Abstract
We present the largest reported consecutive series on robotic partial cystectomy in the management of patients with primary urachal adenocarcinoma. Eight patients with primary urachal adenocarcinoma of the urinary bladder underwent a robotic partial cystectomy. The mean operative time, including trocar placement as well as robotic docking and closure was 184 minutes (range 130–240 minutes). The mean console time was 120 minutes (range 70–170 minutes). The mean estimated blood loss was 50 ml. There were no conversions to open surgery. The mean hospital stay was 4 days (range 3–7 days). Drain removal was performed at postoperative day 2.5 (range 2–3 days). Each patient underwent postoperative cystography on day 10 postoperatively and no patients had evidence of extravasation. There were no major complications. Histological analysis of all tumors confirmed primary urachal adenocarcinoma of the urinary bladder. There were no positive surgical margins. At a mean follow up of 32 months none of the patients have had a disease recurrence with any evidence of disease recurrence. Our initial data indicates that with robotic partial cystectomy for primary urachal adenocarcinoma of the urinary bladder is a safe surgical and oncological procedure. However, longer follow up and larger patient numbers are required to validate this further.
Key Words: Adenocarcinoma, Bladder cancer, Partial cystectomy
Introduction
Primary urachal adenocarcinoma (UA) of the urinary bladder is a rare neoplasm, accounting for less than 1% of all bladder cancers [1]. These tumors are aggressive tumors that occur at the dome or anterior wall of the bladder and have typically been associated with a poor prognosis with many patients found to have distant metastases at the time of diagnosis [2, 3].
Owing to the relatively low incidence of primary UA, there is little data regarding the best definitive management for these patients. Currently, surgery is the only option available to achieve a cure for patients with UA [4], with poor responses to chemotherapy evident in the literature [5]. Traditionally, the surgical option was radical cystectomy (RC) with wide excision of the urachus and umbilicus [6]. This leaves the patient with a host of morbidities, including the requirement of a urinary diversion and potency related issues in male patient. The 5-year survival rate for patients with UA undergoing RC is typically quoted as up to 80% [7, 8].
The less radical option of partial cystectomy (PC) with en bloc resection of the median umbilical ligament and umbilicus, which is bladder-sparing whilst allowing for complete tumour excision with wide surgical margins, is increasingly, and successfully, being used. A PC for urinary bladder cancer lost popularity many years ago as it was originally associated with high rates of recurrence (up to 50–78%) [3]. However, many teams have more recently shown that survival rates in those treated with RC are comparable with those treated with PC [2, 6, 9]. Ashley et al. [2] published the largest patient series of UA to date in 2006; they demonstrated, with the results from 66 patients, that there was no difference in survival between patients who underwent open partial cystectomy versus radical cystectomy.
In 2006 Milhoua et al. [6] marked the advent of a laparoscopic approach for this type of neoplasm with their case report of a patient with primary UA treated with laparoscopic extended PC and en bloc removal of the urachus and umbilicus, remaining disease-free at 18 months. The patient benefited from the reduced morbidity associated with this bladder-sparing technique, as well as the well-documented benefits of laparoscopic surgery, such as reduced blood loss, shorter hospital stay and faster recovery.
Since the first robotic-assisted prostatectomy was performed in 2001 by Rassweiler et al. [10], robotic-assisted surgery has been extended to almost all urological procedures. The benefits include reduced blood loss, reduced postoperative pain, improved cosmetic result and faster postoperative recovery time [11]. Whilst robotic-assisted surgery is commonplace in procedures such as prostatectomy, there are few reports of its use in robotic partial cystectomy (RPC), especially in the treatment of primary UA. Promising results were demonstrated in a paper published in 2010 by Allaparthi et al. [12]; they reported the results of 3 patients undergoing RPC for bladder cancer, one of whom had primary UA. The median follow-up was 6 months and the patient with primary UA was alive with no signs of recurrent or metastatic disease. However, larger cohorts with longer-term follow-up are required. In this case series we present the results of 8 patients with primary UA treated with RPC, extended pelvic lymph node dissection with en bloc resection of the median umbilical ligament and umbilicus. Table 1 summarises the lastest robotic assisted partial cystectomy series.
Table 1.
Robot-assisted partial cystectomy for urachal adenocarcinoma: comparison of Lister series with other adult case series
| Study | Case No. | Age (year) | OT (mins) | Complication (%) | EBL (ml) | LOS (days) | Follow-up (Mo) | Catheter removed (days) | Drain removed (days) |
|---|---|---|---|---|---|---|---|---|---|
| Ours | 8 | 53.5 | 184 | 0 | 50 | 4 | 26 | 8 | 2.5 |
| Spiess et al. [11] | 1 | 55 | 300 | 0 | 150 | 4 | 7 | ||
| Allaparthi et al. [12] | 1 | 24 | 165 | 20 | 2 | 6 | |||
| Kim et al. [13] | 1 | 45 | 175 | 0 | 20 | 7 | 7 | 3 | |
| Monzo Gardiner et al. [14] | 1 | 63 | 148 | 0 | 2 | 7 | 14 |
OT = Operative time; EBL = estimated blood loss; LOS = length of stay.
Patients and Methods
Eight patients underwent RPC between June 2009 and September 2014 by 3 surgeons (J,A.,T.L. and N.V.) using the da Vinci-S/Si robotic system (Intuitive Surgical, Sunnyvale, CA, USA). Five men and 3 women, with a mean age of 53.5 ± 10.3 years and a mean body mass index (BMI) of 25.3 ± 2.8 kg/m2 underwent the operation. Each patient presented with gross hematuria, dysuria, and mucous secretion in the urine. All patients underwent staging with MRI and CT scan pre-operatively. A histological diagnosis of primary UA was confirmed on resection biopsies. All patients were discussed at our Urology Multi-disciplinary team meeting prior to recommending a RPC.
All patients were admitted prior to surgery and had bowel preparation. All patients were consented for an ontable decision to proceed to an open PC/robotic RC and formation of ileal conduit/neobladders formation.
Patients received a standard general anaesthetic consisting of fentanyl 100 µg, midazolam 2 mg and propofol induction and intubation facilitated by atracurium. In addition paracetamol 1 g, ketorolac 30 mg, ondansetrom 4 mg and dexamethasone 6.6 mg were given. Anesthesia was maintained with oxygen, air and desflurane through a circle system and positive pressure ventilation. Muscle relaxation was maintained with atracurium infusion. The caudal block administered contained 40 ml 0.25% bupivicaine, 150 µg clonidine and 100 µg fentanyl. Bupivacaine 0.5% 20 ml were infiltrated to the skin wounds at the end of the operation. Regular paracetomol was prescribed postoperatively and ketorolac 30 mg im and oramorph 20 mg cyclizine were available on an as required basis. Intravenous induction of anesthesia is performed and maintained with an inhalational agent and an intravenous infusion of remifentanil. A naso-gastric tube is not routinely inserted, but, ranitidine may be prescribed as a premedication if there is a history of gastro-oesophageal reflux. A clear view of the face and vigilance in positioning are important preventative measures. The patient is transferred onto a non-slip gel mat on the operating table. Prior to this a sheet is folded around a head pillow and then secured under the gel mat to stop the pillow from moving. A second gel mat is placed transversely across the table at mid arm level. This is used to wrap over the patients arms and under the torso to secure the arms during surgery. This configuration prevents the patient from slipping when in the steep Trendelenburg position and spreads the pressure across all contact points, rather than being focused at the shoulders. It is important to ensure all intravenous cannulae are well padded to protect the skin, connections are secure and fluid is running freely as access is limited during surgery.
Pneumoperitoneum is created using the open Hason technique and a transperitoneal approach was performed with six ports. The placement of the trocars is similar to that in robot-assisted laparoscopic radical prostatectomy but 3 cm superior to ensure that the urachus is accessible. The 12-mm trocar was placed 2 cm superior to the umbilicus. The first robotic arm 8-mm trocar was placed 8 cm left laterally and the second robotic arm 8-mm trocar was placed 8 cm right laterally to the 12-mm camera port. The third robotic arm 8-mm trocar was placed 8 cm laterally to the second robotic arm port. The 12-mm assistant port was placed 8 cm laterally to the first robotic arm port, and the 5-mm assistant suction port was placed 8 cm superior to the camera port and the first robotic arm port After port placement, the patient was placed in the steep Trendelenburg position and the robotic system was docked to the patient. The urinary bladder was filled with 200 ml of air, and a transperitoneal approach was performed with the 0/30 degree robotic camera with the use of monopolar scissors and a bipolar Maryland dissector. Any bowel adhesions were lysed with the monopolar scissors and the medial umbilical ligament was dissected. The bladder was released from the surrounding structure to permit identification of the bladder margins. After the cranial dissection into Retzius' space, the mass of the dome site was identified, and a cystotomy was performed with the monopolar scissors at a distance of 2 cm from the margin of the mass. After careful observation of the intravesical portion of the mass, the mass was excised and removed by use of an EndoCatch® device. We placed a flexible cystoscopy in the bladder at the time of mobilization. The initial incision was made by reducing the telescope light on the robotic camera to 10% and incising where the assistant with the flexible cystoscope indicted normal mucosa away from the location of the tumour. The bladder was closed in two layers with watertight running sutures made with 2-0 Vicryl. The bladder was filled with saline to detect any points of leakage. A Jackson-Pratt drain was positioned in the Retzius' space, and the specimen was removed from the camera port. A leak test was performed with sterile water. A nasogastric tube was removed 1 day after surgery, and an oral liquid diet was started simultaneously. A cystogram is performed at 10 days and a catheter is then removed if no leak is confirmed.
Results
The mean operative time, including trocar placement as well as robotic docking and closure, was 184 minutes (range 130–240 minutes). The mean console time was 120 minutes (range 70–170 minutes). The mean estimated blood loss was 50 ml. There were no conversions to open surgery. The mean hospital stay was 4 days (range 3–7 days). Drain removal was performed at postoperative day 2.5 (range 2–3 days). Each patient underwent postoperative cystography on day 10 postoperatively, and no patients had evidence of extravasation. There were no major complications. Histological analysis of all tumours confirmed primary adenocarcinoma of bladder. There were no positive surgical margins. At a mean follow up of 32 months there is no evidence of disease recurrence in any of the cases.
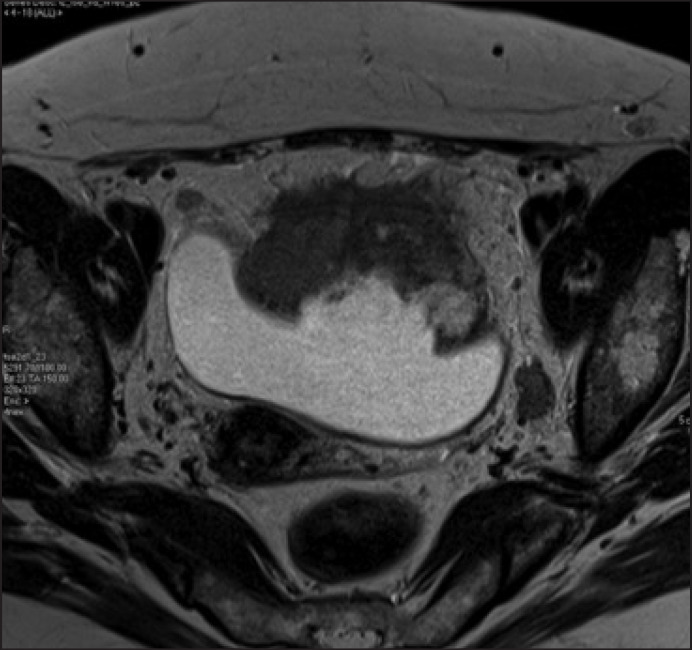
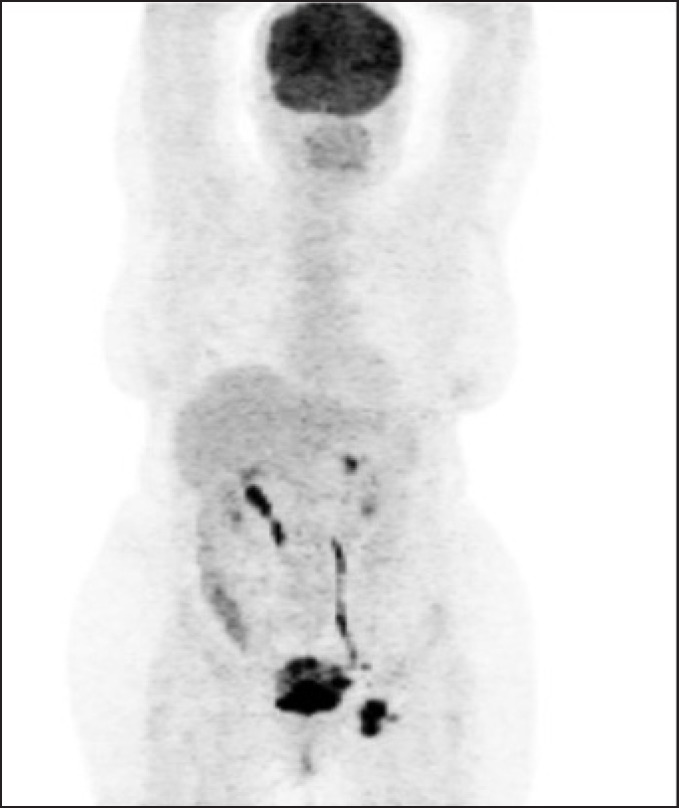
One patient who had advanced disease had a particularly impressive outcome. A 49 year old lady who had been investigated by gynecology for ‘vaginal bleeding’ for 6 months and was eventually found to have a large urachal adenocarcinoma with metastatic nodal disease (T4bN3) in 2010 (fig. 1). The tumor was invading the omentum, so she underwent robotic partial cystectomy, with en bloc removal of a patch of omentum. Twentyfour pelvic nodes were removed, with 3 nodes on the left found to be positive. Estimated blood loss was 150 ml, and she was discharged after three post-operative nights in hospital. Her catheter was removed on day 10, and a cystogram on day 10 showed no leak. She subsequently had four cycles of adjuvant oxaliplatin and capecitabine, and at follow-up at 32 months post-treatment she was disease free with no evidence of recurrence of metastatic disease (fig. 2)
Fig. 1.
MRI scans showing large urachal adenocarcinoma in the urinary bladder.
Fig. 2.
PET scan showing metastatic pelvic lymph node.
At a mean follow up of 32 months there is no evidence of disease recurrence in any of the cases.
Discussion
Primary UA is a rare malignancy with the details of little more than 400 cases reported in the literature. Traditionally the gold standard of treatment has been RC, but a number of teams have reported equally good oncological results with PC over the last few decades; these have generally been open or laparoscopic procedures and there have been very few reports of RPC used to treat UA. In 2010 Allaparthi et al. [12] reported the successful outcomes of one patient with UA who was treated with RPC. Our paper presents the results of the largest series to date of patients undergoing RPC for UA (n = 8). Our series shows that RPC is an oncologically viable option for treating patients with UA, including those patients with widespread metastatic disease. At 32 months all of our patients were alive with no evidence of disease recurrence rate.
What we have demonstrated is important because select patients with some muscle-invasive bladder cancers are no longer necessarily requiring a RC and are having successful oncological outcomes at the same time. These patients therefore enjoy preserved bladder function, preserved sexual function (in the case of male patients), and they also avoid the complications of RC. Early complications following RC are quoted in one study in 2006 as occurring in 28% of patients [15]; these commonly include dehydration and prolonged urinary leakage secondary to urinary diversion. In our series no complications occurred.
Urinary diversion has many consequences, most notably psychological, cosmetic and metabolic drawbacks. Hyperchloraemic metabolic acidosis, one well-recognised metabolic complication of some urinary diversions, can potentially be quite dangerous. The option of partial cystectomy also provides curative treatment to patients with too poor a functional status to undergo a RC, for example elderly patients and those with multiple comorbidities [16].
The patients undergoing RPC reported by Allaparthi et al. [12] had a median follow-up of 6 months and the one patient in the series with UA had no sign of metastatic or recurrent disease at the time of follow-up. In 2007 Herr et al. [17] reported a series of fifty patients with UA who underwent extended PC including the urachal tumor mass and entire urachus; 18% had local recurrence within the first 2 years of follow-up. In our series none of the patient had evidence of any local recurrence at 32 month follow-up.
There are distinct advantages of robotic surgery over laparotomy or simple laparoscopy. Robotic surgery allows for better manoeuvrability and dexterity, improved views of the surgical area, and improved intracorporeal suturing, all of which are particularly important features for RPC for UA because of the need for very precise tumor margins [18, 19]. There were no positive margins in our patient series. Additionally, patients undergoing RPC are more likely to enjoy faster post-operative recovery, improved post-operative cosmetic appearance, and reduced post-operative pain [11]. One study by Ng et al. [20] comparing postoperative complications following open RC with robot RC found that the rate of both overall complications and major complications was higher in patients undergoing open RC than those undergoing robot RC (major complications 29.8% versus 9.6%, p = 0.007). Patients undergoing open RC were more likely to suffer early respiratory complications. This could be attributed to reduced pain in the robotic cohort allowing for earlier pulmonary rehabilitation. Reduced intra-operative blood loss associated with robotic surgery is also postulated to have a role in reducing post-operative complications.
Whilst there is a host of literature supporting the use of robots in prostatic and renal surgery, there is only limited evidence of experience with robots in performing RC, and even more so with PC; this paper adds evidence of success of robotic PC by urologists with advanced robotic skills to the literature base. Studies have shown that robotic RC allows for equivalent lymph node yields when compared to an open approach [21], and this is likely to be extrapolated to include robotic PC. Many would agree that robotic surgery has a relatively steep learning curve, thereby making it potentially more accessible to urologists [20, 22].
Direct comparison between our data and previous data is difficult due to the combination of a rare pathology (urinary bladder UA) and an infrequently used surgical technique (robotic partial cystectomy) in our series. However, when comparing our results with similar series, our results are encouraging and show that RPC for UA is a safe and feasible treatment option for patients with UA. Our survival rates, recurrence rates and perioperative data are comparable with results from other series and studies; all 8 of our patients are alive at 32 months with a recurrence rate of 0%. However, our patients will continue to be followed-up to ensure that our 5-year survival rates are comparable with those of patients undergoing RC. A limitation of our series is the sample size (n = 8), but this is the largest patient series of patients with UA treated with PC to date. Further cohorts with more patients are needed to strengthen the evidence for the successful use of robotic PC to treat UA. Due to the rarity of UA, it is unlikely that a clinical trial will be carried out and the choice of management of these patients will continue to be based on patient series such as this.
Conclusion
We present the largest reported consecutive series on RPC in the management of patients with primary UA. We have demonstrated that RPC is an oncologically viable option for these patients with excellent survival rates at 32 months, rates of recurrence and complication rates. The rarity of UA means that a randomised controlled trial comparing RPC with other surgical techniques is not feasible and as such single institution patient series such as this are currently extremely valuable. A multi-institutional database of patients with UA treated with RPC would improve our knowledge on the long term prognosis of this cohort of patients.
References
- 1.Henly DR, Farrow GM, Zincke H. Urachal cancer: role of conservative surgery. Urology. 1993;42:635–639. doi: 10.1016/0090-4295(93)90526-g. [DOI] [PubMed] [Google Scholar]
- 2.Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, Zincke H. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006;107:712–720. doi: 10.1002/cncr.22060. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead ED, Tessler AN. Carcinoma of the urachus. Br J Urol. 1971;43:468–476. doi: 10.1111/j.1464-410x.1971.tb12070.x. [DOI] [PubMed] [Google Scholar]
- 4.Hong SH, Kim JC, Hwang TK. Laparoscopic partial cystectomy with en bloc resection of the urachus for urachal adenocarcinoma. Int J Urol. 2007;14:963–965. doi: 10.1111/j.1442-2042.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 5.Siefker-Radtke A. Urachal adenocarcinoma: a clinician's guide for treatment. Semin Oncol. 2012;39:619–624. doi: 10.1053/j.seminoncol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Milhoua PM, Knoll A, Bleustein CB, Ghavamian R. Laparoscopic partial cystectomy for treatment of adenocarcinoma of the urachus. Urology. 2006;67:423. doi: 10.1016/j.urology.2005.08.044. e15-e17. [DOI] [PubMed] [Google Scholar]
- 7.Malek RS, Rosen JS, O'Dea MJ. Adenocarcinoma of bladder. Urology. 1983;21:357–359. doi: 10.1016/0090-4295(83)90151-6. [DOI] [PubMed] [Google Scholar]
- 8.el-Mekresh MM, el-Baz MA, Abol-Enein H, Ghoneim MA. Primary adenocarcinoma of the urinary bladder: a report of 185 cases. Br J Urol. 1998;82:206–212. doi: 10.1046/j.1464-410x.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- 9.Santucci RA, True LD, Lange PH. Is partial cystectomy the treatment of choice for mucinous adenocarcinoma of the urachus? Urology. 1997;49:536–540. doi: 10.1016/s0090-4295(96)00574-2. [DOI] [PubMed] [Google Scholar]
- 10.Rassweiler J, Binder J, Frede T. Robotic and telesurgery: will they change our future? Curr Opin Urol. 2001;11:309–320. doi: 10.1097/00042307-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Spiess PE, Correa JJ. Robotic assisted laparoscopic partial cystectomy and urachal resection for urachal adenocarcinoma. Int Braz J Urol. 2009;35:609. doi: 10.1590/s1677-55382009000500014. [DOI] [PubMed] [Google Scholar]
- 12.Allaparthi S, Ramanathan R, Balaji KC. Robotic partial cystectomy for bladder cancer: a single-institutional pilot study. J Endourol. 2010;24:223–227. doi: 10.1089/end.2009.0367. [DOI] [PubMed] [Google Scholar]
- 13.Kim DK, Lee JW, Park SY, Kim YT, Park HY, Lee TY. Initial experience with robotic-assisted laparoscopic partial cystectomy in urachal diseases. Korean J Urol. 2010;51:318–322. doi: 10.4111/kju.2010.51.5.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monzo Gardiner JI, Garcia MF, Albornoz JM, Secin FP. Urachal adenocarcinoma treated with robotic assisted laparoscopy partial cystectomy. Arch Esp Urol. 2013;66:608–613. [PubMed] [Google Scholar]
- 15.Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol. 2006;24:296–304. doi: 10.1007/s00345-006-0061-7. [DOI] [PubMed] [Google Scholar]
- 16.Knoedler JJ, Boorjian SA, Kim SP, Weight CJ, Thapa P, Tarrell RF, Cheville JC, Frank I. Does partial cystectomy compromise oncologic outcomes for patients with bladder cancer compared to radical cystectomy? A matched case-control analysis. J Urol. 2012;188:1115–1159. doi: 10.1016/j.juro.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW, Bochner BH, Sharp D, Dalbagni G, Reuter VE. Urachal carcinoma: contemporary surgical outcomes. J Urol. 2007;178:74–78. doi: 10.1016/j.juro.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Yohannes P, Rotariu P, Pinto P, Smith AD, Lee BR. Comparison of robotic versus laparoscopic skills: is there a difference in the learning curve? Urology. 2002;60:39–45. doi: 10.1016/s0090-4295(02)01717-x. [DOI] [PubMed] [Google Scholar]
- 19.Gettman MT, Blute ML, Peschel R, Bartsch G. Current status of robotics in urologic laparoscopy. Eur Urol. 2003;43:106–112. doi: 10.1016/s0302-2838(02)00579-1. [DOI] [PubMed] [Google Scholar]
- 20.Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, Schwartz MJ, Wang GJ, Scherr DS. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010;57:274–281. doi: 10.1016/j.eururo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 21.De Lorenzis E, Palumbo C, Cozzi G, Talso M, Rosso M, Costa B, Gadda F, Rocco B. Robotics in uro-oncologic surgery. Ecancermedicalscience. 2013;7:354. doi: 10.3332/ecancer.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James K, Vasdev N, Adshead JM. The role of robotic partial cystectomy in a patient with metastatic primary adenocarcinoma of the bladder. Int J Cancer Ther Oncol. 2015;3:030120. [Google Scholar]