Abstract
A new coumarin-based fluorescent probe, containing an allylic esters group, has been designed and synthesized for sensing cysteine in physiological pH. In this fluorescent probe, the coumarin was applied as the fluorophore and an allylic esters group was combined as both a fluorescence quencher and a recognition unit. The probe can selectively and sensitively detect cysteine (Cys) over homocysteine, glutathione, and other amino acids, and has a rapid response time of 30 min and a low detection limit of 47.7 nM. In addition, the probe could be applied for cell imaging with low cytotoxicity.
Keywords: cysteine, fluorescent probe, coumarin, bioimaging
1. Introduction
Biothiols, such as cysteine (Cys), homocysteine (Hcy), and glutathione (GSH), are natural compounds containing sulfydryl, which play crucial roles in the process of intracellular protein turnover [1]. Generally, alternations of the level of cellular biothiols lead to many diseases such as psoriasis, leucocyte loss, cardiovascular disease [2], acquired immunodeficiency syndrome (AIDS) [3], psoriasis-like skin lesions [4], lethargy, liver damage [5], muscle weakness, and wasting [6]. In addition, GSH is the vastest non-protein thiol in cells [7], serving as an important regulator of the redox reaction in vivo and maintaining intracellular redox activities [8,9], which is a key indicator of diseases such as diabetes and Alzheimer’s disease [10]. The level of Hcy has been proved to be connected with neural tube defects, Alzheimer’s disease, osteoporosis, and inflammatory bowel disease [11]. What is more, Cys is a semi-essential amino acid that plays a structural role in many proteins, and its concentration is closely linked with edema, slowed growth [12], and a wide variety of cancers including breast cancer [13], colorectal cancer [14], nasopharyngeal cancer [15], and lung cancer [16]. Therefore, it is essential to develop a reliable method for detecting biothiols [17].
Numerous assays have been carried out to detect biothiols including high performance liquid chromatography [18,19], mass spectrometry [20,21], colorimetric detection [22], electrochemical method [23,24], and capillary electrophoresis [25]. However, these studies faced a number of difficulties such as high facility cost, complicated operation, complex preparation, long detecting time, or poor selectivity. Recently, more attention had been paid to developing fluorescent probes, a cheap and easy method to distinguish biothiols [26,27,28]. For example, Tong et al. synthesized a visible blue-to-green pyrene probe, which could detect polyanion in aqueous media but cannot distinguish Cys from other biothiols [29]. Zhao et al. designed a rapid and sensitive probe for Cys, GSH, and Hcy, but it could not identify them from each other [30]. Only a few reports detected Cys from GSH and Hcy due to their similar structures containing nucleophilic mercapto groups [31,32]; thus, a new kind of fluorescent probe with steady, high selectivity and sensitivity is still needed.
Coumarin is a compound containing the parent nucleus of benzopyrone, an economic chromophore. What is more, coumarin shows high intense fluorescence, good solubility, relatively high fluorescence quantum yield, ease of production, and molar absorption coefficient if an electron donating group, such as hydroxy or unalkylated amino group, is substituted at the 7 position [33]. The fluorescence intensity of coumarins has a close relationship with the substituent groups on the ring. When the intramolecular charge transfer capability was changed by modifying substituent groups, the optical properties of the whole molecule were influenced. So, we can control the optical properties of molecules by adding different substituent groups with pushing or pulling electronics. Moreover, various mechanisms have been used in the design of Cys fluorescent probes, including the cyclization reaction with an aldehyde [34,35,36], Michael addition [37,38,39], and cleavage reaction [40,41,42] by thiols.
In this report, a turn-on fluorescence probe based on coumarins was designed for Cys with highly selectivity and sensitivity. The allylic esters group serves as a blocker of coumarin fluorophore in the probe, which not only functions as a Michael receptor, but also an electrophile [43]. Because Cys has lower steric hindrance to conduct Michael additions, the probe can sharply distinguish Cys from other biothiols within 30 min and has been successfully used in living cell imaging. With the development of fluorescent probes for the discrimination of Cys from other amino acids and biothiols, real-time monitoring of the Cys level of cells, tissues, and animals will be possible.
2. Results and Discussion
2.1. Characterization of the Probe
The synthesis of the probe is outlined in Scheme 1. Compound 1 was further treated with acryloyl chloride to form the probe. The structure of the probe was confirmed by 1H-NMR, 13C-NMR, IR, and MS spectra (Figures S1–S4). The probe was colorless and had two absorption bands centered at 278 nm and 312 nm. It showed weak fluorescence (Φ = 0.0194) due to the quencher acrylamide group.
Scheme 1.
Synthesis of the probe. Reagents and conditions: (a) trimethylamine, CH2Cl2, r.t.
2.2. UV-Vis Absorption and Fluorescence Spectra
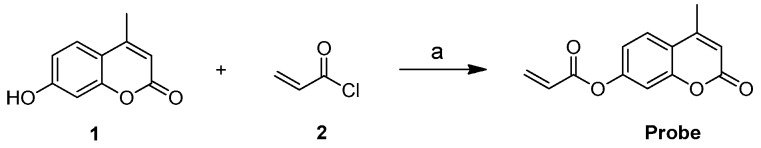
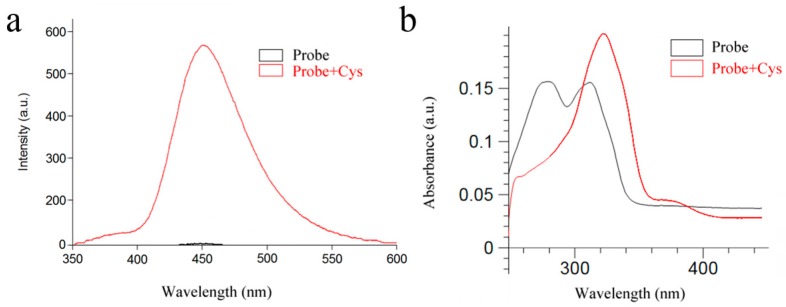
As shown in Figure 1b and Figure S5, the UV-vis absorption spectra of the free probe (10 μM) showed absorption peaks at 278 nm and 312 nm. Upon the addition of Cys, the absorption at 278 nm gradually decreased, while the absorption at 312 nm increased and red-shifted to 323 nm. Furthermore, as shown in Figure 1a, the fluorescence intensity of the probe was enhanced from Φ = 0.0194 to Φ = 0.1725 with Cys.
Figure 1.
(a) The fluorescence intensity of the probe and the probe added to Cys-buffered solution (PBS (phosphate buffered solution):DMSO (dimethyl sulfoxide) = 6:4, pH = 7.4) at room temperature; (b) The absorption spectra of the probe and the probe added to Cys in buffered solution (PBS:DMSO = 6:4, pH = 7.4) at room temperature. λex = 325 nm.
2.3. Selectivity of the Probe for Cys
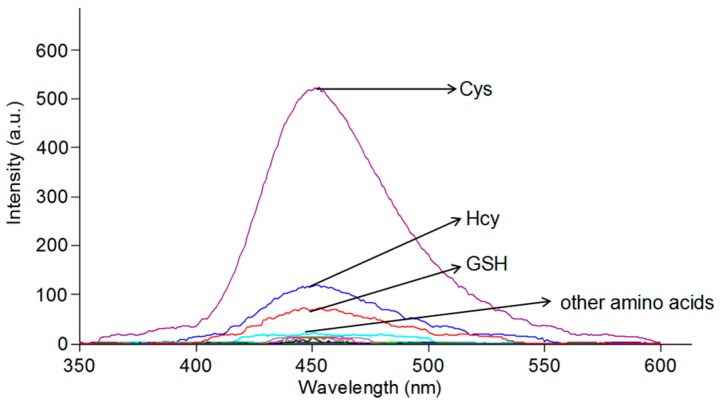
The sensing ability of the probe towards various compounds was detected, including cysteine (Cys), homocysteine (Hcy), glutathione (GSH), glycine (Gly), lysine (Lys), histidine (His), alanine (Ala), tyrosine (Try), cystine (Cys C), valine (Val), isoleucine (Ile), glutamic (Glu), phenylalanine (Phe), serine (Ser), threonine (Thr), aspartic (Asp), methionine (Met), proline (Pro), leucine (Leu), CN−, SCN−, HS−, and SO42− in buffered solution (PBS (phosphate buffered solution):DMSO (dimethyl sulfoxide) = 6:4, pH = 7.4). In Figure 2, the green fluorescence response was sensed upon the addition of Cys (50 equiv.) to the probe (10 μM), and no obvious changes in fluorescence emission were observed in other compounds. The results indicate that Cys is more active in the reaction due to its stronger nucleophilicity compared with that of GSH and Hcy.
Figure 2.
Fluorescence intensity of the probe (10 μM) in the presence of different compounds (50 equiv.); each reaction was in buffered solution (PBS:DMSO = 6:4, pH = 7.4) at room temperature. λex = 325 nm.
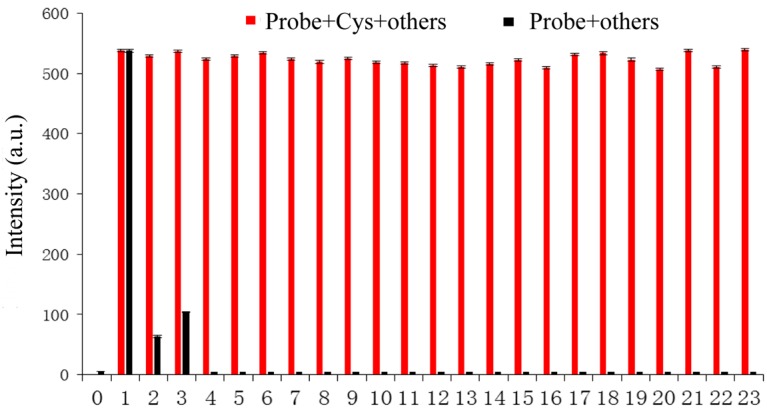
In order to ensure an accurate detection of Cys, the probe could not be obstructed by other compounds. Cys (50 equiv.) and a series of competing compounds (50 equiv.) were added to the probe (10 μM). As depicted in Figure 3, other compounds hardly had an influence on the fluorescence emission. The results showed that the probe could sharply distinguish Cys from the other compounds.
Figure 3.
Black bar represents the fluorescence response of the probe (10 μM) to various compounds (50 equiv.). Red bar represents the fluorescence response of the probe (10 μM) to Cys (50 equiv.) in the presence of other compounds (50 equiv.). Each reaction was in buffered solution (PBS:DMSO = 6:4, pH = 7.4) at room temperature. The numbers represent analytes: 0. Blank; 1. Cys; 2. Hcy; 3. GSH; 4. Asp; 5. Val; 6. Glu; 7. Pro; 8. Gly; 9. Phe; 10. Met; 11. Thr; 12. Ser; 13. Ile; 14. His; 15. Phe; 16. Lys; 17. Try; 18. Leu; 19. Cys C; 20. CN−; 21. SCN−; 22. HS−; 23. SO42−. λex = 325 nm, λem = 450 nm.
2.4. Effect of pH on the Fluorescence Response of Probe
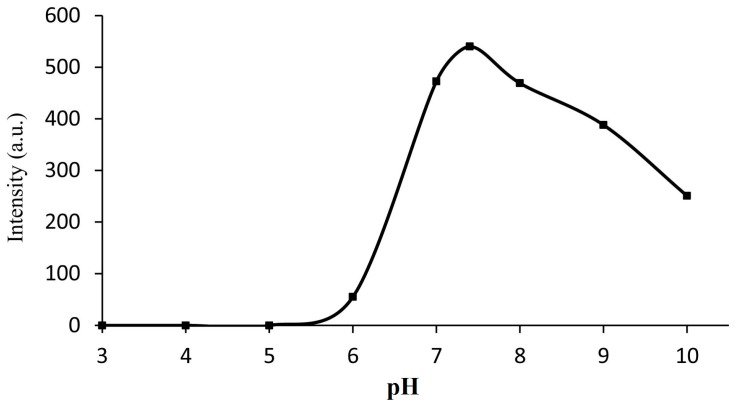
To confirm the appropriate pH scope of the probe, the fluorescence intensity of the probe (10 μM) added to Cys (50 equiv.) with different values of pH (3–10) was detected (Figure 4). The result showed that the range of pH 7–9 fitted the reaction well. Because pH 7.4 is close to physiological conditions, we selected a buffered solution at this level (PBS:DMSO = 6:4, pH = 7.4) for subsequent study.
Figure 4.
The effect of pH on the fluorescence response of the probe (10 μM) in the presence of Cys (500 μM) in buffered solution (PBS:DMSO = 6:4, pH = 3–10), pH was adjusted by NaOH and HCl. λex = 325 nm, λem = 450 nm.
2.5. Effect of Reaction Time
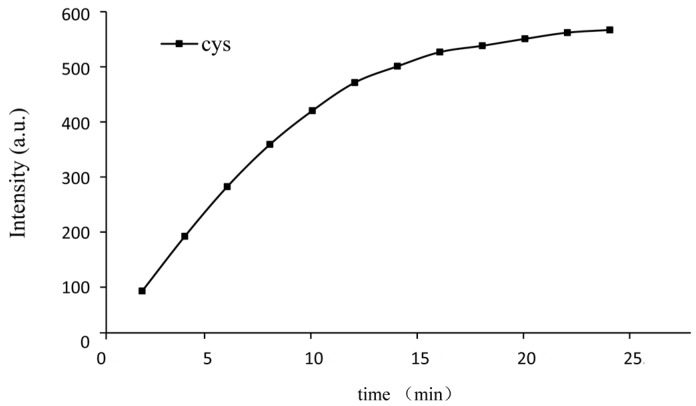
To track the proper time of the response, we traced the fluorescence intensity of the probe (10 μM) added to Cys (50 equiv.) while monitoring the time. The fluorescence intensity almost reached the maximum in 15 min (Figure 5), which was relatively fast. Furthermore, the response obeyed the pseudo-first-order rate, and the rate constant was calculated according to the following equation [44]:
| Ln ((Fmax − F) / Fmax) = −kt, | (1) |
where F is the fluorescence intensity at time t, and Fmax is the fluorescence intensity after the reaction totally completed. The constant k is shown in Figure S6. The value of the rate constant of Cys was 0.1618 min−1.
Figure 5.
Kinetic analysis of the probe towards Cys (10 μM probe with 50 equiv. of Cys) in buffer solution (DMSO:PBS = 4:6, pH = 7.4). λex = 325 nm, λem = 450 nm.
2.6. Quantitative Responses of the Probe for Cys
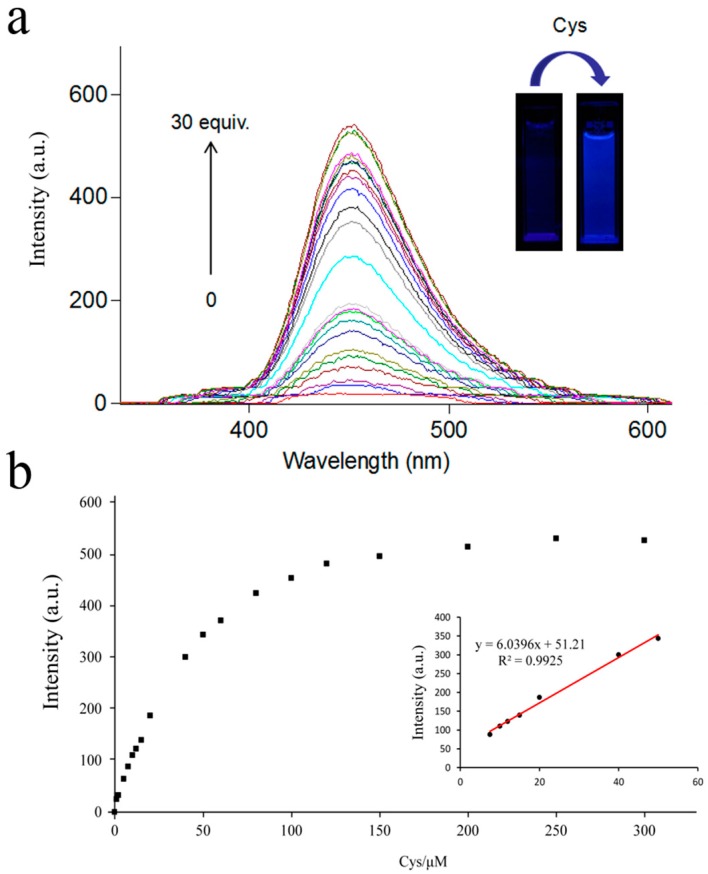
To clarify the sensitivity of the probe, various concentrations of Cys (0–30 equiv.) were added to the probe solution (10 μM) and the fluorescence intensity at 450 nm was recorded after 50 min. The results changed from nearly dark to a strong green fluorescent response as the concentration of Cys increased. Furthermore, the emission intensity at 450 nm was linearly related to the concentration of Cys from 0 to 50 μM (R2 = 0.9925) (Figure 6), which provided useful conditions for the quantitative analysis of Cys. And the detection limit of Cys was calculated to be 47.7 nM based on the 3σ/slope method [45]. The results indicated that the probe remained very sensitive to Cys.
Figure 6.
(a) Fluorescent spectral changes (b) The fluorescence intensity at 450 nm of probe (10 μM) upon addition of Cys (0–300 μM) in buffer solution (DMSO:PBS = 4:6, PH 7.4), incubated 30 min before detected. λex = 325 nm, λem = 450 nm.
As described above, the probe displayed excellent analytical properties, compared with some other fluorescent probes of recent reports, for the detection of Cys. The comparison data is listed in Table 1, indicating that the probe is promising for practical analysis.
Table 1.
Comparison of the probe for the detection of thiols.
| Reference | Response Time | Stokes Shift | Detection Limit | Selectivity for Cys |
|---|---|---|---|---|
| Sensors and Actuators B, 2017 [46] | 12 min | 122 nm | 17.1 nM | no |
| Sensors and Actuators B, 2016 [47] | 3 h | 110 nm | 192 nM | no |
| Biosensors and Bioelectronics, 2017 [48] | 20 min | 85 nm | 14 nM | yes |
| Biosensors and Bioelectronics, 2016 [49] | 30 min | 96 nm | 0.874 μM | no |
| Biosensors and Bioelectronics, 2014 [50] | 40 min | 55 nm | 0.657 μM | yes |
| ChemComm, 2013 [51] | 2 h | 97 nm | - | no |
| Molecules, 2016 [52] | 2.5 h | 174 nm | 0.911 μM | no |
| This work | 30 min | 125 nm | 47.7 nM | yes |
2.7. Reaction Mechanism
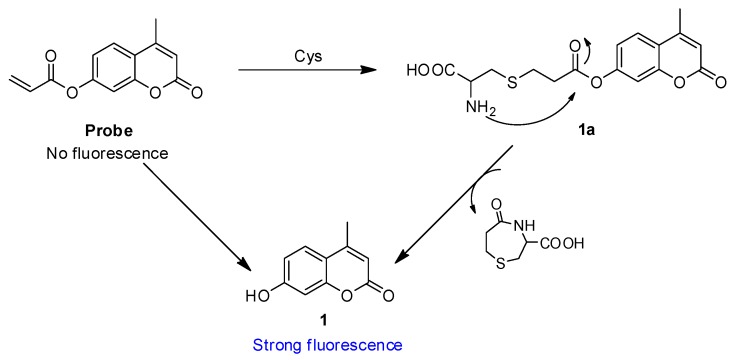
The acrylate group has been applied as a thiols reaction site for detecting thiols [53,54]. Based on the reported conjugate addition/cyclization sensing mechanism, the mechanism of probe responding to Cys involved the next two steps: the conjugate addition of Cys to an unsaturated carbonyl moiety generated thioether (1a), followed by intramolecular cyclization that gave the desired compound 1, which was responsible for the enhancement of the fluorescence intensity. To confirm the mechanism, the probe was incubated with Cys before being isolated by high performance liquid chromatography (see Figure S7 in the supporting information) and analyzed by mass spectra. In HPLC, a peak at 4.54 min corresponded to the compound 1 (coumarin) and a peak at 6.05 min corresponded to the probe. Furthermore, the probe detected compound 1 (coumarin) after being incubated with Cys for 3 h. In MS, a peak at 177.0 corresponded to the resulting compound 1 and a peak at 352.1 corresponded to the additional product compound 1a (see Figure S8 in the supporting information). These data strongly support the mechanism in Scheme 2.
Scheme 2.
Proposed reaction mechanism of the probe with Cys.
2.8. Application of the Probe
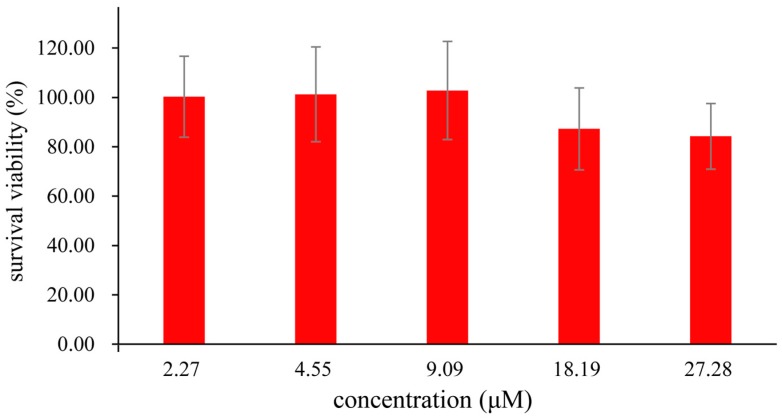
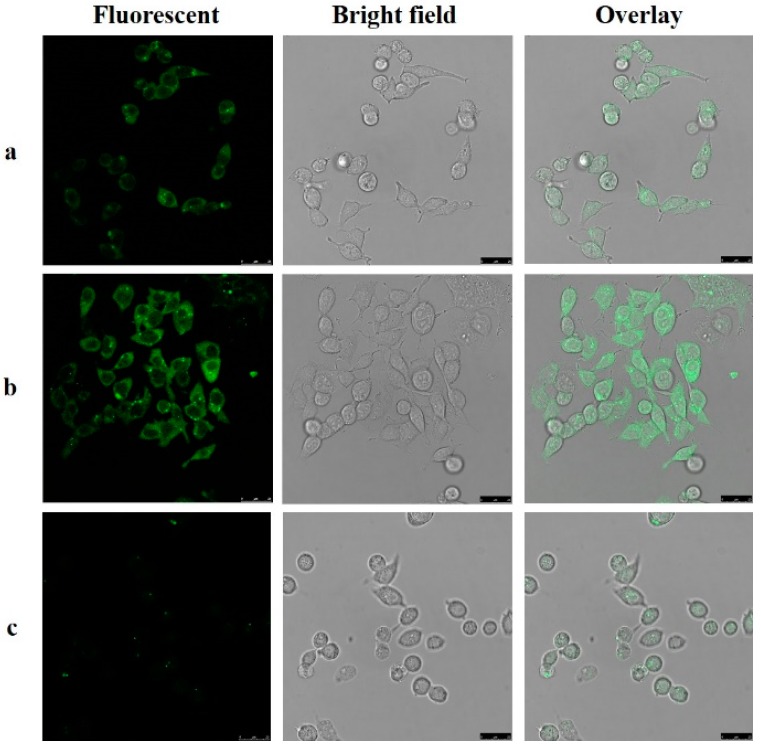
An MTS assay with a HepG2 cell line was applied to estimate the cytotoxicity of the probe. As shown in Figure 7, cellular viability was greater than 80% after 24 h in 27 μM (based on DMSO < 0.1%), which indicated that the probe had low toxicity to HepG2. To investigate the probe’s capability for cell imaging, HepG2 were incubated with the probe (25 μM) in PBS for 2 h at 37 °C and washed three times with PBS. As shown in Figure 8a, green fluorescence response could be observed inside cells under a confocal fluorescence microscope. This observation shows that the probe can detect the biothiols in the cells. Furthermore, in Figure 8b, when the cells were preincubated with Cys (100 μM) for 1 h and then incubated with the probe (25 μM), the fluorescence response was partly enhanced. In the control group, the cells were preincubated with 500 μM N-ethylmaleimide (NEM), a widely used thiol-blocking agent [55] for 1 h, then incubated with the probe (25 μM) for 2 h. As shown in Figure 8c, the fluorescence response was too slight to be detected, indicating the pertinence of Cys. The results obviously indicated that the probe could be used as a probe for both endogenously and exogenously produced Cys in living cells.
Figure 7.
MTS assay of HepG2 cells incubated in the presence of the probe (0–27 μM) at 37 °C for 24 h.
Figure 8.
Fluorescent imaging of HepG2 cells: (Left) fluorescent image; (Middle) bright field image; and (Right) overlay image. λex = 405 nm, λem = 420–480 nm. (a) Images of cells incubated with the probe (25 μM) for 2 h at 37 °C; (b) Images of cells preincubated with Cys (100 μM) for 1 h and then incubated with the probe (25 μM) for 2 h at 37 °C; (c) Images of cells preincubated with N-ethylmaleimide (NEM) (500 μM) for 1 h and then incubated with the probe (25 μM) for 2 h at 37 °C.
3. Materials and Methods
3.1. Materials and Instrumentation
All chemicals (reagent grade) used were purchased from Sino Pharm Chemical Reagent Co., Ltd. (Shanghai, China). Reaction progress was monitored using analytical thin layer chromatography (TLC) on pre-coated silica gel GF254 (Qingdao Haiyang Chemical Plant, Qingdao, China) plates, and spots were detected under UV light (254 nm). Melting point was measured on an XT-4 micromelting point instrument and uncorrected. IR (KBr-disc) spectra were recorded by a Bruker Tensor 27 spectrometer (Bruker, German). 1H-NMR and 13C-NMR spectra were measured on a BRUKER AVANCE III spectrometer at 25 °C and referenced to tetramethyl silane (TMS) (Bruker, German). Mass spectra were obtained on an MS Agilent 1100 Series LC/MSD Trap mass spectrometer (Agilent, Santa Clara, CA, USA). UV-vis spectra were recorded on an Agilent 8454 UV-vis spectrometer (Agilent). Fluorescence spectra measurements were recorded on an Agilent G9800A fluorescence spectrophotometer (Agilent). Fluorescence images were obtained on a Leica TCS-SP8 multiphoton; a confocal microscope and a 63× oil-immersion objective lens was used (Leica, Switzerland). High-resolution EI mass spectra were recorded on an Agilent 6460 triple quad LC-MS mass spectrometer (Agilent).
3.2. Synthesis of the Probe (4-Methyl-2-oxo-2H-chromen-7-yl Acrylate)
A solution of compound 1 (200 mg, 1.14 mmol) and triethylamine (0.12 mL, 0.86 mmol) in dried CH2Cl2 (10 mL) was added to acryloyl chloride (205.5 mg, 2.27 mmol) at 0 °C under nitrogen. After stirring for 12 h at room temperature, the reaction mixture was diluted with CH2Cl2. The CH2Cl2 solution was washed with a saturated aqueous Na2CO3 solution, as well as water, and then dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure. The crude product was purified by column chromatography (SiO2, ethyl acetate/hexane = 1/5, v/v) to give the probe as a pale white solid. Yield: 305 mg (80%). IR νmax/cm−1 (KBr) 1721.36, 1616.37 (C=O), 1388.53, 1164.33; m.p. 151.1–153.4 °C; ESI/MS m/z: 231.0 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.6 Hz, 1H, H5 coumarin), 7.20 (d, J = 2.2 Hz, 1H, H8 coumarin), 7.16 (dd, J = 8.6, 2.3 Hz, 1H, H6 coumarin), 6.68 (dd, J = 17.3, 1.1 Hz, 1H, H3 coumarin), 6.40–6.29 (m, 2H, H3 propionyl-), 6.11 (dd, J = 10.5, 1.1 Hz, 1H, H2 propionyl-), 2.47 (d, J = 1.2 Hz, 3H, -CH3). 13C-NMR (100 MHz, CDCl3) δ 163.8, 160.6, 154.2, 153.0, 152.0, 133.7, 127.3, 125.4, 118.1, 117.9, 114.6, 110.5, 18.7.
3.3. Absorption and Fluorescence Spectroscopy
The probe was dissolved in DMSO (1 mM) for a stock solution. The compounds such as cysteine (Cys), homocysteine (Hcy), glutathione (GSH), glycine (Gly), lysine (Lys), histidine (His), alanine (Ala), tyrosine (Try), cystine (Cys C), valine (Val), isoleucine (Ile), glutamic (Glu), phenylalanine (Phe), serine (Ser), threonine (Thr), aspartic (Asp), methionine (Met), proline (Pro), leucine (Leu), CN−, SCN−, HS−, and SO42− were all dissolved in purified water (10 mM). Tested solutions were prepared by adding 40 μL stock solution and a different analyte solution into the buffer (PBS:DMSO = 6:4, pH = 7.4) to a confirmed volume of 4 mL. The resulting solutions were mixed well and detected after 30 min at room temperature.
Fluorescent quantum yields were determined using Quinine sulphate (Фs = 0.54 in 0.1 M of H2SO4 solution) as a standard, according to a published method [56]. The fluorescent quantum yield was calculated based on the following equation:
| Φx = Φs × (Fx/Fs) × (As/Ax) × (ηx2/ηs2) | (2) |
where Φ is the fluorescent quantum yield, Φs = 0.54 in 0.1 M of H2SO4 solution, Fx and Fs are the integrated fluorescence intensities of the sample and the standard, respectively, at the same excitation wavelength, Ax and As are the absorbance at the excitation wavelength, and η is the refractive index of the respective solvent.
3.4. Cell Culture for HepG2
HepG2 cells were provided by Chinese Academy of Sciences. HepG2 cells were cultured in minimum essential medium (MEM) blended with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution, (100×) at 37 °C under an atmosphere of 5% CO2. Cells were transplanted on confocal dishes and adhered for 24 h before use in an experiment.
3.5. Fluorescence Imaging of Cys in Living Cells
Experiments to evaluate the sense ability of the probe for biothiols were executed in culture medium (90% MEM, 10% FBS). The cells were incubated in the probe (25 μM) for 2 h at 37 °C and washed with 0.1 M PBS (0.6 mL × 3) before observation. To detect exogenously produced biothiols, cells were treated with Cys (100 μM) at 37 °C for 2 h and washed with 0.1 M PBS (0.6 mL × 3). Then, the cells were incubated in the probe (25 μM) for 2 h at 37 °C and washed with 0.1 M PBS (0.6 mL × 3) before observation. For the control experiment, cells were treated with 100 μM NEM for 1 h at 37 °C and washed with 0.1 M PBS (0.6 mL × 3). Then, the cells were incubated in the probe (25 μM) for 2 h at 37 °C and washed with 0.1 M PBS (0.6 mL × 3) before observation. For the positive experiment, cells were treated with coumarin chromophore (25 μM) for 2 h at 37 °C and washed with 0.1 M PBS (0.6 mL × 3) before observation. A fluorescence microscope and a 63× oil-immersion objective lens was used. The cells were excited with UV light below 405 nm, and emission was collected at 450 ± 40 nm.
4. Conclusions
The probe showed a stable, highly selective and sensitive fluorescence response towards Cys over GSH, Hcy, and other compounds. The main mechanism of the pertinence of Cys could be attributed to the nucleophilic addition and its lower steric hindrance. Confocal fluorescence microscopy imaging using HepG2 cells indicated that the probe can be applied for the detection of Cys in living cells.
Acknowledgments
This work was supported by the programs of the National Natural Science Foundation of China (grant numbers 81403175, 81274200); Project from Shanghai Committee of Science and Technology (grant number 13401900301); Youth Talent Sail Plan from Shanghai Committee of Science and Technology (grant number 14YF1411300); Project from Shanghai Municipal Commission of Health and Family planning (grant number 20134Y053, 2017YQ072, 201740152), Research Fund for the Doctoral Program of Shanghai (grant number B201703) and project of Xinglin young talent training system.
Supplementary Materials
The supplementary materials are available online.
Author Contributions
Conceived and designed the experiments: Y.D. and T.Z. Performed research and analyzed the data: J.-S.L., R.-F.Z., X.-D.L., H.-F.L., Y.L. and Y.-J.L.; wrote the paper: J.-S.L. and R.-F.Z. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Bragoszewski P., Wasilewski M., Sakowska P., Gornicka A., Bottinger L., Qiu J., Wiedemann N., Chacinska A. Retro-translocation of mitochondrial intermembrane space proteins. Proc. Natl. Acad. Sci. USA. 2015;112:7713–7718. doi: 10.1073/pnas.1504615112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abby S.L., Harris I.M., Harris K.M. Homocysteine and cardiovascular disease. Curr. Atheroscler. Rep. 2004;6:101–106. doi: 10.3122/15572625-11-5-391. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Zhou Y., Peng X., Yoon J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010;39:2120–2135. doi: 10.1039/b925092a. [DOI] [PubMed] [Google Scholar]
- 4.Hirai T., Kanda T., Sato K., Takaishi M., Nakajima K., Yamamoto M., Kamijima R., Digiovanni J., Sano S. Cathepsin K is involved in development of psoriasis-like skin lesions through TLR-dependent Th17 activation. J. Immunol. 2013;190:4805–4811. doi: 10.4049/jimmunol.1200901. [DOI] [PubMed] [Google Scholar]
- 5.Shahrokhian S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 2001;73:5972–5978. doi: 10.1021/ac010541m. [DOI] [PubMed] [Google Scholar]
- 6.Michel L.Y.M., Hoenderop J.G.J., Bindels R.J.M. Calpain-3-mediated regulation of the Na+-Ca2+ exchanger isoform 3. Pflugers Arch. 2016;468:243–255. doi: 10.1007/s00424-015-1747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin C., Huo F., Zhang J., Martínez-Máñez R., Yang Y., Lv H., Li S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013;42:6032. doi: 10.1039/c3cs60055f. [DOI] [PubMed] [Google Scholar]
- 8.Dalton T.P., Shertzer H.G., Puga A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y., Huo F., Yin C., Zheng A., Chao J., Li Y., Nie Z., Martinez-Manez R., Liu D. Thiol-chromene click chemistry: A coumarin-based derivative and its use as regenerable thiol probe and in bioimaging applications. Biosens. Bioelectron. 2013;47:300–306. doi: 10.1016/j.bios.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Harfield J.C., Batchelor-Mcauley C., Compton R.G. Electrochemical determination of glutathione: A review. Analyst. 2012;137:2285–2296. doi: 10.1039/c2an35090d. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D’Agostino R.B., Wilson P.W., Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. ACC Curr. J. Rev. 2002;11:30. doi: 10.1016/S1062-1458(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 12.Xue S., Ding S., Zhai Q., Zhang H., Feng G. A readily available colorimetric and near-infrared fluorescent turn-on probe for rapid and selective detection of cysteine in living cells. Biosens. Bioelectron. 2015;68:316. doi: 10.1016/j.bios.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Lin W., Tang L.Y., Cen Y.L., Lin Y., Su F.X., Jia W.H., Ren Z.F. Interaction of body mass index and a polymorphism in gene of catalytic subunit of glutamate-cysteine ligase on breast cancer risk among Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34:1115. [PubMed] [Google Scholar]
- 14.Roy N., Nazeem P.A., Babu T.D., Abida P.S., Narayanankutty A., Valsalan R., Valsala P.A., Raghavamenon A.C. EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-l-Cysteine Sulfoxide. Interdiscip. Sci. 2017:1–8. doi: 10.1007/s12539-017-0227-6. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Liu X., Li C.Y., Ding Y., Chau D., Li G., Kung H.F., Lin M.C., Peng Y. Recombinant adeno-associated virus mediated RNA interference inhibits metastasis of nasopharyngeal cancer cells in vivo and in vitro by suppression of Epstein-Barr virus encoded LMP-1. Int. J. Oncol. 2006;4:595–603. doi: 10.3892/ijo.29.3.595. [DOI] [PubMed] [Google Scholar]
- 16.Geng P., Yao J., Zhu Y. Hogg1 Ser326Cys polymorphism and lung cancer susceptibility: A meta-analysis. Mol. Biol. Rep. 2014;41:2299. doi: 10.1007/s11033-014-3083-z. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.H., Tsai J.C., Cheng T.H., Yuan S.S., Wang Y.M. Sensitivity evaluation of NBD-SCN towards cysteine/homocysteine and its bioimaging applications. Biosens. Bioelectron. 2014;56:117–123. doi: 10.1016/j.bios.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Guo X.F., Zhu H., Wang H., Zhang H.S. Determination of thiol compounds by HPLC and fluorescence detection with 1,3,5,7-tetramethyl-8-bromomethyl-difluoroboradiaza-S-indacene. J. Sep. Sci. 2013;36:658–664. doi: 10.1002/jssc.201200936. [DOI] [PubMed] [Google Scholar]
- 19.Kono Y., Iizuka H., Isokawa M., Tsunoda M., Ichiba H., Sadamoto K., Fukushima T. HPLC-fluorescence determination of thiol compounds in the serum of human male and female subjects using HILIC-mode column. Biomed. Chromatogr. 2014;28:589–593. doi: 10.1002/bmc.3086. [DOI] [PubMed] [Google Scholar]
- 20.Capehart S.L., Carlson E.E. Mass spectrometry-based assay for the rapid detection of thiol-containing natural products. Chem. Commun. 2016;52:13229. doi: 10.1039/C6CC07111B. [DOI] [PubMed] [Google Scholar]
- 21.García-Santamarina S., Boronat S., Domènech A., Ayté J., Molina H., Hidalgo E. Monitoring in vivo reversible cysteine oxidation in proteins using ICAT and mass spectrometry. Nat. Protoc. 2014;9:1131–1145. doi: 10.1038/nprot.2014.065. [DOI] [PubMed] [Google Scholar]
- 22.Huo F.-J., Sun Y.-Q., Su J., Chao J.-B., Zhi H.-J., Yin C.-X. Colorimetric detection of thiols using a chromene molecule. Org. Lett. 2009;11:4918–4921. doi: 10.1021/ol901951h. [DOI] [PubMed] [Google Scholar]
- 23.Kruusma J., Benham A.M., Williams J.A., Kataky R. An introduction to thiol redox proteins in the endoplasmic reticulum and a review of current electrochemical methods of detection of thiols. Analyst. 2006;131:459–473. doi: 10.1039/b515874e. [DOI] [PubMed] [Google Scholar]
- 24.Vandeberg P.J., Johnson D.C. Pulsed electrochemical detection of cysteine, cystine, methionine, and glutathione at gold electrodes following their separation by liquid chromatography. Anal. Chem. 2002;65:2713–2718. doi: 10.1021/ac00068a002. [DOI] [Google Scholar]
- 25.Chen G., Zhang L., Wang J. Miniaturized capillary electrophoresis system with a carbon nanotube microelectrode for rapid separation and detection of thiols. Talanta. 2004;64:1018–1023. doi: 10.1016/j.talanta.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z., Nam S.W., Park S., Yoon J. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging. Chem. Sci. 2012;3:2760–2765. doi: 10.1039/c2sc20540h. [DOI] [Google Scholar]
- 27.Yuan L., Lin W., Zhao S., Gao W., Chen B., He L., Zhu S. A unique approach to development of near-infrared fluorescent sensors for in vivo imaging. J. Am. Chem. Soc. 2012;134:13510. doi: 10.1021/ja305802v. [DOI] [PubMed] [Google Scholar]
- 28.Tang B., Xing Y., Li P., Zhang N., Yu F., Yang G. A rhodamine-based fluorescent probe containing a Se-N bond for detecting thiols and its application in living cells. J. Am. Chem. Soc. 2007;129:11666–11667. doi: 10.1021/ja072572q. [DOI] [PubMed] [Google Scholar]
- 29.Tong D., Duan H., Wang J., Yang Z., Lin Y. Aggregation-enhanced excimer emission (AEEE) based on pyrenylchalcone and 2-to-4 molecular decoder by biothiols and polyanions in aqueous media. Sens. Actuators B Chem. 2014;195:80–84. doi: 10.1016/j.snb.2014.01.003. [DOI] [Google Scholar]
- 30.Zhao W., Sun M., Lei T., Liu X., Zhang Q., Zong C. An indicator-displacement assay based on the Murexide-Hg2+ system for fluorescence turn-on detection of biothiols in biological fluids. Sens. Actuators B Chem. 2017;249:90–95. doi: 10.1016/j.snb.2017.04.017. [DOI] [Google Scholar]
- 31.Mei J., Wang Y., Tong J., Wang J., Qin A., Sun J.Z., Tang B.Z. Discriminatory detection of cysteine and homocysteine based on dialdehyde-functionalized aggregation-induced emission fluorophores. Chemistry. 2013;19:613–620. doi: 10.1002/chem.201202969. [DOI] [PubMed] [Google Scholar]
- 32.Song L., Sun Q., Wang N., Chen Z., Zhang W., Qian J. A fluorescent probe for the discrimination between Cys and GSH. Anal. Methods. 2015;7:10371–10375. doi: 10.1039/C5AY02354H. [DOI] [Google Scholar]
- 33.Li J., Zhang C.F., Yang S.H., Yang W.C., Yang G.F. A coumarin-based fluorescent probe for selective and sensitive detection of thiophenols and its application. Anal. Chem. 2014;86:3037–3042. doi: 10.1021/ac403885n. [DOI] [PubMed] [Google Scholar]
- 34.Lin W., Long L., Yuan L., Cao Z., Chen B., Tan W. A ratiometric fluorescent probe for cysteine and homocysteine displaying a large emission shift. Org. Lett. 2008;10:5577. doi: 10.1021/ol802436j. [DOI] [PubMed] [Google Scholar]
- 35.Kim T.K., Lee D.N., Kim H.J. Highly selective fluorescent sensor for homocysteine and cysteine. Tetrahedron Lett. 2008;49:4879–4881. doi: 10.1016/j.tetlet.2008.06.003. [DOI] [Google Scholar]
- 36.Lee K.S., Kim T.K., Lee J.H., Kim H.J., Hong J.I. Fluorescence turn-on probe for homocysteine and cysteine in water. Chem. Commun. 2008;46:6173. doi: 10.1039/b814581d. [DOI] [PubMed] [Google Scholar]
- 37.Jung H.S., Pradhan T., Han J.H., Heo K.J., Lee J.H., Kang C., Kim J.S. Molecular modulated cysteine-selective fluorescent probe. Biomaterials. 2012;33:8495–8502. doi: 10.1016/j.biomaterials.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Jung H.S., Han J.H., Pradhan T., Kim S., Lee S.W., Sessler J.L., Kim T.W., Kang C., Kim J.S. A cysteine-selective fluorescent probe for the cellular detection of cysteine. Biomaterials. 2012;33:945–953. doi: 10.1016/j.biomaterials.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Huo F.J., Sun Y.Q., Su J., Yang Y.T., Yin C.X., Chao J.B. Chromene “lock”, thiol “key”, and mercury(II) ion “hand”: A single molecular machine recognition system. Org. Lett. 2010;12:4756–4759. doi: 10.1021/ol101771j. [DOI] [PubMed] [Google Scholar]
- 40.Pires M.M., Hrycyna C.A., Chmielewski J. Bivalent probes of the human multidrug transporter P-glycoprotein. Biochemistry. 2006;45:11695–11702. doi: 10.1021/bi0608109. [DOI] [PubMed] [Google Scholar]
- 41.Maeda H., Matsuno H., Ushida M., Katayama K., Saeki K., Itoh N. 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman’s reagent in thiol-quantification enzyme assays. Angew. Chem. 2005;44:2922–2925. doi: 10.1002/anie.200500114. [DOI] [PubMed] [Google Scholar]
- 42.Jiang W., Fu Q., Fan H., Ho J., Wang W. A highly selective fluorescent probe for thiophenols. Angew. Chem. 2007;46:8445–8448. doi: 10.1002/anie.200702271. [DOI] [PubMed] [Google Scholar]
- 43.Yang X., Guo Y., Strongin R.M. Conjugate addition/cyclization sequence enables selective and simultaneous fluorescence detection of cysteine and homocysteine. Angew. Chem. 2011;50:10690–10693. doi: 10.1002/anie.201103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma W.-W., Wang M.-Y., Yin D., Zhang X. Facile preparation of naphthol AS-based fluorescent probe for highly selective detection of cysteine in aqueous solution and its imaging application in living cells. Sens. Actuators B Chem. 2017;248:332–337. doi: 10.1016/j.snb.2017.03.169. [DOI] [Google Scholar]
- 45.Liu Y., Xiang K., Tian B., Zhang J. A fluorescein-based fluorescence probe for the fast detection of thiol. Tetrahedron Lett. 2016;57:2478–2483. doi: 10.1016/j.tetlet.2016.04.068. [DOI] [Google Scholar]
- 46.Dai X., Zhang T., Liu Y.Z., Yan T., Li Y., Miao J.Y., Zhao B.X. A ratiometric fluorescent probe for cysteine and its application in living cells. Sens. Actuators B Chem. 2015;207:872–877. doi: 10.1016/j.snb.2014.10.082. [DOI] [Google Scholar]
- 47.Liao Y.C., Venkatesan P., Wei L.F., Wu S.P. A coumarin-based fluorescent probe for thiols and its application in cell imaging. Sens. Actuators B Chem. 2016;232:732–737. doi: 10.1016/j.snb.2016.04.027. [DOI] [Google Scholar]
- 48.Chen Z., Sun Q., Yao Y., Fan X., Zhang W., Qian J. Highly sensitive detection of cysteine over glutathione and homo-cysteine: New insight into the Michael addition of mercapto group to maleimide. Biosens. Bioelectron. 2017;91:553–559. doi: 10.1016/j.bios.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Chen C., Liu W., Xu C., Liu W. A colorimetric and fluorescent probe for detecting intracellular biothiols. Biosens. Bioelectron. 2016;85:46–52. doi: 10.1016/j.bios.2016.04.098. [DOI] [PubMed] [Google Scholar]
- 50.Xu C., Li H., Yin B. A colorimetric and ratiometric fluorescent probe for selective detection and cellular imaging of glutathione. Biosens. Bioelectron. 2015;72:275–281. doi: 10.1016/j.bios.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Wei M., Yin P., Shen Y., Zhang L., Deng J., Xue S., Li H., Guo B., Zhang Y., Yao S. A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem. Commun. 2013;49:4640–4642. doi: 10.1039/c3cc39045d. [DOI] [PubMed] [Google Scholar]
- 52.Na R., Zhu M., Fan S., Wang Z., Wu X., Tang J., Liu J., Wang Y., Hua R. A simple and effective ratiometric fluorescent probe for the selective detection of cysteine and homocysteine in aqueous media. Molecules. 2016;21:1023. doi: 10.3390/molecules21081023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei L.F., Thirumalaivasan N., Liao Y.C., Wu S.P. Fluorescent coumarin-based probe for cysteine and homocysteine with live cell application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017;183:204–208. doi: 10.1016/j.saa.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 54.Yang J., Yu Y., Wang B., Jiang Y. A sensitive fluorescent probe based on coumarin for detection of cysteine in living cells. J. Photochem. Photobiol. A Chem. 2017;338:178–182. doi: 10.1016/j.jphotochem.2017.02.008. [DOI] [Google Scholar]
- 55.Wang P., Wang Y., Li N., Huang J., Wang Q., Gu Y. A novel DCM-NBD conjugate fluorescent probe for discrimination of Cys/Hcy from GSH and its bioimaging applications in living cells and animals. Sens. Actuators B Chem. 2017;245:297–304. doi: 10.1016/j.snb.2017.01.127. [DOI] [Google Scholar]
- 56.Nagaraja D., Melavanki R.M., Patil N.R., Kusanur R.A. Solvent effect on the relative quantum yield and fluorescence quenching of 2DAM. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;130:122–128. doi: 10.1016/j.saa.2014.03.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.