PubMed] [Google Scholar]
- 21.Yasufuku M, Hisano K, Sakata M, Okada M. Arterio-venous extracorporeal membrane oxygenation of fetal goat incubated in artificial amniotic fluid (artificial placenta): influence on lung growth and maturation. Journal of pediatric surgery. 1998;33:442–448. doi: 10.1016/s0022-3468(98)90085-9. [DOI] [PubMed] [Google Scholar]
- 22.Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. The New England journal of medicine. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- 23.Hirschl RB, Philip WF, Glick L, Greenspan J, Smith K, Thompson A, et al. A prospective, randomized pilot trial of perfluorocarbon-induced lung growth in newborns with congenital diaphragmatic hernia. Journal of pediatric surgery. 2003;38:283–289. doi: 10.1053/jpsu.2003.50095. discussion 283-289. [DOI] [PubMed] [Google Scholar]
- 24.Mychaliska G, Bryner B, Dechert R, Kreutzman J, Becker M, Hirschl R. Safety and efficacy of perflubron-induced lung growth in neonates with congenital diaphragmatic hernia: Results of a prospective randomized trial. Journal of pediatric surgery. 2015;50:1083–1087. doi: 10.1016/j.jpedsurg.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Usuda H, Watanabe S, Miura Y, Saito M, Musk GC, Rittenschober-Bohm J, et al. Successful maintenance of key physiological parameters in preterm lambs treated with ex vivo uterine environment therapy for a period of 1 week. American journal of obstetrics and gynecology. 2017 doi: 10.1016/j.ajog.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 26.Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, et al. An extra-uterine system to physiologically support the extreme premature lamb. Nature communications. 2017;8:15112. doi: 10.1038/ncomms15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray BW, El-Sabbagh A, Zakem SJ, Koch KL, Rojas-Pena A, Owens GE, et al. Development of an artificial placenta V: 70 h veno-venous extracorporeal life support after ventilatory failure in premature lambs. Journal of pediatric surgery. 2013;48:145–153. doi: 10.1016/j.jpedsurg.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


