Abstract
Extensive regional droughts are already a major problem on all inhabited continents and severe regional droughts are expected to become an increasing and extended problem in the future. Consequently, extended use of available drought resistant food plants should be encouraged. Bromelia laciniosa, Neoglaziovia variegata and Encholirium spectabile are excellent candidates in that respect because they are established drought resistant edible plants from the semi-arid Caatinga region. From a food safety perspective, increased utilization of these plants would necessitate detailed knowledge about their chemical constituents. However, their chemical compositions have previously not been determined. For the first time, the non-polar constituents of B. laciniosa, N. variegata and E. spectabile have been identified. This is the first thorough report on natural products from N. variegata, E. spectabile, and B. laciniosa. Altogether, 20 non-polar natural products were characterized. The identifications were based on hyphenated gas chromatography-high resolution mass spectrometry (GC-HRMS) and supported by 1D and 2D Nuclear Magnetic Resonance (NMR) plant metabolomics.
Keywords: Bromelia laciniosa, Neoglaziovia variegata, Encholirium spectabile, nonpolar natural products, Hyphenated GC-HRMS, 2D NMR plant metabolomics
1. Introduction
1.1. Bromeliaceae
Several recent severe regional droughts have led to increased interest in exploiting drought resistant edible plants as human food sources and as forage for domesticated animals. Several drought-tolerant plants already utilized for such purposes belong to the Bromeliaceae and display features that make them especially capable of retaining water; the leaves are shaped adaxially concave to channel rainwater down to the overlapping, rosulate base for storage in a central cavity. The surface of the leaves bears absorptive, scale-like trichomes that take up water and nutrients. Bromeliaceae also have a Crassulacean acid metabolism (CAM) photosynthesis, where the stomata remain shut during daytime to avoid evaporation. The Bromeliaceae is a large family of flowering plants within the Monocots, which contains 58 genera and approximately 3200 species. Except for one western African species, all Bromeliaceae are endemic to the American tropics. The family is currently divided into eight subfamilies [1], and the two species, Bromelia laciniosa Mart. ex Schult. & Schult. f. (Figure 1) and Neoglaziovia variegata (Arruda) Mez. (Figure 1), which belong to the subfamily Bromelioideae, and Encholirium spectabile Mart. ex Schult. & Schult. f (Figure 1),which belongs to the Pitcairnioideae subfamily. Bromeliads are used for alimentation (e.g., fruit production of Ananas comosus (L.) Merr., as fiber plants, and cultivated for ornamental and medicinal purposes.
Figure 1.
Bromelia laciniosa (left); Neoglaziovia variegata (middle); and Encholirium spectabile (right) grown in Petrolina, Pernambuco, Brazil. Photos: JRGS Almeida.
1.2. B. laciniosa
B. laciniosa (Portuguese: macambira de porco) is native to Brazil and Argentina. Leaves from B. laciniosa are rich in proteins (4.9%), starch (2.8%) and calcium (1.1%) [2], and are therefore used in alimentation of both humans and domestic animals in northeastern Brazil [3,4]. Farmers in this region use the leaves as supplementary fodder for livestock [3,5,6]. The leaves are dried, powdered and mixed into the local cuisine [7]. A type of bread can be made from masses extracted from the base of the leaves [3]. Flowers, fruit and leaves from B. laciniosa are used in the treatment of infantile colic, diarrhea, fever, jaundice, hepatitis, and dandruff [8]. An aqueous root extract may be drunk as a treatment against intestinal diseases and hepatitis, and as a diuretic [7]. Only limited information is available which could rationalize these medicinal applications. Gum from B. laciniosa has been shown to contain galactose, arabinose and xylose, and an acidic oligosaccharide composed of xylose and galacturonic acid [9]. Quercetin 3,3′,4′-trimethyl ether is the only natural product isolated and characterized from B. laciniosa [10].
1.3. N. variegata
N. variegata (Portuguese: caroá) is one of only three species in the genus Neoglaziovia, all of which are endemic to northeastern Brazil. At the beginning of the rainy season, N. variegata produces edible fleshy fruits [11]. The plant was first described by the Brazilian Manuel Arruda da Câmara (1752–1810), while the genus is named after the French botanist Auguste Francois Marie Glaziou (1828–1906). N. variegata is used as a fiber plant by rural communities in the Caatinga region where a variety of products are made from the white, soft and flexible fibers [12,13,14,15]. Ethanol extracts of N. variegata have been reported to be of low toxicity [16] , and to exhibit antinociceptive effect in experimental models in mice [16], photoprotective potential, antioxidant effect [13,16], gastroprotective effects in a mice model of gastric ulcer [17] and antibacterial effect against both Gram-positive [13] and Gram-negative bacteria [13,18]. There is no report of natural products characterized from N. variegata.
1.4. E. spectabile
E. spectabile (Portuguese: macambira de flexa or macambira de pedra) is one of twenty five species in this genus, which is endemic to Brazil. This species is used as a supplementary food supply in famine emergencies by rural communities in the semi-arid Caatinga region [19]. The edible part of E. spectabile is the leaf base, which is rich in carbohydrates (28.7%), and contains some proteins (0.7%) and lipids (0.8%) [19]. Flour made from the dried leaves is used to prepare a somewhat bitter tasting couscous. The nutritional value of E. spectabile (124.6 kcal/100 g) is in the same range as commercial grains such as rice (Oryza sativa L.) with 130 kcal/100 g [19]. Extracts have exhibited no signs of toxicity towards mice [20]; have been reported to exhibit antioxidant [21,22], photoprotective [22], and anti-nociceptive activity in mice models [20]; gastroprotective activity in a mice model of gastric ulcer [23]; and antibacterial activity towards Gram-negative [18,21] and Gram-positive bacteria [21]. No natural products have been characterized from this species.
Very little authoritative information is available about natural products from Bromelia laciniosa, and there is absolutely no previous information about Encholirium spectabile and Neoglaziovia variegata. In this paper, we report for the first time on the natural products characterized from hexane extracts of Bromelia laciniosa, Encholirium spectabile and Neoglaziovia variegata.
2. Results and Discussion
As part of our ongoing work on the characterization of natural products from food and medicinal plants aimed at rationalizing the molecular basis of their applications, the constituents of non-polar extracts of B. laciniosa, N. variegata and E. spectabile have been characterized. All identifications were based on hyphenated GC-HRMS. Altogether, 20 compounds were for the first time identified in the chromatograms of the hydrophobic crude extracts of B. laciniosa, N. variegata and E. spectabile (Table 1 and Figure 2, Figure 3 and Figure 4). Compounds of each class are treated in separate paragraphs below.
Table 1.
Compounds identified from hexane extracts of leaves of B. laciniosa, N. variegata and E. spectabile.
| Nr. | Compounds According to Group | MF | Exact Mass * | Retention Time (min) | Content (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | B. L. | N. V. | E.S. | B.L. | N.V. | E.S. | |||
| Fatty acids and their derivatives | ||||||||||
| 1 | n-Hexadecanoic acid (Palmitic acid) | C16H32O2 | 256.24110 | 256.24023 | 57.52 | 57.30 | 57.38 | 11.3 | 7.4 | 6.1 |
| 2 | Octadeca-(9,12)dienoic acid | C18H32O2 | 280.24398 | 280.24023 | 64.38 | 3.1 | ||||
| 3 | (9Z)-Octadec-9-enoic acid (Oleic acid) | C18H34O2 C18H32O a |
n.a. 264.2434 |
264.24532 |
65.10 | 64.86 | 64.75 | 8.8 | 4.5 | 1.7 |
| 4 | Octadecanoic acid (Stearic acid) | C18H36O2 | 284.27187 | 284.27153 | 66.05 | 65.88 | 65.88 | 3.2 | 1.4 | 1.1 |
| Alkanes | ||||||||||
| 5 | n-Pentacosane | C25H52 | 79.41 | 0.8 | ||||||
| 6 | n-Hexacosane | C26H54 | 83.03 | 1.1 | ||||||
| 7 | n-Heptacosane | C27H56 | 86.54 | 5.7 | ||||||
| 8 | n-Octacosane | C28H58 | 89.90 | 1.4 | ||||||
| 9 | n-Nonacosane | C29H60 | 93.16 | 93.14 | 93.18 | 2.0 | 1.0 | 9.3 | ||
| 10 | n-Triacontane | C30H62 | 99.36 | 99.34 | 99.35 | 18.9 | 13.6 | 3.5 | ||
| Vitamins | ||||||||||
| 11 | β-Tocopherol | C28H48O2 | 416.36392 | 416.36543 | 96.67 | 4.9 | ||||
| 12 | α-Tocopherol | C29H50O2 | 430.38089 | 430.38108 | 99.60 | 99.56 | 99.52 | 1.8 | 1.5 | 0.9 |
| Phytol | ||||||||||
| 13 | (2E,7R,11R)-3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) | C20H40O1 | 296.30982 | 296.30791 | 100.76 | 16.1 | ||||
| Triterpenoids and derivatives | ||||||||||
| 14 | Stigmastan-3-one | C29H50O1 | 414.38688 | 414.38616 | 90.62 | 2.6 | ||||
| 15 | Campesterol ((3β,24R)-Ergost-5-en-3-ol) | C27H44O2 | 400.33890 | 400.33413 | 102.33 | 102.33 | 102.37 | 4.5 | 3.9 | 9.4 |
| 16 | Ergostanol | C28H50O1 | 402.38723 | 402.38616 | 102.60 | 4.5 | ||||
| 17 | Stigmasta-4,22-dien-3-β-ol | C29H48O1 | 412.37123 | 412.37051 | 103.24 | 103.24 | 103.25 | 0.7 | 2.0 | 3.1 |
| 18 | β-Sitosterol | C29H50O1 | 414.38655 | 414.38616 | 105.18 | 105.14 | 105.16 | 25.8 | 19.8 | 17.8 |
| 19 | Stigmastanol | C29H52O1 | 416.40276 | 416.40181 | 105.42 | 17.6 | ||||
| 20 | 24-Methyl-3-β-9,19-cyclolanost-24-en-3-ol | C31H52O1 | 440.40234 | 440.40181 | 108.67 | 3.2 | ||||
| Unidentified | 23.0 | 14.7 | 16.3 | |||||||
Abbreviations: B. L. = B. laciniosa; N. V. = N. variegata; E. S. = E. spectabile; n.d. = not detected; MF = Molecular Formula. a Only the pseudomolecular ion [M − H2O]+ observed for this compound. * All exact masses are calculated for lowest monoisotopic mass.
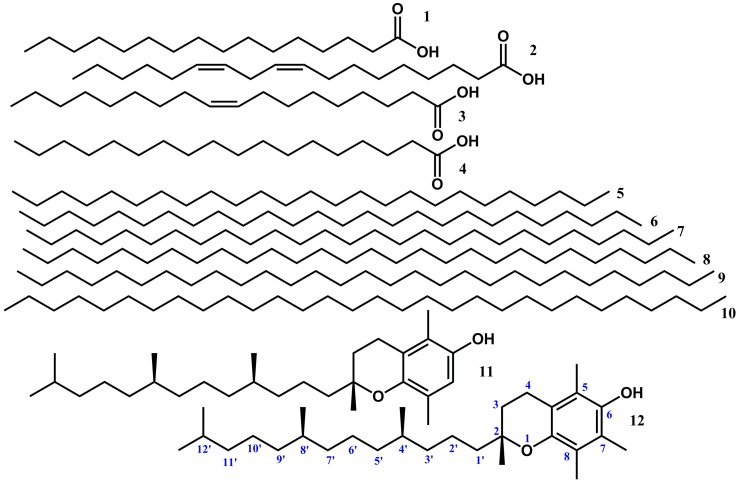
Figure 2.
Structures of non-polar compounds identified from the leaves of B. laciniosa, N. variegata and E. spectabile. 1 n-Hexadecanoic acid (Palmitic acid); 2 9Z,12Z-Octadecadienoic acid; 3 9Z-Octadecenoic acid (Oleic acid); 4 Octadecanoic acid (Stearic acid); 5 n-Pentacosane; 6 n-Hexacosane; 7 n-Heptacosane; 8 n-Octacosane; 9 n-Nonacosane; 10 n-Triacontane; 11 β-Tocopherol; and 12 α-Tocopherol (Vitamin E).
Figure 3.
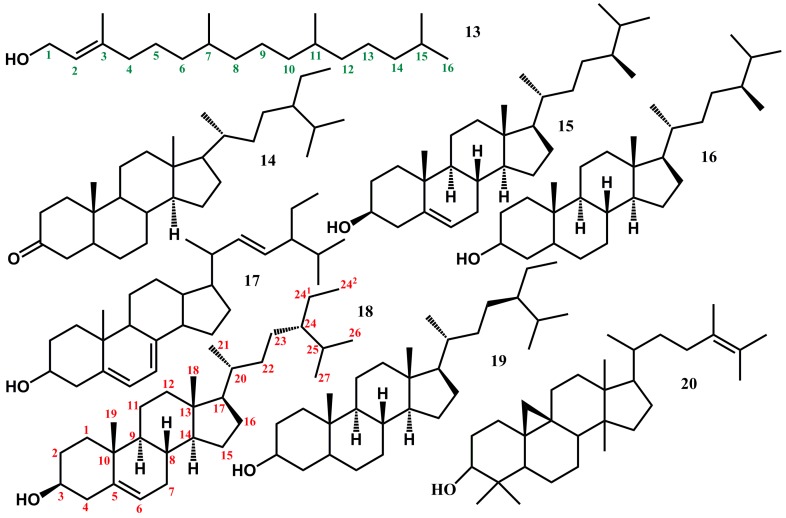
Structures of non-polar compounds identified from the leaves of B. laciniosa, N. variegata and E. spectabile. 13 Phytol; 14 Stigmastan-3-one; 15 Campesterol; 16 Ergostanol; 17 Stigmasta-4,22-dien-3-β-ol; 18 β-Sitosterol; 19 Stigmastanol; and 20 24-Methyl-3-β -9,19-cyclolanost-24-en-3-ol.
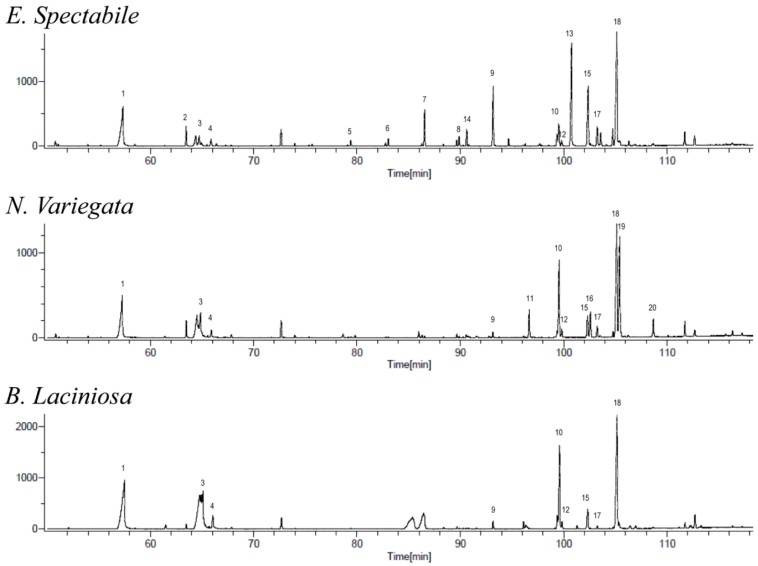
Figure 4.
Comparison of TIC for B. laciniosa, N. variegata and E. spectabile.
2.1. Fatty Acids and Their Derivatives
Four fatty acids, palmitic acid, oleic acid, stearic acid and (9,12)-octadecadienoic acid, were identified from all three investigated species (Figure 3). These fatty acids are common phytochemical constituents from several plant species with significant nutritional value including wheat [24].
2.2. Very Long-Chain Alkanes (VLCA)
Very long-chain alkanes (VLCA) are considered to be of interest from a dietary point of view because their long-chain alcohol metabolites may contribute to the cholesterol-lowering effect associated with the intake of plant waxes [25]. The efficiency with which alkanes might contribute to a cholesterol-lowering effect of waxes is regulated by limited absorption and the need for hydroxylation of these compounds. Conversion to long-chain alcohols in vivo is achieved by the action of different cytochrome P450 enzymes, which hydroxylate alkanes at several positions [26]. Altogether, six very long-chain n-alkanes were identified (Figure 3). The identifications were initially based on the fragmentation pattern observed in the mass spectra. To further support these identifications, a standard solution containing alkanes with chain lengths varying from C9–C40 was co-chromatographed with the samples. The results confirmed the presence and identities of several long-chain alkanes. The six n-alkanes identified in this work have a chain length ranging from C25 to C30. E. spectabile differs from the two other species since the crude extract from this species contain all six n-alkanes from n-pentacosane (C25) to n- triacontane (C30). Meanwhile, only the two longest n-alkanes, n-nonacosane (C29) and n-triacontane (C30), were identified from the crude extracts from B. laciniosa and N. variegata.
The quantitative amount of the two n-alkanes identified from all of the species seems to be largest in the crude extract from E. spectabile while the amount in the crude extracts from B. laciniosa and N. variegata was much lower and at a comparable level. Seven alkanes with considerably shorter chain lengths, ranging C12–C18, have previously been identified from Bromeliaceae spp. [27]. Of particular importance may be that the chain lengths of the alkanes identified in B. laciniosa, N. variegata and E. spectabile were considerably longer than for those that have previously been reported to occur in Bromeliaceae species.
2.3. Vitamins
α-Tocopherol was present in all three investigated species (Figure 2). The discovery of the presence of an active form of vitamin E in the crude extracts from B. laciniosa and E. spectabile underlines the nutritional value of these plants as food for both humans and animals. Previously, α-tocopherol has not been identified from any plant source belonging to genus Bromelia. However, the vitamin has been found in the distant relative A. erectifolius belonging to genus Ananas in Bromeliaceae [28]. Moreover, another vitamin E, namely β-tocopherol (Figure 2), was identified in the crude extract of N. variegata.
2.4. Other Compounds
The relatively common natural product phytol (Figure 3) was identified in the crude extract from E. spectabile. According to Vetter et al. (2012), the presence of phytol in human food is mainly restricted to spinach, beans, raw vegetables, and asparagus [29,30]. It may be mentioned that intake of food plants containing free phytol should be restricted for individuals suffering from Refsum’s disease [30].
2.5. Triterpenoids and Derivatives Therefrom
Altogether, seven triterpenoids were identified from the investigated Bromeliaceae species (Figure 4). While all seven were detected in N. variegata, only three (Compounds 15, 17 and 18) were identified in B. laciniosa and E. spectabile. With twice as many identified triterpenoids, N. variegata differs markedly from the two other species (Table 1). Five of the seven triterpenoids have previously been identified from other species of the family Bromeliaceae (Compounds 15–19). The two triterpenoids stigmastan-3-one (Compound 14) and 24-methyl-β-9,19-cyclolanost-24-en-3-ol (Compound 20) are identified from species of the family Bromeliaceae for the first time. 24-Methyl-β-9,19-cyclolanost-24-en-3-ol (Compound 20) is also known as 24-methyl-cycloartenol. Campesterol (Compound 15), ergostanol (Compound 16), stigmasta-4,22-dien-3-β-ol (Compound 17), β-sitosterol (Compound 18) and stigmastanol (Compound 19) have all been detected previously from species of the family Bromeliaceae. Although stigmastan-3-one (Compound 14) has not been identified from Bromeliaceae previously the unsaturated form stigmast-4-en-3-one has previously been identified from Ananas erectofolius [28]. Campesterol (Compound 15) is a common phytosterol found in many edible plants. It is therefore unsurprising that campesterol has been identified from six Bromeliaceae species. The species are A. comosus [31], A. erectofolius [28], Tillandsia fasciculata [32], Tillandsia pohliana (T. pohliana has been examined by Caiado and co-workers in an unpublished work according to Manetti et al. [33]), Tillandsia streptocarpa [34] and T. usneoides [35]. Ergostanol (Compound 16) has previously been identified from the two species A. comosus [31] and A. erectofolius [28] from the family Bromeliaceae. Stigmasta-4,22-dien-3-β-ol (Compound 17) is an unsaturated derivative of stigmastanol, which is known to inhibit absorption of cholesterol from the diet. Stigmasta-4,22-dien-3-β-ol has previously been identified from the three species T. fasciculata [32], T. streptocarpa [34] and T. usneoides [35]. All three species belongs to Tillandsia, a genus of the Bromeliaceae family. β-Sitosterol (Compound 18) is the major compound from the non-polar (hydrophobic) extracts in all three investigated species. β-Sitosterol (Compound 18) is a phytosterol with wide distribution throughout the plant kingdom including several plants used for human nutrition. Several dietary plant sterols including β-sitosterol exhibit significant cholesterol-lowering effects [36,37,38]. In the Bromeliaceae family β-sitosterol has been identified from the eight species: A. comosus [31,39] commonly known as pineapple, A. erectofolius [28], Hechtia rosea [40], H. scariosa [40], T. fasciculata [32], T. pohliana [33], T. streptocarpa [34], T. usneoides [35,41]. Stigmastanol (Compound 19) is previously known from two species of the Bromeliaceae family, namely A. comosus [31] and A. erectofolius [28]. Stigmastanol is also known as sitostanol and is a phytosterol commonly found in many edible plants. 9,19-Cyclolanost-24-en-3-ol-3-β (Compound 20) is not previously identified from species of the Bromeliaceae family. However, similar cycloartanol triterpenoids such as cyclolaudenol [32] and 24-methylenecycloartanol [35,42] are commonly found in Bromeliaceae species [33]. Identification of phytosterols with documented potential beneficial health effects, such as campesterol and β-sitosterol, from the leaves of B. laciniosa and E. spectabile strengthens the nutritional value of these plants.
2.6. NMR Plant Metabolomics
Even though GC-HRMS is an excellent method for characterizing mixtures of natural products, there are some limitations in connection with the application of this method. Some compounds may avoid detection because they are either not sufficiently volatile or insufficiently ionized. To further support the identifications of natural products achieved by GC-HRMS, we proceeded with a NMR metabolomics strategy by directly analyzing the dried extract of N. variegata (when dissolved in deuterated chloroform) on 600 MHz NMR equipped with a cryogenic probe, without any requirements for further workup of the sample. NMR plant metabolomics is, among others, a complementary strategy for identification of known plant metabolites of plant-derived extracts and allows for the detection of signals of all compounds present in the sample at sufficient quantities to be detected. Recent development in cryoprobe technology has made it possible to characterized complex natural products with concentrations as low as in the micromolar range. In current literature, NMR spectroscopy has been successfully applied in the evaluation of metabolites of plant extracts (NMR plant metabolomics) [43]. NMR metabolomics has gained importance because this strategy provides insight into complex systems of mixtures of natural products occurring at their natural relative abundance. NMR is able to provide a “holistic view” of the metabolites under certain conditions, and thus is advantageous for metabolomic studies [44]. Although most publications about NMR metabolomics, including plant metabolomics, only include application of 1D 1H NMR (reviewed by Kim et al. 2011 [44]), several applications of 2D NMR exist in plant metabolomics [45]. Recent development in cryoprobe technology has led to a four-fold increase of sensitivity and thus a 16-fold reduction of experiment time for 2D inverse experiments compared with those for similar NMR experiments recorded on analogous instruments equipped with conventional probes. This allows for applications of a broad selection of 2D NMR spectroscopic experiments, which are now accessible within an acceptable time scale of approximately 15–30 min per experiment.
To support the identifications achieved with GC-HRMS, 1D 1H (Figure S1) and the 2D NMR experiments 2D 1H-13C Heteronuclear Single Quantum Coherence (HSQC), 2D 1H-13C Heteronuclear Multiple Bond Correlation (HMBC) (Figure S2), 2D 1H-13C Heteronuclear Single Quantum Coherence-Total Correlation Spectroscopy (HSQC-TOCSY), 2D 1H-13C Heteronuclear 2 Bond Correlation (H2BC), 2D 1H-1H Correlation Spectroscopy (COSY) and 2D 1H-1H Rotating frame Overhauser enhancement spectroscopy (ROESY) of the dried hexane extract of B. laciniosa dissolved in deuterated chloroform were recorded. The same NMR experiments were also recorded on samples of pure β-sitosterol, stigmasterol, α-tocopherol and phytol. The combined information from the 2D 1H-13C edited HSQC, HSQC-TOCSY, HMBC and H2BC were particularly helpful for assignment of 1H and 13C signals of both reference compounds (Tables S1–S3) and the analogous signals belonging to the mixture containing these compounds comprising the extract sample. The overlaid 2D NMR spectra of extract of N. variegata and pure β-sitosterol, α-tocopherol and phytol confirmed the presence of these compounds in the plant extract and allowed for identifications of individual signals (Figure 5). The multidimensional NMR data provided supportive evidence for the presence of the above-mentioned compounds, as well as significant amounts of long-chain alkanes and fatty acids identified by hyphenated GC-HRMS in N. variegata (Table 1).
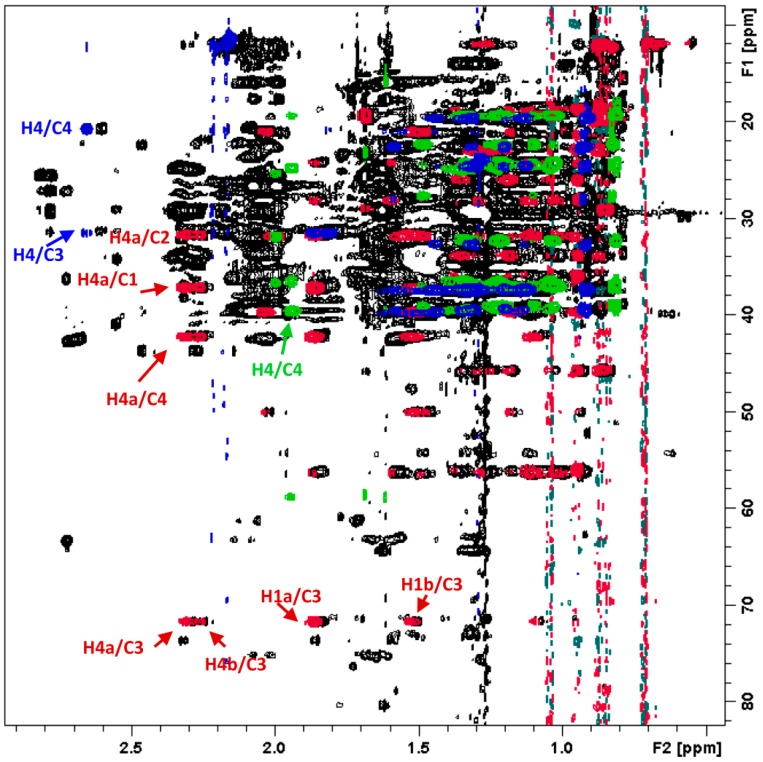
Figure 5.
Expanded region of the superimposed 2D 1H-13C HSQC-TOCSY NMR spectra of N. variegata (black signals), β-sitosterol (red signals), phytol (green signals) and α-tocopherol (blue signals). Selected crosspeaks are assigned. Complete assignments are presented in Tables S1–S3. Notice that the characteristic signal patterns of the pure standard compounds matches the corresponding signals of the same compounds present as part of mixture comprising the extract of N. variegata.
3. Materials and Methods
3.1. Plant Material
Leaves of B. laciniosa, E. spectabile and N. variegata were collected within the municipality borders of Petrolina, Pernambuco, Brazil, in January 2013. Voucher specimens were deposited in the Herbarium Vale do São Francisco (HVASF) of the Federal University of Vale do São Francisco. The site for collecting the leaves of B. laciniosa was at 08°59′16.90′′ S and 40°35′20.60′′ W and the voucher specimen is No. 6442. The leaves of E. spectabile were collected at the coordinates 09°07′54.30′′ S and 40°26′21.00′′ W and the voucher specimen is No. 6443. N. variegata leaves were collected at the coordinates 08°59′16.90′′ S and 40°35′20.60′′ W and the voucher specimen is No. 6441. Identification of the collected plant species was done by the botanist André Paviotti Fontana from Centro de Recuperação de Áreas Degradadas da Caatinga (CRAD). Prior to shipment to Norway the leaves were dried in an oven with air circulation at a temperature of 50 °C for seven days. After drying, the plant materials were powdered in a mill.
3.2. Extraction and Concentration
Dried and pulverized leaves of B. laciniosa (100.86 g), E. spectabile (100.22 g) and N. variegata (100.49 g) were separately macerated in 800 mL of hexane for 89 h at room temperature. After extraction, solutions were filtered through glass wool before being concentrated under reduced pressure on a rotary evaporator. The volumes were reduced to 140 mL (B. laciniosa), 75 mL (E. spectabile) and 145 mL (N. variegata), respectively. Before examination by GC-FID and GC-HRMS the concentrated samples were filtered through a 0.45 µm Micropore Membrane Filter.
3.3. GC-FID
To optimize conditions for the GC-MS analysis, GC-FID was performed. These investigations of the composition and concentration of the extracts were performed on a Gas Chromatograph (GC) with a Flame Ionization Detector (FID). A Trace GC Ultra instrument (Thermo Electron Corporation S.p.A., Milan, Italy) fitted with an Ultra 1 column (crosslinked methyl siloxane, ID = 0.200 mm, L = 25 m, film thickness = 0.33 μm) (Santa Clara, CA, USA). Samples were dissolved in hexane and splitless mode was used for injection. The applied temperature gradient (initial temperature = 50 °C, holding for 2.5 min, then heating at 20 °C/min to 100 °C, holding for 10 min, finally heating at 2 °C/min to 300 °C applying a 15 min holding time provided both good separation of the compounds and the necessary information about the concentration of the extracts. Helium was used as carrier gas with a flow rate of 0.7 mL/min. An injector temperature of 260 °C was used.
3.4. GC-MS (TOF)
All samples were analyzed on an AccuTOF T100GC mass spectrometer from JEOL Ltd. (Tokyo, Japan) interfaced with an Agilent 6890 N gas chromatograph (Santa Clara, CA, USA). Samples dissolved in hexane were injected on a VF-50 MS GC column from Varian Inc. (Palo Alto, CA, USA), (silica column (5% phenyl)-metylpolysiloxane, ID = 0.200 mm, L = 25 m, film thickness = 0.33 μm) using splitless injection at 250 °C (injector temperature). Helium (5.0) was used as carrier gas at a constant gas flow rate of 0.7 mL/min (ʋ = 33.0 cm/s), and the following GC temperature program was applied; initial temperature = 40 °C, holding for 2.5 min, then heating at 20 °C/min to 100 °C, holding for 10 min, finally heating at 2 °C/min to 300 °C applying a 15 min holding time. The GC-MS interface was heated to 260 °C introducing the column flow into the electron ionization source four minutes after injection. The ion source operated at 260 °C generating positive ions at an ionization potential and ionization current of 70 eV and 300 μA, respectively. Settings for the time of flight mass analyser were optimized for ions in the mass range 40–800 amu, acquiring mass spectra after the following acquisition settings; spectral recording interval = 0.3 s, wait time = 0.005 s, spectra accumulation time = 0.295 s and data sampling interval = 1 ns. A total ion chromatogram (TIC) was acquired during the whole GC run and mass spectra were generated and transformed to centroided spectra using baseline correction and smoothing using a weighted moving average. All mass spectra were calibrated against one of several polysiloxane background ions (m/z = 147.03290, 207.03290, 281.05169 or 355.07048) originating from the column and acquired during the same set of experiments. The relative quantities of each compound were calculated based on the peak heights in the total ion chromatogram. Mass spectral fragmentation patterns of individual compounds was compared with that of analogous standard compounds in NIST standard reference Mass Spectral library. Absolute configurations are based on mass spectral matches against known library mass spectra (NIST07), when applicable. Individual mass spectra are shown in Figures S3–S16.
3.5. NMR Spectroscopy
NMR spectroscopy was performed on samples of 19.1 mg dried heptane extract of B. laciniosa and on 164.9 mg dried hexane extract of N. variegata. In addition to pure samples of 20.0 mg of β-sitosterol, stigmasterol, 25 volume % of phytol and 76.2 mg of α-tocopherol, each individual sample was dissolved in, or mixed with, 0.75 mL chloroform-D. The 1D 1H and the 2D 1H-1H COSY, 2D 1H-1H ROESY, the 2D 1H-13C HSQC, the 2D 1H-13C Edited HSQC, the 2D 1H-13C HSQCTOCSY, the 2D 1H-13C HMBC and the 2D 1H-13C H2BC NMR experiments were recorded on a Bruker Avance 600 MHz spectrometer (Bruker BioSpin AG, canton of Zürich, Switzerland) equipped with a 1H-13C-15N triple resonance cryoprobe at 298 K. All 2D NMR experiments were recorded without spinning.
4. Conclusions
Using a combination of hyphenated GC-HRMS and NMR plant metabolomics, 20 natural products have been identified in the drought-resistant plants B. laciniosa, N. variegata and E. spectabile for the first time. A total of 13 natural products including six triterpenoids were identified from N. variegata. From the edible leaves of B. laciniosa and E. spectabile, 9 and 16 natural products were identified, respectively. The presence of significant amounts of vitamin E in leaves of B. laciniosa and E. spectabile, as well as nutrients such as fatty acids, and phytosterols with well documented potential beneficial health effects, as well as the absence of compounds with significant toxicity, underlines the nutritional values of the plants as food sources for humans and livestock.
Acknowledgments
This work was supported by grants from Brazilian agencies CNPq (Process 476770/2010-6) and FACEPE (Process APQ-0542-4.03/10). The authors are grateful to Centre for Pharmacy, University of Bergen, for financial support.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
T.F., B.H., G.W.F., J.R.G.d.S.A. and O.J.J. conceived and designed the experiments; O.J.J. and B.H. performed the experiments; T.F., B.H., G.W.F. and O.J.J. analyzed the data; J.R.G.d.S.A., R.G.d.O.J. and A.P.d.O. collected, identified and processed the plant material; O.J.J., G.W.F., H.L.A., J.R.G.d.S.A and T.F. wrote the paper.
Conflicts of Interest
The authors report no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds phytol, β-sitosterol and α-tocopherol are available from the authors.
References
- 1.Givnish T.J., Barfuss M.H.J., Van Ee B., Riina R., Schulte K., Horres R., Gonsiska P.A., Jabaily R.S., Crayn D.M., Smith J.A.C., et al. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: Insights from an eight-locus plastid phylogeny. Am. J. Bot. 2011;98:872–895. doi: 10.3732/ajb.1000059. [DOI] [PubMed] [Google Scholar]
- 2.Manera G., Nunes W. Ed. UEFS; Feira de Santana, Brazil: 2001. Convivendo com a Seca: Plantas Forrageiras; pp. 7–8. [Google Scholar]
- 3.Angelim A.E.S., De Moraes J.P.S., Da Silva J.A.B., Gervásio R.d.C.R.G. Germinação e aspectos morfológicos de plantas de macambira (Bromelia laciniosa), encontradas na região do vale do são francisco [Germination and morphological aspects of macambira (Bromelia laciniosa), found in the region of Vale do São Francisco] Rev. Bras. Biocienc. 2007;5:1065–1067. [Google Scholar]
- 4.Dutra A.S., Teófilo E.M., Filho S.M. Germinação de sementes de macambira (Bromelia laciniosa mart. Ex schult) [Germination of macambira (Bromelia laciniosa mart. Ex schult. & schult. F.) seeds] Rev. Caatinga. 2010;23:12–17. [Google Scholar]
- 5.Santo F.D.S.D.E., Maciel J.R., de Siqueira Filho J.A. Impacto da herbivoria por caprinos sobre as populacos naturais de Bromelia laciniosa mart. Ex schult. F. (bromeliaceae) [Impact of herbivory by goats on natural populations of Bromelia laciniosa mart. Ex. Schult & schult. F.] Rev. Arvore. 2012;36:143–149. [Google Scholar]
- 6.De Lima J.L.S. In: Plantas Forrageiras das Caatingas, Usos e Potencialidades. Rocha P., editor. EMBRAPA-CPATSA/PNE/RBG-KEW; Petrolina, Brazil: 1996. pp. 1–45. [Google Scholar]
- 7.Agra M.F., Baracho G.S., Nurit K., Basilio I.J., Coelho V.P. Medicinal and poisonous diversity of the flora of “cariri paraibano”, Brazil. J. Ethnopharmacol. 2007;111:383–395. doi: 10.1016/j.jep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 8.De Albuquerque U.P., De Medeiros P.M., De Almeida A.L.S., Monteiro J.M., Neto E.M.D.F.L., De Melo J.G., Dos Santos J.P. Medicinal plants of the Caatinga (semi-arid) vegetation of ne Brazil: A quantitative approach. J. Ethnopharmacol. 2007;114:325–354. doi: 10.1016/j.jep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Bongiorno de Pfirter G.M., Buttazzoni de Cozzarin M.S., Caffini N.O. Otros exudados gomosos en especies argentinas del genero bromelia. I. Las gomas de Bromelia serra gris. Y Bromelia laciniosa mart. (Bromeliaceae) Rev. Farm. 1973;115:98–99. [Google Scholar]
- 10.De Oliveira-Júnior R.G., De Oliveira A.P., Guimarães A.L., Araújo E.C.C., Braz-Filho R., Øvstedal D.O., Fossen T., da Silva Almeida J.R.G. The first flavonoid isolated from Bromelia laciniosa (Bromeliaceae) J. Med. Plants Res. 2014;8:558–563. [Google Scholar]
- 11.Mayo S. Neoglaziovia variegata, Bromeliaceae. Curtis’s Bot. Mag. 1992;9:124–127. doi: 10.1111/j.1467-8748.1992.tb00082.x. [DOI] [Google Scholar]
- 12.D’Almeida J.R.M., d’Almeida A.L.F.S., de Carvalho L.H. Mechanical, morphological, and structural characteristics of caroa (Neoglaziovia variegata) fibers. Polym. Polym. Compos. 2008;16:589–595. [Google Scholar]
- 13.De Oliveira-Júnior R.G., Araújo C.D.S., Santana C.R.R., Souza G.R., de Lima-Saraiva S.R.G., Guimarães A.L., de Oliveira A.P., de Siqueira Filho J.A., Pacheco A.G.M., da Silva Almeida J.R.G. Phytochemical screening, antioxidant and antibacterial activity of extracts from the flowers of Neoglaziovia variegata (Bromeliaceae) J. Chem. Pharm. Res. 2012;4:4489–4494. [Google Scholar]
- 14.Silveira D.G., Santana J.R.F., Souza F.V.D., Ledo C.A.S., Cunha E.C. Development of micropropagated shoots and plants of caroá in different substrates. Acta Hortic. 2010;865:305–313. doi: 10.17660/ActaHortic.2010.865.42. [DOI] [Google Scholar]
- 15.Silveira D.G., Souza F.V.D., Pelacani C.R., Souza A.D.S., Ledo C.A.d.S., Ferreira de Santana J.R. Micropropagation and in vitro conservation of Neoglaziovia variegata (arr. Cam.) mez, a fiber producing bromeliad from Brazil. Braz. Arch. Biol. Technol. 2009;52:923–932. doi: 10.1590/S1516-89132009000400016. [DOI] [Google Scholar]
- 16.De Lima-Saraiva S.R.G., Guimarães A.L., de Oliveira A.P., Saraiva H.C.C., de Oliveira-Junior R.G., de Barros V.R.P., Menezes V.G., de Oliveira R.A., Silva F.S., de Lima R.S., et al. Antioxidant activity and acute toxicity of Neoglaziovia variegata (Bromeliaceae) Afr. J. Biotechnol. 2012;11:13998–14006. doi: 10.5897/AJB12.1913. [DOI] [Google Scholar]
- 17.Machado F.D.F., Silva F.V., Fernandes H.B., Freitas F.F.B.P., Arcanjo D.D.R., Lima J.T., da Silva Almeida J.R.G., Oliveira F.A., Oliveira R.C.M. Gastroprotective effect of an ethanolic extract from Neoglaziovia variegata (arruda) mez (Bromeliaceae) in rats and mice. Z. Naturforsch. (C) 2013;68:97–107. doi: 10.5560/ZNC.2013.68c0097. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva V.F., Franco I., Damasceno T.E.F., da Silva Almeida J.R.G., da Costa M.M. Antimicrobial potential of ethanol extracts of plants against Gram-negative bacilli isolated from cervicovaginal mucosa of sheep bred in the region of Petrolina-pe. Semin. Cienc. Agrar. 2014;35:883–890. [Google Scholar]
- 19.Do Nascimento V.T., Vasconcelos M.A.D.S., Maciel M.I.S., Albuquerque U.P. Famine foods of Brazil’s seasonal dry forests: Ethnobotanical and nutritional aspects. Econ. Bot. 2012;66:22–34. doi: 10.1007/s12231-012-9187-2. [DOI] [Google Scholar]
- 20.De Lima-Saraiva S.R.G., Silva J.C., Branco C.R.C., Branco A., Amorim E.L.C., da Silva Almeida J.R.G. Antinociceptive effect of Encholirium spectabile: A Bromeliaceae from the Brazilian Caatinga biome. Pharmacogn. Mag. 2014;10:655–660. doi: 10.4103/0973-1296.139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santana C.R.R., De Oliveira-Junior R.G., Araújo C.D.S., Souza G.R., De Lima-Saraiva S.R.G., Guimarães A.L., De Oliveira A.P., Filho J.A.D.S., Pacheco A.G.M., Da Silva Almeida J.R.G. Phytochemical screening, antioxidant and antibacterial activity of Encholirium spectabile (Bromeliaceae) Int. J. Sci. 2012;1:1–19. [Google Scholar]
- 22.De Oliveira-Junior R.G., Souza G.R., Guimarães A.L., de Oliveira A.P., Silva Morais A.C., Araújo E.C.D.C., Nunes X.P., da Silva Almeida J.R.G. Dried extracts of Encholirium spectabile (Bromeliaceae) present antioxidant and photoprotective activities in vitro. J. Young Pharm. 2013;5:102–105. doi: 10.1016/j.jyp.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Carvalho K.I.M., Fernandes H.B., Machado F.D.F., Oliveira I.S., Oliveira F.A., Nunes P.H.M., Lima J.T., da Silva Almeida J.R.G., Oliveira R.C.M. Antiulcer activity of ethanolic extract of Encholirium spectabile mart. Ex schult & schult f. (Bromeliaceae) in rodents. Biol. Res. 2010;43:459–465. [PubMed] [Google Scholar]
- 24.Prinsen P., Gutiérrez A., Faulds C.B., del Rio J.C. Comprehensive study of valuable lipophilic phytochemicals in wheat bran. J. Agric. Food. Chem. 2014;62:1664–1673. doi: 10.1021/jf404772b. [DOI] [PubMed] [Google Scholar]
- 25.Hargrove J.L., Greenspan P., Hartle D.K. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med. 2004;229:215–226. doi: 10.1177/153537020422900301. [DOI] [PubMed] [Google Scholar]
- 26.Morohashi K.-I., Sadano H., Okada Y., Omura T. Position specificity in n-hexane hydroxylation by two forms of cytochrome P-450 in rat liver microsomes. J. Biochem. 1983;93:413–419. doi: 10.1093/oxfordjournals.jbchem.a134195. [DOI] [PubMed] [Google Scholar]
- 27.Parada F., Duque C. Studies on the aroma of piñuela fruit pulp (Bromelia plumieri): Free and bound volatile composition and characterization of some glucoconjugates as aroma precursors. J. High Resol. Chromatogr. 1998;21:577–581. doi: 10.1002/(SICI)1521-4168(19981001)21:10<577::AID-JHRC577>3.0.CO;2-V. [DOI] [Google Scholar]
- 28.Marques G., Gutierrez A., del Rio J.C. Chemical characterization of lignin and lipophilic fractions from leaf fibers of curaua (Ananas erectifolius) J. Agric. Food Chem. 2007;55:1327–1336. doi: 10.1021/jf062677x. [DOI] [PubMed] [Google Scholar]
- 29.Vetter W., Schröder M., Lehnert K. Differentiation of refined and virgin edible oils by means of the trans- and cis-phytol isomer distribution. J. Agric. Food Chem. 2012;60:6103–6107. doi: 10.1021/jf301373k. [DOI] [PubMed] [Google Scholar]
- 30.Coppack S.W., Evans R., Gibberd F.B., Clemens M.E., Billimoria J.D. Can patients with refsum’s disease safely eat green vegetables? Br. Med. J. (Clin. Res. Ed.) 1988;296:828. doi: 10.1136/bmj.296.6625.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pakrashi S.C., Achari B., Majumdar P.C. Studies on indian medicinal-plants: Part XXXII. constituents of Ananas comosus (linn) merr leaves. Indian J. Chem. 1975;13:755–756. [PubMed] [Google Scholar]
- 32.Cantillo-Ciau Z., Brito-Loeza W., Quijano L. Triterpenoids from Tillandsia fasciculata. J. Nat. Prod. 2001;64:953–955. doi: 10.1021/np0100744. [DOI] [PubMed] [Google Scholar]
- 33.Manetti L.M., Delaporte R.H., Laverde-Junior A. Metabólitos secundários da família Bromeliaceae [Secondary metabolites from Bromeliaceae family] Quim. Nova. 2009;32:1885–1897. doi: 10.1590/S0100-40422009000700035. [DOI] [Google Scholar]
- 34.Delaporte R.H., Sarragiotto M.H., Takemura O.S., Sanchez G.M., Filho B.P.D., Nakamura C.V. Evaluation of the antioedematogenic, free radical scavenging and antimicrobial activities of aerial parts of Tillandsia streptocarpa baker—Bromeliaceae. J. Ethnopharmacol. 2004;95:229–233. doi: 10.1016/j.jep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera G.M., Gallo M., Seldes A.M. Cycloartane derivatives from Tillandsia usneoides. J. Nat. Prod. 1996;59:343–347. doi: 10.1021/np960075+. [DOI] [Google Scholar]
- 36.Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003;78:965–978. doi: 10.1016/S0025-6196(11)63144-3. [DOI] [PubMed] [Google Scholar]
- 37.Farquhar J.W., Sokolow M. Response of serum lipids and lipoproteins of man to beta-sitosterol and safflower oil—A long term study. Circulation. 1958;17:890–899. doi: 10.1161/01.CIR.17.5.890. [DOI] [PubMed] [Google Scholar]
- 38.AbuMweis S.S., Marinangeli C.P.F., Frohlich J., Jones P.J. Implementing phytosterols into medical practice as a cholesterol—Lowering strategy: Overview of efficacy, effectiveness, and safety. Can. J. Cardiol. 2014;30:1225–1232. doi: 10.1016/j.cjca.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., Ding Y., Xing D.-M., Wang J.-P., Du L.-J. Studies on phenolic constituents from leaves of pineapple (Ananas comosus) China J. Chin. Mater. Med. 2006;31:1242–1244. [PubMed] [Google Scholar]
- 40.Marker R.E., Wagner R.B., Ulshafer P.R., Wittbecker E.L., Goldsmith D.P.J., Ruof C.H. Sterols. CLVII. Sapogenins. LXIX. Isolation and structures of thirteen new steroidal sapogenins. New sources for known sapogenins. J. Am. Chem. Soc. 1943;65:1199–1209. doi: 10.1021/ja01246a051. [DOI] [Google Scholar]
- 41.Djerassi C., McCrindle R. 789. Terpenoids. Part LI. The isolation of some new cyclopropane-containing triterpenes from spanish moss (Tillandsia usneoides, L.) J. Chem. Soc. 1962:4034–4039. doi: 10.1039/jr9620004034. [DOI] [Google Scholar]
- 42.Cabrera G.M., Seldes A.M. Hydroperoxycycloartanes from Tillandsia recurvata. J. Nat. Prod. 1995;58:1920–1924. doi: 10.1021/np50126a020. [DOI] [Google Scholar]
- 43.Palomino-Schätzlein M., Escrig P.V., Boira H., Primo J., Pineda-Lucena A., Cabedo N. Evaluation of nonpolar metabolites in plant extracts by 13C NMR spectroscopy. J. Agric. Food Chem. 2011;59:11407–11416. doi: 10.1021/jf2030902. [DOI] [PubMed] [Google Scholar]
- 44.Kim H.K., Choi Y.H., Verpoorte R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011;29:267–275. doi: 10.1016/j.tibtech.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Kontogianni V.G., Exarchou V., Troganis A., Gerothanassis I.P. Rapid and novel discrimination and quantification of oleanolic and ursolic acids in complex plant extracts using two-dimensional nuclear magnetic resonance spectroscopy-Comparison with HPLC methods. Anal. Chim. Acta. 2009;635:188–195. doi: 10.1016/j.aca.2009.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.