Abstract
Objective
Cognitive deficits in schizophrenia are the strongest predictor of disability and effective treatment is lacking. This reflects our limited mechanistic understanding and consequent lack of treatment targets. In schizophrenia, impaired sleep-dependent memory consolidation correlates with reduced sleep spindle activity, suggesting sleep spindles as a potentially treatable mechanism. In the present study we investigated whether sleep-dependent memory consolidation deficits in schizophrenia are selective.
Methods
Schizophrenia patients and healthy individuals performed three tasks that have been shown to undergo sleep-dependent consolidation: the Word Pair Task (verbal declarative memory), the Visual Discrimination Task (visuoperceptual procedural memory), and the Tone Task (statistical learning). Memory consolidation was tested 24h later, after a night of sleep.
Results
Compared with controls, schizophrenia patients showed reduced overnight consolidation of word pair learning. In contrast, both groups showed similar significant overnight consolidation of visuoperceptual procedural memory. Neither group showed overnight consolidation of statistical learning.
Conclusion
The present findings extend the known deficits in sleep-dependent memory consolidation in schizophrenia to verbal declarative memory, a core, disabling cognitive deficit. In contrast, visuoperceptual procedural memory was spared. These findings support the hypothesis that sleep-dependent memory consolidation deficits in schizophrenia are selective, possibly limited to tasks that rely on spindles. These findings reinforce the importance of deficient sleep-dependent memory consolidation among the cognitive deficits of schizophrenia and suggest sleep physiology as a potentially treatable mechanism.
Keywords: schizophrenia, sleep, memory consolidation, declarative memory, procedural memory, statistical learning
1. Introduction
It is now well-established that sleep plays a critical role in memory consolidation – processes that stabilize and enhance recently encoded memories, integrate them into existing associative networks and extract generalities from their content (Stickgold, 2005; Walker and Stickgold, 2010). Recent work in both humans and animal models has demonstrated the importance of specific brain oscillations, including sleep spindles, in the consolidation of different memory types during sleep (Dudai et al., 2015; Rasch and Born, 2013; Stickgold and Walker, 2013). Schizophrenia (SZ) patients show a specific reduction in sleep spindles (for review see Manoach et al., 2016), a thalamocortical oscillation characterizing non-rapid eye movement (NREM) Stage 2 sleep (N2). Sleep spindles correlate with IQ and are thought to both promote long-term potentiation and enhance memory consolidation (Fogel and Smith, 2011). Chronic medicated SZ patients also have reduced sleep-dependent consolidation of motor procedural memory (Genzel et al., 2015, 2011, Manoach et al., 2010, 2004; Wamsley et al., 2012) and declarative memory for pictures (Göder et al., 2015), deficits that correlate with reduced spindle activity (Göder et al., 2015; Wamsley et al., 2013, 2012). Reduced spindle activity that correlates with cognitive function is also seen in non-psychotic first-degree relatives of SZ patients and early-course antipsychotic drug-naïve SZ patients (Manoach et al., 2014; Schilling et al., 2017) suggesting that the spindle deficit is an endophenotype of SZ (Manoach et al., 2016). The goal of the present study was to better define the scope of the sleep-dependent memory deficit in SZ. Findings of dissociations (e.g., reduced sleep-dependent consolidation of motor procedural memory in the context of intact consolidation of memory for a complex figure; Seeck-Hirschner et al., 2010), suggest that only certain memory types are affected. In the present study we tested the hypothesis that deficits in sleep-dependent memory consolidation of memory are selective and present on tasks that have previously been associated with spindles.
A challenge to studying sleep-dependent memory consolidation in SZ is identifying tasks that allow patients to achieve a comparable level of baseline (pre-sleep) performance to controls. This makes it less likely that any observed group differences in sleep-dependent consolidation reflect encoding differences at baseline. The three tasks utilized meet this criterion and have shown sleep-dependent consolidation in healthy adults (i.e., better performance after sleep than after an equivalent interval of daytime wake). We probed declarative memory with a Word Pair Task (WPT) that requires learning of semantically unrelated word pairs. Previous work shows that sleep “protects” newly learned word pairs (i.e., results in reduced forgetting and less susceptibility to interference (Ellenbogen et al., 2006; Payne et al., 2012; Wilson et al., 2012). Sleep-dependent protection of WPT performance correlates with the percentage of NREM Stage 3 sleep (N3) during a daytime nap (Baran et al., 2016), percentage of NREM (N2 plus N3) during nocturnal sleep (Mantua et al., 2015) and with sleep spindles (Gais et al., 2002; Lustenberger et al., 2015; Schabus et al., 2008, 2004; Schmidt et al., 2006; Studte et al., 2017). Transcranial direct current stimulation that increases time spent in N3, slow oscillations and the number of slow spindles (8–12Hz) enhances WPT recall (Marshall et al., 2006, 2004) while transcranial alternate current stimulation disrupting slow wave activity impairs it (Garside et al., 2015).
The visual discrimination task (VDT) measures visuoperceptual procedural memory, requiring participants to determine the arrangement of a target embedded in a background of distractors (Karni et al., 1994). The target screen is presented briefly and is followed, after a variable interstimulus interval (ISI), by a mask screen, allowing determination of a threshold ISI – the minimum ISI required for 80% accuracy. After sleep, the threshold ISI is reduced (Karni et al., 1994; Stickgold et al., 2000a) and this improvement in performance correlates with percent of time in N3 in the first quarter of the night and rapid eye movement (REM) sleep in the last quarter of the night, but is best predicted by the product of these two sleep parameters, suggesting a two-step consolidation process (Stickgold et al., 2000b).
Finally, we investigated sleep-dependent consolidation of statistical learning using the Tone Task (Durrant et al., 2011). Participants hear a probabilistically determined sequence of notes. They are then asked to identify which of two brief sequences sounds similar. Sleep improves the recognition of novel sequences with the same structure presumably by facilitating the abstraction of statistical rules embedded in the sequences. Post-sleep improvement correlates with time in N3 (Durrant et al., 2013, 2011).
2. Materials and Methods
2.1. Participants
Schizophrenia outpatients (n=28), diagnosed with a Structured Clinical Interview for DSM-IV (SCID; First et al., 1997) and characterized with the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987)) were recruited from an urban mental health center. Two patients were unmedicated, and the rest were maintained on stable doses of antipsychotic medications and adjunctive agents (including antidepressants, benzodiazepines and mood stabilizers; Supplemental Table 1) for at least six weeks.
Healthy controls (n=32), screened to exclude personal histories of mental illness with a SCID-Non-Patient Edition; First et al., 2002) or family histories of SZ spectrum disorders or psychosis, were recruited from the community through advertisements.
Potential participants with current diagnoses of sleep disorders, treatment with sleep medications, a history of significant head injury or neurological illness or substance abuse or dependence within the past six months were excluded.
Controls and patients did not differ in age or parental education but there were more males in the schizophrenia group (trend-level difference: p=0.06; Table 1).
Table 1.
Participant Characteristics. Means ± standard deviations and group comparisons of demographic data.
| Schizophrenia (n=28) | Healthy Controls (n=32) | t | p | |
|---|---|---|---|---|
| Age (yrs) | 31.6 ± 7.2 | 31.9 ± 6.3 | .19 | .84 |
| Parental Education (yrs) | 14.4 ± 3.2 | 14.7 ± 3.3 | .42 | .68 |
| Sex | 6F/22M | 15F/17M | χ2=4.41 | .06 |
| Average Level of Severity | ||||
| PANSS Total | 71 ± 13 | mild | ||
| PANSS Positive | 17 ± 5 | mild | ||
| PANSS Negative | 19 ± 5 | mild | ||
| PANSS General | 35 ± 6 | mild | ||
PANNS, Positive and Negative Syndrome Scale (Kay et al., 1987)
The study was approved by the Partners Human Research Committee and participants gave written informed consent. In addition to a base rate of pay, participants received a bonus of $0.05 for each correct response on the memory tasks as an incentive to enhance attention and motivation.
2.2. General Procedure
Training and Test sessions were separated by 24h to avoid circadian effects. Tasks were presented in a fixed order (Figure 1A) and each session lasted approximately 2h. At the beginning of each session, participants filled out the Stanford Sleepiness Scale (SSS; Hoddes et al., 1973)) to measures alertness. At the end of the Test session, participants completed a questionnaire about sleep duration and quality for the previous night, daytime caffeine use and how well-rested they felt.
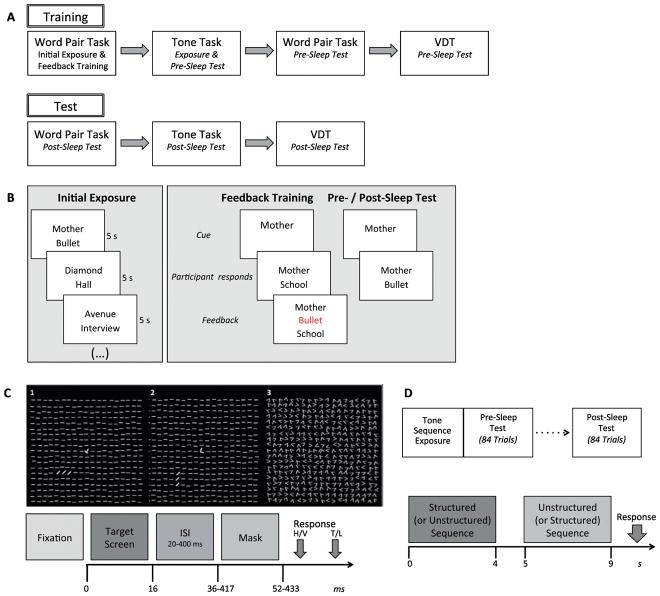
Figure 1. Study Procedures.
(A) Design. Participants were trained on three tasks in a fixed order during Training and were Tested 24h later. (B) Word Pair Task. During Initial Exposure, each word pair was displayed for 5s, with an interstimulus interval (ISI) of 100ms, and participants were instructed to “remember which words go together.” During subsequent phases, the middle 20 pairs were presented in a random order to avoid primacy and recency effects. During Feedback Training, participants were shown the first word of each pair (cue) and asked to verbally report its associate (target). The experimenter typed their response and it was displayed on the screen below the cue for 2s. If the response was incorrect, the correct target word was displayed in red above the incorrect response for 2s. This procedure was repeated until at least 12 of the targets (60%) were recalled correctly or until the list had been repeated 10 times. The Pre-Sleep Test started approximately 30m later, after training on the Tone Task. (C) Visual Discrimination Task. After a jittered interval (200–280ms), a target screen (Panels 1, 2) that contained a rotated “T” or “L” at the center and a horizontal or vertical array of three diagonal bars in the lower-left quadrant was presented for 16ms (fixation points and target arrays are displayed in bold for illustration purposes only). The array of diagonal bars was ~2.5° in length and was located ~5° from the center. The target screen was followed by a blank screen for an ISI of 20–400ms and then a mask for 16ms (Panel 3). Upon termination of the mask screen, the screen went black and participants indicated with button presses whether the diagonal lines were arranged horizontally (H) or vertically (V), and whether there was a T or an L at fixation. This was followed by the return of the fixation screen. (D) Tone Task. The task consisted of three phases: Exposure, Pre-Sleep Test and Post-Sleep Test. Exposure involved listening to a single structured stream of 1818 tones lasting 400s. Each tone lasted 200ms, with a 20ms gap between tones. Pre and Post-Sleep tests took place immediately and 24h after Exposure, respectively, and consisted of a forced-choice recognition test. Bottom: In each of 84 trials participants were presented with two 18-tone sequences (4s each) and asked to indicate which sequence sounded more familiar.
2.3. Word-Pair Task (WPT)
Stimuli were 24 semantically unrelated word pairs (Payne et al., 2012). The task consisted of four phases: Initial Exposure, Feedback Training, Pre-Sleep Test and Post-Sleep Test (Figure 1B). Participants were presented with 24 word pairs and instructed to “remember which words go together.” During Initial Exposure, participants studied cue-target word pairs. During Feedback Training, participants were presented with the cue and asked to recall the target; feedback was provided. Pre- and Post-Sleep recall were tested with no feedback.
2.4. Visual Discrimination Task (VDT)
The VDT (Stickgold et al., 2000a) was adapted for a population with SZ by adding a task demonstration, having subjects report the peripheral target orientation before rather than after the letter, and monitoring fixation. Participants were tested in a dark room while resting their head on a chin rest 35–40cm from the computer screen. Participants completed the task demonstration to preview the trial structure and practice response key mapping. To initiate each trial, participants fixated a white cross in the center of a black screen and after a jittered interval the target screen would appear (Figure 1C). Fixation was monitored with an eye tracker (EyeLink II, SR Research, Ontario, CA). Target screens contained a rotated “T” or “L” at the center and a horizontal or vertical array of three diagonal bars in the lower-left quadrant. Following the target screen, a blank screen appeared for an ISI of 20–400ms and then a mask. After the mask, participants indicated whether the three diagonal bars were arranged horizontally or vertically and whether the central letter was a T or an L. Each block consisted of 50 trials with a constant ISI. Both Pre and Post-Sleep tests consisted of one block each at ISIs of 400, 300, 200 and 160ms, followed by three blocks each at ISIs of 120 down to 20ms, in decrements of 20ms. The task was discontinued when a participant failed to correctly identify the arrangement of the diagonal lines on at least 66% of the trials for two consecutive blocks. The outcome measure was the detection threshold defined as the interpolated ISI at which the participant’s accuracy for reporting the arrangement of the diagonal lines dropped to 80%.
2.5. Tone Task
The task was adapted from Durrant et al (2011). Stimuli consisted of sequences of pure tones taken from the Bohlen-Pierce scale (frequencies 262Hz, 301Hz, 345Hz, 397Hz and 456Hz). During Exposure, participants listened to a single, structured, continuous tone stream (Figure 1D). Pre- and Post-Sleep tests took place immediately and 24h after Exposure, respectively. During each test trial, participants were presented with a structured and an unstructured 18-tone sequence and asked which sounded “more familiar.” One sequence was randomly generated and the other was a structured sequence that adhered to the statistical pattern of the exposure stream. The sequence structure is based on a probabilistic transition matrix similar to Durrant et al. (2011) except using first-order rather than second-order transitions. Difficulty was manipulated by varying the number of “likely” transitions in the structured test sequences, with harder sequences having fewer. Equal numbers of easy, medium and hard sequences were pseudorandomly presented during the test. While Durrant et al (2011) set the number of likely transitions in each 18-tone sequence to 14, 11 and 8 for easy, medium and hard sequences, respectively, we used 16, 15 and 14 likely transitions to make the task easier for older controls and patients with SZ.
2.6. Data Analysis
We investigated overnight performance changes using repeated measures ANOVA with the within-subjects factor Session (Pre-Sleep vs. Post-Sleep) and the between-subjects factor Group (patients vs. controls). For the Tone Task we added a within-subjects factor of Difficulty (Easy, Medium or Hard). We used post-hoc t-tests to compare groups on overnight change in performance calculated as the percent change in performance from the Pre- to Post-Sleep test.
3. Results
3.1. Self-report of sleep quality and alertness
Patients reported greater total sleep time, longer latency to initiate sleep, deeper sleep and better sleep quality than controls (Table 2). The groups did not differ significantly in alertness upon awakening, alertness during the day, caffeine consumption or number of arousals during sleep. They did not differ in SSS ratings of alertness at either session (Training – controls: 2.0±1.0[SD], patients: 1.6±0.7, t(58)=1.5, p=0.13; Test – controls: 2.5±1.3, patients: 2.2±1.4, t(58)=.93, p=0.36).
Table 2.
Means ± standard deviations and group comparisons of subjective ratings of sleep and daytime alertness for the interval between Training and Testing sessions
| Schizophrenia | Healthy Controls | t | p | |
|---|---|---|---|---|
| Sleep latency (min)** | 21 ± 14 | 12 ± 10 | 2.9 | 0.006 |
| Arousals (number) | 0.5 ± 0.8 | 0.9 ± 1.1 | 1.4 | 0.15 |
| Sleep depth (1–5)* | 1.6 ± 0.8 | 2.2 ± 1.1 | 2.1 | 0.04 |
| Sleep quality (1–5)* | 2.5 ± .85 | 3 ± .89 | 2.0 | 0.05 |
| Total sleep time (hr)** | 8.4 ± 1.8 | 7.1 ± 1.2 | 3.1 | 0.003 |
| Well-rested (1–5)* | 3.5 ± 0.8 | 3.0 ± 0.9 | 2.0 | 0.05 |
| Alertness upon awakening (1–5) | 2.2 ± 1.1 | 2.4 ± 1.2 | 0.7 | 0.48 |
| Alertness during the day (1–5) | 2.2 ± 1.0 | 2.5 ± 0.9 | 1.0 | 0.32 |
| Caffeinated beverages (cups) | 0.9 ± 0.9 | 1.2 ± 1.6 | 0.8 | 0.45 |
1–5 scales: 1 = very high (good), 5 = very low (poor).
p ≤ .01,
p ≤ .05.
3.2. Word Pair Task
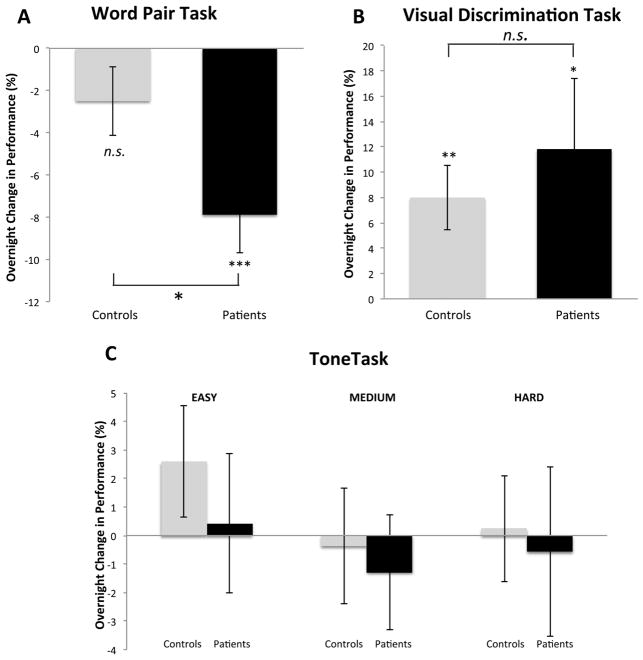
SZ patients required more Feedback Training to reach criterion (5.4±2.9 vs. 3.1±2.1 trials, t(56)=3.47, p=0.001), but did not differ from controls on the Pre-Sleep Test (controls: 68±11%, patients: 62±14%; t(56)=1.6, p=0.12). Patients, but not controls, showed a significant decline in performance (i.e. forgetting) at the Post-Sleep Test (Figure 2A; Group by Session interaction: F(1,56)=5.0, p=0.03; controls: −3.1±13.5%, t(30)=1.3, p=0.19; patients: −11.5±15.8%, t(26)=3.8, p<.001), and significantly greater forgetting than controls (t(56)=2.2, p=0.03). Thus, despite similar Pre-Sleep Test performance, patients averaged more than three times the forgetting seen in controls (11.5% vs. 3.1%). Similar results were obtained when absolute, rather than relative, forgetting was measured (word pairs forgotten: 0.47 controls vs.1.56 patients; t(56)=2.2, p=0.03). Consolidation of the WPT was not correlated with either the number of Feedback Training trials (r=.16, p=0.42) or Pre-Sleep Test performance (r=−.27, p=0.16) in SZ. In summary, despite comparable pre-sleep recall, unlike controls, patients failed to show evidence of overnight consolidation of word pair learning.
Figure 2. Overnight change in performance.
Y axes show percent change in performance from the Pre- to Post-Sleep test in healthy controls (gray bars) and SZ patients (black bars) for (A) Word Pair Task, (B) Visual Discrimination Task and (C) Tone Task. Error bars are standard errors of the mean. *p<.05, **p<.01, ***p<.001, n.s.: not significant.
3.3. Visual Discrimination Task
Both groups showed significant overnight improvement (Session main effect: F(1,44)=11.7, p=0.001), and performed comparably on all measures of learning and overnight improvement (all p’s >0.4). At training, patients displayed an average detection threshold of 133ms, compared to 134ms for controls (t(44)=0.10, p=0.93), while the next day thresholds were 110ms and 120ms, respectively (t(44)=0.93, p=0.36). Overnight, patients improved by 12±25% (Figure 2B; t(19)=2.1, p=0.049; absolute improvement: 23±49ms; t(19)=2.1, p=0.047), while controls improved by 8±13% (t(25)=3.1, p=0.004; absolute improvement: 14±23ms; t(25)=3.1, p=0.004). Improvement in the two groups did not differ significantly (t=0.63, p=0.50; absolute improvement: t(44)=.75, p=0.42). Overall, no group differences were found in either VDT learning or overnight consolidation.
3.4. Tone Task
Both groups learned to discriminate between structured and unstructured sequences; Pre-Sleep Test performance was above chance for both groups at all difficulty levels (all p’s<0.001). Across difficulty levels, performance ranged from 67% to 81% correct for controls and from 63% to 79% for patients, with higher accuracy on easier trials (main effect of difficulty: F(2,51)=78.2, p<.001). However, contrary to expectation, there was no significant overnight improvement in either group and no group differences at any difficulty level (Session main effect: F(1,52)=.03, p=0.87; Group main effect: F(1,52)=.76, p=0.39; all interactions: p>0.14).
3.5. Correlation of sleep-dependent changes in performance across tasks
In general, overnight changes on one task did not predict changes on other tasks in the same individual (pairwise regression models; all task effects p>0.21, all group by task interactions, p>0.17).
3.6. Control analyses
Because there were more males in the schizophrenia group, we repeated our main analyses in males only. Restricting the sample to males did not substantially change our findings (Supplement). Neither antipsychotic dosage, measured as chlorpromazine equivalents (Woods, 2003) (all p’s≥.48) nor age correlated with overnight improvement on any task. For age these relations did not differ by group (regression of age, group and age by group interaction, all p’s≥.12).
4. Discussion
On three tasks of sleep-dependent memory consolidation, SZ patients reached similar performance levels as healthy controls after training, but showed a selective deficit in the sleep-dependent consolidation of word pair learning (WPT). This deficit in sleep-dependent verbal declarative memory consolidation occurred in the context of intact consolidation of visuoperceptual procedural memory (VDT). Previous studies of SZ have shown deficits in sleep-dependent memory consolidation of motor procedural memory on the finger tapping motor sequence task (Genzel et al., 2015, 2011, Manoach et al., 2010, 2004; Wamsley et al., 2012) and visual declarative memory for a picture recognition task (Göder et al., 2015). Sleep-dependent consolidation of both these tasks correlates with sleep spindle density in SZ patients and healthy individuals (Albouy et al., 2013; Barakat et al., 2011; Göder et al., 2015; Nishida and Walker, 2007; Wamsley et al., 2012) and, for the motor sequence task, with the temporal coordination of spindles with cortical slow waves in SZ (Demanuele et al., 2017). Since overnight consolidation of the WPT, but not the VDT, has been shown to correlate with sleep spindles (Gais et al., 2002; Lustenberger et al., 2015; Schabus et al., 2008, 2004; Schmidt et al., 2006; Studte et al., 2017) the present findings are consistent with the hypothesis that deficits in sleep-dependent memory consolidation in SZ are mediated by sleep spindle deficits (for review see Manoach et al., 2016)).
Verbal declarative memory, along with attention and executive function, are the most impaired neurocognitive domains in SZ. Deficits in verbal declarative memory are present in individuals at high-risk for SZ, including non-psychotic relatives (Whyte et al., 2005), and in antipsychotic-naïve first episode patients and chronic patients (Saykin et al., 1994) and remain fairly stable over time, suggesting that they are a trait reflecting genetic risk for SZ (for review see Cirillo and Seidman, 2003). Previous studies have been restricted to studying verbal declarative memory deficits within a single session, typically with delays of approximately 30 min (e.g., Saykin et al., 1994) similar to the delay in the WPT Pre-sleep Test. The present study demonstrates for the first time a seemingly independent deficit in the sleep-dependent consolidation of verbal declarative memory in SZ. Although patients needed more exposure to the word pairs to reach criterion, they showed a comparable level of recall 30 minutes after the end of training and their encoding deficit did not correlate with their overnight deficit. It is possible that sleep-dependent deficits in verbal memory exacerbate the effects of verbal encoding deficits to worsen functional disability.
Despite the impairment of sleep-dependent verbal declarative memory consolidation in the present study and of motor procedural memory in prior work, SZ patients showed intact sleep-dependent consolidation of visuoperceptual procedural memory. Examination of performance indicates that the absence of a group difference is unlikely to reflect ceiling or floor effects or reduced sensitivity of this task compared with the WPT. Rather than correlating with sleep spindles, this type of memory has mostly been shown to correlate with N3 early in the night and rapid eye movement (REM) sleep late in the night (Aeschbach et al., 2008; Karni et al., 1994; Stickgold et al., 2000b).
Unexpectedly, neither group showed significant consolidation of statistical learning on the Tone Task. Previous reports in healthy young adults reported significant sleep-dependent enhancement with both overnight sleep and daytime naps, which was associated with N3 (Durrant et al., 2013, 2011). Although the participants in the present study were older (31±7 vs. 22±6 yrs), age explained less than 1% of total variance. Another possible source of the failure of overnight improvement is that modifications made the task too easy. Several studies have found sleep-dependent improvement on memory tasks to depend on task difficulty or the success of individual subjects in learning it (Peters et al., 2007; Tucker and Fishbein, 2008). Indeed, Durrant et al. (2011) only observed sleep-dependent improvement when baseline performance was 60% or lower and not when 70% or higher. In the current study, both groups showed baseline performances greater than 60% at all difficulty levels, and above 70% for medium and easy levels.
The present findings of impaired overnight preservation of word pair associate learning in the context of intact visuoperceptual learning add to the previous literature showing deficient sleep-dependent consolidation of motor procedural learning and declarative memory for pictures. Although we did not have a wake control condition in the present study, we attribute the preserved post-sleep word pair performance in controls to sleep-dependent consolidation of the memory that protects it from wake-related deterioration and hypothesize that this was absent in patients. This is based on the finding that patients did not show greater forgetting of word pairs after a 30m interval of wake than controls, and on previous studies showing greater forgetting of word pairs after 12 hours of wake versus sleep in healthy individuals (Wilson et al., 2012). Our finding of reduced sleep-dependent consolidation of verbal declarative memory in SZ complements a previous report that only controls, not SZ patients, showed better declarative memory for pictures after 12 hours of sleep versus wake (Göder et al., 2015).
Importantly, sleep-dependent memory deficits in SZ may be treatable. Sleep-dependent memory consolidation is thought to rely on the precise temporal coordination of three cardinal oscillations of NREM sleep: spindles, hippocampal sharp wave ripples and cortical slow waves (Mölle et al., 2011; Siapas and Wilson, 1998). In healthy individuals zolpidem enhances sleep-dependent protection of WPT encoding while increasing spindle density and spindle-cortical slow wave coupling (Mednick et al., 2013; Niknazar et al., 2015). In SZ, both spindle density and the temporal coordination of spindles with cortical slow waves correlate with better sleep-dependent consolidation of motor procedural memory (Demanuele et al., 2017). This raises the possibility that increasing spindles and their coordination with other NREM oscillations may improve sleep-dependent memory deficits.
Several study limitations warrant consideration. First, because we did not record sleep on the night between the training and test sessions, we could not investigate the relations of sleep with overnight memory consolidation. We therefore base our hypothesis that reduced overnight consolidation on the WPT in SZ reflects abnormal sleep on the literature showing spindle deficits in SZ (reviewed in Manoach et al., 2016) and that overnight WPT consolidation correlates with spindles (Gais et al., 2002; Lustenberger et al., 2015; Schabus et al., 2008, 2004; Schmidt et al., 2006; Studte et al., 2017). While we did not include a wake control condition to demonstrate that overnight changes in task performance were sleep-dependent, this has been demonstrated in previous studies of healthy individuals for the tasks that we employed. Studies now in progress are assessing the relation of sleep parameters to WPT consolidation in both SZ and health. Another limitation is that patients were treated with a variety of antipsychotic drugs (APDs) and adjunctive agents that may have affected sleep and sleep-dependent memory consolidation. APDs are sedating and may also have contributed to the longer sleep times reported by SZ patients. Chronic use of APDs tends to normalize sleep architecture (Hinze-Selch et al., 1997; Krystal et al., 2008; Maixner et al., 1998; Taylor et al., 1991), and withdrawal is associated with a progressive deterioration of sleep quality (Nofzinger et al., 1993), but their effects on the spectral content of sleep, including spindles, is largely unexplored. Previous findings that early course antipsychotic-naïve patients with SZ and non-psychotic first-degree relatives of SZ patients have spindle deficits that correlate with cognitive performance make it unlikely that the spindle deficits and consequent memory consolidation deficits are due to chronicity or treatment with APDs (Manoach et al., 2014; Schilling et al., 2017).
In summary, the present findings extend the known deficits in sleep-dependent memory consolidation in SZ to verbal declarative memory, a core, disabling cognitive deficit, and demonstrate that sleep-dependent consolidation of a visuoperceptual procedural memory task is unaffected. They support the hypothesis that sleep-dependent memory consolidation deficits in SZ are selective and limited to those tasks for which consolidation depends on spindles. Our findings reinforce the importance of deficient sleep-dependent memory consolidation among the cognitive deficits of SZ and suggest abnormal sleep as a potentially treatable mechanism.
Supplementary Material
Acknowledgments
Role of funding sources
This work was supported by NIH grants MH092638 to DSM and RS, MH048832 to RS, HL007901 to BB and 1UL1TR001102 to Harvard Clinical and Translational Science Center.
Footnotes
Contributors
BB analyzed data and wrote the manuscript. DC and TCV collected data and edited the manuscript. AM programmed and implemented experimental tasks and edited the manuscript. SJD designed the tone task, aided in data analysis and edited the manuscript. DSM and RS conceived and designed the study, aided in data analysis and contributed to writing the manuscript.
Conflicts of Interest
The authors report no financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci Off J Soc Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PloS One. 2013;8:e52805. doi: 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217:117–121. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Baran B, Mantua J, Spencer RMC. Age-related Changes in the Sleep-dependent Reorganization of Declarative Memories. J Cogn Neurosci. 2016:1–11. doi: 10.1162/jocn_a_00938. [DOI] [PMC free article] [PubMed]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Demanuele C, Bartsch U, Baran B, Khan S, Vangel MG, Cox R, Hämäläinen M, Jones MW, Stickgold R, Manoach DS. Coordination of Slow Waves With Sleep Spindles Predicts Sleep-Dependent Memory Consolidation in Schizophrenia. Sleep. 2017:40. doi: 10.1093/sleep/zsw013. [DOI] [PMC free article] [PubMed]
- Dudai Y, Karni A, Born J. The Consolidation and Transformation of Memory. Neuron. 2015;88:20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Durrant SJ, Cairney SA, Lewis PA. Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cereb Cortex. 2013;23:2467–2478. doi: 10.1093/cercor/bhs244. [DOI] [PubMed] [Google Scholar]
- Durrant SJ, Taylor C, Cairney S, Lewis PA. Sleep-dependent consolidation of statistical learning. Neuropsychologia. 2011;49:1322–1331. doi: 10.1016/j.neuropsychologia.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol CB. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. https://doi.org/20026697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P, Arizpe J, Lau CI, Goh C, Walsh V. Cross-hemispheric Alternating Current Stimulation During a Nap Disrupts Slow Wave Activity and Associated Memory Consolidation. Brain Stimulat. 2015;8:520–527. doi: 10.1016/j.brs.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Ali E, Dresler M, Steiger A, Tesfaye M. Sleep-dependent memory consolidation of a new task is inhibited in psychiatric patients. J Psychiatr Res. 2011;45:555–560. doi: 10.1016/j.jpsychires.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Cornu M, Jäger E, Konrad B, Adamczyk M, Friess E, Steiger A, Czisch M, Goya-Maldonado R. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77:177–186. doi: 10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Göder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, Prehn-Kristensen A. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16:564–569. doi: 10.1016/j.sleep.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Hinze-Selch D, Mullington J, Orth A, Lauer CJ, Pollmächer T. Effects of clozapine on sleep: a longitudinal study. Biol Psychiatry. 1997;42:260–266. doi: 10.1016/S0006-3223(96)00347-2. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Goforth HW, Roth T. Effects of antipsychotic medications on sleep in schizophrenia. Int Clin Psychopharmacol. 2008;23:150–160. doi: 10.1097/YIC.0b013e3282f39703. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Wehrle F, Tüshaus L, Achermann P, Huber R. The Multidimensional Aspects of Sleep Spindles and Their Relationship to Word-Pair Memory Consolidation. Sleep. 2015;38:1093–1103. doi: 10.5665/sleep.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner S, Tandon R, Eiser A, Taylor S, DeQuardo JR, Shipley J. Effects of antipsychotic treatment on polysomnographic measures in schizophrenia: a replication and extension. Am J Psychiatry. 1998;155:1600–1602. doi: 10.1176/ajp.155.11.1600. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, Kupfer D, Buysse D, Stickgold R, Keshavan MS. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. doi: 10.3389/fnhum.2014.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry. 2016;80:599–608. doi: 10.1016/j.biopsych.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantua J, Mahan KM, Henry OS, Spencer RMC. Altered sleep composition after traumatic brain injury does not affect declarative sleep-dependent memory consolidation. Front Hum Neurosci. 2015;9:328. doi: 10.3389/fnhum.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SPA. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci Off J Soc Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknazar M, Krishnan GP, Bazhenov M, Mednick SC. Coupling of Thalamocortical Sleep Oscillations Are Important for Memory Consolidation in Humans. PloS One. 2015;10:e0144720. doi: 10.1371/journal.pone.0144720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PloS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, van Kammen DP, Gilbertson MW, Gurklis JA, Peters JL. Electroencephalographic sleep in clinically stable schizophrenic patients: two-weeks versus six-weeks neuroleptic-free. Biol Psychiatry. 1993;33:829–835. doi: 10.1016/0006-3223(93)90024-8. [DOI] [PubMed] [Google Scholar]
- Payne JD, Tucker MA, Ellenbogen JM, Wamsley EJ, Walker MP, Schacter DL, Stickgold R. Memory for semantically related and unrelated declarative information: the benefit of sleep, the cost of wake. PloS One. 2012;7:e33079. doi: 10.1371/journal.pone.0033079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KR, Smith V, Smith CT. Changes in sleep architecture following motor learning depend on initial skill level. J Cogn Neurosci. 2007;19:817–829. doi: 10.1162/jocn.2007.19.5.817. [DOI] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, Sauter C, Kloesch G, Klimesch W, Saletu B, Zeitlhofer J. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling C, Schlipf M, Spietzack S, Rausch F, Eisenacher S, Englisch S, Reinhard I, Haller L, Grimm O, Deuschle M, Tost H, Zink M, Meyer-Lindenberg A, Schredl M. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. Eur Arch Psychiatry Clin Neurosci. 2017;267:213–224. doi: 10.1007/s00406-016-0725-2. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Münch M, de Quervain DJF, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Göder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2010;44:42–47. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000a;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000b;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Studte S, Bridger E, Mecklinger A. Sleep spindles during a nap correlate with post sleep memory performance for highly rewarded word-pairs. Brain Lang. 2017;167:28–35. doi: 10.1016/j.bandl.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Tandon R, Shipley JE, Eiser AS. Effect of neuroleptic treatment on polysomnographic measures in schizophrenia. Biol Psychiatry. 1991;30:904–912. doi: 10.1016/0006-3223(91)90004-6. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci. 2010;11:218. doi: 10.1038/nrn2762-c1. author reply 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36:1369–1376. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;78:13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RMC. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging. 2012;33:991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.