Abstract
Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus are key factors in the fermentation process and the final quality of dairy products worldwide. This study was performed to investigate the effects of the proportions of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus isolated from traditionally fermented dairy products in China and Mongolia on the profile of volatile compounds produced in samples. Six proportional combinations (1:1, 1:10, 1:50, 1:100, 1:1000, and 1:10,000) of L. delbrueckii subsp. bulgaricus IMAU20401 to S. thermophilus ND03 were considered, and the volatiles were identified and quantified by solid-phase microextraction and gas chromatography–mass spectrometry (SPME-GC-MS) against an internal standard. In total, 89 volatile flavor compounds, consisting of aldehydes, ketones, acids, alcohols, esters, and aromatic hydrocarbons, were identified. Among these, some key flavor volatile compounds were identified, including acetaldehyde, 3-methylbutanal, acetoin, 2-heptanone, acetic acid, butanoic acid, and 3-methyl-1-butanol. The of L. delbrueckii subsp. bulgaricus IMAU20401 to S. thermophilus ND03 influenced the type and concentration of volatiles produced. In particular, aldehydes and ketones were present at higher concentrations in the 1:1000 treatment combination than in the other combinations. Our findings emphasize the importance of selecting the appropriate proportions of L. delbrueckii subsp. bulgaricus and S. thermophilus for the starter culture in determining the final profile of volatiles and the overall flavor of dairy products.
Keywords: fermented milk, volatile compounds (VOCs), solid phase microextraction (SPME), gas chromatography-mass spectrometry (GC-MS)
1. Introduction
Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus are lactic acid bacteria (LAB) that have been isolated from a variety of habitats, particularly traditionally fermented food [1,2]. They play important roles in the production of dairy products, particularly yogurt, where they are the keys to final product quality. Lactobacillus delbrueckii subsp. bulgaricus and S. thermophilus used in mixed culture have a symbiotic relationship in milk due to the exchange of metabolites [3]. For example, L. delbrueckii subsp. bulgaricus can easily utilize the pyruvic acid, formic acid, folic acid, and long-chain fatty acids produced by S. thermophilus, whereas the peptides, free amino acids, and putrescine produced by L. delbrueckii subsp. bulgaricus stimulate the growth of S. thermophilus [4,5].
Yogurt production is perhaps one of the most complex milk fermentation processes; milk fermentation with L. delbrueckii subsp. bulgaricus and S. thermophilus produces yogurt with good flavor, acidity, and viscosity. Flavor is achieved by the integration of a variety of volatile compounds (VOCs), including acids, aldehydes, ketones, alcohols, esters, and hydrocarbons. These compounds can impart favorable flavors to yogurt [6,7]. Using solid-phase microextraction coupled with gas chromatography–mass spectrometry (SPME-GC-MS), Settachaimongkon et al. identified VOCs produced in milk by L. delbrueckii subsp. bulgaricus and S. thermophilus, including fatty acids, alcohols, and sulfur compounds [8]. Dan et al. evaluated the VOCs produced by L. delbrueckii subsp. bulgaricus and S. thermophilus from traditional fermented milk and reported similar results; these VOCs included acids, alcohols, aldehydes, ketones, and hydrocarbons [9].
The introduction of GC-MS has accelerated the field of flavor chemistry, especially when linked to SPME as a pretreatment method [10,11]. The main advantages of SPME are its simplicity, low cost, ease of automation, and in situ sampling [12]. SPME coupled with GC-MS has been used widely to evaluate the flavor chemical profiles of volatile aromas produced by a wide variety of substances, including fermented milk [13,14], the fruit and sap of mango cultivars [15], grapes and wine [16], dry fermented sausage [17], and alcoholic beverages [18].
Lactobacillus delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 were isolated from traditional fermented dairy products in China and Mongolia and selected based on their excellent processing properties, such as flavor, acidity, viscosity, and water-holding capacity [9,19]. This study was performed to quantify variations in the profile of volatiles produced using different proportional combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03.
2. Results and Discussion
2.1. Microbiological Counts
The viable counts of the different proportional combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 (1:1, 1:10, 1:50, 1:100, 1:1000, and 1:10,000) in the samples were 5 × 109, 4 × 109, 2.8 × 109, 3.3 × 109, 1.60 × 109, and 0.09 × 109 CFU/mL−1, respectively, at pH 4.5.
2.2. Extraction Temperature and Time Effect
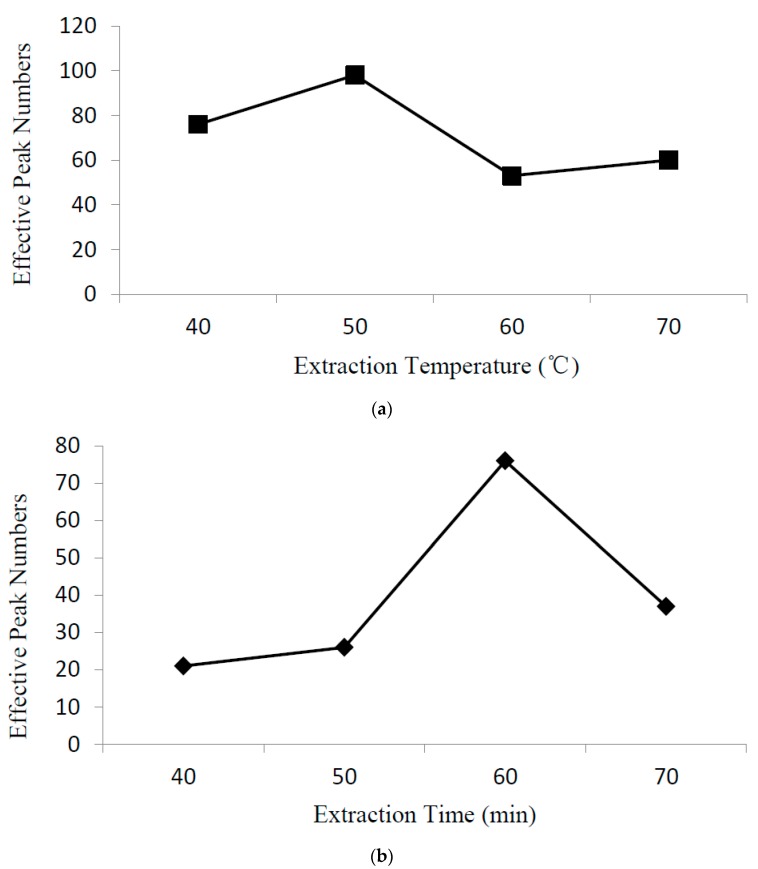
A study to optimize extraction conditions, including the sample temperature and extraction time of volatile compounds present in samples, was performed using SPME fiber (50/30 μm DVB/Carboxen/PDMS). The sample temperature and extraction time are important parameters in the SPME sampling process and can increase the extraction efficiency when optimized. The sample temperature and extraction time are discussed for the 1:1 proportional combination of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03. Extraction was compared at temperatures of 40 °C, 50 °C, 60 °C, and 70 °C. The effective peak numbers were enhanced at temperatures up to 50 °C and then began to decline (Figure 1a). Therefore, a temperature of 50 °C was used to study extraction time. Using extraction times ranging from 40 to 70 min, the effective peak numbers were enhanced as time increased up to 60 min and then began to decline (Figure 1b). Therefore, the optimum extraction conditions were 50 °C for 60 min.
Figure 1.
Effects of extraction temperature and time on extraction efficiency.
2.3. Volatile Composition of Samples
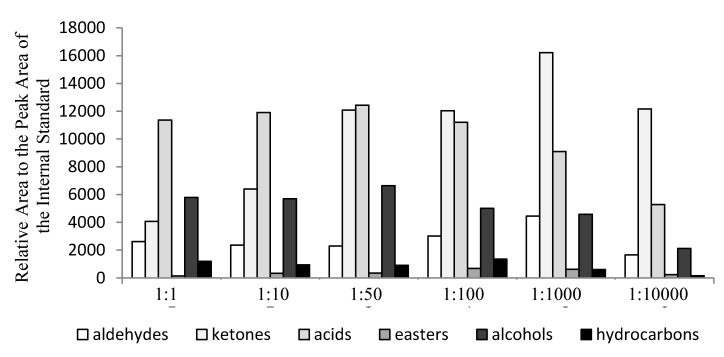
Figure 2 shows the categories and relative peak areas compared to those of the internal standards for the volatiles in milk fermented with different proportions of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03. The relative peak areas to those of the internal standards of aldehyde and ketone compounds were higher for 1:1000 treatment than for the other combinations, reaching 4456 and 16,219, respectively. With 1:50 treatment, the relative peak areas to those of the internal standards for acids, esters, and alcohols were higher than in the other combinations and reached 12,430, 4435, and 6633, respectively, and then began to decline. These observations indicated that smaller initial proportions of L. delbrueckii subsp. bulgaricus in samples result in a reduction of post-acidification. In contrast, the relative peak areas to those areas of internal standards for aromatic hydrocarbons were higher with 1:100 treatment than with the other combinations, reaching a value of 1353. This observation indicated that there are significant differences between the samples fermented with different proportions of L. delbrueckii subsp. bulgaricus IMAU 20,401 and S. thermophilus ND03 in terms of the composition of volatile compounds.
Figure 2.
The categories and relative peak areas compared to those of the internal standards for the volatiles in milk fermented with different proportions of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03.
2.4. Profiles of Aldehyde Compounds
Volatile compounds detected using SPME pretreatment combined with GC-MS included 16 aldehyde compounds (Table 1). Acetaldehyde is the key aroma compound in fermented milk products and can improve flavor [20]. Lactobacillus delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 are known to play important roles in producing aromatic compounds in fermented milk [9,19]. Acetaldehyde can be produced directly from ethanol by the activity of alcohol dehydrogenase as an intermediate in the metabolism of sugar [21,22]. Several metabolic pathways in L. delbrueckii subsp. bulgaricus and S. thermophiles have been reported to result in the production of acetaldehyde in fermented milk [21]. For example, threonine is readily converted to acetaldehyde by catalysis of threonine aldolase [23,24,25]. In this study, high levels of acetaldehyde were present in all combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03, particularly 1:1000 treatment. Similar results were reported by Hamdan et al. (1973), who reported that a more abundant S. thermophiles population stimulated the production of aldehyde compounds in yogurt [26].
Table 1.
Volatile compounds produced by milk fermented with different proportions of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03.
| No. | Volatile Compound | Chemical Formula | RT (min) 1 | RI 2 | RI 3 | Method 4 | Ratio of L. delbrueckii subsp. bulgaricus to S. thermophiles (μg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:1 | 1:10 | 1:50 | 1:100 | 1:1000 | 1:10,000 | |||||||
| Aldehyde Compounds | ||||||||||||
| 1 | Acetaldehyde | C2H4O | 1.26 | - | - | MS, STD | 6.86 | 7.11 | 7.77 | 11.15 | 14.33 | - |
| 2 | 3-Hydroxybutanal | C4H8O2 | 2.37 | 605 | - | MS | - | 1.33 | 2.39 | 1.16 | 6.96 | 5.46 |
| 3 | 3-Methylbutanal | C5H10O | 2.82 | 639 | 634 | MS, RI | - | 5.55 | 5.38 | 8.23 | 7.80 | 5.31 |
| 4 | Pentanal | C5H10O | 3.19 | 668 | 668 | MS, RI | - | - | 0.32 | 1.36 | 1.07 | - |
| 5 | (E)-2-Pentenal | C5H8O | 5.14 | 753 | 754 | MS, RI | - | 3.89 | - | 1.27 | 5.44 | - |
| 6 | Hexanal | C6H12O | 5.61 | 768 | 769 | MS, RI | - | 1.43 | 1.29 | 2.51 | 5.20 | 1.24 |
| 7 | (E)-2-Hexenal | C6H10O | 8.35 | 848 | 848 | MS, RI | 2.58 | 2.01 | 1.95 | 2.29 | - | - |
| 8 | Heptanal | C7H14O | 8.66 | 857 | 860 | MS, RI | 1.55 | 1.95 | 1.66 | 1.86 | 2.23 | - |
| 9 | Benzaldehyde | C7H6O | 11.77 | 932 | 933 | MS, RI | - | - | 0.31 | - | - | 3.05 |
| 10 | (E)-2-Heptenal | C7H12O | 12.28 | 953 | 953 | MS, RI | 3.82 | - | - | - | - | 0.23 |
| 11 | (E,E)-2,4-Heptadienal | C7H10O | 14.23 | 1006 | 1007 | MS, RI | - | - | 1.32 | - | - | - |
| 12 | (E)-2-Octenal | C8H14O | 15.82 | 1054 | 1055 | MS, RI | 1.26 | - | 0.32 | - | - | - |
| 13 | Nonanal | C9H18O | 16.85 | 1085 | 1086 | MS, RI | 2.92 | 0.25 | 0.31 | 0.30 | 0.86 | 1.22 |
| 14 | (E)-2-Nonenal | C9H16O | 19.03 | 1155 | 1157 | MS, RI | 3.93 | - | - | - | - | - |
| 15 | Decanal | C10H20O | 19.91 | 1184 | 1185 | MS, RI | 1.93 | - | - | - | 0.67 | - |
| 16 | (Z)-2-Decenal | C10H18O | 22.01 | 1257 | 1252 | MS, RI | 1.26 | - | - | - | - | - |
| Ketone Compounds | ||||||||||||
| 17 | 2,3-Butanedione | C4H6O2 | 2.25 | - | - | MS, STD | - | 1.06 | 2.11 | 2.33 | 2.13 | 5.88 |
| 18 | 2-Pentanone | C5H10O | 2.97 | 651 | 653 | MS, RI | - | - | 3.89 | 1.67 | 5.27 | 5.04 |
| 19 | 3-Methyl-2-butanone | C5H10O | 3.01 | 654 | 654 | MS, RI | 1.65 | 3.26 | 3.45 | 4.21 | 5.28 | 2.61 |
| 20 | 1-Hydroxy-2-propanone | C3H6O2 | 3.33 | 679 | 674 | MS, RI | - | 5.23 | - | - | - | - |
| 21 | Acetoin | C4H8O2 | 3.42 | 686 | - | MS, STD | - | 41.18 | 87.38 | 94.26 | 111.89 | 68.57 |
| 22 | 3-Methyl-(S)-2-butanol | C5H12O | 5.35 | 760 | - | MS | - | - | - | - | - | 4.30 |
| 23 | 2-Heptanone | C7H14O | 9.23 | 871 | 863 | MS, RI | 21.79 | 9.00 | 17.18 | 11.27 | 25.56 | 27.48 |
| 24 | 5-Methyl-3-heptanone | C8H16O | 12.43 | 957 | 962 | MS, RI | 1.31 | - | - | - | - | - |
| 25 | 2-Propyl-1-heptanol | C10H22O | 15.29 | 1033 | - | MS, RI | - | - | 0.25 | 0.18 | - | - |
| 26 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | C6H8O3 | 16.08 | 1062 | 1060 | MS, RI | - | 0.53 | - | - | - | - |
| 27 | 2-Nonanone | C9H18O | 16.45 | 1071 | 1070 | MS, RI | 14.04 | 2.11 | 5.43 | 5.84 | 10.67 | 6.77 |
| 28 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | C6H8O4 | 18.56 | 1140 | 1149 | MS, RI | - | 0.70 | - | - | - | - |
| 29 | 2-Undecanone | C11H22O | 22.48 | 1273 | 1273 | MS, RI | 1.84 | 0.85 | 1.07 | 0.62 | 1.39 | 1.03 |
| Acid Compounds | ||||||||||||
| 30 | Acetic acid | C2H4O2 | 2.56 | 619 | 622 | MS, RI, STD | 24.65 | 18.48 | 17.08 | 14.48 | 13.28 | 7.89 |
| 31 | 2-Oxopropanoic acid | C3H4O3 | 3.08 | 659 | - | MS | - | 1.70 | 1.34 | 1.18 | 1.67 | 1.79 |
| 32 | 2-Methylpropanoic acid | C4H8O2 | 5.12 | 752 | 753 | MS, RI | 2.88 | 5.68 | 6.64 | 5.32 | 4.85 | - |
| 33 | Butanoic acid | C4H8O2 | 6.30 | 793 | 793 | MS, RI | 16.83 | 12.07 | 11.40 | 11.14 | 4.02 | 0.62 |
| 34 | 3-Methylbutanoic acid | C5H10O2 | 8.22 | 845 | 845 | MS, RI | - | 3.35 | 8.33 | 6.79 | 5.04 | 4.09 |
| 35 | 2-Methylhexanoic acid | C7H14O2 | 8.93 | 863 | - | MS | - | 5.31 | 8.15 | 5.33 | 2.74 | 3.60 |
| 36 | 2-Methylbutanoic acid | C5H10O2 | 9.10 | 868 | - | MS | - | 7.16 | 12.42 | 10.24 | 9.29 | - |
| 37 | Pentanoic acid | C5H10O2 | 9.27 | 872 | 875 | MS, RI | - | 10.27 | 11.86 | 11.03 | 8.38 | - |
| 38 | Lactic acid | C3H6O3 | 10.82 | 914 | - | MS, STD | 6.67 | 5.41 | - | - | - | - |
| 39 | Hexanoic acid | C6H12O2 | 13.16 | 974 | 974 | MS, RI | 40.57 | 34.38 | 30.15 | 35.00 | 25.29 | 22.88 |
| 40 | 7-Oxo-Octanoic acid | C8H14O3 | 13.56 | 987 | - | MS | 3.94 | - | - | - | - | - |
| 41 | Heptanoic acid | C7H14O2 | 16.77 | 1083 | 1085 | MS, RI | 2.02 | 1.98 | 1.29 | 1.04 | - | - |
| 42 | Octanoic acid | C8H16O2 | 19.70 | 1177 | 1178 | MS, RI | 13.43 | 7.85 | 10.13 | 6.08 | 12.43 | 8.11 |
| 43 | Nonanoic acid | C9H18O2 | 21.08 | 1224 | 1226 | MS, RI | - | 2.99 | 1.85 | 1.69 | 1.78 | - |
| 44 | 2-Undecenoic acid | C11H20O2 | 22.31 | 1267 | - | MS | 0.73 | 0.67 | - | 0.35 | 1.11 | - |
| 45 | n-Decanoic acid | C10H20O2 | 24.54 | 1349 | 1349 | MS, RI | 2.01 | 1.74 | 3.66 | 2.40 | 1.07 | 3.79 |
| Ester Compounds | ||||||||||||
| 46 | Formic acid, hexyl ester | C7H14O2 | 8.94 | 864 | 870 | MS, RI | 1.44 | 1.89 | 1.97 | 2.65 | 1.87 | 2.29 |
| 47 | Heptanoic acid, 2-methyl-2-butyl ester | C12H24O2 | 14.10 | 1002 | - | MS | - | - | - | - | 0.89 | - |
| 48 | Sec-butyl nitrite | C4H9NO2 | 17.02 | 1090 | - | MS | - | 1.27 | - | - | - | - |
| 49 | Allyl 2-ethyl butyrate | C9H16O2 | 20.39 | 1200 | - | MS | - | - | - | 0.73 | 1.50 | - |
| 50 | Butanoic acid, 2-ethyl-,1,2,3-propanetriyl ester | C21H38O6 | 20.40 | 1200 | - | MS | - | - | 1.48 | 1.49 | 1.94 | - |
| 51 | Pentanoic acid, heptyl ester | C12H24O2 | 25.22 | 1375 | 1376 | MS, RI | - | 0.14 | - | 1.93 | - | - |
| Alcohol Compounds | ||||||||||||
| 52 | Cyclobutanol | C4H8O | 1.81 | - | - | MS | - | 4.22 | 5.49 | 6.49 | - | - |
| 53 | Trans-4-methylcyclohexanol | C7H14O | 3.18 | 667 | - | MS | 9.57 | - | - | - | - | - |
| 54 | 3-Methyl-2-butanol | C5H12O | 3.38 | 682 | 700 | MS, RI | 3.92 | 4.69 | 5.03 | 4.99 | - | - |
| 55 | 3-Methyl-3-buten-1-ol | C5H10O | 3.53 | 694 | 726 | MS, RI | - | - | - | - | 4.92 | - |
| 56 | 3-Methylbutanol | C5H12O | 4.54 | 732 | 732 | MS, RI | - | 23.12 | 20.55 | 14.89 | 15.86 | - |
| 57 | 2,2-Dimethyl-1-butanol | C6H14O | 4.83 | 742 | - | MS | - | - | - | - | 3.06 | - |
| 58 | Trans-1,2-cyclopentanediol | C5H10O2 | 5.49 | 765 | - | MS | - | - | - | - | 1.73 | 0.92 |
| 59 | 1-Propoxy-2-propanol | C6H14O2 | 5.67 | 771 | - | MS | - | - | - | - | - | 1.13 |
| 60 | 2-Methyl-3-pentanol | C6H14O | 6.67 | 804 | 805 | MS, RI | - | - | 2.09 | - | - | - |
| 61 | 1-Hexanol | C6H14O | 8.50 | 852 | 858 | MS, RI | 16.29 | 7.45 | 6.92 | 5.90 | 3.05 | 5.54 |
| 62 | 2-Methyl-3-pentanol | C8H18O | 9.40 | 876 | - | MS | - | 1.66 | 12.14 | - | - | - |
| 63 | 3-Methyl-2-hexanol | C7H16O | 10.30 | 900 | 906 | MS, RI | - | 0.24 | 1.96 | 2.96 | - | - |
| 64 | 5-Methyl-2-heptanol | C8H18O | 11.26 | 925 | - | MS | - | 0.71 | - | - | - | - |
| 65 | 2-Heptanol | C7H16O | 11.47 | 931 | 915 | MS, RI | - | 0.93 | - | - | - | 0.42 |
| 66 | 1-Heptanol | C7H16O | 13.17 | 976 | 974 | MS, RI | 12.49 | 1.86 | 2.92 | 3.10 | 8.37 | 4.38 |
| 67 | 1-Octen-3-ol | C8H16O | 13.26 | 979 | 979 | MS, RI | 2.15 | - | - | - | - | - |
| 68 | 3,5-Octadien-2-ol | C8H14O | 15.21 | 1036 | 1037 | MS, RI | 0.82 | - | - | - | - | - |
| 69 | 2-Nonen-1-ol | C9H18O | 15.79 | 1053 | - | MS | - | 3.46 | 1.93 | 1.42 | 2.33 | 2.58 |
| 70 | (E)-2-Octen-1-ol | C8H16O | 16.21 | 1066 | 1067 | MS, RI | 1.24 | - | - | - | - | - |
| 71 | 5-Ethyl-2-heptanol | C9H20O | 16.76 | 1082 | - | MS | - | - | - | - | 1.16 | - |
| 72 | 2-Nonanol | C9H20O | 17.24 | 1097 | 1098 | MS, RI | 1.14 | 1.59 | 2.38 | 3.98 | - | - |
| 73 | (E)-2-Nonen-1-ol | C9H18O | 19.32 | 1165 | 1171 | MS, RI | 1.26 | - | - | - | - | - |
| 74 | 1-Nonanol | C9H20O | 19.41 | 1168 | 1168 | MS, RI | 7.45 | 6.39 | 4.92 | 6.12 | 5.29 | 6.21 |
| 75 | (E)-2-Decen-1-ol | C10H20O | 22.23 | 1265 | - | MS | 1.64 | - | - | - | - | - |
| 76 | 2-Undecanol | C11H24O | 23.15 | 1297 | 1303 | MS, RI | - | 0.62 | - | 0.28 | - | - |
| Aromatic Hydrocarbons | ||||||||||||
| 77 | Toluene | C7H8 | 5.08 | 751 | 757 | MS, RI | - | 0.23 | 5.13 | 4.16 | 3.45 | - |
| 78 | 1,3,5-Cycloheptatriene | C7H8 | 5.38 | 761 | 765 | MS, RI | - | - | - | - | - | 0.91 |
| 79 | 2,4-Dimethylhexane | C8H18 | 6.15 | 787 | - | MS | 7.39 | 1.13 | - | - | - | - |
| 80 | 1-Octene | C8H16 | 6.17 | 788 | 791 | MS, RI | 1.35 | 0.98 | - | - | - | - |
| 81 | (E)-5-Methyl-2-hexene | C7H14 | 8.48 | 852 | - | MS | - | - | - | 1.52 | - | - |
| 82 | (Z)-2-Heptene | C7H14 | 9.17 | 870 | - | MS | - | 4.84 | - | - | - | - |
| 83 | Dodecane | C12H26 | 15.29 | 1038 | - | MS | - | - | - | 2.04 | - | 0.69 |
| 84 | 1-Nonyne | C9H16 | 15.78 | 1049 | - | MS | - | 1.91 | 1.89 | 2.17 | - | - |
| 85 | 2,4,6-Trimethyldecane | C13H28 | 17.91 | 1118 | 1121 | MS, RI | - | - | 1.54 | 1.62 | 1.65 | - |
| 86 | 4,6-Decadiene | C10H18 | 17.92 | 1119 | - | MS | 2.56 | - | - | - | - | - |
| 87 | 4-Ethylphenol | C8H10O | 19.12 | 1158 | 1161 | MS, RI | 0.64 | - | - | - | - | - |
| 88 | 2-Methylundecane | C12H26 | 19.33 | 1162 | 1164 | MS, RI | - | - | - | 1.66 | - | - |
| 89 | 2,3,5,8-Tetramethyldecane | C14H30 | 21.85 | 1251 | - | MS | - | 0.32 | 0.48 | 0.36 | 0.96 | - |
1 Retention time; 2 Retention index of unknown compounds on an HP-5MS column calculated against the gas chromatography-mass spectrometry (GC-MS) retention time of n-alkanes (C3–C25); 3 RI from database (http://webbook.nist.gov/chemistry); 4 RI, agreed with retention index in the literature; MS, compared with NIST 11 Mass Spectral Database; STD, agreed with the mass spectrum of standard chemical. ‘-’ = not detected.
3-Methylbutanal is a branched-chain aldehyde compound derived from isoleucine and leucine by the action of enzymes [27]. As this aldehyde compound has a low taste threshold (1.2 μg /L), trace concentrations can be important characteristics in fermented milk [28,29]. In this study, a high concentration of 3-methylbutanal was present in the volatile fraction of milk fermented by all combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03, particularly in the 1:100 and 1:1000 treatments, where the relative concentrations reached 8.23 and 7.8 μg/L, respectively.
Hexanal and heptanal are also important aroma compounds that contribute to good flavor in fermented milk products [23]. In this study, hexanal concentration reached 5.2 μg/L in the 1:1000 combination; the levels reached 1.43, 1.29, 2.51, and 1.24 μg/L in the 1:1, 1:10, 1:50, 1:100, and 1:10,000 combinations, respectively. As an important flavor compound, hexanal is derived from the oxidation of unsaturated fatty acids and is regularly reported in dairy products, such as fermented milk [30]. In this study, the concentration of heptanal ranged from 1.55 to 2.23 μg/L in the different combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03; there was no detectable heptanal only in the 1:10,000 combination, which may have been due to the smaller initial proportions of L. delbrueckii subsp. bulgaricus in the samples. Similar results were reported by Dan et al. (2017), who reported that heptanal was produced at high levels by L. delbrueckii subsp. bulgaricus during storage [9].
The concentrations of 3-hydroxybutanal and (E)-2-pentenal ranged from 1.16 to 6.96 and from 1.27 to 5.44 μg/L, respectively, reaching maximum values in the 1:1000 combination. Although 3-hydroxybutanal and (E)-2-pentenal had lower threshold values of 27 and 1.2 μg/L, respectively, only the level of (E)-2-pentenal in samples was above the limit of detection. In addition to the above-mentioned aldehyde compounds, the concentrations of (E)-2-heptenal, (E,E)-2,4-heptadienal, (E)-2-nonenal, and (E)-2-hexenal reached maximum values in the 1:1 and 1:50 treatment combinations, respectively. These observations were similar to the results of Ning et al., (2011), who observed changes in (E)-2-heptenal, (E,E)-2,4-heptadienal, and (E)-2-nonenal in fermented camels’ milk, and of Fortini et al. who reported changes in levels of (E)-2-hexenal in olive oil [14,31].
2.5. Profiles of Ketone Compounds
In total, 13 ketone compounds were identified in the samples fermented with different proportions of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 (Table 1). Among these, some are important flavor VOCs and were present at relatively high levels: 2,3-butanedione, 2-pentanone, 3-methyl-2-butanone, acetoin, 2-heptanone, and 2-nonanone. 2,3-Butanedione and acetoin are common metabolic products of citrate metabolism and contribute positively to the perception of buttery and creamy flavors in dairy products [32,33]. The concentration of acetoin was generally higher than those of the other ketone compounds, particularly in the 1:1000 combination. This was similar to the results reported by Rincondelgadillo et al. [34]. 2-Pentanone and 3-methyl-2-butanone were detected in all samples with concentrations of 1.67–5.27 and 1.65–5.28 μg/L, respectively, peaking in the 1:1000 combination. Similarly, the values for 2-heptanone and 2-nonanone reached 25.56 and 10.67 μg/L, respectively; in particular, the 2-nonanone concentration was above the detection limit (5 μg/L) in the 1:1000 combination. These compounds have frequently been reported in dairy products, including milk, fermented milk, and cheese [11,35,36].
2.6. Profiles of Acid Compounds
Sixteen acid compounds were identified in the different combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 (Table 1). Hexanoic acid is a saturated fatty acid with six carbons and one carboxylic group [37]. This acid compound is an important aroma compound that has been detected frequently in a number of different dairy products [14,38]. In this study, hexanoic acid was the most abundant acid compound and was found in all treatment combinations at concentrations ranging from 22.88 to 40.57 μg/L. The next most abundant was acetic acid, followed by butanoic acid and octanoic acid. Acetic acid is the main subproduct of LAB fermentation [39,40]. In this study, acetic acid concentrations ranged from 7.89 to 24.65 μg/L, with the highest value of 24.65 μg/L seen in the 1:1 treatment combination. Rincon-Delgadillo et al. reported similar results [34]. In the present study, high levels of butanoic acid and octanoic acid were detected in all treatment combinations and ranged from 0.62 to 16.83 and from 6.08 to 13.43 μg/L, respectively. These acid compounds are frequently found in dairy products, including fermented milk [33].
3-Methylbutanoic acid and its corresponding alcohols (3-methylbutanol) and aldehydes (3-methylbutanal) are amino acid degradation products. 3-Methylbutanal can be converted to 3-methylbutanoic acid via oxidation [40]. In this study, 3-methylbutanoic acid concentrations ranged from 3.35 to 8.33 μg/L, peaking in the 1:50 treatment combination. The concentrations of 2-methylhexanoic acid, 2-methylbutanoic acid, and pentanoic acid were 2.74–8.15, 7.16–12.42, and 8.38–11.86 μg/L, respectively. These values also peaked in the 1:50 treatment combination.
Low levels of heptanoic acid, nonanoic acid, 2-undecenoic acid, and n-decanoic acid were also detected at concentrations of 1.04–2.02, 1.69–2.99, 0.35–1.11, and 1.07–3.79 μg/L, respectively. Although these concentrations were relatively low compared with those of the other acid compounds, they have also been reported frequently in dairy products by other groups [14,16,23].
2.7. Profiles of Ester Compounds
Esterification reactions occur during lactose fermentation or amino acid catabolism [41]. These ester compounds have low taste thresholds, and therefore low levels can still contribute to good flavor in fermented dairy products. The formation of ester compounds has been studied in dairy products, and some ester compounds were detected in milk fermented by lactococci [32]. Six ester compounds were identified in the present study (Table 1). Among these, traces of formic acid hexyl ester were detected in all treatment combinations, particularly in the 1:100 treatment, where the concentration reached 2.65 μg/L, which was higher than those in the other treatment combinations.
2.8. Profiles of Alcohol Compounds
Similar to ketone compounds, alcohol compounds are important for flavor in dairy products [8]. In general, alcohol compounds are generated by reduction from the corresponding aldehydes [27]. In total, 25 alcohol compounds were detected in the present study (Table 1). High levels of 3-methylbutanol, 1-hexanol, and 1-heptanol were detected in all samples, with concentrations of 14.89–23.12, 3.05–16.29, and 1.86–12.49 μg/L, respectively. The concentrations of these alcohol compounds were higher in the 1:1, 1:10, and 1:50 treatment combinations compared with in the other treatments. 3-Methylbutanol is present in relatively large quantities in dairy products and imparts an “alcoholic and floral” flavor to fermented milk [41]. In the present study, 3-methylbutanol concentrations were calculated in the 1:10 treatment combination and showed a maximum value of 23.12 μg/L. 1-Hexanol and 1-heptanol have also been reported in dairy products [9,14,42]. In the present study, their concentrations reached 16.29 and 12.49 μg/L, respectively, in the 1:1 treatment combination.
Methylalcohols can be generated from corresponding methylketones by reductase activity. Six methylalcohols were detected in the different treatment combinations: 3-methyl-2-butanol, 3-methyl-3-buten-1-ol, 2-methyl-3-pentanol, 3-methyl-2-heptanol, 3-methyl-2-hexanol, and 5-methyl-2-heptanol. Among these, high levels of 3-methyl-2-heptanol were detected, reaching 12.14 μg/L in the 1:50 treatment combination.
2-Heptanol and 2-nonanol have been reported previously at relatively high concentrations in dairy products [43]. However, only low levels of 2-heptanol and 2-nonanol were detected in the present study: the 2-heptanol concentration varied between 0.42 and 0.93 μg/L in the 1:10 and 1:10,000 treatment combinations, respectively, and the 2-nonanol concentration ranged from 1.14 to 3.98 μg/L in the 1:1, 1:10, 1:50, and 1:100 treatment combinations.
1-Octen-3-ol has a low threshold value (1.5 μg/L) and contributes to good flavor in dairy products, even at low concentrations [41]. In the present study, 1-octen-3-ol was present at levels above the detection limit, although it was present only in the 1:1 treatment combination. Friedrich and Acree reported this alcohol compound previously [44].
2.9. Profiles of Aromatic Hydrocarbons
Aromatic hydrocarbons consist of a wide variety of natural and synthetic low molecular mass compounds [45]. Thirteen aromatic hydrocarbons were detected in the present study (Table 1). Among these, a number of important aromatic hydrocarbons were found in the 1:10, 1:50, and 1:1000 treatment combinations, including toluene, 2,4-dimethylhexane, and (Z)-2-heptene, at concentrations of 0.23–5.13, 1.13–7.39, 0–4.84 μg/L, respectively. Toluene has been detected previously in fermented milk using the SPME technique [9,36]. Although high levels of 2,4-dimethylhexane and (Z)-2-heptene were detected in the present study, the influence of these compounds on the flavor of samples is not clear. Future work will target these aromatic hydrocarbons by examining samples to obtain a better understanding of their roles in fermented milk.
2.10. Principal Component Analysis of Volatile Compounds
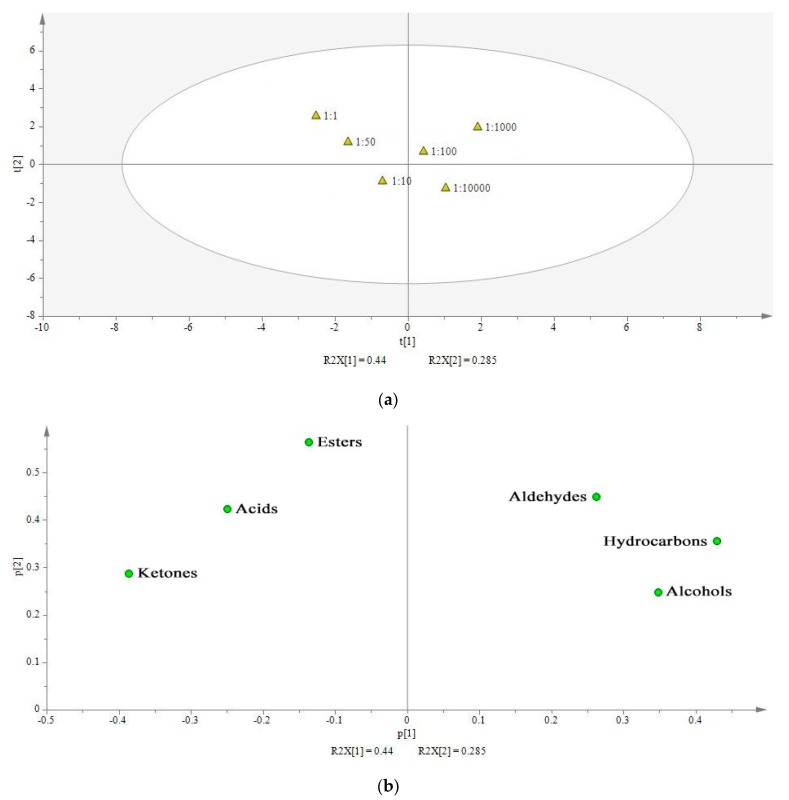
Principal component analysis (PCA) was performed to examine the differences in volatile compounds from milk fermented with different proportions of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03. Figure 3a indicates the score scatter plot for six treatments (1:1, 1:10, 1:50, 1:100, 1:1000, and 1:10,000). The samples could be divided into two distinctive groups. The first group included treatments with a lower proportion of L. delbrueckii subsp. bulgaricus IMAU20401 in the samples (1:100, 1:1000, and 1:1000), whereas the second group included treatments with a higher proportion of L. delbrueckii subsp. bulgaricus IMAU20401 in the samples (1:1, 1:10, 1:50). The results revealed differences in flavor compounds between the two groups. Figure 3b shows a loading scatter plot of six classes of volatile components. There was a positive correlation between flavor and aldehyde compounds in the 1:1000 treatment combination. Nevertheless, a negative correlation was observed between flavor and acid compounds in the 1:1 treatment combination. These results also indicated that the proportions of L. delbrueckii subsp. bulgaricus and S. thermophilus influence the flavor of samples.
Figure 3.
Principal component analysis (PCA) analysis: (a) Score scatter plot of six groups of samples with different strain ratios; (b) Loading scatter plot of six classes of volatile components.
3. Experimental
3.1. Bacterial Isolates and Reagents
Lactobacillus delbrueckii subsp. bulgaricus IMAU20401 was originally isolated from yogurt collected in Huvsgel province, Mongolia; S. thermophilus ND03 was isolated from kurut collected in Qinghai province, China. C3–C25 n-alkanes were purchased from Accustandard (New Haven, CT, USA). 1,2-Dichlorobenzene (internal standard, ISTD) was obtained from Sigma-Aldrich (Steinheim, Germany). de Man Rogosa Sharpe (MRS) and M17 broths were acquired from OXOID (Hampshire, UK). Whole milk powder was purchased from NZMP Ltd. (Wellington, New Zealand). Furthermore, acetaldehyde (from Dr. Ehrenstorfer), acetic acid (from Dr. Ehrenstorfer), lactic acid (from Sigma-Aldrich), 2,3-butanedione (from Sigma-Aldrich), and acetoin (from Sigma-Aldrich) were also used as standards to confirm identifications.
3.2. Sample Production
Whole milk powder was mixed with water at 50 °C to a total solids content of 11.5 g/100 g and supplemented with 6.5 g/100 g of sucrose. The prepared medium was stored at 4 °C prior to use.
Frozen cells of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 were activated by three subcultures in MRS broth and then inoculated into the milk/sucrose medium. Six different inoculation ratios were used: 1:1, 1:10, 1:50, 1:100, 1:1000, and 1:10,000 of L. delbrueckii subsp. bulgaricus IMAU20401 to S. thermophilus ND03. In all combinations, S. thermophilus ND03 was inoculated at a concentration of 5 × 107 colony-forming units (CFU)/mL. After inoculation, fermentation was allowed to proceed at 42 °C until the pH fell to 4.5. Samples were taken and stored at −20 °C prior to analysis of the volatile compounds.
3.3. Microbiological Counts
Viable bacterial counts in the different proportional combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03 (1:1, 1:10, 1:50, 1:100, 1:1000, and 1:10,000) in samples were recorded at pH 4.5 by plating on MRS agar and incubating at 37 °C for 48 h.
3.4. HS-SPME-GC-MS Analysis
The headspace solid-phase microextraction–gas chromatography-mass spectrometry (HS-SPME-GC-MS) technique was used to analyze the volatile compounds produced in each of the combinations of L. delbrueckii subsp. bulgaricus IMAU20401 to S. thermophilus ND03 according to the methods of Aunsbjerg et al. [46]. Briefly, as ISTD, 1,2-dichlorobenzene and 5 mL of sample were mixed in 20-mL glass vials (CNW Technologies, Germany) fitted with a PTFE/silicone septum. The final concentration of ISTD in each sample was 10 μg/L. The samples were stirred for 5 min at 50 °C using microstirring bars to allow the samples to reach equilibrium. Subsequently, a SPME fiber (50/30 um DVB/Carboxen/PDMS; Supelco, Inc. Bellefonte, PA, USA) was exposed in the headspace for 60 min under the same conditions; the fiber was then immediately inserted into the injection port of a 7890 B GC (Agilent Technologies, Inc., Palo Alto, CA, USA) for 5 min at 270 °C to desorb volatile compounds into the GC. The optimum extraction conditions were selected based on preliminary experiments on SPME extraction of samples (1:1 proportional combinations of L. delbrueckii subsp. bulgaricus IMAU20401 and S. thermophilus ND03) at different extraction temperatures (40 °C, 50 °C, 60 °C, and 70 °C) and for different times (40, 50, 60, and 70 min).
3.5. Identification of Volatile Compounds
The volatile compounds from each combination were identified using a 7890 B GC equipped with a 5977 A mass-selective detector (MSD; both Agilent Technologies, Inc.) equipped with an HP-5MS column (length, 30 m; i.d., 0.25 mm; film thickness, 0.25 μm; Agilent Technologies, Inc.). Helium was used as the carrier gas at 1 mL/min. The GC temperature was initially maintained at 35 °C for 5 min and then increased to 140 °C at a rate of 4 °C/min for 5 min, heated to 250 °C at a rate of 10 °C/min, and, finally, held at 250 °C for 5 min. The MSD was made according to the manufacturer’s recommendations in the full scan mode. The ion source and transfer line temperatures were 230 °C and 250 °C, respectively. The mass spectra from each sample were recorded using a scan range of 40–400 m/z with electron impact mode set at a voltage of 70 eV.
Volatile compounds were identified by comparing their mass spectra and retention times with those in the National Institute of Standards and Technology database (NIST version 11 mass spectral database; Agilent Technologies Inc.). The retention indexes (RIs) of detected compounds were calculated by injection of a standard mixture containing C3–C25 n-alkanes in pure hexane under the same chromatographic conditions and then compared with the RI in the database (http://webbook.nist.gov/chemistry). Each sample measurement was carried out in triplicate.
3.6. Statistical Analysis
PCA is commonly used for complex data analysis to summarize variation. It provides a way to characterize multidimensional data and identify similarities and differences. In the present study, the PCA of the data was performed using the SPSS for Windows statistical software package (SPSS Inc., Chicago, IL, USA). Figures were drawn using Origin 7.5 software (OriginLab, Northampton, MA, USA).
4. Conclusions
The increasing demand for flavor in various industrial applications has prompted a great deal of interest in the effects of different ratios of L. delbrueckii subsp. bulgaricus to S. thermophilus in fermented milk products. In the present study, 89 volatile flavor compounds were identified using the HS-SPME-GC-MS technique with DVB/Carboxen/PDMS. These included aldehydes, ketones, acids, alcohols, esters, alcohols, and aromatic compounds. There were significant changes in the profiles of volatile flavor compounds depending on the ratio of the initial proportion of L. delbrueckii subsp. bulgaricus IMAU20401 to that of S. thermophilus ND03. In particular, aldehyde and ketone compound concentrations were higher in the 1:1000 treatment combination than in the other combinations. Our results indicated that selecting the appropriate proportions of L. delbrueckii subsp. bulgaricus and S. thermophilus for the starter culture is important for determination of the final profile of volatiles and overall flavor of milk products.
Acknowledgments
This research was supported by National Natural Science Foundation of China (Beijing; No. 31471711, 31460446, 31671871).
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “T.D. and T.S. conceived and designed the experiments; D.W. and S.W. performed the experiments; W.L. analyzed the data; W.R. contributed reagents/materials/analysis tools; T.D. and T.S. wrote the paper.” Authorship must be limited to those who have contributed substantially to the work reported.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Bernardeau M., Vernoux J.P., Henri-Dubernet S., Gueguen M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008;126:278–285. doi: 10.1016/j.ijfoodmicro.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Yu J., Sun Z., Liu W., Xi X., Song Y., Xu H., Lv Q., Bao Q., Menghe B., Sun T. Multilocus sequence typing of Streptococcus thermophilus from naturally fermented dairy foods in China and Mongolia. BMC Microbiol. 2015;15:1–13. doi: 10.1186/s12866-015-0551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki I., Kato S., Kitada T., Yano N., Morichi T. Growth of Lactobacillus bulgaricus in Milk. 1. Cell Elongation and the Role of Formic Acid in Boiled Milk. J. Dairy Sci. 1986;69:311–320. doi: 10.3168/jds.S0022-0302(86)80407-6. [DOI] [PubMed] [Google Scholar]
- 4.Sieuwerts S., Bok F.A.M.D., Hugenholtz J., Vlieg J.E.T.V.H. Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Appl. Environ. Microbiol. 2008;74:4997–5007. doi: 10.1128/AEM.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko D., Igarashi T., Aoyama K. Reduction of the off-flavor volatile generated by the yogurt starter culture including Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus in soymilk. J. Agric. Food Chem. 2014;62:1658–1663. doi: 10.1021/jf404567e. [DOI] [PubMed] [Google Scholar]
- 6.Beshkova D.M., Simova E.D., Frengova G.I., Simov Z.I., Dimitrov Z.P. Production of volatile aroma compounds by kefir starter cultures. Int. Dairy J. 2003;13:529–535. doi: 10.1016/S0958-6946(03)00058-X. [DOI] [Google Scholar]
- 7.Lin J., Hua B., Xu Z., Li S., Ma C. The impact of proteolytic pork hydrolysate on microbial, flavor and free 388 amino acids compounds of yogurt. Korean J. Food Sci. Anim. Resour. 2016;36:558–565. doi: 10.5851/kosfa.2016.36.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Settachaimongkon S., Nout M.J., Antunes Fernandes E.C., Hettinga K.A., Vervoort J.M., van Hooijdonk T.C., Zwietering M.H., Smid E.J., van Valenberg H.J. Influence of different proteolytic strains of Streptococcus thermophilus inco-culture with Lactobacillus delbrueckii subsp. bulgaricus on themetabolite profile of set-yoghurt. Int. J. Food Microbiol. 2014;177:29–36. doi: 10.1016/j.ijfoodmicro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Dan T., Wang D., Jin R.L., Zhang H.P., Zhou T.T., Sun T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017;100:2488–2500. doi: 10.3168/jds.2016-11528. [DOI] [PubMed] [Google Scholar]
- 10.Arthur C.L., Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990;62:2145–2148. doi: 10.1021/ac00218a019. [DOI] [Google Scholar]
- 11.Chiofalo B., Zumbo A.R., Liotta L., Mondello L., Dugo P., Chiofalo V. Characterization of Maltese goat milk cheese flavour using SPME-GC/MS. S. Afr. J. Anim. Sci. 2004;34:176–180. [Google Scholar]
- 12.Yang X., Peppard T. Solid-phase microextraction for flavor analysis. J. Agric. Food Chem. 1994;42:1925–1930. doi: 10.1021/jf00045a018. [DOI] [Google Scholar]
- 13.Lubbers S., Decourcelle N., Vallet N., Guichard E. Flavor release and rheology behavior of strawberry fat free stirred yogurt during storage. J. Agric. Food Chem. 2004;52:3077–3082. doi: 10.1021/jf0352374. [DOI] [PubMed] [Google Scholar]
- 14.Li N., Zheng F.-P., Chen H.-T., Liu S.-Y., Chen G., Song Z.-Y., Sun B.-G. Identification of volatile components in chinese sinkiang fermented camel milk using SAFE, SDE, and HS-410 SPME-GC/MS. Food Chem. 2011;129:1242–1252. doi: 10.1016/j.foodchem.2011.03.115. [DOI] [PubMed] [Google Scholar]
- 15.San A.T., Joyce D.C., Hofman P.J., Macnish A.J., Webb R.I., Matovic N.J., Williams C.M., Voss J.J.D., Wong S.H., Smyth H.E. Stable isotope dilution assay (SIDA) and HS-SPME-GCMS quantification of key aroma volatiles for fruit and sap of Australian mango cultivars. Food Chem. 2017;221:613–619. doi: 10.1016/j.foodchem.2016.11.130. [DOI] [PubMed] [Google Scholar]
- 16.Panighel A., Flamini R. Applications of solid-phase microextraction and gas chromatography/mass spectrometry (SPME-GC/MS) in the study of grape and wine volatile compounds. Molecules. 2014;19:21291–21309. doi: 10.3390/molecules191221291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corral S., Leitner E., Siegmund B., Flores M. Determination of sulfur and nitrogen compounds during the processing of dry fermented sausages and their relation to amino acid generation. Food Chem. 2016;190:657–664. doi: 10.1016/j.foodchem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues F., Caldeira M., Câmara J.S. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC-qMSD for evaluation the chemical profile in alcoholic beverages. Anal. Chim. Acta. 2008;609:82–104. doi: 10.1016/j.aca.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z.H., Chen X., Wang J.C., Zhao W.J., Shao Y.Y., Wu L., Zhou Z.M., Sun T.S., Wang L., Meng H., Zhang H.P., Chen W. Complete genome sequence of Streptococcus thermophilus strain ND03. J. Bacteriol. 2011;193:793–794. doi: 10.1128/JB.01374-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zha M., Yu J., Zhang Y., Wang H., Bai N., Qin Y., Liangliang D., Liu W., Zhang H., Bilige M. Study on Streptococcus thermophilus isolated from Qula and associated characteristic of acetaldehyde and 432 diacetyl in their fermentedmilk. J. Gen. Appl. Microbiol. 2015;61:50–56. doi: 10.2323/jgam.61.50. [DOI] [PubMed] [Google Scholar]
- 21.Chaves A.C.S.D., Fernandez M., Lerayer A.L.S., Mierau I., Kleerebezem M., Hugenholtz J. Metabolic engineering of acetaldehyde production by Streptococcus thermophilus. Appl. Environ. Microbiol. 2002;68:5656–5662. doi: 10.1128/AEM.68.11.5656-5662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M., Mcintee E.J., Cheng G., Shi Y., Villalta P.W., Hecht S.S. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010;50:938–950. doi: 10.1080/10408390903044081. [DOI] [PubMed] [Google Scholar]
- 24.Ott A., Germond J.E., Germond A. Vicinal diketone formation in yogurt: 13C precursors and effect of branched-chain amino acids. J. Agric. Food Chem. 2000;48:724–731. doi: 10.1021/jf990487z. [DOI] [PubMed] [Google Scholar]
- 25.Ramya I., Tomar S.K., Uma M.T., Rameshwar S. Streptococcus thermophilus strains: Multifunctional lactic acid bacteria. Int. Dairy J. 2010;20:133–141. [Google Scholar]
- 26.Hamdan I.Y., Kunsman J.E., Jr., Deanne D.D. Acetaldehyde production by combined yogurt cultures. J. Dairy Sci. 1971;54:1080–1082. doi: 10.3168/jds.S0022-0302(71)85975-1. [DOI] [Google Scholar]
- 27.Valero E., Villamiel M., Miralles B., Sanz J., MartíNez-Castro I. Changes in flavour and volatile components during storage of whole and skimmed UHT milk. Food Chem. 2001;72:51–58. doi: 10.1016/S0308-8146(00)00203-X. [DOI] [Google Scholar]
- 28.Gadaga T.H., Viljoen B.C., Narvhus J.A. Volatile organic compounds in naturally fermented milk and milk fermented using yeasts, lactic acid bacteria and their combinations as starter cultures. Food Technol. Biotechnol. 2007;45:195–200. [Google Scholar]
- 29.Sheldon R.M., Lindsay R.C., Libbey L.M., Morgan M.E. Chemical nature of malty flavor and aroma produced by Streptococcus lactis var. maltigenes. Appl. Microbiol. 1971;22:263–266. doi: 10.1128/am.22.3.263-266.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang H.Z., Yang S.P., Liu W.J., Yuan L.I. In situ real-time monitoring of volatile metabolites of fermented milk by dynamic headspace sampling-atmospheric pressure ionization mass spectrometry. Food Sci. 2012;33:307–310. [Google Scholar]
- 31.Fortini M., Migliorini M., Cherubini C., Cecchi L., Calamai L. Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile organic compounds (VOO-VOCs) profile. Talanta. 2017;165:641–652. doi: 10.1016/j.talanta.2016.12.082. [DOI] [PubMed] [Google Scholar]
- 32.Alemayehu D., Hannon J.A., Mcauliffe O., Ross R.P. Characterization of plant-derived lactococci on the basis of their volatile compounds profile when grown in milk. Int. J. Food Microbiol. 2014;172:57–61. doi: 10.1016/j.ijfoodmicro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 33.Pan D.D., Wu Z., Peng T., Zeng X.Q., Li H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014;97:624–631. doi: 10.3168/jds.2013-7131. [DOI] [PubMed] [Google Scholar]
- 34.Rincon-Delgadillo M.I., Lopez-Hernandez A., Wijaya I., Rankin S.A. Diacetyl levels and volatile profiles of commercial starter distillates and selected dairy foods. J. Dairy Sci. 2012;95:1128–1139. doi: 10.3168/jds.2011-4834. [DOI] [PubMed] [Google Scholar]
- 35.Bok F.A.M.D., Janssen P.W.M., Bayjanov J.R., Sieuwerts S., Lommen A., Vlieg J.E.T.V.H., Molenaar D. Volatile compound fingerprinting of mixed-culture fermentations. Appl. Environ. Microbiol. 2011;77:6233–6239. doi: 10.1128/AEM.00352-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereda J., Jaramillo D.P., Quevedo J.M., Ferragut V., Guamis B., Trujillo A.J. Characterization of volatile compounds in ultra-high-pressure homogenized milk. Int. Dairy J. 2008;18:826–834. doi: 10.1016/j.idairyj.2007.12.002. [DOI] [Google Scholar]
- 37.Jeon B.S., Moon C., Kim B.C., Kim H., Um Y., Sang B.I. In situ extractive fermentation for the production of hexanoic acid from galactitol by Clostridium sp. BS-1. Enzym. Microb. Technol. 2013;53:143–151. doi: 10.1016/j.enzmictec.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Güler Z. Changes in salted yoghurt during storage. Int. J. Food Sci. Technol. 2007;42:235–245. doi: 10.1111/j.1365-2621.2006.01505.x. [DOI] [Google Scholar]
- 39.Hettinga K.A., van Valenberg H.J., Lam T.J., van Hooijdonk A.C. Detection of mastitis pathogens by analysis of volatile bacterial metabolites. J. Dairy Sci. 2008;91:3834–3839. doi: 10.3168/jds.2007-0941. [DOI] [PubMed] [Google Scholar]
- 40.Smit B.A., Engels W.J., Smit G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009;81:987–999. doi: 10.1007/s00253-008-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molimard P., Spinnler H.E. Review: Compounds involved in the flavor of surface mold-ripened cheeses: Origins and properties. J. Dairy Sci. 1996;79:169–184. doi: 10.3168/jds.S0022-0302(96)76348-8. [DOI] [Google Scholar]
- 42.Van A.M., Duncan S.E., Marcy J.E., Long T.E., O’Keefe S.F., Nielsen-Sims S.R. Aroma analysis of light-exposed milk stored with and without natural and synthetic antioxidants. J. Dairy Sci. 2005;88:881–890. doi: 10.3168/jds.S0022-0302(05)72754-5. [DOI] [PubMed] [Google Scholar]
- 43.Moinas M., Groux M., Horman I. Flaveur des fromages. iii. mise en evidencede quelques constituants mineurs de l’arome du camembert. Dairy Sci. Technol. 1975;55:414–417. doi: 10.1051/lait:197554724. [DOI] [Google Scholar]
- 44.Friedrich J.E., Acree T.E. Gas chromatography olfactometry (GC/O) of dairy products. Int. Dairy J. 1998;8:235–241. doi: 10.1016/S0958-6946(98)80002-2. [DOI] [Google Scholar]
- 45.Fuchs G. Anaerobic metabolism of aromatic compounds. Ann. N. Y. Acad. Sci. 2008;1125:82–99. doi: 10.1196/annals.1419.010. [DOI] [PubMed] [Google Scholar]
- 46.Aunsbjerg S.D., Honoré A.H., Marcussen J., Ebrahimi P., Vogensen F.K., Benfeldt C., Skov T., Knøchel S. Contribution of volatiles to the antifungal effect of Lactobacillus paracasei in defined medium and yogurt. Int. J. Food Microbiol. 2015;194:46–53. doi: 10.1016/j.ijfoodmicro.2014.11.007. [DOI] [PubMed] [Google Scholar]