Abstract
Background
To characterize glucose patterns with continuous glucose monitoring (CGM) in cystic fibrosis (CF) and assess relationships between CGM and clinical outcomes.
Methods
110 CF youth and healthy controls (HC), 10–18 years, wore CGM up to 7 days. Correlations between CGM and lung function and BMI z-score change over the prior year were determined.
Results
Multiple CGM measures were higher in CF Normal Glycemic (CFNG) youth versus HC (peak glucose, excursions >140 mg/dl/day, %time >140 mg/dl, standard deviation (SD) and mean amplitude of glycemic excursions (MAGE)). Hypoglycemia was no different among groups. In CF, decline in FEV1% and FVC% correlated with maximum CGM glucose, excursions >200 mg/dl/day, SD, and MAGE.
Conclusions
CFNG youth have higher glucoses and glucose variability than HC on CGM. Higher and more variable glucoses correlate with lung function decline. Whether earlier treatment of CGM abnormalities improves lung function in CF requires further study.
Keywords: Cystic fibrosis related diabetes, continuous glucose monitoring, pediatrics
Introduction
Cystic fibrosis-related diabetes (CFRD) is the most common comorbidity in CF, present in up to 20% of adolescents and 50% of adults (1). CF guidelines recommend annual screening with an oral glucose tolerance test (OGTT) starting at 10 years of age, when CFRD prevalence rises. Current gold standard criteria for diagnosing CFRD are based on the OGTT (1) and extrapolated from American Diabetes Association (ADA) type 2 diabetes (T2D) guidelines. However, these ADA cut points [fasting glucose (FG ≥126 mg/dl, 2-hour glucose ≥200, and hemoglobin A1c (HbA1c) ≥6.5% (48 mmol/mol)] are intended to identify the glucose value or HbA1c at which the risk for microvascular complications (largely retinopathy) increases in adults with T2D (2). Whether these cut-points are optimal for detecting changes in CF-relevant clinical outcomes is unclear. Neither FG nor HbA1c are recommended for CFRD screening, as both appear to underestimate hyperglycemia in this population (3–6) and whether the OGTT is the best test for diabetes screening in this population has been questioned (7–9).
Given the limitations of current approaches to CFRD screening and diagnosis, continuous glucose monitoring (CGM) is increasingly being used because it can detect glycemic abnormalities earlier than other screening tests (10–12). Studies have found correlations between CGM glucoses >200 mg/dl and lung function impairment (12) and between CGM time spent >140 mg/dl and poor weight gain in CF (13). However, more robust characterizations of glycemic profiles in individuals with CF are lacking. In our clinical practice, CGM is often used as an adjunct to the OGTT in CFRD screening, but it remains unclear how best to interpret CGM profiles in CF. Furthermore, data on CGM in CF youth with normal FG and OGTT results are limited. Therefore, our first objective was to characterize CGM profiles among CF youth across the glycemic spectrum, from normal glycemia (CFNG, defined as FG <100 mg/dl, 1hr ≤200 mg/dl, AND 2hr ≤140 mg/dl), abnormal glycemia (CFAG, defined as impaired fasting glucose of 100–125 mg/dl, or impaired glucose tolerance with 2 hour OGTT glucose of 140–199 mg/dl, or indeterminate glucose with 1hr ≥200 mg/dl), to CFRD (defined as FG ≥126 mg/dl or 2hr OGTT ≥200 mg/dl), and to compare these findings to CGM in a cohort of relatively age-matched healthy controls.
Studies have demonstrated declines in pulmonary function and body mass index (BMI) up to 5 years prior to a diagnosis of CFRD by OGTT (14, 15), suggesting that abnormalities in glucose metabolism occur earlier than clinically recognized and suggesting an opportunity for earlier intervention to reduce associated morbidity and mortality. However, most of these studies were in young adults with more advanced lung disease. It is uncertain whether similar findings occur in CF children with milder lung disease and whether or not clinical decline may be associated with CGM abnormalities. Our second aim therefore was to perform an analysis of the relationship between CGM measures and clinical outcomes in our CF cohort, as measured by rate of change in lung function (forced expiratory volume in 1 sec % predicted (FEV1%) and forced vital capacity % predicted (FVC%)), weight z-score, and body mass index (BMI) z-score, over the 12 months preceding CGM wear.
Methods
Participants with CF, ages 10–18 years, with CGM collected from 2011–2016 were included. CF participants had either worn CGM in the clinical setting for further evaluation of glucoses after an abnormal OGTT or hyperglycemia detected during hospitalization, or through an ongoing research study collecting CGM and OGTTs that included CFNG youth and healthy controls (HC). A subset of participants classified as CFRD were diagnosed after persistent hyperglycemia during hospitalization, with glucoses >200 mg/dl for >48 hrs. Collection of CGM data typically occurred >6 weeks after hospitalization, in order to capture glycemic patterns during a period of clinical stability when patients were at baseline health. Some participants wore CGM more than once; in this scenario, the most recent CGM obtained prior to insulin start was analyzed. HC were identified from our general endocrine clinics and recruitment flyers and emails sent to faculty, staff, and students on campus. Those with BMI >85th%ile, known type 1 or type 2 diabetes, use of medications affecting glucose (ex. insulin, systemic steroids) in the prior 3 months, or hospitalization in the prior 6 weeks were excluded.
All participants wore a blinded iPro®2 continuous glucose monitor (Medtronic, Minimed, Inc Northridge, CA) for a minimum of 3 days and up to 7 days. They were provided a glucometer (OneTouch, LifeScan) and trained to collect capillary blood glucoses four times daily - prior to meals and at bedtime - and to keep a food log during the week of CGM wear. Participants recruited after October 2015 underwent an OGTT with HbA1c as well as height and weight measurements at the time of CGM wear. For participants with CGM data available prior to October 2015, their most recent OGTT and HbA1c relative to date of CGM were collected. In CF participants, pulmonary function data from the most recent office visit relative to date of CGM wear, as well as CF genotype, use of CFTR modulators, and presence of pancreatic insufficiency were noted. To generate the slope of weight z-score, BMI z-score and lung function over the preceding year, anthropometric and lung function data from clinical visits over the 12 months prior to CGM wear were collected.
This study was approved by the Colorado Multiple Institutional Review Board (Aurora, CO) and appropriate consent and assent were obtained.
CGM calculations
CGM variables were generated with R-code after manual review of raw glucose values downloaded from CGM software. Our approach to generating CGM variables was defined a priori and similar to that outlined in CGM studies in other populations (16). To allow time for CGM calibration, the first 4 hours of CGM data were excluded from analysis. To account for small gaps (<45 min) of missing sensor glucoses, data were populated by averaging the last and next available glucose reading during periods of relative glucose stability. CGM data, including time spent above/under a glucose cutpoint, area under the curve (AUC), and number of excursions, were averaged over the total days of CGM wear. We also determined CGM AUC above the HC-mean sensor glucose, as another measure of biologically relevant glucose abnormalities in our CF cohort. CGM measures were calculated in each participant in contiguous 24-hour intervals to include an equal percentage of daytime versus nighttime sensor glucoses (288 sensor glucose values per day). Mean amplitude of glycemic excursions (MAGE) was calculated using EasyGV version 9.0.R2 (© University of Oxford). Excursions over a set glucose cutpoint were defined using the same equation used by the software’s accompanying CGM summary output. In brief, the formula counts one excursion each time the sensor glucose exceeds a specified cutpoint and drops below that cutpoint. Further glucose elevations above that cutpoint are not counted again until 25 minutes have lapsed, to avoid repeatedly counting multiple small glucose fluctuations around a cutpoint.
Statistical analyses
BMI z-score was calculated using the CDC SAS program; if a participant was over 227 months of age, then 227 months was used as age for purposes of calculating BMI z-score. The slopes of weight z-score, BMI z-score, FEV1 absolute and % predicted, and FVC absolute and % predicted were calculated as the value at the most recent pulmonary office visit minus the value at an office visit 12 months prior, divided by the number of days between visits. CGM placements were recommended a minimum of 6 weeks after a hospitalization, and chart review was performed to ensure BMI and pulmonary function data were obtained during periods of relative clinical stability. Two subjects were excluded because they had data from only one visit. Descriptive statistics, clinical characteristics, and CGM variables were calculated by group. Categorical variables were compared using Fisher’s exact test and continuous variables were compared using t-tests or ANOVA. Pearson correlation coefficients were calculated for CGM variables with clinical outcome variables. No corrections for multiple comparisons were performed.
Results
A total of 110 patients with CGM data were included (Table 1). There were no significant differences in age, sex, Tanner stage, weight z-score nor BMI z-score among CF glycemic groups and HC youth, and there were no differences in FEV1% nor FVC% among CF glycemic groups (Table 1). Of those with CFRD, 6 had the diagnosis made based on hyperglycemia during hospitalization, and of these participants, 4 had AGT and 2 had NGT on a recent OGTT. The CGMs were collected in these participants an average of 4.7±5.2 months from hospitalization. In the overall cohort, CGM was collected (mean±SD) 1.4±3.9 months from the most recent OGTT. All CF participants had pulmonary function testing within 3 months of CGM. The mean FEV1% and BMI z-score of our CF cohort were comparable to measures in our overall CF population of 10–18 year olds (CF Registry). HbA1c was higher in those with CFRD compared to those with AG, NG, and HC. Those with CFAG had higher HbA1c than HC. There was no significant difference in HbA1c between HC and CFNG youth. The CFRD cohort included a greater number of patients with CFTR Class I–III mutations than those with CFNG, although there were no significant differences in use of CFTR modulators among glycemic groups. Three pancreatic sufficient individuals were included in the CF NGT group while all with AGT and CFRD were pancreatic insufficient.
Table 1.
Subject Characteristics at enrollment
| HC (n=22) |
CFNG (n=23) |
CFAG (n=42) |
CFRD (n=23) |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 15.5 (0.9) | 13.6 (0.7) | 14.4 (0.4) | 13.5 (0.6) | 0.15 |
|
| |||||
| Male, n (%) | 9 (41) | 7 (30) | 22 (52) | 8 (35) | 0.31 |
|
| |||||
| Race, n (%) | |||||
| White | 18 (82) | 21 (91) | 38 (90) | 18 (78) | 0.21 |
| Hispanic | 2 (9) | 1 (4.4) | 4 (10) | 5 (22) | |
| Other | 2 (9) | 1 (4.4) | 0 (0) | 0 (0) | |
|
| |||||
| Tanner stage, n (%) | 0.88 | ||||
| I | 2 (10) | 2 (12.5) | 7 (29) | 1 (9) | |
| II | 2 (10) | 2 (12.5) | 1 (4) | 2 (18) | |
| III | 3 (15) | 3 (18.8) | 3 (12.5) | 2 (18) | |
| IV | 1 (5) | 1 (6.25) | 2 (8) | 0 (0) | |
| V | 12 (60) | 8 (50) | 11 (46) | 6 (55) | |
|
| |||||
| HbA1c (%) | 5.1 (0.04) | 5.3 (0.08) | 5.5 (0.05) | 5.8 (0.14) | <.001* |
|
| |||||
| Genotype, n (%) | 0.29 | ||||
| F508del/F508del | - | 13 (57) | 29 (69) | 10 (43.5) | |
| F508del/other | 7 (30) | 11 (26) | 10 (43.5) | ||
| Other | 3 (13) | 2 (5) | 3 (13) | ||
|
| |||||
| CFTR mutation class, n (%) | - | 0.02† | |||
| Class I–III (−/−) | 17 (74) | 40 (95) | 21 (91) | ||
| Class IV–V | 4 (17) | 0 (0) | 0 (0) | ||
| Unknown | 2 (9) | 2 (5) | 2 (9) | ||
|
| |||||
| On CFTR modulator, n (%) | - | 8 (35) | 9 (21) | 3 (13) | 0.22 |
|
| |||||
| Pancreatic insufficient, n (%) | - | 20 (87) | 42 (100) | 23 (100) | 0.03‡ |
|
| |||||
| G-Tube feedings, n(%) | - | 4 (17) | 6 (14) | 6 (26) | 0.52 |
|
| |||||
| Weight z-score | −0.14 (0.18) | −0.32 (0.16) | −0.25 (0.13) | −0.14 (0.18) | 0.86 |
|
| |||||
| Weight z-score slope | - | 0.0002 (0.0002) | −0.00 (0.0002) | 0.0005 (0.0004) | 0.43 |
|
| |||||
| BMI z-score | −0.19 (0.14) | −0.30 (0.16) | −0.08 (0.11) | −0.14 (0.17) | 0.70 |
|
| |||||
| BMI z-score slope | - | 0.0001 (0.0002) | 0.00 (0.0003) | 0.0008 (0.0006) | 0.31 |
|
| |||||
| FEV1% | - | 92.8 (2.5) | 94.5 (2.3) | 95.0 (3.8) | 0.87 |
|
| |||||
| FEV1% slope | - | −0.002 (0.008) | −0.001 (0.005) | −0.004 (0.009) | 0.95 |
|
| |||||
| FVC% | - | 99.7 (2.3) | 102.4 (2.1) | 102.8 (3.5) | 0.69 |
|
| |||||
| FVC% slope | - | 0.001(0.007) | −.003(0.004) | −.001(0.01) | 0.88 |
Data presented as Mean (SE) unless otherwise indicated
CFAG significantly different from HC; CFRD significantly different from all other groups
CFRD significantly different from CFNG
CFNG significantly different fom CFAG and CFRD
HC=Healthy Controls; CFNG=CF normal glycemia; CFAG=CF abnormal glycemia; CFRD=CF-related diabetes; CFTR = cystic fibrosis transmembrane regulator; HbA1c=hemoglobin A1c; BMI=body mass index; FEV1=forced expiratory volume in 1 sec; FVC=forced vital capacity
CGM findings
An average of 5.1±1.1 days of usable CGM data was generated per participant. Table 2 presents CGM variables by group. As expected, youth with CFRD and CFAG had more glucose abnormalities by multiple measures (maximum sensor glucose, average sensor glucose, AUC >98 mg/dl, number of excursions >140 and >200 mg/dl/day, % time >140 mg/dl, SD, and MAGE) compared to CFNG and HC. Youth with CFRD had a higher average glucose and AUC as well as greater number of excursions >140 mg/dl/day than CFAG participants, but the two groups were not significantly different by other CGM measures. Notably, CFNG youth also had more CGM abnormalities (peak glucose, number of daily excursions >140 mg/dl, % time >140 mg/dl, SD, and MAGE) than HC. Although youth with CFRD appeared to have more % time >200 mg/dl than the other groups, the difference was not statistically significant. Furthermore, there were no significant differences in any measure of hypoglycemia (minimum glucose, % time <70 mg/dl, % time <60 mg/dl) among any of the groups.
Table 2.
CGM Variables by Groups
| CGM Sensor Glucose | HC N=22 |
CFNG N=23 |
CFAG N=42 |
CFRD N=23 |
p-value |
|---|---|---|---|---|---|
| Average (mg/dl) | 98 (1.6) | 106 (2.4) | 109 (1.9) | 118 (4.5) | <.001* |
| Maximum (mg/dl) | 142 (4.2) | 195 (5.9) | 213 (8.8) | 227 (11.7) | <.001† |
| Minimum (mg/dl) | 64 (1.8) | 64 (2.8) | 63 (2.0) | 64 (2.9) | 0.946 |
| Average AUC | 1.4 ×105 (2.2 ×103) | 1.5 ×105 (3.4×103) | 1.6 ×105 (2.8 ×103) | 1.7 ×105 (6.5 ×103) | <.001* |
| AUC >98 mg/dl | 8.1 ×103 (1.3 ×103) | 1.9 ×104 (2.2 ×103) | 2.2 ×104 (2.3 ×103) | 3.3 ×104 (6.0 ×103) | <.001* |
| # Excursions >140/day | 0.5 (0.2) | 2.5 (0.3) | 2.3 (0.2) | 3.1 (0.2) | <.001‡ |
| # Excursions >200/day | 0.0 (0.0) | 0.2 (0.1) | 0.4 (0.1) | 0.6 (0.2) | 0.009§ |
| % time >140 mg/dl | 0.6 (0.3) | 7.4 (1.2) | 9.5 (1.7) | 14.3 (3.2) | <.001† |
| % time >200 mg/dl | 0.0 (0.0) | 0.2 (0.1) | 9.5 (1.6) | 15.0 (3.8) | 0.125 |
| % time <70 mg/dl | 1.9 (0.6) | 3.2 (1.3) | 2.1 (0.5) | 2.0 (0.8) | 0.655 |
| % time <60 mg/dl | 0.3 (0.2) | 1.6 (1.0) | 0.6 (0.3) | 0.6 (0.3) | 0.306 |
| Standard deviation | 12 (0.6) | 21 (0.9) | 22.2 (1.3) | 25.4 (2.0) | <.001† |
| MAGE | 25 (1.5) | 48 (2.7) | 54.0 (3.6) | 61.5 (5.9) | <.001† |
Data presented as Mean (SE); AUC >98 mg/dl is the AUC above the average sensor glucose in HCs.
CGM=continuous glucose monitoring; HC=healthy controls; CFNG=cystic fibrosis normal glycemia; CFAG=cystic fibrosis abnormal glycemia; CFRD=cystic fibrosis related diabetes; AUC = area under the curve; MAGE = mean amplitude of glycemic excursions
CFRD different from all other groups, HC different from CFAG and CFRD.
CFRD different from CFNG, HC different from all other groups.
CFRD different from CFAG, HC different from all groups.
CFRD different from CFNG, HC different from CFAG and CFRD
Correlations between CGM abnormalities and clinical outcomes
Correlations between CGM and clinical outcomes were examined for the entire CF cohort (n=88). Table 3 presents the correlations between CGM variables and the rate of change in weight z-score, BMI z-score, FEV1%, and FVC% over the year preceding CGM wear. No measure of CGM correlated significantly with rate of change in weight or BMI z-score. Multiple CGM measures, however, were associated with decline in the slopes of both FEV1% and FVC% over the prior year, including measures of hyperglycemia (max glucose, and excursions >200 mg/dl/day) and glycemic variability (SD, MAGE).
Table 3.
Correlations Between CGM and Change in Nutritional and Pulmonary Outcomes over the prior 12 months (n=88)
| CGM Variables | Weight z-score slope | BMI z-score slope | FEV1% predicted slope | FVC% predicted slope |
|---|---|---|---|---|
| Average (mg/dl) | −0.05 | −0.01 | −0.13 | −0.15 |
| Maximum (mg/dl) | −0.09 | 0.01 | −0.14 | −0.22* |
| Minimum (mg/dl) | −0.01 | −0.05 | 0.12 | 0.11 |
| AUC | −0.05 | −0.01 | −0.13 | −0.15 |
| AUC >98 mg/dl | −0.05 | −0.01 | −0.16 | −0.17 |
| Excursions >140/day | −0.03 | 0.03 | −0.08 | −0.01 |
| Excursions >200/day | −0.01 | 0.02 | −0.22* | −0.30* |
| % time >140 mg/dl | −0.07 | −0.03 | −0.20 | −0.21 |
| % time >200 mg/dl | −0.01 | −0.01 | −0.14 | −0.18 |
| % time <70 mg/dl | 0.05 | −0.001 | −0.12 | −0.09 |
| % time <60 mg/dl | 0.06 | −0.001 | −0.12 | −0.09 |
| Standard deviation | 0.01 | 0.03 | −0.20 | −0.26* |
| MAGE | −0.02 | 0.002 | −0.24* | −0.27* |
p<0.05
CGM=Continuous glucose monitoring; AUC=area under the curve; BMI=body mass index; FEV1=forced expiratory volume in 1sec; FVC=forced vital capacity; MAGE=mean amplitude of glycemic excursions
CGM Measures of Hyperglycemia: A greater number of excursions >200 mg/dl/day correlated with greater declines in FEV1% slope (r= −0.22, p=0.047) and FVC% slope (r= −0.30, p=0.005). Max glucose correlated with decline in FVC% (r= −0.22, p=0.04). Greater %time >140 mg/dl trended with greater decline in FEV1% (r= −0.20, p=0.07) and FVC% (r= −0.21, p=0.06) but did not reach statistical significance.
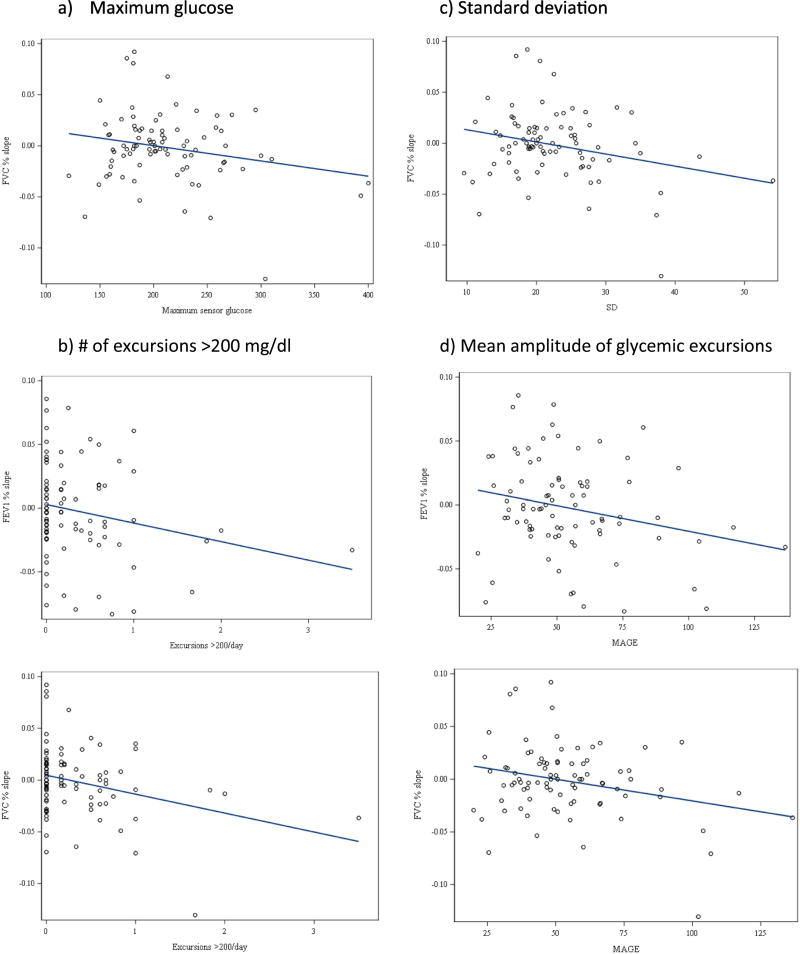
CGM Measures of glycemic variability: Higher MAGE correlated with greater declines in FEV1% (r= −0.24, p=0.03) and FVC% (r= −0.27, p=0.01). Greater SD also correlated with decline in FVC% (r= −0.26, p=0.01). Higher SD also trended with greater decline in FEV1% slope (r= −0.20, p=0.06). (Figure 1).
Figure 1.
We then repeated this analysis after removing patients with a diagnosis of CFRD to determine whether CGM abnormalities in those with CFNG or CFAG were associated with clinical decline. There were no statistically significant relationships between CGM and clinical parameters (change in weight z-score, BMI z-score and FEV1% and FVC% predicted) in this subgroup (data not shown).
Discussion
In this study of relatively healthy CF youth, multiple CGM abnormalities, including measures of hyperglycemia and glucose variability, correlated with lung function decline, as measured by changes in FEV1% and FVC% over the preceding year. In contrast to previous studies that have examined the relationship between glucose metabolism in CF and clinical outcomes (12–14, 17), our cohort has notable differences. The average HbA1c of our CFRD patients was 5.8%±0.1 (40±1 mmol/mol) reflecting our earlier diagnoses of CFRD, particularly given our inclusion into this category of patients with hyperglycemia detected during hospitalization. Despite these more inclusive CFRD criteria, patients with CFNG and CFAG were still found to have abnormalities in glucose metabolism on CGM. Our CF participants also had relatively good lung function, reflective of averages seen at our CF center as a whole. Even those with CFRD had an average FEV1% of 95±3.8% and FVC% of 102.8±3.5%, reflecting a healthier cohort with less impaired lung function than previously reported; in most published CFRD studies to date, average FEV1%s were as low as 50–70% (10, 12, 14, 17–19). This study, and others in even younger cohorts (20, 21), demonstrates the presence of glucose abnormalities in young children despite normal fasting and OGTT-defined glucoses and relatively good lung function. As treatment regimens for CF continue to improve, including increasing use of cystic fibrosis transmembrane conductance regulator (CFTR) modulators, overall clinical outcomes and lifespan for patients with CF will continue to improve. Our study population therefore represents a newer generation of healthier youth with longer projected lifespans in which it is critical to reassess the impact of early glucose metabolism abnormalities on clinical outcomes.
This study is one of the largest to characterize multiple components of CGM variability in CF youth along the glycemic spectrum, from those with normal fasting and OGTT glucoses to CFRD. Unlike prior studies assessing the potential utility of one or two selected CGM measures, this study extensively characterizes CGM variability by OGTT-grouped categories and compares these measures to CGM in relatively age-, sex-, and BMI-matched HC. As expected, patients with recently diagnosed CFRD and CFAG had higher CGM glucoses than HC on multiple measures. However, CFNG individuals also had higher average and peak sensor glucoses, AUC, % time >140 mg/dl, >200 mg/dl, SD, and MAGE than HC, indicating that young, relatively healthy CF youth with good lung function have multiple glycemic abnormalities at this early stage.
Even with improving treatments for CF, latest estimates find that CFRD continues to be associated with increased mortality compared to CF patients without diabetes (22). Abnormalities in glucose metabolism (by OGTT or CGM) detectable before a diagnosis of CFRD have been associated with poorer clinical outcomes. Brodsky et al found elevations of 1-hour plasma glucose during OGTT to be associated with reduced FEV1, even after adjustment for BMI%ile, with a decrease of 1% for every 10 mg/dl increase in 1-hour plasma glucose (23). A prospective study of pulmonary function changes in individuals with CF prior to a diagnosis of CFRD with fasting hyperglycemia (FH), published 16 years ago when a distinction was made between CFRD with FH versus CFRD without FH, found a pattern of FEV1 and FVC decline over 4 years that was directly proportional to the severity of glucose intolerance and to the degree of insulin deficiency, as assessed by 2hr AUC (24).
Very few studies have looked at the relationship between CGM abnormalities and clinical outcomes in CF. Leclercq et al found that CFNG patients with peak glucoses >200 mg/dl on CGM had lower lung function and increased Pseudomonas aeruginosa infections compared to those with a peak glucose <200 mg/dl, despite no differences in BMI SDS (12). Hameed et al examined peak sensor glucose and CGM time >140 mg/dl in 25 children with CF and found both to be associated with %FVC decline in the prior year. Increased CGM time spent >140 mg/dl was also associated with declining weight SDS in the preceding year (13), although, BMI SDS, a more reliable measure of nutritional status in growing children, was not reported. Our findings more comprehensively characterize CGM and its relationship with clinical outcomes and, although no correlations with weight nor BMI z-score were detected, possibly due to minimal changes in both over the prior year, similar to prior studies we found that measures of hyperglycemia – specifically peak glucose and excursions over 200 mg/dl - correlated with FEV1% and FVC% decline. Nutritional status is correlated with pulmonary function in CF (25), and insulin insufficiency, the primary pathophysiologic defect leading to CFRD (26, 27), is associated with compromised nutritional status resulting in excess protein and fat catabolism (28, 29). However, hyperglycemia also directly affects airway glucose and evidence suggests that blood glucoses >140 mg/dl lead to elevations in airway glucose (30, 31) that have been linked to increased growth of S. aureus and P. aeruginosa (32). Diabetes has also been shown to increase lung stiffness, cause changes in pulmonary small blood vessels, and reduce diffusion capacity (8, 33, 34). Our findings support the hypothesis that hyperglycemia is associated with negative impacts on lung function, independent of nutritional status.
Novel findings in our study include the relationships detected between measures of CGM glycemic variability and FEV1% and FVC% decline in the prior year. In individuals with type 2 diabetes, glycemic variability is associated with measures of oxidative stress involved in the development of diabetic cardiovascular complications (35, 36). Whether or not glycemic variability is associated with markers of oxidative stress leading to pulmonary function decline in CF requires further study.
Notably, when we repeated our analysis assessing the relationship between CGM and lung function and nutrition after removing those with CFRD, the relationships were no longer significant. This may imply either that hospital and OGTT-based criteria for diagnosing CFRD, as defined by CFRD guidelines (37), sufficiently identified youth with glycemia-associated declining lung function or that the study was underpowered to detect a significant correlation in this smaller sample. Larger studies in CF populations pre-CFRD diagnoses are needed to verify these findings.
Anecdotally, hypoglycemia is reported by many CF patients (38), commonly attributed to reactive hypoglycemia and impairments in first-phase insulin secretion (39–41). Low blood sugars have been reported in 6–15% of patients undergoing routine OGTT (42–44) and as many as 45% when the OGTT is extended to 3 hours (45). Notably, these rates of hypoglycemia on OGTT are not higher than that seen in the general population, particularly with extension of the OGTT beyond 2 hours (46). A recent review found few systematic studies characterizing hypoglycemia, as well as a dearth of evidenced-based studies assessing the significance of hypoglycemia and clinical outcomes in CF (38). Findings from our data reveal that hypoglycemia, as measured by CGM minimum glucose, time <70 mg/dl and <60 mg/dl, were not significantly different in any of the CF groups compared to HC. From these findings one might speculate that hypoglycemic symptoms in this young population, not yet on insulin therapy, result from counterregulatory hormone increases resulting from acute decreases in glucose from the hyperglycemic range into the normal range, rather than an absolute low glucose threshold. Further studies examining changes in counterregulatory hormones under experimental conditions controlling glycemia in CF participants are needed to explore this hypothesis.
Limitations of this report include the lack of prospective clinical data. These results indicate that a period of clinical decline of at least one year appears to precede hyperglycemia and glycemic variability detected on CGM. However, whether or not clinicians can use CGM to predict who may be at most risk for development of future clinical decline will require prospective studies. Furthermore, most of the data were collected from individuals not yet on CFTR modulators, and more research is required to understand the potential impact of these medications on clinical outcomes and glucose metabolism.
In summary, the ability for CGM to record consecutive glucoses over a 24-hour period in both fasting and prandial states allows greater insight into glycemic patterns in CF. To better understand the impact of these early changes on CF outcomes, a comprehensive approach to description and analyses of CGM variables will advance the understanding of both researchers and practitioners as to which glycemic abnormalities most significantly impact CF outcomes. Whether a single CGM variable or a combination of CGM variables best predicts clinically relevant CF outcomes, potentially redefining CFRD, and whether insulin intervention for these glucose abnormalities is warranted, requires ongoing study.
Highlights.
Characterization of CGM patterns across the glycemic spectrum in CF youth
Hyperglycemia and greater glucose variability are present in ‘normal glycemic’ youth
Objectively measured hypoglycemia was no different between any CF group vs controls
CGM measures of hyperglycemia and glucose variability are associated with FEV1 and FVC decline
Acknowledgments
This research was supported by NIH grants DK094712-04, TR000154 (CCTSI), UL1 TR001082 (REDCap), and Cystic Fibrosis Foundation Therapeutics grant CHAN16A0. Research reported in this publication was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ADA
American Diabetes Association
- CGM
Continuous Glucose Monitoring
- CFRD
Cystic Fibrosis Related Diabetes
- CFNG
Cystic Fibrosis Normal Glycemia
- CFAG
Cystic Fibrosis Abnormal Glycemia
- FEV1
Forced Expiratory Volume in 1 second
- FVC
Forced Vital Capacity
- HbA1c
Hemoglobin A1c
- MAGE
Mean amplitude of glycemic excursions
- OGTT
Oral Glucose Tolerance Testing
- T2D
Type 2 Diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: C.C. designed the study, researched data, and wrote the manuscript. T.V. and L.P. researched data, reviewed and edited the manuscript. P.Z., K.N., and S.S. designed the study, contributed to the discussion, reviewed and edited the manuscript. C.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no relevant conflicts of interest to disclose.
References
- 1.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardin DSGK, Baron B, Hale KA. Accelerated Red Blood Cell Turover Can Invalidate the Use of Hemoglobin A1c as a Diagnostic Test for Cystic Fibrosis Related Diabetes. The American Pediatric Society and the Society for Pediatric Research. 1999 May 4; 1999. [Google Scholar]
- 4.Kelly A, Moran A. Update on cystic fibrosis-related diabetes. J Cyst Fibros. 2013;12(4):318–31. doi: 10.1016/j.jcf.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis: five year prospective study. BMJ. 1995;311(7006):655–9. doi: 10.1136/bmj.311.7006.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frohnert BI, Ode KL, Moran A, Nathan BM, Laguna T, Holme B, et al. Impaired fasting glucose in cystic fibrosis. Diabetes Care. 2010;33(12):2660–4. doi: 10.2337/dc10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walshaw M. Routine OGTT screening for CFRD - no thanks. J R Soc Med. 2009;102(Suppl 1):40–4. doi: 10.1258/jrsm.2009.s19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waugh N, Royle P, Craigie I, Ho V, Pandit L, Ewings P, et al. Screening for cystic fibrosis-related diabetes: a systematic review. Health Technol Assess. 2012;16(24):iii–iv. 1–179. doi: 10.3310/hta16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuing N, Holl RW, Dockter G, Hermann JM, Junge S, Koerner-Rettberg C, et al. High variability in oral glucose tolerance among 1,128 patients with cystic fibrosis: a multicenter screening study. PLoS One. 2014;9(11):e112578. doi: 10.1371/journal.pone.0112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzese A, Valerio G, Buono P, Spagnuolo MI, Sepe A, Mozzillo E, et al. Continuous glucose monitoring system in the screening of early glucose derangements in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab. 2008;21(2):109–16. doi: 10.1515/jpem.2008.21.2.109. [DOI] [PubMed] [Google Scholar]
- 11.Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med. 2004;21(7):691–6. doi: 10.1111/j.1464-5491.2004.01219.x. [DOI] [PubMed] [Google Scholar]
- 12.Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2014;13(4):478–84. doi: 10.1016/j.jcf.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Hameed S, Morton JR, Jaffe A, Field PI, Belessis Y, Yoong T, et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010;33(2):221–6. doi: 10.2337/dc09-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanng S, Thorsteinsson B, Nerup J, Koch C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr. 1992;151(9):684–7. doi: 10.1007/BF01957574. [DOI] [PubMed] [Google Scholar]
- 15.Mohan K, Israel KL, Miller H, Grainger R, Ledson MJ, Walshaw MJ. Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration. 2008;76(2):181–6. doi: 10.1159/000110206. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez TL, Barbour LA. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol Ther. 2013;15(2):172–9. doi: 10.1089/dia.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferies C, Solomon M, Perlman K, Sweezey N, Daneman D. Continuous glucose monitoring in adolescents with cystic fibrosis. J Pediatr. 2005;147(3):396–8. doi: 10.1016/j.jpeds.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Moreau F, Weiller MA, Rosner V, Weiss L, Hasselmann M, Pinget M, et al. Continuous glucose monitoring in cystic fibrosis patients according to the glucose tolerance. Horm Metab Res. 2008;40(7):502–6. doi: 10.1055/s-2008-1062723. [DOI] [PubMed] [Google Scholar]
- 19.Schiaffini R, Brufani C, Russo B, Fintini D, Migliaccio A, Pecorelli L, et al. Abnormal glucose tolerance in children with cystic fibrosis: the predictive role of continuous glucose monitoring system. Eur J Endocrinol. 2010;162(4):705–10. doi: 10.1530/EJE-09-1020. [DOI] [PubMed] [Google Scholar]
- 20.Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013;1(1):52–8. doi: 10.1016/S2213-8587(13)70015-9. [DOI] [PubMed] [Google Scholar]
- 21.Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, et al. Abnormal Glucose Tolerance in Infants and Young Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2016;194(8):974–80. doi: 10.1164/rccm.201512-2518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, et al. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191(2):194–200. doi: 10.1164/rccm.201403-0576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care. 2011;34(2):292–5. doi: 10.2337/dc10-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891–5. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 25.Cystic Fibrosis Foundation Patient Registry. 2015 Annual Data Report [Google Scholar]
- 26.Mohan K, Miller H, Dyce P, Grainger R, Hughes R, Vora J, et al. Mechanisms of glucose intolerance in cystic fibrosis. Diabet Med. 2009;26(6):582–8. doi: 10.1111/j.1464-5491.2009.02738.x. [DOI] [PubMed] [Google Scholar]
- 27.Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care. 2010;33(12):2677–83. doi: 10.2337/dc10-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kien CL, Zipf WB, Horswill CA, Denne SC, McCoy KS, O'Dorisio TM. Effects of feeding on protein turnover in healthy children and in children with cystic fibrosis. Am J Clin Nutr. 1996;64(4):608–14. doi: 10.1093/ajcn/64.4.608. [DOI] [PubMed] [Google Scholar]
- 29.Moran A, Milla C, Ducret R, Nair KS. Protein metabolism in clinically stable adult cystic fibrosis patients with abnormal glucose tolerance. Diabetes. 2001;50(6):1336–43. doi: 10.2337/diabetes.50.6.1336. [DOI] [PubMed] [Google Scholar]
- 30.Garnett JP, Baker EH, Baines DL. Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. Eur Respir J. 2012;40(5):1269–76. doi: 10.1183/09031936.00052612. [DOI] [PubMed] [Google Scholar]
- 31.Wood DM, Brennan AL, Philips BJ, Baker EH. Effect of hyperglycaemia on glucose concentration of human nasal secretions. Clin Sci (Lond) 2004;106(5):527–33. doi: 10.1042/CS20030333. [DOI] [PubMed] [Google Scholar]
- 32.Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, et al. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros. 2007;6(2):101–9. doi: 10.1016/j.jcf.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Goldman MD. Lung dysfunction in diabetes. Diabetes Care. 2003;26(6):1915–8. doi: 10.2337/diacare.26.6.1915. [DOI] [PubMed] [Google Scholar]
- 34.Kaminsky DA. Spirometry and diabetes: implications of reduced lung function. Diabetes Care. 2004;27(3):837–8. doi: 10.2337/diacare.27.3.837. [DOI] [PubMed] [Google Scholar]
- 35.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–8. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch IB. Glycemic Variability and Diabetes Complications: Does It Matter? Of Course It Does! Diabetes Care. 2015;38(8):1610–4. doi: 10.2337/dc14-2898. [DOI] [PubMed] [Google Scholar]
- 37.Moran A, Pillay K, Becker DJ, Acerini CL, International Society for P. Adolescent D. ISPAD Clinical Practice Consensus Guidelines 2014. Management of cystic fibrosis-related diabetes in children and adolescents. Pediatric diabetes. 2014;15(Suppl 20):65–76. doi: 10.1111/pedi.12178. [DOI] [PubMed] [Google Scholar]
- 38.Armaghanian N, Brand-Miller JC, Markovic TP, Steinbeck KS. Hypoglycaemia in cystic fibrosis in the absence of diabetes: A systematic review. J Cyst Fibros. 2016;15(3):274–84. doi: 10.1016/j.jcf.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Cucinotta D, De Luca F, Arrigo T, Di Benedetto A, Sferlazzas C, Gigante A, et al. First-phase insulin response to intravenous glucose in cystic fibrosis patients with different degrees of glucose tolerance. J Pediatr Endocrinol. 1994;7(1):13–7. doi: 10.1515/jpem.1994.7.1.13. [DOI] [PubMed] [Google Scholar]
- 40.De Schepper J, Hachimi-Idrissi S, Smitz J, Dab I, Loeb H. First-phase insulin release in adult cystic fibrosis patients: correlation with clinical and biological parameters. Horm Res. 1992;38(5–6):260–3. doi: 10.1159/000182555. [DOI] [PubMed] [Google Scholar]
- 41.Moran A, Diem P, Klein DJ, Levitt MD, Robertson RP. Pancreatic endocrine function in cystic fibrosis. J Pediatr. 1991;118(5):715–23. doi: 10.1016/s0022-3476(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 42.Battezzati A, Battezzati PM, Costantini D, Seia M, Zazzeron L, Russo MC, et al. Spontaneous hypoglycemia in patients with cystic fibrosis. Eur J Endocrinol. 2007;156(3):369–76. doi: 10.1530/eje.1.02344. [DOI] [PubMed] [Google Scholar]
- 43.Haliloglu B, Gokdemir Y, Atay Z, Abali S, Guran T, Karakoc F, et al. Hypoglycemia is common in children with cystic fibrosis and seen predominantly in females. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12470. [DOI] [PubMed] [Google Scholar]
- 44.Radike K, Molz K, Holl RW, Poeter B, Hebestreit H, Ballmann M. Prognostic relevance of hypoglycemia following an oral glucose challenge for cystic fibrosis-related diabetes. Diabetes Care. 2011;34(4):e43. doi: 10.2337/dc10-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch IB, Janci MM, Goss CH, Aitken ML. Hypoglycemia in adults with cystic fibrosis during oral glucose tolerance testing. Diabetes Care. 2013;36(8):e121–2. doi: 10.2337/dc12-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lev-Ran A. Nadirs of oral glucose tolerance tests are independent of age and sex. Diabetes Care. 1983;6(4):405–8. doi: 10.2337/diacare.6.4.405. [DOI] [PubMed] [Google Scholar]