Abstract
Background
Mycoplasma pneumoniae pneumonia (MPP) is one of the most common forms of community-acquired pneumonia in children. The objective of this study was to explore potential changes in levels of serum tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) associated with pediatric MPP.
Methods
This protocol has been registered (PROSPERO 2017: CRD42017077979). A literature search was performed in October 2017 using PubMed, Embase, the Cochrane Library, and other Chinese medical databases to identify studies. The meta-analysis was performed using Review Manager 5.3 software. Random-effect models were used to estimate mean differences (MDs) and 95% confidence intervals (CIs) of cytokine levels.
Results
Twelve studies were included in the meta-analysis, encompassing 2,422 children with MPP and 454 healthy control children. Serum TNF-α levels were significantly higher in children with MPP compared with healthy children (MD = 22.5, 95% CI = 13.78–31.22, P < 0.00001), and there was significant heterogeneity across studies (I2 = 100%, P < 0.00001). Subgroup analyses showed no evidence for a difference in serum TNF-α levels between children with refractory and nonrefractory MPP. Serum IFN-γ levels did not significantly differ in children with MPP compared with healthy children (MD = 4.83, 95% CI = −3.27–12.93, P=0.24).
Conclusions
Our meta-analysis showed that serum TNF-α and IFN-γ levels were significantly elevated and unchanged, respectively, in pediatric MPP. Because infection by different pathogens has variable effects on serum TNF-α and IFN-γ levels, the finding could be helpful in developing novel diagnostic methods.
1. Introduction
Mycoplasma pneumoniae (MP) is one of the most prevalent etiological agents of community-acquired pneumonia in children [1, 2]. Infection by MP is traditionally thought to be self-limiting [3], but under some circumstances, it can result in severe life-threatening diseases such as acute respiratory distress syndrome, necrotizing pneumonitis, and fulminant pneumonia. The incidence of MP pneumonia (MPP) has been increasing in recent years [2]. However, symptoms and radiographic findings in children with MPP are often similar to those associated with other respiratory infections [4]. Thus, early diagnosis of MPP is crucial for initiating appropriate antibiotic therapy. Proinflammatory cytokines play an important role in the mechanism of MP infection [5–7].
The proinflammatory cytokine tumor necrosis factor-alpha (TNF-α) is an important initiator of inflammatory and bactericidal processes [8, 9]. In bacterial pneumonia, macrophage-derived TNF-α is elevated, resulting in recruitment of inflammatory cells to sites of infection [8, 10, 11]. However, TNF-α may not play a significant antiviral role, and its levels in serum do not change significantly during viral pneumonia [8, 12, 13]. Serum levels of TNF-α in patients with MPP are less well defined, with some studies showing elevated levels and others no difference [14, 15]. Another proinflammatory cytokine, interferon-gamma (IFN-γ), is a critical mediator of antiviral immunity and is often associated with chronic inflammation [16, 17]. Serum levels of IFN-γ are typically elevated during both viral and bacterial infections [18, 19], but its levels in pediatric MPP patients have been inconsistent across studies [20, 21].
Thus, the purpose of this meta-analysis was to analyze levels of serum TNF-α and IFN-γ in pediatric MPP and compare these with levels in healthy children.
2. Methods
2.1. Literature Search and Study Selection
A literature search was performed in October 2017 using PubMed, Embase, the Cochrane Library, and other Chinese medical databases to identify studies. The following search terms were used: (“cytokine”) or (“tumor necrosis factor-α” or “TNF-α”) or (“interferon-γ” or “IFN-γ”) and (“Mycoplasma pneumoniae pneumonia” or “MPP”). The searches were restricted to studies whose subjects were children; no language restrictions were applied. The reference lists and supplemental materials associated with the search results were manually inspected to identify additional relevant publications. A meta-analysis of the mean differences (MDs) of serum TNF-α and IFN-γ levels in pediatric MPP was undertaken. The Prospero registration number is CRD42017077979.
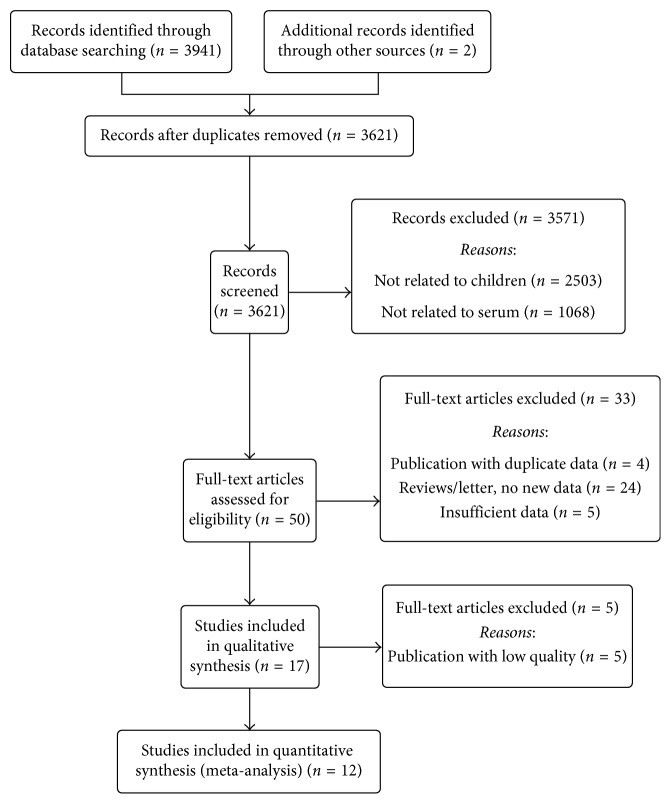
All studies aiming to explore the association between serum TNF-α and IFN-γ levels and pediatric MPP were included. The study inclusion criteria were as follows: (i) study subjects included children with MPP; (ii) study subjects included a group of healthy control children; (iii) serum TNF-α and IFN-γ levels were measured; and (iv) full text, original research articles could be obtained. The study exclusion criteria were as follows: (i) lack of data on serum TNF-α and IFN-γ levels in pediatric MPP; (ii) not a primary study or not a case-control design; (iii) insufficient data extracted from the articles or the full text could not be obtained; and (iv) duplicate studies. The study inclusion and exclusion procedures are summarized in Figure 1.
Figure 1.
Flow diagram of included studies for this meta-analysis.
2.2. Data Extraction
Two investigators (Y Wang and YS Zhang) independently performed the data extraction. The general characteristics of the study were extracted using a standardized data extraction form on which publication information (first author's name, publication year, and country) and subject characteristics (serum cytokine measurement method, MPP type, control type, and sample size) were noted. If no standard deviations for serum TNF-α and IFN-γ concentrations were available, these values were calculated using confidence intervals and medians [22]. If multiple published reports of the same study population were available, we included only the report with the largest sample size and the most complete data. Discrepancies were resolved by discussion with other investigators (WT Lu and L Wang).
2.3. Statistical Analysis
The meta-analysis was performed using Review Manager 5.3 software. Serum TNF-α and IFN-γ levels were extracted as the means ± standardized deviations (SDs) of each study. MDs with 95% CIs were used to determine the strength and directionality of the association between serum TNF-α and IFN-γ levels and pediatric MPP. The pooled MDs for TNF-α and IFN-γ concentrations associated with pediatric MPP were calculated. Subgroup analyses were performed to compare levels in children with refractory and nonrefractory MPP. Heterogeneity was assessed using Cochran's Q test and the I-squared statistic. If P < 0.1 or I2 > 50%, heterogeneity was considered significant, and a random-effect model was used.
3. Results
3.1. Search Results
The steps for screening and the study selection procedure are presented in Figure 1. A total of 3,621 relevant articles were initially identified from PubMed, Embase, the Cochrane Library, and other Chinese medical databases. Through screening of titles and abstracts, 50 publications met the study inclusion criteria. After reading the full text, 12 studies were included in the meta-analysis [14, 15, 20, 21, 23–30].
3.2. Levels of Serum TNF-α and IFN-γ in Pediatric MPP
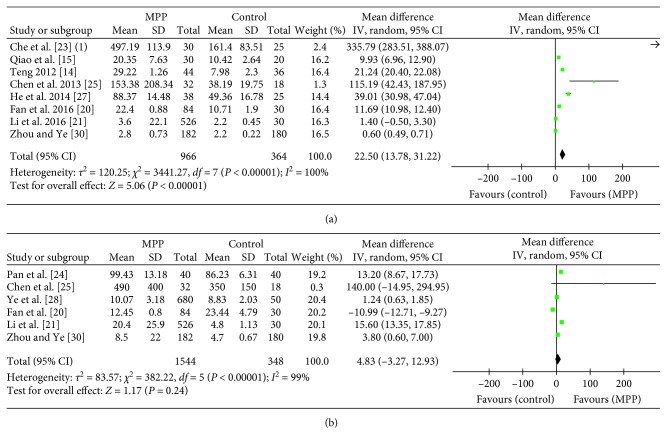
The results of 12 studies of serum TNF-α and IFN-γ levels in children with MPP are summarized in Table 1. The pooled MDs revealed that serum TNF-α levels were higher in the pediatric MPP group as compared with age-matched healthy controls (MD = 22.5, 95% CI = 13.78–31.22, P < 0.00001) (Figure 2(a)). Substantial heterogeneity was observed among studies (I2 = 100%; P < 0.00001). As shown in Figure 2(b), serum IFN-γ levels did not significantly differ between children with MPP and healthy control children (MD = 4.83, 95% CI = −3.27–12.93; P=0.24), with significant heterogeneity across studies (I2 = 99%; P < 0.00001).
Table 1.
Characteristics of studies included in the meta-analysis.
| Author/year of publication | Country | Plasma cytokines assay method | Mycoplasma pneumoniae pneumonia group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | Source of pneumonia type | TNFα (pg/ml) | IFNγ (pg/ml) | Total N | Source of control | TNFα (pg/ml) | IFNγ (pg/ml) | |||
| Che et al. [23] (1) | China | Radioimmunoassay | 30 | MPP | 497.19 ± 113.90 | — | 25 | Healthy | 161.40 ± 83.51 | — |
| Che et al. [23] (2) | China | Radioimmunoassay | 20 | RMPP | 520.42 ± 67.90 | — | 10 | NRMPP | 280.12 ± 26.72 | — |
| Pan et al. [24] | China | ELISA | 40 | MPP | — | 99.43 ± 13.18 | 40 | Healthy | — | 86.23 ± 6.31 |
| Teng [14] | China | ELISA | 44 | MPP | 29.22 ± 1.26 | — | 36 | Healthy | 7.98 ± 2.30 | — |
| Qiao et al. [15] | China | ELISA | 30 | MPP | 20.35 ± 7.63 | — | 20 | Healthy | 10.42 ± 2.64 | — |
| Chen et al. [25] | China | ELISA | 32 | MPP | 153.38 ± 208.34 | 490 ± 400 | 18 | Healthy | 38.19 ± 19.75 | 350 ± 150 |
| Wang et al. [26] | China | ELISA | 76 | RMPP | 114.5 ± 112.8 | 376.9 ± 296 | 26 | NRMPP | 43 ± 28 | 180 ± 154 |
| He et al. [27] | China | ELISA | 38 | MPP | 88.37 ± 14.48 | — | 25 | Healthy | 49.36 ± 16.78 | — |
| Ye et al. [28] | China | Flow cytometry | 680 | MPP with acute phase | — | 10.07 ± 3.18 | 50 | Healthy | — | 8.83 ± 2.03 |
| Zhang et al. [29] | China | Flow cytometry | 145 | RMPP | 3.0 ± 0.47 | 16.3 ± 6.8 | 489 | NRMPP | 2.9 ± 0.35 | 7.9 ± 1.27 |
| Fan et al. [20] | China | ELISA | 84 | MPP | 22.4 ± 0.88 | 12.45 ± 0.8 | 30 | Healthy | 10.71 ± 1.90 | 23.44 ± 4.79 |
| Li et al. [21] | China | Flow cytometry | 526 | MPP | 3.6 ± 22.1 | 20.4 ± 25.9 | 30 | Healthy | 2.2 ± 0.45 | 4.8 ± 1.13 |
| Zhou and Ye [30] | China | Flow cytometry | 182 | MPP | 2.8 ± 0.73 | 8.5 ± 22.0 | 180 | Healthy | 2.2 ± 0.22 | 4.7 ± 0.67 |
RMPP: refractory Mycoplasma pneumoniae pneumonia; NRMPP: nonrefractory Mycoplasma pneumoniae pneumonia.
Figure 2.
Forest plots showing mean difference (MD) and confidence intervals (CI) of the serum TNF-α and IFN-γ levels to diagnose pediatric MPP. (a) TNF-α and (b) IFN-γ related results have been shown.
3.3. Subgroup Analysis
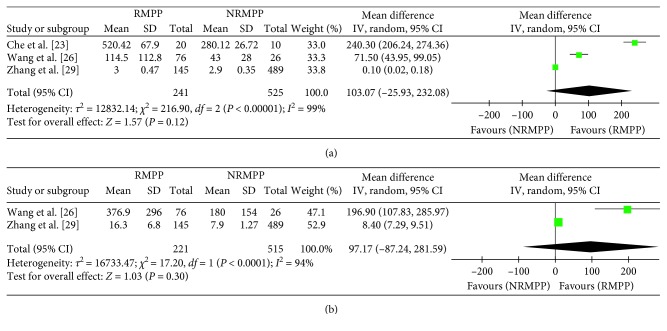
Although the result above demonstrated that serum TNF-α levels significantly differed between children with MPP and healthy children, levels in different pediatric MPP subgroups were still unknown. We found that serum TNF-α and IFN-γ levels were not significantly different in children with refractory and nonrefractory MPP (TNF-α: MD = 103.07, 95% CI = −25.93–232.08, P=0.12; IFN-γ: MD = 97.17, 95% CI = −87.24–281.59, P=0.30) (Figure 3). In the subgroup analysis, significant heterogeneity was observed both for TNF-α levels (I2 = 99%; P < 0.00001) and IFN-γ levels (I2 = 94%; P < 0.00001) (Figure 3).
Figure 3.
Forest plots analysis change rule in the serum TNF-α and IFN-γ levels between refractory and nonrefractory pediatric MPP. (a) TNF-α and (b) IFN-γ related results have been shown.
3.4. Sensitivity Analysis and Reporting Bias
Sensitivity analysis was performed by excluding studies one by one. No obvious changes were found in the results, which confirmed their stability. We did not construct a funnel plot as they are known to be unreliable when constructed using fewer than 10 studies [31].
4. Discussion
TNF-α is involved in normal inflammatory reactions and immune responses and is crucial for bactericidal processes [10, 11]. Serum TNF-α levels are usually elevated in bacterial pneumonia and not significantly altered in viral pneumonia [8, 12, 13]. Our results revealed that serum TNF-α levels were higher in children with MPP compared with healthy controls. This finding suggests that a large amount of TNF-α is released systemically in children infected by MP to combat the pathogen via inflammatory processes. We speculate that MP and other bacteria use similar signals to promote inflammatory responses. Previously, it was reported that the actin-like protein of MP and other bacterial species might share a common evolutionary ancestor [32]. Due to the limited study of serum TNF-α levels in different pediatric MPP subgroups, we could not obtain sufficient data to perform a rigorous statistical analysis; this may be one explanation for our finding that serum TNF-α levels did not differ in refractory and nonrefractory MPP.
IFN-γ is a major mediator of antiviral immunity and inflammatory responses [16, 17]. Serum IFN-γ levels are consistently elevated in both viral and bacterial pneumonia [18, 19]. In our meta-analysis, serum IFN-γ levels were not significantly different in children with MPP and healthy controls. We speculate that IFN-γ may be produced predominantly at sites of local inflammation rather than systemically. Consistent with this hypothesis, some studies have reported elevated IFN-γ levels in sputum and alveolar lavage fluid of children with MPP [33, 34]. Our results showed that serum levels of TNF-α and IFN-γ during infection with MP differed from those observed during infection by other bacterial and viral pathogens causing pneumonia. Thus, we speculate that serum TNF-α and IFN-γ levels in pediatric MPP may be potential diagnostic markers of infection by this pathogen.
The main limitation of this study was the significant heterogeneity observed across included studies. This heterogeneity remained in subgroup analyses of children stratified by disease severity, which indicated that variation in test methods, measurement reagents, or MPP onset time might be potential sources. Of the 12 included studies, cytokine testing was performed using radioimmunoassay, flow cytometry, and ELISA in 1, 4, and 7 studies, respectively (Table 1). Each measurement method yielded different cutoff values, which may have contributed to greater heterogeneity. Moreover, even for the same measurement method (such as ELISA), reagents were purchased from different manufacturers in each study; the reagents used may have affected cutoff values, also contributing to heterogeneity. Early-onset or late-onset MPP can induce varying degrees of inflammatory responses, and thus variation in time of testing could also introduce heterogeneity. Finally, most of the included studies had relatively small sample sizes. Further studies with larger sample size are needed to reduce statistical heterogeneity in TNF-α and IFN-γ levels and improve their prognostic value as diagnostic biomarkers for MPP, as well as to make meta-analyses more valuable. Due to the limited number of included studies, we did not attempt to analyze covariates as possible sources of heterogeneity. Although significant heterogeneity was detected, sensitivity analysis showed that no single study influenced the pooled results.
In conclusion, although few studies have analyzed serum TNF-α and IFN-γ levels in pediatric MPP, our meta-analysis showed that serum TNF-α levels in children with MPP were higher than those in healthy children. However, serum IFN-γ levels were not different in children with MPP and healthy controls. Based on the varying trends of serum TNF-α and IFN-γ levels during infection by different pathogens, this finding may be useful for distinguishing pathogens in clinical settings.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Korppi M., Heiskanen-Kosma T., Kleemola M. Incidence of community- acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology. 2004;9(1):109–114. doi: 10.1111/j.1440-1843.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 2.Yan C., Sun H., Zhao H. Latest surveillance data on Mycoplasma pneumoniae infections in children, suggesting a new epidemic occurring in Beijing. Journal of Clinical Microbiology. 2016;54(5):1400–1401. doi: 10.1128/jcm.00184-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae pneumonia. Frontiers in Microbiology. 2016;7:p. 800. doi: 10.3389/fmicb.2016.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu C. Y., Chen C. J., Wong K. S., Tsai M.-H., Chiu C.-H., Huang Y.-C. Impact of bacterial and viral coinfection on mycoplasmal pneumonia in childhood community-acquired pneumonia. Journal of Microbiology, Immunology and Infection. 2015;48(1):51–56. doi: 10.1016/j.jmii.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Yang J., Hooper W. C., Phillips D. J., Talkington D. F. Cytokines in Mycoplasma pneumoniae infections. Cytokine and Growth Factor Reviews. 2004;15(2-3):157–168. doi: 10.1016/j.cytogfr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H., Koba H., Honma S., Sugaya F., Abe S. Relationships between radiological pattern and cell-mediated immune response in Mycoplasma pneumoniae pneumonia. European Respiratory Journal. 1996;9(4):669–672. doi: 10.1183/09031936.96.09040669. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Hooper W. C., Phillips D. J., Talkington D. F. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infection and Immunity. 2002;70(7):3649–3655. doi: 10.1128/iai.70.7.3649-3655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C. M., Tang R. B., Chung R. L., Hwang B. T. Tumor necrosis factor-alpha and interleukin-6 profiles in children with pneumonia. Journal of Microbiology, Immunology and Infection. 1999;32(4):233–238. [PubMed] [Google Scholar]
- 9.O’Brien D. P., Briles D. E., Szalai A. J., Tu A. H., Sanz I., Nahm M. H. Tumor necrosis factor alpha receptor i is important for survival from Streptococcus pneumoniae infections. Infection and Immunity. 1999;67(2):595–601. doi: 10.1128/iai.67.2.595-601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strieter R. M., Belperio J. A., Keane M. P. Host innate defenses in the lung: the role of cytokines. Current Opinion in Infectious Diseases. 2003;16(3):193–198. doi: 10.1097/00001432-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Bordon J. M., Fernandez-Botran R., Wiemken T. L., et al. Bacteremic pneumococcal pneumonia: clinical outcomes and preliminary results of inflammatory response. Infection. 2015;43(6):729–738. doi: 10.1007/s15010-015-0837-z. [DOI] [PubMed] [Google Scholar]
- 12.Peper R. L., Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microbial Pathogenesis. 1995;19(3):175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- 13.Narita M., Tanaka H., Abe S., Yamada S., Kubota M., Togashi T. Close association between pulmonary disease manifestation in Mycoplasma pneumonia infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon. Clinical and Vaccine Immunology. 2000;7(6):909–914. doi: 10.1128/cdli.7.6.909-914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng N. Y. Detection of TNF-a, IL-6, IL-8 levels in serum of children with Mycoplasma pneumoniae and its clinical significance. Chinese Journal of Nosocomiology. 2012;22:533–535. [Google Scholar]
- 15.Qiao H. M., Pang H. X., Zhang Y. F., et al. Changes of IL-6, IL-10 and TNF-α in children with Mycoplasma pneumoniae pneumonia. Journal of Clinical Pediatrics. 2012;1:59–61. [Google Scholar]
- 16.Finke D., Brinckmann U. G., ter Meulen V., Liebert U. G. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. Journal of Virology. 1995;69(9):5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szkaradkiewicz A., Karpiński T. M., Zeidler A., Szkaradkiewicz A. K., Masiuk H., Giedrys-Kalemba S. Cytokine response in patients with chronic infections caused by Staphylococcus aureus strains and diversification of their Agr system classes. European Journal of Clinical Microbiology and Infectious Diseases. 2012;31(10):2809–2815. doi: 10.1007/s10096-012-1633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano T., Tajiri H., Kashiwagi Y., Kimura S., Kawashima H. Cytokine and chemokine response in children with the 2009 pandemic influenza A (H1N1) virus infection. European Journal of Clinical Microbiology and Infectious Diseases. 2011;30(1):117–120. doi: 10.1007/s10096-010-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan S., Young E., Luce M. J., Reiser J., Kolls J. K., Shellito J. E. Conditional expression of interferon-gamma to enhance host responses to pulmonary bacterial infection. Pulmonary Pharmacology and Therapeutics. 2006;19(4):251–257. doi: 10.1016/j.pupt.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Fan Q., Gu T., Li P., Yan P., Chen D., Han B. Roles of T-cell immunoglobulin and mucin domain genes and toll-like receptors in wheezy children with Mycoplasma pneumoniae pneumonia. Heart, Lung and Circulation. 2016;25(12):1226–1231. doi: 10.1016/j.hlc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Li W., Liu Y. J., Zhao X. L., et al. Th1/Th2 cytokine profile and its diagnostic value in Mycoplasma pneumoniae pneumonia. Iranian Journal of Pediatrics. 2016;26(1) doi: 10.5812/ijp.3807.e3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hozo S. P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology. 2005;5(1):p. 13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Che L., Lu J., Lu J. Measurement of serum tumor necrosis factor-alpha and interleukin-8 in children with low respiratory tract infection (LRTI) caused by Mycoplasma pneumonia. Chinese Journal of Epidemiology. 1999;20:50–52. [PubMed] [Google Scholar]
- 24.Pan W., Xu Z., Zheng B. H. Serum levels of IFN-gamma and IL-4 in children with Mycoplasmal pneumonia at the acute phase. Chinese Journal of Contemporary Pediatrics. 2006;8:373–375. [PubMed] [Google Scholar]
- 25.Chen Z. R., Zhang G. B., Wang Y. Q., et al. Soluble B7-H3 elevations in hospitalized children with Mycoplasma pneumoniae, pneumonia. Diagnostic Microbiology and Infectious Disease. 2013;77(4):362–366. doi: 10.1016/j.diagmicrobio.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Wang M., Wang Y., Yan Y., et al. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. International Journal of Infectious Diseases. 2014;29:18–23. doi: 10.1016/j.ijid.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 27.He J. E., Gao C. Y. Correlation between plasma D-Dimer level and serum TNF - A levels in children with mycoplasma pneumonia. Journal of Ningxia Medical University. 2014;7:796–798. [Google Scholar]
- 28.Ye Q., Xu X. J., Shao W. X., Pan Y.-X., Chen X.-J. Mycoplasma pneumoniae infection in children is a risk factor for developing allergic diseases. The Scientific World Journal. 2014;2014:11. doi: 10.1155/2014/986527.986527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Zhou Y., Li S., Yang D., Wu X., Chen Z. The clinical characteristics and predictors of refractory Mycoplasma pneumonia pneumonia in children. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0156465.e0156465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J. M., Ye Q. Utility of assessing cytokine levels for the differential diagnosis of pneumonia in a pediatric population. Pediatric Critical Care Medicine. 2017;18(4):e162–e166. doi: 10.1097/pcc.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.01. London, UK: Cochrane Collaboration; 2011. [Google Scholar]
- 32.Neimark H. Mycoplasma and bacterial proteins resembling contractile proteins: a review. The Yale Journal of Biology and Medicine. 1983;56:419–423. [PMC free article] [PubMed] [Google Scholar]
- 33.Kang Y. M., Ding M. J., Han Y. L., et al. Th1/Th2 immune response in bronchoalveolar lavage fluid in children with severe Mycoplasma pneumoniae pneumonia. Chinese Journal of Contemporary Pediatrics. 2011;13:188–190. [PubMed] [Google Scholar]
- 34.Sun J., Cheng Y., Cai H., et al. Significance of detecting interleukin-4 and interferon-γ in sputum induction of children with mycoplasma pneumoniae pneumonia. Journal of Clinical Medicine. 2013;17(17):45–47. [Google Scholar]