Abstract
Phthalates exposure has been linked to multiple health risks, and US immigrants may have different exposures to phthalates due to lifestyle differences. Urinary concentrations of eight phthalate metabolites (mono-ethyl phthalate [MEP], mono-n-butyl phthalate [MnBP], mono-isobutyl phthalate [MiBP], mono-(3-carboxypropyl) phthalate [MCPP], mono-benzyl phthalate [MBzP], mono-2-ethylhexyl phthalate [MEHP], mono-(2-ethyl-5-hydroxyhexyl) phthalate [MEHHP], mono-(2-ethyl-5-oxohexyl) phthalate [MEOHP]) were measured in 10318 US-born and 3511 foreign-born individuals from NHANES 1999-2014. Using multivariate adjusted linear regression, we assessed whether phthalate metabolite levels differed by nativity in the whole population, within racial/ethnic groups, and by years in the US. We also tested whether immigrant demographics predicted phthalate metabolite levels. In fully adjusted models, MEP, MnBP, and MiBP were significantly higher, and MBzP significantly lower, among immigrants than US-born participants. Among immigrants, MnBP and MiBP significantly declined with longer time in the US (Ptrend = 0.029 and Ptrend =0.039, respectively), while MCPP and MBzP significantly rose (Ptrend = 0.019 and Ptrend =0.043, respectively). Results within each racial/ethnic group were consistent with the whole population. Among immigrants, women had significantly higher metabolite levels than men (all p<0.01), and MEP, MnBP, and MCPP differed by race/ethnicity. Due to higher phthalate exposures, immigrants may be especially vulnerable to phthalate-associated health problems.
Keywords: Phthalates, endocrine disruptors, population-based studies, biomonitoring
Introduction
Immigrants are a sizable and growing fraction of the United States’ (US) population. According to the US Census Bureau, in 2015 more than 42 million immigrants were living in the US. By 2060, the immigrant population is projected to grow to close to 80 million people, comprising nearly 20% of the US population. (1) There are many reasons why the health of foreign-born populations may differ in important ways from the health of the US-born population. Immigrants are less likely to have health insurance, more likely to be employed, and more likely to live in poverty than the US-born population, though there is substantial heterogeneity within the immigrant population by region of birth. (2–6) Health may also be influenced by exposures to environmental pollutants that vary by immigration status. For example, immigrant populations have been shown to have higher exposure to persistent organic pollutants like polychlorinated biphenyls and organochlorine pesticides, and heavy metals like mercury, arsenic, lead, and cadmium, with implications for adverse health outcomes. (7–10)
While persistent chemicals and metals have been evaluated, less is known about immigrant exposures to non-persistent chemicals. Non-persistent chemicals, including phthalates and phenols, have been associated with a number of adverse health outcomes, (11–14) including some conditions (e.g., allergic disease, preterm birth) that vary by immigrant status. (15–17) Therefore, understanding sources of these chemicals, their health effects, and potential vulnerability among immigrant populations are important public health goals.
Phthalates are industrial chemicals widely used in consumer and household products. (18) Low molecular weight phthalates are frequently found in personal care products such as cosmetics and fragrances, while high molecular weight phthalates are used in polyvinyl chloride (PVC) products, floor and wall coverings, and in food packaging. (19) Phthalates migrate from these products into the environment and into humans; (20, 21) because phthalates are so widely used, most of the US population is continuously exposed to multiple phthalates. (22) In humans, phthalates are quickly metabolized, and the metabolites rather than the parent compounds are used as biomarkers of exposure. Phthalate metabolites have half-lives in humans under 24 hours, and therefore represent short-term, recent exposure to phthalates. (23, 24) Details of common phthalate exposure sources and metabolism have been reviewed elsewhere. (12, 18–22) In some human and animal studies, certain phthalates and urinary phthalate metabolites have been associated with health risks including allergic sensitization, reproductive toxicity, impaired neurodevelopment and preterm delivery. (11–14) Identifying highly exposed subgroups of the population is important for reducing environmental health disparities.
Phthalate exposure in the US population has been shown to vary by a number of demographic factors, including sex, race/ethnicity, and socioeconomic status. Women have higher levels of exposure than men, and non-white populations have higher levels of exposure than whites to some phthalates. (25, 26) Additionally, variables reflecting low socioeconomic status, including lower educational attainment, poverty status, and greater food insecurity, have been associated with higher phthalate concentrations. (27, 28) Although the reasons underlying these demographic differences in phthalate metabolite levels are not entirely known, these higher levels of exposure may contribute to poorer health in vulnerable populations that are also subjected to many other social and biological stressors. (29)
Immigrants may have different exposure to phthalates than individuals born in the US for a variety of reasons. For example, immigrants may use different personal care products (30), work in different occupational environments (4, 31, 32), or have differing dietary patterns (33), among other possible differences, compared to US-born individuals. Several studies support the idea of different phthalate exposure patterns among immigrants. For example, a small study of Hmong women that included immigrants (n=45 women) noted that Hmong women had much lower mono-ethyl phthalate (MEP), and much higher mono-isobutyl phthalate (MiBP) and mono-benzyl phthalate (MBzP) than background exposure levels in the US. (34) Similarly, a study of phthalate exposures in the CHAMACOS cohort of pregnant Mexican-Americans reported that women born in the US and women who had lived in the US for a longer time had higher MBzP levels than recent Mexican immigrants. (35) However, to our knowledge, our study is the first to describe phthalate exposures among an ethnically diverse, nationally representative sample of the immigrant population in the US.
In this study, we report levels of eight phthalate metabolites (MEP, mono-n-butyl phthalate [MnBP], MiBP, mono-(3-carboxypropyl) phthalate [MCPP], MBzP, mono-2-ethylhexyl phthalate [MEHP], mono-(2-ethyl-5-hydroxyhexyl) phthalate [MEHHP], mono-(2-ethyl-5-oxohexyl) phthalate [MEOHP]) in 13829 individuals surveyed by the National Health and Nutrition Examination Survey (NHANES) 1999-2014. We hypothesize that foreign-born participants will be exposed to different levels of phthalates than US-born participants. We also expect that the nativity-related differences in phthalate exposure will be more pronounced among those who have been in the US for a shorter period of time.
Methods
Study Population
We used data from 8 two-year survey sampling cycles of the National Health and Nutrition Examination Survey (NHANES years 1999-2014), a nationally representative survey of the non-institutionalized US population conducted by Centers for Disease Control and Prevention (CDC)’s National Center for Health Statistics. Survey protocols were approved by the National Center for Health Statistics Research Ethics Review Board and informed consent was obtained from all participants. NHANES uses a complex multi-stage probability sampling strategy and over-samples low-income individuals, adults over age 60, and members of some minority racial/ethnic groups to increase the precision of estimates for these subpopulations. About 10000 individuals are sampled each cycle, and about one-third of these are selected to contribute urinary phthalate metabolite data. In this study, we included all adults over age 20 years who contributed urinary phthalate metabolite data (n=13842). Individuals missing data on immigrant status (n=13) were excluded, and analyses were run on the remaining 13829 individuals.
NHANES conducts interviews in English and Spanish and attempts to hire local interpreters for other languages spoken by participants when needed. (36) Interview language information was collected beginning in 2003. In our sample, approximately 6% of participants completed the interview in Spanish, and another 1.5% used an interpreter.
Urinary Phthalate Metabolite Measurements
Urine samples were collected by NHANES technicians in the mobile examination unit during the participant’s visit. Samples were frozen at −20°C (1999–2010) or −40°C (2011–2014) until analysis. Sample processing and analyte quantification is described in detail in the Centers for Disease Control documentation. (37) Briefly, samples were processed by CDC technicians using enzymatic deconjugation of the glucuronidated analytes followed by solid-phase extraction. In all cycles, a labeled internal standard was incorporated for each analyte to monitor precision. From 1999 to 2002 phthalate metabolites were quantitatively detected using APCI-tandem mass spectrometry (MS/MS). (38) From 2003-2014 phthalate metabolites were quantitatively detected using high performance liquid chromatography-electrospray ionization-tandem mass spectrometry. (39)
We chose five urinary phthalate metabolites (MEP, MnBP, MiBP, MCPP, MBzP) that were measured in at least 7 cycles and detected in at least 75% of samples across all cycles. We also included three metabolites of di-(2-ethylhexyl)phthalate (DEHP). DEHP metabolites represent a single pathway, in which MEHP is the first monoester metabolite that is oxidized to form secondary metabolites MEHHP and MEOHP. (23) MEP, MnBP, MBzP, and MEHP were measured in all study years, while MiBP, MCPP, MEHHP, and MEOHP were measured beginning in 2001. We used MEHP, MEHHP, and MEOHP to calculate a molar sum of DEHP (ΣDEHP), dividing each metabolite by its molecular weight, using the following equation: . Metabolite measurements below the metabolite-and cycle-specific limit of detection (LOD) were replaced with .
Nativity Ascertainment
Nativity was ascertained with the question, “In what country were you born?”. Individuals answering that they were born in the 50 US states or Washington, DC were recorded as US-born. Those answering that they were born in another country or US territory were recorded as foreign-born. Those who declined to answer or did not know where they were born were recorded as missing (n=13). Foreign-born individuals were also asked how many years they had been living in the US.
Statistical Analysis
All phthalate metabolite levels were natural log-transformed to account for non-normality (right skewed distributions). All analytical models were adjusted for urinary creatinine, age (continuous), age squared, sex, education (Less than high school, High school graduate or equivalent, Some college or associate’s degree, College graduate or above), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, Other Hispanic, Other), income to poverty ratio (IPR; 0-0.99, 1-1.99, 2-3.99, 4-4.99, ≥5), and survey year. IPR was calculated by NHANES as a ratio of family income to poverty based on the poverty guidelines used by the Department of Health and Human Services. Covariates were chosen a priori as likely confounders based on previous literature. (26–28) We did not adjust for body mass index (BMI) because the direction of the relationship between phthalate exposure and body mass is unclear in cross sectional data. Additionally, including BMI in statistical models produced nearly identical results but reduced the sample size due to some individuals missing BMI. Models predicting metabolites of dibutyl phthalate (MCPP and MnBP) were additionally adjusted for current smoking status because dibutyl phthalate is used in cigarette filters. (40)
We first assessed whether phthalate metabolite levels differed by nativity (US-born or foreign-born) in the whole population. We further assessed whether length of time in the US predicted phthalate metabolite levels within the immigrant population. We calculated least squares geometric mean (LSGM) phthalate metabolite levels for immigrants who had lived 0-4 years, 5-9 years, 10-19 years, 20-29 years, and ≥30 years in the US. Least square geometric means are predicted geometric mean values of the dependent variable from fully adjusted regression models. The 5-category ordinal years in US variable was also entered as a continuous variable in the multivariate model to test for a linear trend.
To separate participant age from length of time in the US, we also divided the study population by age (<40 years [young] and ≥40 years [older]) and nativity status (US born, <10 years in US (recent immigrant), and ≥10 years in US (long-term immigrant) to create a 6-category variable combining age and nativity: Young US-born, Older US-born, Young recent immigrant, Older recent immigrant, Young long-term immigrant, and Older long-term immigrant. For every phthalate metabolite, we estimated percent difference in urinary phthalate metabolite concentrations by comparing each Young immigrant group to the Young US-born referent, and each Older immigrant group to the Older US-born referent. Percent difference was calculated as (eβ-1) * 100%.
Finally, we tested whether demographic characteristics within the immigrant population predicted levels of each phthalate metabolite. We estimated percent difference in urinary phthalate metabolite concentrations for age, sex, education, and higher quintiles of IPR compared to the lowest quintile. We also calculated LSGM values by race/ethnicity, using only data from 2011-2014 to allow division into 6 groups and to present current LSGM estimates of metabolite levels (Non-Hispanic Asians were measured as a separate group beginning in 2011). We compared phthalate metabolite levels in foreign-born to US-born individuals within each racial/ethnic group. IPR was modeled as a 2-category variable (above vs below 1) to accommodate the smaller sample size used in this analysis.
Because phthalate metabolite levels in the US population have changed over time (19), as a sensitivity analysis, we evaluated the demographic profile of the immigrant and US-born populations in four 4-year sub-populations (1999–2002, 2003–2006, 2007–2010, 2011–2014) to assess whether the demographic profile of the population had substantially changed over the study period. We also repeated the main analyses using data from the most recent 4-year sub-population (2011-2014) only, to ensure that differences by nativity were robust to shifts in phthalate metabolite levels over time. Because the racial/ethnic makeup of US-born and foreign-born populations was quite different, we also repeated the main analyses limiting the study population to Mexican American participants only (the group with the largest immigrant sample size) to eliminate potential confounding of the effect of nativity by race/ethnicity.
All analyses used NHANES multistage sub-sample weights to ensure that the sample was representative of the US adult population over age 20 years. Because some phthalate metabolites were measured in 7 sampling cycles (14 years), while others were measured in 8 cycles (16 years), we calculated 8-cycle and 7-cycle sample weights for each participant according to National Center for Health Statistics guidelines (41) and used each when appropriate. All analyses were conducted using SAS 9.4 (Cary, NC).
Code availability
The code used to generate the findings of this paper is available in its entirety in the Supplementary Information.
Results
Immigrant demographics
Compared to those born in the US, foreign-born individuals were younger, more likely to have less than a high school education, less likely to be current smokers, and more likely to have a lower IPR (all p<0.0001). The racial/ethnic makeup of the foreign-born population also differed substantially from the US-born population. Foreign-born individuals were much less likely to be non-Hispanic White or non-Hispanic Black, and much more likely to report their race/ethnicity as Mexican American, Other Hispanic, or Other. In fact, 73.2% of foreign-born individuals reported membership in these latter 3 groups versus 8.4% of US-born individuals (p<0.0001; Table 1).
Table 1.
Demographic characteristics of the study population.
| Characteristic | US-born n (%) | Foreign-born n (%) | P |
|---|---|---|---|
| Age (years) | <0.0001 | ||
| 20-29 | 1887 (18.7%) | 626 (19.8%) | |
| 30-39 | 1681 (17.9%) | 701 (24.9%) | |
| 40-49 | 1636 (20.1%) | 696 (22.6%) | |
| 50-59 | 1471 (18.0%) | 522 (15.8%) | |
| 60-69 | 1608 (12.5%) | 526 (8.3%) | |
| 70-79 | 1204 (8.3%) | 292 (6.0%) | |
| ≥ 80 | 831 (4.6%) | 148 (2.6%) | |
| Sex | 0.0011 | ||
| Male | 4912 (47.4%) | 1772 (51.1%) | |
| Female | 5406 (52.6%) | 1739 (48.9%) | |
| Race/Ethnicity | <0.0001 | ||
| Non-Hispanic White | 6120 (79.3%) | 346 (20.3%) | |
| Non-Hispanic Black | 2621 (12.2%) | 279 (6.5%) | |
| Mexican American | 973 (3.6%) | 1475 (29.1%) | |
| Other Hispanic | 292 (2.2%) | 714 (21.9%) | |
| Other1 | 312 (2.6%) | 697 (22.2%) | |
| Education | <0.0001 | ||
| < High School | 2321 (15.4%) | 1629 (36.3%) | |
| High School | 2637 (25.3%) | 582 (18.0%) | |
| Some College | 3139 (32.2%) | 626 (21.1%) | |
| ≥ College | 2205 (27.1%) | 666 (24.6%) | |
| Smoking | <0.0001 | ||
| Current | 2495 (24.1%) | 497 (15.8%) | |
| Past | 2693 (25.0%) | 707 (19.5%) | |
| Never | 5125 (50.9%) | 2300 (64.7%) | |
| Income/Poverty Ratio | <0.0001 | ||
| 0-0.99 | 1738 (12.8%) | 914 (24.4%) | |
| 1-1.99 | 2346 (19.3%) | 948 (26.9%) | |
| 2-2.99 | 1463 (15.2%) | 439 (15.6%) | |
| 3-4.99 | 2078 (25.9%) | 434 (18.2%) | |
| ≥ 5 | 1928 (26.8%) | 353 (15.0%) | |
| Time in US (years) | – | ||
| 0-4 | – | 460 (15.2%) | |
| 5-9 | – | 502 (15.7%) | |
| 10-19 | – | 832 (26.4%) | |
| 20-29 | – | 684 (20.1%) | |
| ≥ 30 | – | 893 (22.6%) |
Other includes Non-Hispanic Asians, who were not reported by NHANES as a separate racial/ethnic group until 2011.
Over the study period, the proportion of foreign-born individuals in the NHANES population grew from about 15% to over 18%, and also became somewhat older, less likely to currently smoke, and more likely to have lived in the US longer than 10 years during the study period (see Table S1). Compared to those who lived in the US longer than 10 years, foreign-born individuals who lived in the US less than 10 years were much more likely to be under the age of 40, and more likely to be below the poverty line (IPR < 1) (see Table S2).
Phthalate metabolites by nativity
The frequency of detection and concentration distribution of measured phthalate metabolites stratified by nativity are shown in Table 2. With the exception of MEHP, all phthalate metabolites were detected in over 93% of samples in both the US- and foreign-born populations. In fully adjusted models, levels of MEP, MnBP, and MiBP were significantly higher, and levels of MBzP were significantly lower, among immigrants compared to those born in the US (Figure 1). Levels of MnBP and MiBP significantly declined with longer time in the US (Ptrend = 0.029 and Ptrend =0.039, respectively), while levels of MCPP and MBzP significantly rose with length of time in the US (Ptrend = 0.019 and Ptrend =0.043, respectively). The time trend for MEP did not achieve statistical significance (p=0.60). No differences by nativity were found for ΣDEHP metabolites (Figure 1).
Table 2.
Percent detected and distribution of urinary phthalate metabolites (ng/mL).
| Metabolite1 | n | % Detected | 25% | 50% | 75% | 95% |
|---|---|---|---|---|---|---|
| MEP | ||||||
| US-born | 9931 | 99.9% | 25.61 | 72.01 | 223.08 | 1219.42 |
| Foreign-born | 3432 | 99.9% | 29.60 | 87.32 | 299.31 | 1672.60 |
| MnBP | ||||||
| US-born | 9936 | 98.2% | 6.80 | 15.30 | 30.50 | 87.60 |
| Foreign-born | 3432 | 98.0% | 7.30 | 16.60 | 35.10 | 102.10 |
| MiBP | ||||||
| US-born | 8891 | 93.8% | 2.20 | 5.00 | 10.64 | 28.00 |
| Foreign-born | 3018 | 95.8% | 3.20 | 6.90 | 14.10 | 40.76 |
| MCPP | ||||||
| US-born | 8891 | 95.7% | 1.10 | 2.50 | 5.10 | 17.30 |
| Foreign-born | 3018 | 93.4% | 0.90 | 2.10 | 4.30 | 16.00 |
| MBzP | ||||||
| US-born | 9936 | 98.2% | 2.81 | 6.98 | 15.98 | 52.13 |
| Foreign-born | 3432 | 97.5% | 2.30 | 5.40 | 12.40 | 38.70 |
| MEHP | ||||||
| US-born | 9936 | 69.3% | – | 1.80 | 4.70 | 22.70 |
| Foreign-born | 3432 | 73.1% | – | 2.20 | 5.20 | 25.30 |
| MEHHP | ||||||
| US-born | 8891 | 99.3% | 5.76 | 13.30 | 30.50 | 147.90 |
| Foreign-born | 3018 | 99.3% | 5.50 | 12.20 | 26.30 | 120.70 |
| MEOHP | ||||||
| US-born | 8891 | 98.6% | 3.70 | 8.40 | 19.07 | 84.10 |
| Foreign-born | 3018 | 98.6% | 3.40 | 7.66 | 16.64 | 73.80 |
| ΣDEHP2 | ||||||
| US-born | 8891 | – | 0.037 | 0.083 | 0.19 | 0.88 |
| Foreign-born | 3018 | – | 0.035 | 0.078 | 0.16 | 0.75 |
Metabolites were included in this analysis only if they were measured in at least 7 cycles and detected in at least 75% of samples across cycles, or are a metabolite of DEHP. Parent phthalates are as follows: diethyl phthalate (parent of MEP); di-n-butyl phthalate (parent of MnBP); di-isobutyl phthalate (parent of MiBP); di-n-octyl phthalate (parent of MCPP); butyl benzyl phthalate (parent of MBzP); and DEHP (parent of MEHP, MEHHP, and MEOHP).
.
Figure 1.
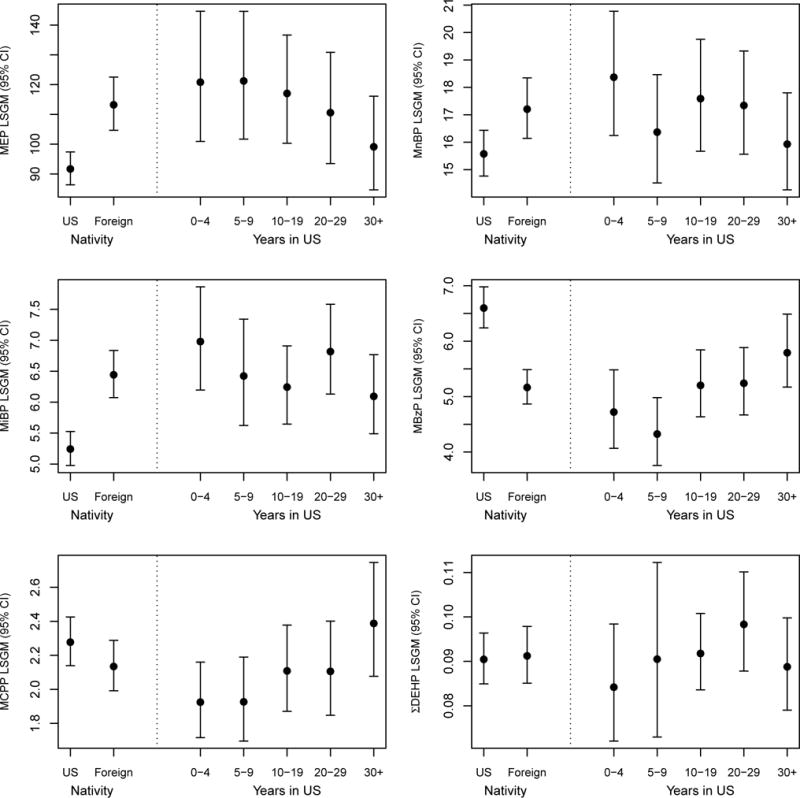
LSGM values and 95% confidence intervals of each phthalate metabolite (ng/mL) by nativity and length of time in the US. Levels of MEP, MnBP and MiBP were significantly higher in foreign-born individuals (p<0.0001, p=0.0067, p<0.0001, respectively). Levels of MBzP were significantly higher in US-born individuals (p<0.0001). MnBP and MiBP levels among immigrants significantly declined with longer time in the US (Ptrend = 0.029 and Ptrend =0.039, respectively). MCPP and MBzP levels among immigrants significantly increased with longer time in the US (Ptrend = 0.019 and Ptrend =0.043, respectively). All models were adjusted for creatinine, age (continuous), age2, sex, race, survey year, education (4 categories), and IPR (5 categories). MnBP and MCPP models additionally adjusted for current smoking.
In Table 3, we show percent differences in phthalate metabolite levels comparing immigrant and US-born populations by participant length of time in the US and age. Among young individuals, MEP levels were 38.2% (95% CI: 15.1, 66.0) higher in recent immigrants and 28.5% (95% CI: 10.4, 49.6) higher in long-term immigrants compared to those born in the US (both p<0.0001). Among older individuals, MEP was 19.1% (95% CI: −6.2, 51.1) higher in recent immigrants and 15.0% (95% CI: 0.5, 31.6, p<0.05) higher in long-term immigrants than in those born in the US. MiBP levels were 21.9% (95% CI: 6.6, 39.5) higher in young recent immigrants and 15.5% (95% CI: 3.7, 28.7) higher in young long-term immigrants than in young US-born individuals. In older individuals, MiBP levels were 41.4% (95% CI: 20.2, 66.4) higher in recent immigrants and 24.9% (95% CI: 14.0, 36.8) higher in long-term immigrants compared to US-born individuals (all p<0.0001). MBzP levels were 29.3% (95% CI: 19.0, 38.3) lower in young recent immigrants and 21.7% (95% CI: 10.9, 31.2) lower in young long-term immigrants than in young US-born individuals. In older individuals, MBzP levels were 36.3% (95% CI: 22.8, 47.5) lower in recent immigrants and 16.2% (95% CI: 7.7, 23.9) lower in long-term immigrants compared to US-born individuals (all p<0.0001; Table 3).
Table 3.
Percent difference in urinary phthalate metabolite levels by age group, nativity, and length of time in the United States.
| <40 years old | ≥40 years old | |||
|---|---|---|---|---|
|
|
||||
| n | % Difference (95% CI) | n | % Difference (95% CI) | |
| MEP | ||||
| US-born | 3268 | Ref11 | 5945 | Ref22 |
| Recent immigrant (<10 yr) | 575 | 38.2 (15.1, 66.0)** | 264 | 19.1 (−6.2, 51.1) |
| Long-term immigrant (≥10 yr) | 546 | 28.5 (10.4, 49.6)** | 1556 | 15.0 (0.5, 31.6)* |
| MnBP | ||||
| US-born | 3267 | Ref1 | 5947 | Ref2 |
| Recent immigrant (<10 yr) | 572 | 10.6 (−2.1, 24.9) | 263 | 11.6 (−4.4, 30.2) |
| Long-term immigrant (≥10 yr) | 545 | 3.3 (−5.8, 13.3) | 1554 | 11.8 (1.4, 23.2)* |
| MiBP | ||||
| US-born | 2927 | Ref1 | 5374 | Ref2 |
| Recent immigrant (<10 yr) | 500 | 21.9 (6.6, 39.5)** | 236 | 41.4 (20.2, 66.4)** |
| Long-term immigrant (≥10 yr) | 487 | 15.5 (3.7, 28.7)** | 1372 | 24.9 (14.0, 36.8)** |
| MCPP | ||||
| US-born | 2926 | Ref1 | 5372 | Ref2 |
| Recent immigrant (<10 yr) | 499 | −15.0 (−23.6, −5.4)** | 235 | −14.8 (−28.5, 1.4) |
| Long-term immigrant (≥10 yr) | 486 | −4.9 (−18.0, 10.3) | 1370 | −2.7 (−12.6, 8.2) |
| MBzP | ||||
| US-born | 3268 | Ref1 | 5949 | Ref2 |
| Recent immigrant (<10 yr) | 575 | −29.3 (−38.3, −19.0)** | 264 | −36.3 (−47.5, −22.8)** |
| Long-term immigrant (≥10 yr) | 546 | −21.7 (−31.2, −10.9)** | 1556 | −16.2 (−23.9, −7.7)** |
| ΣDEHP | ||||
| US-born | 2927 | Ref1 | 5374 | Ref2 |
| Recent immigrant (<10 yr) | 500 | −7.5 (−23.0, 11.2) | 236 | 4.3 (−17.3, 31.6) |
| Long-term immigrant (1≥10 yr) | 487 | −7.7 (−18.1, 4.1) | 1372 | 8.1 (−3.0, 20.4) |
In models stratified by race/ethnicity, MEP levels were significantly higher in foreign-born Mexican American, Other Hispanic, Non-Hispanic White, and Other participants compared to members of those groups born in the US (Table 4). MnBP and MiBP levels were significantly higher among foreign-born Mexican Americans and non-Hispanic Asians than US-born members of these groups; MiBP levels were also significantly higher among non-Hispanic blacks. Finally, MBzP levels were lower among foreign-born Other Hispanics, non-Hispanic blacks and Other races than among US-born members of these groups. In general, non-Hispanic blacks, both US-born and foreign-born, had higher phthalate metabolite levels than other racial/ethnic groups, particularly for MEP and MiBP. In contrast, non-Hispanic Asians had much lower levels of MEP, MnBP, and MBzP compared to the other racial/ethnic groups. Overall, levels of MEP, MnBP, MBzP, and ΣDEHP metabolites were lower in the analysis limited to 2011-2014 than in the analysis spanning 1999-2014 (Table 4 and Figure 1, respectively).
Table 4.
Least square geometric mean values of each phthalate metabolite (ng/mL) by nativity in the years 2011–2014 only, stratified by race/ethnicity.
| Mexican Americans | Other Hispanics | NH Whites | NH Blacks | NH Asians | Other/Multiracial | |
|---|---|---|---|---|---|---|
|
| ||||||
| LSGM (95% CI) | LSGM (95% CI) | LSGM (95% CI) | LSGM (95% CI) | LSGM (95% CI) | LSGM (95% CI) | |
| MEP | ||||||
| US-born | 40.8 (31.1, 53.5)** | 47.7 (34.8, 65.2)** | 30.4 (27.5, 33.5)* | 89.0 (78.2, 101.2) | 16.9 (10.8, 26.4) | 60.7 (43.8, 84.2)* |
| Foreign-born | 74.7 (51.6, 108.2) | 76.0 (61.1, 94.6) | 53.4 (33.3, 85.8) | 115.8 (78.7, 170.4) | 22.8 (19.2, 26.9) | 111.1 (64.7, 191.0) |
| MnBP | ||||||
| US-born | 7.5 (6.3, 8.9)** | 12.2 (9.7, 15.4) | 6.9 (6.2, 7.7) | 12.0 (11.0, 12.9)# | 4.4 (3.1, 6.2)** | 11.8 (9.4, 14.9) |
| Foreign-born | 11.2 (9.0, 13.9) | 10.4 (8.8, 12.3) | 8.4 (6.5, 10.9) | 15.5 (11.8, 20.4) | 8.4 (7.0, 10.1) | 9.4 (5.3, 16.8) |
| MiBP | ||||||
| US-born | 6.3 (5.2, 7.5)** | 8.3 (6.7, 10.4) | 5.2 (4.8, 5.5)# | 9.4 (8.5, 10.4)* | 4.3 (2.9, 6.3)* | 7.7 (6.1, 9.8) |
| Foreign-born | 8.5 (7.2, 10.0) | 7.6 (6.5, 8.9) | 6.7 (5.0, 9.0) | 12.6 (9.9, 16.1) | 6.6 (5.6, 7.7) | 7.6 (4.7, 12.3) |
| MCPP | ||||||
| US-born | 2.0 (1.6, 2.4) | 2.7 (1.7, 4.3) | 2.4 (2.1, 2.8) | 2.6 (2.4, 2.9) | 1.8 (1.0, 3.3) | 3.1 (2.4, 3.9)** |
| Foreign-born | 2.0 (1.5, 2.5) | 2.8 (1.9, 4.1) | 2.4 (1.8, 3.2) | 2.2 (1.6, 3.1) | 1.7 (1.2, 2.3) | 1.6 (1.0, 2.5) |
| MBzP | ||||||
| US-born | 3.8 (3.3, 4.3) | 4.5 (3.2, 6.3)# | 4.2 (3.8, 4.6) | 6.5 (5.9, 7.2)* | 1.9 (1.3, 3.0) | 5.7 (4.4, 7.4)* |
| Foreign-born | 4.3 (3.6, 5.3) | 3.5 (3.0, 4.1) | 3.9 (2.6, 5.8) | 4.5 (3.3, 6.2) | 2.5 (2.1, 3.0) | 3.4 (2.2, 5.2) |
| ΣDEHP | ||||||
| US-born | 0.044 (0.036, 0.054)# | 0.059 (0.046, 0.076) | 0.039 (0.036, 0.043) | 0.058 (0.053, 0.064) | 0.031 (0.024, 0.041) | 0.051 (0.044, 0.060) |
| Foreign-born | 0.057 (0.048, 0.069) | 0.050 (0.044, 0.057) | 0.047 (0.037, 0.060) | 0.054 (0.045, 0.066) | 0.038 (0.032, 0.045) | 0.042 (0.030, 0.059) |
Abbreviations: NH: Non-Hispanic.
P <0.01,
P <0.05;
P <0.10
Phthalate metabolites by immigrant demographics
Demographic characteristics within the immigrant population predicted some variation in phthalate metabolite levels. Among foreign-born participants, women had significantly higher phthalate metabolite levels than men (all p<0.01). The magnitude of the sex difference varied by metabolite, with the largest difference seen in MnBP (52.0% higher levels in women than in men). Levels of MEP, MnBP, and MCPP differed by immigrant race/ethnicity. Compared to non-Hispanic White participants, non-Hispanic Black, Mexican American, and Other Hispanic participants had significantly higher MEP levels while Other participants had significantly lower MEP levels. Compared to non-Hispanic White participants, MCPP levels were similar in Mexican American and Other Hispanic participants and much lower in non-Hispanic Black (p<0.05) and Other participants (p<0.01), and MnBP levels were significantly higher in Other Hispanic participants (p<0.01). College-educated participants had lower levels of MBzP than those with less than a high school education (p<0.01), and participants in the highest quintile of IPR (wealthiest) had significantly lower MEP levels than those in the lowest quintile (poorest) (p<0.01, see Table S3).
Sensitivity analysis
In the models restricted to Mexican American participants, the pattern of results was similar to the pattern seen in the whole study population. Results for most phthalate metabolites remained in the same direction, though some estimates were attenuated or had wider confidence intervals due to the smaller sample size (see Table S4). In the 2011-2014 sensitivity analysis, MBzP levels no longer differed significantly by nativity status, though this may have been due to the smaller sample size. Also, ΣDEHP metabolite levels were significantly (20-24%) higher in older foreign-born individuals compared to older US-born individuals (see Table S5).
Discussion
In this cross-sectional study of the US population, we report associations between nativity and phthalates exposure levels that were robust to adjustment for demographic differences between US- and foreign-born populations. Specifically, we found that immigrants had 23% higher levels of MEP, 23% higher levels of MiBP, 10% higher levels of MnBP, and 28% lower levels of MBzP when compared to individuals born in the US. Among foreign-born individuals, longer time living in the US reduced the difference between foreign-born and US-born levels of MnBP, MiBP, MCPP, and MBzP. To our knowledge, this is the first study to examine phthalate exposures in the US immigrant community.
We reported significantly higher MEP among immigrants than US-born individuals. MEP’s parent phthalate is commonly found in fragranced products such as soap, lotion, hair spray, and air fresheners, so these differences are likely due to different patterns of personal care product use. (20, 42, 43) Racial/ethnic differences in hair care, skin care, and feminine care product usage have been documented in the US population. (44–47) Due to cultural preferences, beauty norms, or different consumer options linked to lower socioeconomic status, immigrants may use different products or different amounts of product than US-born individuals. (30, 48–51) Alternatively, recent immigrants may prefer to use products from their country of origin, which may contain different concentrations of phthalates. (52, 53) Unfortunately, personal care product use is not well measured in NHANES, so we were not able to evaluate these possible differences.
We found significantly lower levels of MBzP among immigrants than among US-born individuals. Previous work has associated MBzP levels with housing characteristics such as vinyl or linoleum flooring. (54) However, a connection between these housing characteristics and nativity is not clear. It is possible that other sources of exposure to butyl benzyl phthalate (BBzP; MBzP’s parent phthalate) such as having other plastic or synthetic leather items in the home (55) may contribute more to higher US-born individual exposures.
In addition to personal care product and housing variables, differences in dietary pattern may be a driver of the observed differences in phthalate metabolite levels. Higher MEP has been associated with higher consumption of vegetables (56), while MiBP may be found in fish. (20, 43, 57) Interestingly, however, no differences by nativity status were found in ΣDEHP metabolite levels. Because DEHP is used in food processing and packaging, has been detected in meats and fats (56,58) and has been temporally associated with eating meals (59), we anticipated that if there were dietary differences, these would be reflected in ΣDEHP exposures. Since no differences in ΣDEHP exposure were seen in the main analyses, we cannot be sure that dietary differences are truly driving differences in phthalate metabolite levels.
Our results partially align with those reported in two other recent studies. Using data from pregnant Canadian women, the MIREC study investigators reported significantly higher MEP and DEHP metabolites, and significantly lower MBzP, among foreign-born participants compared to Canadian-born participants. (60) This Canadian study provides evidence that the pattern of results reported here may be generalizable to immigrants in other Western countries. Significantly lower MBzP in foreign-born participants was also reported among pregnant, low-income Mexican-Americans in the CHAMACOS study. (35) These findings together suggest that the exposure source of higher MBzP levels may be linked to some specific aspects of US or Canadian culture.
There was a linear trend between duration of residence in the US and levels of MnBP, MiBP, MCPP, and MBzP, such that immigrants who had lived longer in the US had levels that were more similar to US-born individuals’ levels. Phthalate metabolites have a very short half-life. (23, 24) Therefore, measured phthalate metabolites reflect at most the previous few days of exposures. For this reason, we suspect that measured changes in phthalate metabolite levels associated with longer time living in the US reflect changes in everyday lifestyle, behaviors, and environmental exposures among immigrants. Since immigrants’ exposures become more similar to the exposures of those born in the US, the process underlying exposure changes may be related to acculturation rather than changes in underlying biology. (61, 62) This shift aligns with the well documented ‘negative acculturation hypothesis,’ which predicts that health benefits observed in immigrants compared to non-immigrants are attenuated with increased time in the US. (63, 64) Changes in non-persistent chemical exposures may be one mechanism driving the shift in health outcomes associated with nativity and duration of residence in the US.
The observed association between nativity and phthalate exposure was not entirely driven by differences in the ethnic makeup of US-born and foreign-born populations, and the association was not specific to only a few groups. Indeed, within each ethnic group, the pattern of phthalate exposure differences by nativity was consistent with the results in the whole population. That is, the racial/ethnic groups whose US-born members were highly exposed to some phthalate metabolites were typically also the groups with highly exposed foreign-born members. This suggests that exposure factors correlated with race/ethnicity may be further amplified among immigrant members of these groups. For the first time, we also report lower urinary phthalate metabolite LSGM levels in a population of US-born and foreign-born non-Hispanic Asians compared to other racial/ethnic groups. Though the non-Hispanic Asian population sampled by NHANES is small and represents a heterogeneous group, this finding deserves further examination to identify reasons for these differences.
We found some demographic factors that have been linked to high phthalates exposure in the whole US population also predicted higher exposures in foreign-born individuals living in the US. Specifically, within foreign-born individuals, female sex predicted higher levels of all phthalate metabolites, non-white race predicted higher MEP and lower MCPP levels, and factors associated with higher socioeconomic status (education and IPR) were associated with lower MBzP and lower MEP, respectively. These results are consistent with previous studies using the whole NHANES population, which associated higher MEP, MnBP, and MBzP with female sex, and higher MEP with non-white race. (25, 26) Other studies have also linked higher MBzP and MnBP (or their parent compounds, butyl benzyl phthalate and di-n-butyl phthalate) with lower levels of income and education. (28, 60)
Previous work has indicated that phthalate exposure is associated with cardiovascular, reproductive, allergic, and neurodevelopmental health effects (11–15), and no safe level of exposure has been identified. Additionally, individuals are likely co-exposed to multiple phthalates and other endocrine disrupting chemicals, and this mixture of exposures may produce greater than expected toxicity. (21) Therefore, identifying highly exposed groups and preventing these exposures is an environmental health priority.
Our analysis was subject to several important limitations. First, although we utilized precise data on time living in the US, NHANES does not collect other variables that would be useful to evaluate acculturation, such as the country where participants attended school, social ties with Americans, cultural identity as an American, American media consumption, and generational status among those born in the US (e.g., identifying participants whose parents were born outside the US). (65–67) We would expect more acculturated immigrants to have phthalate exposures similar to US-born individuals. Similarly, NHANES does not collect detailed information on housing, which prevented us from being able to explore contributions from this possible exposure source. Second, we chose to treat socioeconomic factors (IPR and education) as confounders and adjust for them appropriately in analyses, but socioeconomic factors may also act as mediators between immigration status and phthalate exposures. We were not able to evaluate mediation due to the cross-sectional study design. Third, because NHANES provides crude data on country of origin and race/ethnicity, we were not able to pinpoint highly exposed subpopulations within the immigrant community. For example, better detail on the countries of origin and exposures experienced by groups within the broad racial/ethnic categories reported by NHANES would permit us to evaluate culturally specific factors leading to exposure. Finally, because immigrant participants composed a relatively small percentage of the NHANES sample, analyses restricted to 2011-2014 or to specific racial/ethnic groups had limited power.
This study also had many strengths. We were able to test our hypotheses using 16 years of continuously collected cross-sectional data from a large, nationally representative dataset. Nativity status and duration of time in the US was recorded for nearly all participants, and interviews were conducted in multiple languages, reducing concerns of selection bias. We were able to adjust for the effects of major demographic factors, including age, IPR, education, and race/ethnicity. Additionally, most phthalate metabolites of interest were very highly detected. Finally, sensitivity analyses in Mexican Americans and 2011-2014 largely affirmed our major findings.
Conclusions
We showed that foreign-born participants across all racial/ethnic groups are exposed to different levels of phthalate metabolites than US-born participants, and that nativity-related differences in phthalate exposure were more pronounced among those who had been in the US for a shorter amount of time. Our finding that immigrants are exposed to significantly higher levels of MEP, MiBP, and MnBP suggest that immigrants may be especially vulnerable to adverse health outcomes associated with higher concentrations of these phthalate parent compounds (i.e. diethyl phthalate, diisobutyl phthalate, di-n-butyl phthalate). Future research should establish modifiable exposure sources and reduction strategies specific to this vulnerable population.
Supplementary Material
Acknowledgments
This study was funded by the National Institute of Environmental Health Sciences (R01ES026166 and T32ES007069).
Footnotes
Conflict of interest declaration: The authors declare that they have no actual or potential competing financial interests and that their freedom to design, conduct, interpret, and publish research is not compromised by any controlling sponsor.
Supplementary Information:
Supplementary information is available at the Journal of Exposure Science and Environmental Epidemiology’s website.
References
- 1.US Census. Projections of the Size and Composition of the US Population: 2014 to 2060. 2015 Available: https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf [accessed 14 June 2017]
- 2.Carrasquillo O, Carrasquillo AI, Shea S. Health insurance coverage of immigrants living in the United States: differences by citizenship status and country of origin. Am J Pub Health. 2000;90(6):917–923. doi: 10.2105/ajph.90.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derose KP, Escarce JJ, Lurie N. Immigrants and health care: Sources of vulnerability. Health Affairs. 2007;26(5):1258–1268. doi: 10.1377/hlthaff.26.5.1258. [DOI] [PubMed] [Google Scholar]
- 4.Fan W, Qian Y. Native-immigrant occupational segregation and worker health in the United States, 2004-2014. Social Sci Med. 2017;183:130–141. doi: 10.1016/j.socscimed.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Kandula NR, Kersey M, Lurie N. Assuring the health of immigrants: what the leading health indicators tell us. Ann Rev Pub Health. 2004;25:357–376. doi: 10.1146/annurev.publhealth.25.101802.123107. [DOI] [PubMed] [Google Scholar]
- 6.Lopéz G, Radford J. Statistical portrait of the foreign-born population in the United States. Pew Research Center; 2017. Available: http://www.pewhispanic.org/2017/05/03/statistical-portrait-of-the-foreign-born-population-in-the-united-states-2015/ [accessed 23 May 2017] [Google Scholar]
- 7.Bradman A, Schwartz JM, Fenster L, Barr DB, Holland NT, Eskenazi B. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expo Sci Env Epid. 2007;17:388–399. doi: 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SX, Wiseman CLS, Chakravartty D, Cole DC. Metal concentrations in newcomer women and environmental exposures: a scoping review. Int J Env Res Pub Health. 2017;14:277. doi: 10.3390/ijerph14030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petreas M, She J, Winkler J, Windham G, Rogers E, Zhao G, et al. High body burdens of 2,2’4,4’-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Persp. 2003;111:1175–1179. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quandt SA, Jones BT, Talton JW, Whalley LE, Galván L, Vallejos QM, et al. Heavy metals exposures among Mexican farmworkers in eastern North Carolina. Environ Res. 2010;100(1):83–88. doi: 10.1016/j.envres.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children’s health. Curr Opin Pediatr. 2013;25(2):247–254. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. Phthalate exposure and children’s neurodevelopment: a systematic review. Environ Res. 2015:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariana M, Feiteiro J, Verde I, Cairrao E. The effect of phthalates in the cardiovascular and reproductive system: a review. Environ Int. 2016;24:758–776. doi: 10.1016/j.envint.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Eldeirawi K, McConnell R, Freels S, Persky VW. Associations of place of birth with asthma and wheezing in Mexican American children. J Allergy Clin Immun. 2005;116(1):42–48. doi: 10.1016/j.jaci.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg JI, Simpson EL, Durkin HG, Joks R. Prevalence of allergic disease in foreign-born American children. JAMA Pediatrics. 2013;167(6):554–560. doi: 10.1001/jamapediatrics.2013.1319. [DOI] [PubMed] [Google Scholar]
- 17.Urquia ML, Glazier RH, Blondel B, Zeitlin J, Gissler M, Macfarlane A, et al. International migration and adverse birth outcomes: role of ethnicity, region of origin and destination. J Epidemiol Commun Health. 2010;64:243–251. doi: 10.1136/jech.2008.083535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phthalates Factsheet. National Biomonitoring Program: Centers for Disease Control and Prevention. 2016 Available: https://www.cdc.gov/biomonitoring/phthalates_factsheet.html [accessed 22 May 2017]
- 19.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Heath and Nutrition Examination Survey, 2001-2010. Environ Health Persp. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentration of some phthalates. Environ Health Persp. 2005;113:1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, et al. Consumer product chemicals in indoor dust: a quantitative meta-analysis of U.S. studies. Environ Sci Technol. 2016;50(19):10661–10672. doi: 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—an update and latest results. Int J Andrology. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson WAC, Castle L, Scotter MJ, Massey RC, Springall C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18(12):1068–1074. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- 25.Huang T, Saxena AR, Isganaitis E, James-Todd T. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001-2008. Environ Health. 2014;13(1):6. doi: 10.1186/1476-069X-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Persp. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ Res. 2012;115:11–17. doi: 10.1016/j.envres.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Koo JW, Parham F, Kohn MC, Masten SA, Brock JW, Needham LL, et al. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environ Health Persp. 2002;110:405–410. doi: 10.1289/ehp.02110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Affairs. 2011;30(5):879–887. doi: 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- 30.Mahé A. The practice of skin-bleaching for a cosmetic purpose in immigrant communities. J Travel Med. 2014;21(4):282–287. doi: 10.1111/jtm.12106. [DOI] [PubMed] [Google Scholar]
- 31.Hines CJ, Nilsen Hopf NB, Deddens JA, Calafat AM, Silva MJ, Grote AA, et al. Urinary phthalate metabolite concentrations among workers in selected industries: a pilot biomonitoring study. Ann Occup Hyg. 2009;53(1):1–17. doi: 10.1093/annhyg/men066. [DOI] [PubMed] [Google Scholar]
- 32.Quach T, Gunier R, Tran A, Von Behren J, Doan-Billings PA, Nguyen KD, et al. Characterizing workplace exposures in Vietnamese women working in California nail salons. Am J Public Health. 2011;101:S271–S276. doi: 10.2105/AJPH.2010.300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhouser ML, Thompson B, Coronado GD, Solomon CC. Higher fat intake and lower fruit and vegetables intakes are associated with greater acculturation among Mexicans living in Washington state. J Am Diet Assoc. 2004;104:51–57. doi: 10.1016/j.jada.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Peck JD, Sweeney AM, Symanski E, Gardiner J, Silva MJ, Calafat AM, et al. Intra- and inter-individual variability of urinary phthalate metabolite concentrations in Hmong women of reproductive age. J Expo Sci Env Epid. 2010;20(10):90–100. doi: 10.1038/jes.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holland N, Huen K, Tran V, Street K, Nguyen B, Bradman A, et al. Urinary phthalate metabolites and biomarkers of oxidative stress in a Mexican-American cohort: variability in early and late pregnancy. Toxics. 2016;4(1):7. doi: 10.3390/toxics4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NHANES (National Health and Nutrition Examination Survey) Interpretation Guidelines. 2006 Available: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/Interpretation_Guidelines.pdf.
- 37.Laboratory Procedures Manual. National Health and Nutrition Examination Survey. 2004 Jan; Available: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/manuals/lab.pdf.
- 38.CDC Laboratory Procedure Manual. Phthalate Monoesters. 1999–2000 Available: https://wwwn.cdc.gov/nchs/data/nhanes/1999-2000/labmethods/phpypa_met_phthalates.pdf.
- 39.CDC Laboratory Procedure Manual. Phthalate Monoesters. 2003–2004 Available: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l24ph_c_met.pdf.
- 40.Phillip Morris USA. PM USA Cigarette Non-Tobacco Ingredients. Available: http://www.altria.com/our-companies/philipmorrisusa/our-products-and-ingredients/Documents/PM%20USA%20Non%20Tobacco%20Ingredients.pdf [accessed 31 May 2017]
- 41.NHANES (National Health and Nutrition Examination Survey) Environmental Chemical Data Tutorial. Overview of NHANES survey design and weights. 2013 Available: https://www.cdc.gov/Nchs/tutorials/environmental/orientation/sample_design/index.htm.
- 42.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Persp. 2012;120:935–943. doi: 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111:329–336. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Branch F, Woodruff TJ, Mitro SD, Zota AR. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: national health and Nutrition Examination Survey 2001-2004. Environ Health. 2015;14:57. doi: 10.1186/s12940-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James-Todd T, Senie R, Terry MB. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immig Minor Health. 2012;3:506–511. doi: 10.1007/s10903-011-9482-5. [DOI] [PubMed] [Google Scholar]
- 46.Taylor KW, Baird DD, Herring AH, Engel LS, Nichols HB, Sandler DP, et al. Associations among personal care product use patterns and exogenous hormone use in the NIEHS Sister Study. J Expo Sci Env Epi. 2017;00:1–7. doi: 10.1038/jes.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.07.020. e-pub ahead of print 16 Aug 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campaign for Healthier Solutions. A day late and a dollar short: discount retailers are falling behind on safer chemicals. 2015 Available: http://ej4all.org/assets/media/documents/Report_ADayLateAndADollarShort.pdf [accessed 29 June 2017]
- 49.Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, et al. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ Health Persp. 2016;124(10):1600–1607. doi: 10.1289/ehp.1510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley DM, Newby CA, Leal-Aleraz TO, Thomas VM. Assessing elemental mercury vapor exposure from cultural and religious practices. Environ Health Persp. 2001;109:779–784. doi: 10.1289/ehp.01109779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiseman CLS, Parnia A, Chakravartty D, Archbold J, Zawar N, Copes R, et al. Blood cadmium concentrations and environmental exposure sources in newcomer South and East Asian women in the Greater Toronto Area, Canada. Environ Res. 2017;154:19–27. doi: 10.1016/j.envres.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Al-Saleh I, Elkhatib R. Screening of phthalate esters in 47 branded perfumes. Environ Sci Pollut Res. 2016;23:455–468. doi: 10.1007/s11356-015-5267-z. [DOI] [PubMed] [Google Scholar]
- 53.Yano K, Hirosawa N, Sakamoto Y, Katayama H, Moriguchi T, Asaoka K. Phthalate levels in baby milk powders sold in several countries. Bull Environ Contam Toxicol. 2005;74:373–379. doi: 10.1007/s00128-004-0594-7. [DOI] [PubMed] [Google Scholar]
- 54.Just AC, Miller RL, Perzanowski MS, Rundle AG, Chen Q, Jung KH, et al. Vinyl flooring in the home is associated with children's airborne butylbenzyl phthalate and urinary metabolite concentrations. J Expo Sci Env Epid. 2015;25(6):574–579. doi: 10.1038/jes.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, Sundell J. Phthalates in indoor dust and their association with building characteristics. Environ Health Persp. 2005;113:1399–1404. doi: 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13:43. doi: 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Persp. 2010;118(7):998–1003. doi: 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and Bisphenol A and phthalates exposures among the US population in NHANES, 2003-2010. Environ Health Persp. 2016;124:1521–1528. doi: 10.1289/ehp.1510803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch HM, Lorber M, Christensen KLY, Pälmke C, Koslitz S, Brüning T. Identifying sources of phthalate exposure with human biomonitoring: results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Env Health. 2013;216:672–681. doi: 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Lewin A, Arbuckle TE, Fisher M, Liang CL, Marro L, Davis M, et al. Univariate predictors of maternal concentrations of environmental chemicals: the MIREC study. Int J Hyg Env Health. 2017;220:77–85. doi: 10.1016/j.ijheh.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Ogden DT, Ogden JR, Schau HJ. Exploring the impact of culture and acculturation on consumer purchase decisions: toward a microcultural perspective. Acad Marketing Sci Rev. 2004;3:1–22. [Google Scholar]
- 62.Kara A, Kara NR. Ethnicity and consumer choice: a study of Hispanic decision processes across different acculturation levels. J Appl Business Res. 1996;12(2):22–34. [Google Scholar]
- 63.Cho Y, Frisbie WP, Hummer RA, Rogers RG. Nativity, duration of residence, and the health of Hispanic adults in the United States. Intl Migr Rev. 2004;38(1):184–211. [Google Scholar]
- 64.Goel MS, McCarthy EP, Phillips RS. Obesity among US immigrant subgroups by duration of residence. JAMA. 2004;292(33):2860–2867. doi: 10.1001/jama.292.23.2860. [DOI] [PubMed] [Google Scholar]
- 65.Cuéllar I, Arnold B, Maldonaldo R. Acculturation rating scale for Mexican Americans-II: A revision of the original ARSMA Scale. Hispanic J Behav Sci. 1995;17(3):275–304. [Google Scholar]
- 66.Johnson-Agbakwu CE, Flynn P, Asiedu GB, Hedberg E, Radecki C. Adaptation of an acculturation scale for African refugee women. J Immigr Minor Health. 2016;18(1):252–262. doi: 10.1007/s10903-014-9998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kukaswadia A, Janssen I, Pickett W, Bajwa J, Georgiades K, Lalonde RN, et al. Development and validation of the bicultural youth acculturation questionnaire. PLoS ONE. 2016;11(8):e0161048. doi: 10.1371/journal.pone.0161048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


