Abstract
Background
Neurovascular coupling, the relationship between cerebral blood flow and neuronal activity, is attenuated in patients with impaired executive function. We tested the hypothesis that peripheral vascular function may associate with executive function in older subjects with cardiovascular risk factors, and that treatment with the antioxidant L-arginine would improve both vascular and executive function.
Methods
Nineteen subjects with type 2 diabetes mellitus and/or controlled hypertension were enrolled. Subjects were treated with L-arginine or placebo for 4 days in a randomized, double-blinded cross-over study. Brachial artery vascular function, peripheral artery tonometry, and Trail Making Test Part B (TMTB) testing were performed on day 1 and day 4 during each condition.
Results
L-arginine signficiantly reduced the digital reactive hyperemia index, and the comparison of changes against placebo was significant (p=0.01). With executive function testing, we observed a significant interaction between treatment and order. Restricting the analysis to the first treatment period, subjects treated with placebo decreased their TMTB times by 57.3 ± 52.5 seconds from day 1 to day while those treated with arginine had no significant change (6.4 ± 18.4 seconds worse, p = 0.37). In addition, L-arginine was associated with increased mean arterial pressure from 88 ± 9 mm Hg to 92 ± 11 mm Hg, which trended toward significance.
Conclusions
L-arginine treatment worsened digital microvascular and executive function in older subjects with cardiovascular risk factors. These data further support a link between vascular and executive function.
Keywords: Aging, Nitric Oxide, Endothelial Function, Cognitive Impairment
We have previously reported on the close relationship between cerebral blood flow and neuronal activity in response to cognitive work, termed neurovascular coupling (NVC) (1). NVC was intact in healthy patients and attenuated in patients with impaired executive function, suggesting a relationship between vascular and executive function (2). This linkage between vascular function and health has been noted in a large number of domains. For example, a reduction in conduit artery nitric oxide bioavailability (endothelial dysfunction) has been associated with worse cardiovascular health, such that abnormal endothelial function associates with the presence of coronary artery disease, the risk of myocardial infarction and death (3). Patients with executive function decline are commonly older with risk factors for atherosclerosis (4, 5). Diminished middle cerebral artery blood flow velocity in response to cognitive stress, coupled to a risk factor state, suggests that an attenuation in endothelium-derived nitric oxide bioavailability may represent a common pathophysiology underlying vascular and executive function.
In a cohort of older subjects with cardiovascular risk factors, we tested the hypothesis that two measures of systemic vascular function, brachial (conduit) artery flow-mediated vasodilation and digital pulse amplitude tonometry, would improve with the endothelial nitric oxide synthase co-factor L-arginine. Further, we tested whether vascular function in these separate vascular beds associates with neurocognitive function and whether treatment with L-arginine would improve executive function in association with improved endothelial function.
Methods
Nineteen subjects aged 70 and older with diabetes mellitus type 2 and/or controlled Stage 1 hypertension by JNC7 guidelines (SBP <160 and DBP <100mm Hg) were enrolled. Hypertension was defined by blood pressure ≥140/90 mm Hg on two separate visits, or by preexisting diagnosis of hypertension and subject taking anti-hypertensive medication. Type 2 diabetes mellitus was defined by HbA1C ≥6.5% or by preexisting diagnosis of diabetes and subject taking oral hypoglycemia agents. Screening blood pressure was taken as the median of three consecutive recordings performed manually with a sphygmomanometer, with participants seated. Participants were recruited from the local Boston community through IRB-approved advertisements placed online and in print media. To obtain the 19 subjects who completed the protocol, after consent was obtained, a screening transcranial ultrasound was performed; 13 people whose cerebral arteries could not be insonated were excluded. Other exclusion criteria included intact executive function, as measured by a TMTB test above the 25th percentile based on normative reference data (1, 6) (19 people excluded for intact function); history of stroke, myocardial infarction, or diagnosis of dementia. Studies were performed in accordance with Declaration of Helsinki, approved by the institutional review committee, and all subjects signed informed consent prior to participation.
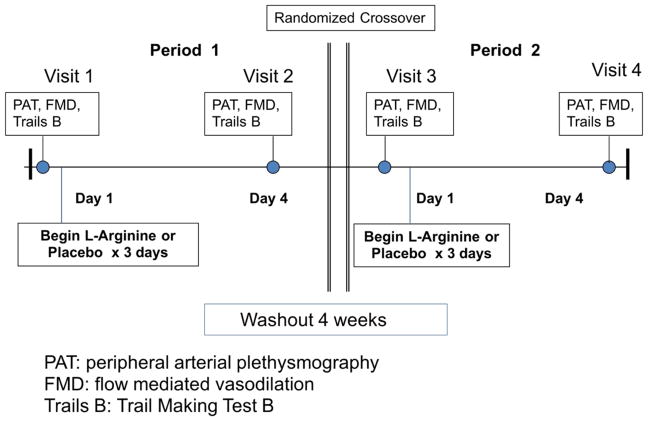
Once enrolled, subjects were randomized to receive one of two sequences of oral L-arginine and placebo in this double-blinded crossover study (Figure 1). Arginine was chosen for this study because of its rapid effect on eNOS (7, 8). Outpatient visits were performed at the Clinical Trials Center and Vascular Research Laboratory at the Brigham and Women’s Hospital. During the study period, subjects continued to take their usual daily medications, including those for diabetes and for hypertension. This study was registered on clinicaltrials.gov (NCT01482247).
Figure 1.
Study Schema
Study Visits
Peripheral and cerebral vascular function was measured on four outpatient visits, before and after separate three-day trials of oral L-arginine and placebo (Figure 1). Vascular studies included peripheral plethysmography (PAT) and brachial artery flow mediated vasodilation (FMD). After the first baseline study, subjects were given an equal number of identically-appearing placebo or L-arginine packets dosed 7g each (BioKyowa Inc., Cape Girardeau, MO.). Subjects began the study drug after their first baseline study, and consumed two doses of study supplement on Day 1 (14 g), three doses on Days 2 and 3 (21 g) and two doses on Day 4 (21g). On Day 4, subjects took the final dose of study medication in the hospital and then again had PAT and FMD responses measured. There was a four week washout, followed by two final study days, before and after whichever drug (L-arginine or placebo) they did not initially receive. Subjects refrained from chocolate, red wine, and tea during the study, and avoided caffeine on the four study visit days. Drug order was determined by the Investigational Pharmacy who maintained the investigators’ blinding to study condition during data acquisition and analysis.
Finger Blood Flow - Plethysmography (PAT)
Pulsatile blood volume responses were assessed by peripheral arterial tonometry (PAT) using a plethysmographic device (PAT 1000RD; Itamar Medical, Inc., Caesarea, Israel) designed to reflect only pulsatile arterial volume changes. Pulse wave amplitude (PWA) readings were followed by 5 minutes of radial artery occlusion at 30 mmHg above systolic blood pressure. Flow-mediated vasodilation (FMD), measured as reactive hyperemia, was assessed one minute post release from occlusion. This peripheral tonometry method has been described in detail (9). A single technician, blinded to subject, study day, time and treatment calculated pulsatile volume, employing area under the curve. The intra-observer variability for repeated measurements was 0.2 ± 0.7 μL. Reactive hyperemia index (RHI) was calculated as PAT ratio_ln[(Xh90–120/Xh0)].
Flow Mediated Dilation (FMD)
Brachial (conduit) artery vascular function was assessed according to standard methods and as we have previously performed (10–12). Participants were studied in a quiet, temperature-controlled, dimly lit room after resting supine for a minimum of 5 minutes. High-resolution B-mode ultrasonography of the brachial artery was performed with a 7.5-MHz linear array probe (GE VIVID 7; GE Healthcare). The brachial artery was imaged longitudinally just proximal to the antecubital fossa. The transducer position was adjusted to obtain optimal images of the near and far walls of the intima. The video output and electrocardiographic signal of the ultrasound machine were connected to a computer equipped with a Data Translation Frame-Grabber video card, (Dataviz). The R wave on the electrocardiogram served as a trigger to acquire frames at end diastole. After baseline image acquisition, a sphygmomanometric cuff placed on the upper arm was inflated to suprasystolic pressure (200 mm Hg or 50 mm Hg above systolic) for 5 minutes. On cuff release, reactive hyperemia caused flow to increase through the brachial artery subserving the forearm. Flow-induced endothelium-dependent vasodilation of the brachial artery was determined by acquiring images from 60 to 70 seconds after cuff deflation. Flow-mediated vasodilation at this time point is largely endothelium dependent and nitric oxide mediated and can be inhibited by administration of the nitric oxide synthase antagonist NG-monomethyl L-arginine.5 Ten minutes after cuff release, the brachial artery was imaged again to reestablish basal conditions. Subsequently, to determine endothelium-independent vasodilation, participants received 0.4 mg nitroglycerin sublingually. The brachial artery was imaged 3 minutes later. Brachial artery blood flow velocity was determined via velocity–time integral measurement. Nitroglycerin was not administered if the systolic blood pressure was <100 mm Hg or if the participant refused nitroglycerin, usually to avoid a severe headache during the second visit. Flow mediated dilation (FMD) is calculated as FMDmax = (DDFmax - DBL)/DBL and FMD60 = (DDF60 - DBL)/DBL.
Executive Function Testing
Subjects underwent executive function assessment with Trail Making Test Part B (TMTB), a measure of executive function involving mental flexibility, sequencing, visual search, and set shifting. This test assesses executive function, visual search speed, and information processing pace (13). Baseline testing was performed at screening, and follow-up on day 4 of each treatment arm, in order to reduce learning effect. The test was performed by a single, trained, blinded-to-treatment researcher.
Statistical Methods
Basic statistical methods for crossover studies were employed. Randomized sequence groups were initially compared for balance in pre-treatment measures, followed by within-subject statistical comparisons including paired t-tests and exact Wilcoxon signed rank tests. The interaction between the effect of treatment and the order in which the treatments were given was assessed before testing treatment effects. Associations were tested using Spearman rank correlation. With the relatively small sample size, effect sizes should be taken into consideration along with the statistical tests.
Results
Nineteen subjects completed the protocol. The subjects had a mean age of 75 years, included 11 women, and were 84% Caucasian (Table 1). Fifty-three percent of the patients had diabetes, and 17 of 19 were hypertensive; screening blood pressure readings revealed good control. The mean baseline executive function test score, TMTB, was 105 +/− 52 seconds, consistent with reduced executive function.
Table 1.
Demographics
| Variable (SD) | N |
|---|---|
| Age, y | 75 (4) |
| Sex, M:F | 8:11 |
| Race, % Caucasian | 84 |
| Education yrs past high school | 4.1 (1.0) |
| Diabetes, % | 53 |
| Hypertension, % | 89 |
| BMI, kg/m2 | 29.2 (7.4) |
| Systolic BP mm Hg | 129 (13) |
| Diastolic BP mm Hg | 68 (8) |
Effect of L-Arginine on Peripheral Vascular Function
Blood Pressure
Administration of L-arginine was associated with a significantly increased mean arterial pressure from day 1 (88 ± 9 mm Hg) to day 4 (92 ± 11 mm Hg, p = 0.01), whereas there was no change in blood pressure with administration of placebo (91 ± 9 mm Hg on day 1 to 90 ± 11 mm Hg on day 4, p = 0.7). There was a trend toward a significant difference in blood pressure response to arginine versus placebo; mean within-subject difference between the blood pressure change within each condition was 3.9 ± 10.0 mm Hg (p=0.12).
Conduit Artery
Baseline brachial artery diameter did not differ significantly in either pre-treatment condition or after L-arginine and placebo therapy (all p > 0.2) (Table 2). Flow-mediated vasodilation decreased significantly from day 1 to day 4 during the L-arginine treatment period (7.9 ± 1.0 vs 6.4 ± 0.9%, for baseline and post-arginine treatment; p = 0.02). Flow-mediated vasodilation did not change significantly from day 1 to day 4 during the placebo period (7.8 ± 4.3 vs 7.2 ± 4.2; p= 0.710 for baseline and post-placebo treatment; p = 0.71; comparing change during placebo with change during arginine p=0.5). Comparing end of placebo to end of L-arginine treatment, flow-mediated vasodilation trended lower after L-arginine treatment, but did not reach statistical significance (7.2 ± 1.0 vs 6.4 ± 0.9%, for placebo and L-arginine, respectively; p = 0.15). Nitroglycerin-mediated vasodilation differed neither from day 1 to 4 in either condition nor when comparing placebo to L-arginine day 4 (all p > 0.2).
Table 2.
Vascular Functional Assessments
| Placebo | Arginine | |||
|---|---|---|---|---|
| Day 1 | Day4 | Day 1 | Day 4 | |
| Baseline Diameter (mm) | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.6 ± 0.2 | 3.7 ± 0.2 |
| Reactive Hyperemia (fold VTI) | 6.1 ± 0.7 | 6.9 ± 0.8 | 6.9 ± 0.8 | 6.9 ± 1.5 |
| Flow-Mediated Vasodilation | 7.8 ± 1.0 | 7.2 ± 1.0 | 7.9 ± 1.0 | 6.4 ± 0.9* |
| Nitroglycerin-Mediated Vasodilation | 9.8 ± 1.7 | 7.9 ± 1.0 | 9.5 ± 1.7 | 11.4 ± 2.0 |
| Ln RHI | 0.27 ±0.27 | 0.52±0.35 | 0.43 ±0.32 | 0.32 ± .37# |
p = 0.02 for comparison of FMD from day 1 to day 4 during arginine treatment
p = 0.01 for the comparison of the change during placebo versus the change during arginine
Digital Arteriolar
Baseline Ln RHI was not statistically different at the beginning of each treatment condition (Table 2). LnRhI after arginine treatment (0.32 ± 0.37) was lower than after placebo (0.52 ± 0.35; p=0.06). Further, the comparison of the changes in Ln RHI in each condition was significant (p=0.01). There was no significant correlation between conduit artery flow-mediated vasodilation and the pulse amplitude tonometry (PAT) response to hyperemia at baseline.
Effect of Arginine on Executive Function
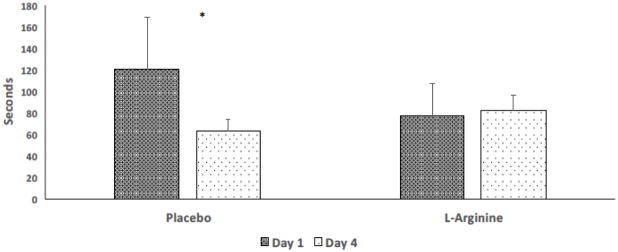
TMTB scores were significantly affected by L-arginine. We observed a significant interaction between treatment and order, indicating that the effect of treatment depended on the order in which the treatments were given (p = 0.045). Restricting the analysis to the first treatment period, subjects treated with placebo completed the TMTB 57.3 ± 52.5 seconds faster on day 4 than day 1 (p = 0.01). In contrast, subjects treated with L-arginine had no significant change from day 1 to day 4 (6.4 ± 18.4 seconds worse, p = 0.37).
We sought to understand if L-arginine prevented the subjects susceptibility to the effects of practice by looking at performance during the second period. We determined whether the subjects had reached their maximum performance (fastest TMTB time) and could not further improve. TMTB times in visits 2 and 3 were compared (end of treatment period 1 and beginning of treatment period 2) in subjects who completed every visit to determine if a peak speed of completion (time “floor”) had been reached with both treatments in the first treatment period (Figure 2). In the group treated initially with placebo, the time at the end of placebo treatment (visit 2) was 60 ± 13 seconds, not significantly different from time at the start of the arginine treatment (visit 3): 66 ±17 seconds (p = 0.11). In contrast, subjects who were treated with L-arginine first completed the TMTB test in 73 ± 8 seconds at the end of treatment period 1 (visit 2), but their time decreased significantly to 53 ± 9 seconds at the beginning of treatment period 2 (visit 3, p < 0.001) four weeks later, indicating that the test time in the first treatment period did not represent a peak speed of completion in the L-arginine first subjects.
Figure 2.
The Effect of L-Arginine Therapy on Time to Completion of Trail-Making B Testing Subjects were tested on day 1 with the Trail-Making Test B, were administered L-arginine 14 to 21 g daily or matching placebo, and then returned on day 4 for repeat testing. Subjects treated with placebo demonstrated the expected improvement in time to completion whereas those treated with L-arginine had no change at all (* p = 0.01).
Discussion
Previous investigations have demonstrated a strong association between peripheral and coronary artery endothelial function in health and disease (14). Similarly, in some disease states, there is evidence of an association between peripheral and intracranial microvascular function(15–17). This study sought to determine if there was a relationship between conduit artery, digital artery, and executive function in older subjects. In addition, it sought to determine whether administration of L-arginine would improve vascular function, either large or small vessel, and executive function in this cohort of patients with baseline impairment of executive function. This study demonstrated that L-arginine, in patients with risk factors for atherosclerosis, attenuated vascular reactivity in the digital microarterioles, as well as executive functioning compared to placebo treatment, and was further associated with increased blood pressure and decreased flow-mediated vasodilated.
Vascular Function Linkage in Regional Circulations
In this cohort, there was no correlation of endothelial function between the two vascular beds. Baseline conduit artery flow-mediated vasodilation was less than 8%, consistent with our prior results in patients with risk factors for atherosclerosis using an upper cuff technique. Similarly, the digital reactive hyperemic index was attenuated, as seen in people with this risk factor mix. This pilot study of L-arginine is consistent with prior work in the Third Generation Framingham cohort demonstrating no correlation between conduit artery FMD and digital artery PAT ratio (18). Although both studies of vasodilation depend, in part, on endothelium-derived nitric oxide, the discordance reconfirms the concept that more than one vasodilator likely participates in flow-mediated vasodilation and there is heterogeneity in function between macro and microcirculations (19).
In addition to the lack of association between the brachial conduit artery and digital microarterioles, there was no association between reactive hyperemia in the forearm skeletal muscle and digital arteriolar response. The bioavailability of nitric oxide, in part, also contributes to the amount of reactive hyperemia induced by the 5 minutes of forearm ischemia (20). Here again, our finding no correlation between the forearm muscular microvascular response and the digital microvascular response is consistent with previous reports (18).
L-Arginine and Vascular Function
Administration of L-arginine was associated with negative vascular effects by three separate measures. Comparing mean arterial response to arginine and to placebo, the difference between the two conditions, 3.9 mm Hg, represents a clinically significant value. A similar increase in BP was associated with 58% increase in mortality with torcetrapib treatment (21).
In addition, L-arginine attenuated both brachial artery and digital microvascular function when compared to placebo. While the change in FMD between the two conditions did not reach statistical significance, FMD fell by 19% with arginine; there is no known intervention that worsened FMD and was not associated with an increase in adverse cardiovascular events. Finally, the change in a measure of arteriolar vascular function by peripheral arterial tonometry, LnRHI, was significantly worse after arginine compared with placebo.
In these older patients with risk factors for atherosclerosis, we expected administration of L-arginine, an amino acid used by endothelial nitric oxide synthase to increase the bioavailability of nitric oxide, to restore attenuated endothelial function, either by acting as an antioxidant or by restoring its level to normal. Many studies have demonstrated that nitroarginine, an inhibitor of nitric oxide synthase, reduces the bioavailability of nitric oxide. Administration of L-arginine has been shown to increase endothelium-dependent vasodilation in healthy humans and those with hypercholesterolemia, but not to change it significantly in subjects with hypertension or type 1 diabetes (22–25). In one study of 10 pre-menopausal women with type 2 diabetes, administration of 9 g of L-arginine increased flow-mediated vasodilation (26). These results are at odds with our own, however, and the different findings may have arisen because of differences in the subjects’ ages, risk factor burden, or dose of L-arginine. Our subjects were older and had a worse risk factor profile than in previous studies. Our data find support in a recent trial of longer term L-arginine therapy. Administration of 3 g daily of L-arginine for 6 months has been shown to reduce vascular function and walking distance in patients with peripheral artery disease (27).
The mechanism for these findings may reside in the underlying coupling state of endothelial nitric oxide synthase. Administration of L-arginine increases the substrate for eNOS; however, the oxidative stress conditions and availability of tetrahydrobiopeterin (BH4) determine what is elaborated (28). In disease conditions, the increase in eNOS uncoupling from its healthy dimer to monomer state changes production from nitric oxide to superoxide anion. In the eNOS uncoupled state, nicotinamide adenine dinucleotide phosphate (NADPH) reacts with oxygen instead of BH4, increasing oxidative stress and further reducing the bioavailability of nitric oxide. Thus, in an older population of subjects with diabetes, hypertension, and attenuated conduit artery and digital microvascular function, it is possible that eNOS uncoupling explains the adverse effect of L-arginine administration.
Our physiological findings find support, as well, from the increase in blood pressure during L-arginine treatment. Most studies have shown that administration of L-arginine in relatively healthy or modestly unhealthy populations, including pregnant women (29), healthy volunteers (30), patients with mild hypertension (31), and obese hypertensive men in their 40s (32), improves blood pressure. In a meta-analysis of mostly healthy subjects with a mean age of 40 years, L-arginine reduced mean blood pressure by 3.6 mm Hg (33). However, in the study by Wilson and coworkers (27), where the mean age was 73 years, administration of L-arginine reduced urinary nitrogen oxide, flow-mediated endothelium-dependent vasodilation, and showed a trend towards a decrease in treadmill time walked. This population was most like ours, with mean age 75 years, and suggests that the underlying disease state affects significantly the vascular and blood pressure effect of L-arginine therapy.
L-Arginine and Executive Function
Executive function encompasses a domain of higher cognitive skills responsible for the planning, initiation, sequencing, and monitoring of complex goal-directed behavior. Executive function is considered a salient feature of vascular executive function impairment (34) and, together with memory and attention, is among the cognitive domains particularly vulnerable to microvascular disease (35–37). Perhaps the most unexpected and most significant finding in our study is that administration of L-arginine significantly impaired executive function as measured by the TMTB test. It has been demonstrated that repetitive executive function testing results in improved TMTB test time, with improvements occurring two to three months out (38). The practice effect has been reported in healthy older people as well (39), albeit with some decline when compared to younger subjects (40). Thus the expected outcome of re-testing at 4 days was an improved time, as seen in the placebo group. However, the similar speed of test completion in visit 3 by both the placebo-first and arginine-first subjects was reached because of a significant reduction in time in the arginine-first group. This pattern suggests L-arginine treatment decreased the participants’ susceptibility to the effects of practice which recovered when the L-arginine was removed.
These findings were surprising based on previous animal investigation. L-arginine restored memory formation in morphine-treated mice (41), and increased the concentration of NO in the hippocampus and shortened the time of pole-climbing shock avoidance task in rats (42). In another mouse model, inhibition of arginine catabolism also reduced progression of Alzheimer’s-like changes (43). There is also evidence that arginine metabolism is significantly altered in humans with Alzheimer’s disease (44). In fact, studies using metabolomics have shown that L-arginine metabolism is differentially disrupted in subjects with mild executive impairment, MCI subjects who went on to develop Alzheimer’s, and healthy subjects (45).
Our study is the first to show that L-arginine administration impairs executive function in older adults with mild impairment of executive function. These findings may suggest a direct neurotoxic effect of L-arginine in patients with impaired executive function. Changes in nitric oxide bioavailability may affect memory and cognition through its production in hippocampal and other brain tissues (46). Close observation of L-arginine clinical trials currently in progress (47) may help illuminate the mechanisms of this effect, which require further investigation. Another possibility is that we are now substantiating our previous report where we showed that cocoa improved neurovascular coupling and executive function and linked vascular and executive function (1). In this case, by giving a harmful agent, we show that vascular function and executive function both worsen in the face of L-arginine administration. Thus, these two reports, when viewed as a whole, suggest a significant coupling between vascular and executive function and avenues for future research to reduce executive function decline.
Limitations
This study was a pilot, designed primarily to assess effect sizes in a small sample in a controlled setting, and potentially to inform the design of a larger future study. The demonstration of impaired peripheral vascular function either met or trended towards dysfunction. The observed trend toward impaired peripheral vascular function reported here is consistent with the literature, but the size of our cohort likely limited our ability to demonstrate these changes unambiguously. The demonstration of an adverse effect of L-arginine on executive function limits its ethical use in a research setting, suggests further administration in this population would be unsafe, and prevents additional recruitment that would likely make the changes in blood pressure and peripheral conduit artery endothelial function reach statistical significance.
Conclusions
Arginine therapy significantly impairs digital microvascular endothelial function and executive function, and causes a trend toward increased blood pressure and reduced peripheral conduit artery endothelial function. We have previously demonstrated a link between executive function and blood flow velocity responses in the middle cerebral artery in response to cocoa. We now demonstrate that L-arginine therapy impairs digital microvascular and executive function, providing further support for a link between vascular function and cognition.
Highlights.
Arginine impaired digital microvascular function in subjects with cardiovascular risk.
Arginine worsened executive function in subjects with cardiovascular risk.
These findings suggest a significant coupling between vascular and executive function.
Acknowledgments
Source of Funding:
This research was supported by the NHLBI (RO1HL089570)
Footnotes
Disclosures/Conflicts of Interest: None
Clinical Trial Registration: www.clinicaltrials.gov (NCT01482247)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81(10):904–9. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS, et al. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab. 2014;34(2):228–34. doi: 10.1038/jcbfm.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 4.Vincent C, Hall PA. Executive Function in Adults With Type 2 Diabetes: A Meta-Analytic Review. Psychosomatic medicine. 2015;77(6):631–42. doi: 10.1097/PSY.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 5.Schillerstrom JE, Horton MS, Royall DR. The impact of medical illness on executive function. Psychosomatics. 2005;46(6):508–16. doi: 10.1176/appi.psy.46.6.508. [DOI] [PubMed] [Google Scholar]
- 6.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 7.Jablecka A, Checinski P, Krauss H, Micker M, Ast J. The influence of two different doses of L-arginine oral supplementation on nitric oxide (NO) concentration and total antioxidant status (TAS) in atherosclerotic patients. Med Sci Monit. 2004;10(1):CR29–32. [PubMed] [Google Scholar]
- 8.Jabecka A, Ast J, Bogdaski P, Drozdowski M, Pawlak-Lemaska K, Cielewicz AR, et al. Oral L-arginine supplementation in patients with mild arterial hypertension and its effect on plasma level of asymmetric dimethylarginine, L-citruline, L-arginine and antioxidant status. European review for medical and pharmacological sciences. 2012;16(12):1665–74. [PubMed] [Google Scholar]
- 9.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19(1):6–11. doi: 10.1016/j.tcm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen PL, Jarolim P, Basaria S, Zuflacht JP, Milian J, Kadivar S, et al. Androgen deprivation therapy reversibly increases endothelium-dependent vasodilation in men with prostate cancer. Journal of the American Heart Association. 2015;4(4) doi: 10.1161/JAHA.115.001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milian J, Goldfine AB, Zuflacht JP, Parmer C, Beckman JA. Atazanavir improves cardiometabolic measures but not vascular function in patients with long-standing type 1 diabetes mellitus. Acta Diabetol. 2015;52(4):709–15. doi: 10.1007/s00592-014-0708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nohria A, Kinlay S, Buck JS, Redline W, Copeland-Halperin R, Kim S, et al. The effect of salsalate therapy on endothelial function in a broad range of subjects. Journal of the American Heart Association. 2014;3(1):e000609. doi: 10.1161/JAHA.113.000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22(4):518–28. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 14.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75(6):71B–4B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara Y, Mizuno T, Okuyama C, Nagakane Y, Watanabe-Hosomi A, Kondo M, et al. Simultaneous impairment of intracranial and peripheral artery vasoreactivity in CADASIL patients. Cerebrovasc Dis. 2012;33(2):128–34. doi: 10.1159/000334185. [DOI] [PubMed] [Google Scholar]
- 16.Pretnar-Oblak J, Sabovic M, Vidmar G, Zaletel M. Evaluation of L-arginine reactivity in comparison with flow-mediated dilatation and intima-media thickness. Ultrasound Med Biol. 2007;33(10):1546–51. doi: 10.1016/j.ultrasmedbio.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Rajan R, Khurana D, Lal V. Interictal cerebral and systemic endothelial dysfunction in patients with migraine: a case-control study. J Neurol Neurosurg Psychiatry. 2015;86(11):1253–7. doi: 10.1136/jnnp-2014-309571. [DOI] [PubMed] [Google Scholar]
- 18.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57(3):390–6. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 2006;101(2):545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 20.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270(4 Pt 2):H1435–40. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 21.Barter PJ, Caulfied M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. NEJM. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 22.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90(4):1248–53. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panza JA, Casino PR, Badar DM, Quyyumi AA. Effect of increased availability of endothelium-derived nitric oxide precursor on endothelium-dependent vascular relaxation in normal subjects and in patients with essential hypertension. Circulation. 1993;87(5):1475–81. doi: 10.1161/01.cir.87.5.1475. [DOI] [PubMed] [Google Scholar]
- 24.Mullen MJ, Wright D, Donald AE, Thorne S, Thomson H, Deanfield JE. Atorvastatin but not L-arginine improves endothelial function in type I diabetes mellitus: a double-blind study. J Am Coll Cardiol. 2000;36(2):410–6. doi: 10.1016/s0735-1097(00)00743-9. [DOI] [PubMed] [Google Scholar]
- 25.Jude EB, Dang C, Boulton AJ. Effect of L-arginine on the microcirculation in the neuropathic diabetic foot in Type 2 diabetes mellitus: a double-blind, placebo-controlled study. Diabet Med. 2010;27(1):113–6. doi: 10.1111/j.1464-5491.2009.02876.x. [DOI] [PubMed] [Google Scholar]
- 26.Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, et al. Oral L-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med. 2003;8(3):169–75. doi: 10.1191/1358863x03vm489oa. [DOI] [PubMed] [Google Scholar]
- 27.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116(2):188–95. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 28.Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, et al. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47(1):87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Q, Yue X, Tian QY, Saren G, Wu MH, Zhang Y, et al. Effect of L-arginine supplementation on blood pressure in pregnant women: a meta-analysis of placebo-controlled trials. Hypertens Pregnancy. 2013;32(1):32–41. doi: 10.3109/10641955.2012.697952. [DOI] [PubMed] [Google Scholar]
- 30.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am J Hypertens. 2000;13(5 Pt 1):547–51. doi: 10.1016/s0895-7061(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 31.Ast J, Jablecka A, Bogdanski P, Smolarek I, Krauss H, Chmara E. Evaluation of the antihypertensive effect of L-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Med Sci Monit. 2010;16(5):CR266–71. [PubMed] [Google Scholar]
- 32.Castejon AM, Hoffmann IS, Jimenez E, Cubeddu RJ, Baldonedo RM, Cubeddu LX. Differential blood pressure effects of oral glucose and intravenous L-arginine in healthy lean normotensive and obese hypertensive subjects. J Hum Hypertens. 2002;16(Suppl 1):S133–6. doi: 10.1038/sj.jhh.1001359. [DOI] [PubMed] [Google Scholar]
- 33.Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162(6):959–65. doi: 10.1016/j.ahj.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelick PB, Nyenhuis D, Materson BJ, Calhoun DA, Elliott WJ, et al. American Society of Hypertension Writing G. Blood pressure and treatment of persons with hypertension as it relates to cognitive outcomes including executive function. Journal of the American Society of Hypertension: JASH. 2012;6(5):309–15. doi: 10.1016/j.jash.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension. 2012;60(2):260–8. doi: 10.1161/HYPERTENSIONAHA.111.186429. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet neurology. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 38.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC neuroscience. 2010;11:118. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krenk L, Rasmussen LS, Siersma VD, Kehlet H. Short-term practice effects and variability in cognitive testing in a healthy elderly population. Experimental gerontology. 2012;47(6):432–6. doi: 10.1016/j.exger.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 40.McComb E, Brewster P, Chou PH, Crossley M, Simard M, Tuokko H. Telephone administration of the Mental Alternation Test: sensitivity to cognitive decline and practice effects across midlife and late life. Neuroepidemiology. 2010;35(4):298–302. doi: 10.1159/000321176. [DOI] [PubMed] [Google Scholar]
- 41.Khavandgar S, Homayoun H, Zarrindast MR. The effect of L-NAME and L-arginine on impairment of memory formation and state-dependent learning induced by morphine in mice. Psychopharmacology. 2003;167(3):291–6. doi: 10.1007/s00213-002-1377-7. [DOI] [PubMed] [Google Scholar]
- 42.Paul V, Reddy L, Ekambaram P. A reversal by L-arginine and sodium nitroprusside of ageing-induced memory impairment in rats by increasing nitric oxide concentration in the hippocampus. Indian journal of physiology and pharmacology. 2005;49(2):179–86. [PubMed] [Google Scholar]
- 43.Kan MJ, Lee JE, Wilson JG, Everhart AL, Brown CM, Hoofnagle AN, et al. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35(15):5969–82. doi: 10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, Waldvogel HJ, et al. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiology of aging. 2014;35(9):1992–2003. doi: 10.1016/j.neurobiolaging.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Graham SF, Chevallier OP, Elliott CT, Holscher C, Johnston J, McGuinness B, et al. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS One. 2015;10(3):e0119452. doi: 10.1371/journal.pone.0119452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul V, Ekambaram P. Involvement of nitric oxide in learning & memory processes. The Indian journal of medical research. 2011;133:471–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Calabro RS, Gervasi G, Baglieri A, Furnari A, Marino S, Bramanti P. Is high oral dose L-arginine intake effective in leukoaraiosis? Preliminary data, study protocol and expert’s opinion. Current aging science. 2013;6(2):170–7. doi: 10.2174/1874609811306020005. [DOI] [PubMed] [Google Scholar]