Abstract
Linear, dimeric, tetrameric, and cyclic peptides derived from lactoferricin B, containing the RRWQWR motif, were designed, synthesized, purified, and characterized using RP-HPLC chromatography and MALDI-TOF mass spectrometry. The antibacterial activity of the designed peptides against E. coli (ATCC 11775 and 25922) and their cytotoxic effect against MDA-MB-468 and MDA-MB-231 breast cancer cell lines were evaluated. Dimeric and tetrameric peptides showed higher antibacterial activity in both bacteria strains than linear peptides. The dimeric peptide (RRWQWR)2K-Ahx exhibited the highest antibacterial activity against the tested bacterial strains. Furthermore, the peptides with high antibacterial activity exhibited significant cytotoxic effect against the tested breast cancer cell lines. This cytotoxic effect was fast and dependent on the peptide concentration. The tetrameric molecule containing RRWQWR motif has an optimal cytotoxic effect at a concentration of 22 µM. The evaluated dimeric and tetrameric peptides could be considered as candidates for developing new therapeutic agents against breast cancer. Polyvalence of linear sequences could be considered as a novel and versatile strategy for obtaining molecules with high anticancer activity.
Keywords: lactoferricin B, E. coli, breast cancer, cytotoxic effect, antibacterial activity, synthetic peptides
1. Introduction
According to the World Health Organization (WHO), breast cancer is the most frequent cancer, impacting over 1.5 million women each year, and also it causes the greatest number of cancer-related deaths among women. In 2015, 570,000 women died from breast cancer, which is approximately 15% of all female deaths causes by cancer [1]. The treatment of the disease consists of surgery, chemotherapy, radiotherapy, and drugs. However, the side effects are harmful, decreasing the quality of life of the patient. These treatments prolong the survival time, but recurrence of the disease is frequent. Chemotherapy causes several adverse effects, such as neutropenia, nausea and vomiting, amenorrhea, alopecia, neurological toxicity, weight gain, secondary leukemia, and cardiotoxicity [2,3]. Hormone therapy also causes adverse effects, such as hot flashes, musculoskeletal pain, fatigue, mood disturbances, nausea, vomiting, and fractures, among others [4].
Antimicrobial peptides (AMPs) exhibit a cytotoxic effect on cancer cell lines and antimicrobial activity because of the electrostatic interaction between the amino acid side chain and the negative charge of the membrane surface [5,6]. AMPs are able to discriminate between neoplastic and non-neoplastic cells, interacting specifically with negatively-charged membrane components such as phosphatidylserine, sialic acid, or heparan sulfate, which differ between cancer and non-cancer cells [7]. AMPs are considered to be a main source of molecules that are candidates for developing new drugs based on peptides. Bovine lactoferrin (BLF) is a milk protein with great biological activity. LF exhibits antimicrobial activity against pathogenic bacteria, fungi, parasites, and viruses. Furthermore, BLF inhibits colon, esophagus, lung, and bladder carcinogenesis in rats when administered orally in the post-initiation stage. BLF has the capacity to reduce the metastatic properties of both MDA-MB-231 and MCF-7 cell lines [8]. The bovine lactoferricin LfcinB: 17FKCRRWQWRMKKLGAPSITCVRRAF41 is a 25 amino acid-peptide belonging to the N-terminal region of BLF [9,10,11,12]. It has been suggested that LfcinB is responsible for the antimicrobial activity of BLF, and LfcinB exhibits greater antimicrobial and anticancerigenic activity than BLF itself [13,14,15]. LfcinB contains aromatic amino acids, such as tryptophan and phenylalanine, and basic residues (arginine and lysine) that confer amphipathic properties [9,16]. These positively-charged residues interact electrostatically with the negative charges of the bacterial cell wall lipopolysaccharide (LPS), allowing the peptide to approach the bacterial membrane [9,16]. Thereupon, hydrophobic residues interact with the membrane lipid bilayer, causing its disruption and cell lysis [9,16]. LfcinB exhibits a cytotoxic effect against human cancer cell lines of breast, gastric, and colorectal cancer, leukemia, fibrosarcomas, melanomas, and colon cancer [17,18,19,20,21]. Subcutaneous administration of LfcinB to mice inhibits lymphoma cell metastasis to the liver and lungs [17,22]. The minimal motif (RRWQWR) exhibited cytotoxic activity in leukemia cells, and the dead cells were related to both Cathepsin B and caspase activity [17,23,24].
In the present paper, linear, dimeric, and tetrameric peptides were synthesized via solid-phase peptide synthesis (SPPS) and purified and characterized using reverse phase high performance liquid chromatography (RP-HPLC) and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS). Peptide antibacterial activity (against E. coli strains) and cytotoxicity against two breast cancer cell lines were evaluated. Our results indicate that peptides derived from LfcinB that exhibit antibacterial activity also exhibit a cytotoxic effect in breast cancer cell lines. Tetrameric and dimeric peptides containing the minimal motif have the greatest cytotoxic effect against both MDA-MB-468 and MDA-MB-231 breast cancer cell lines. This activity was fast and dependent on peptide concentration.
2. Results and Discussion
The aim of this study was to establish if the polyvalence or molecular restriction of LfcinB-derived peptides increases its antibacterial activity and/or cytotoxic effect in breast cancer cell lines. Therefore, linear, dimeric, tetrameric, and cyclic peptides containing sequences derived from LfcinB were designed and synthesized through SPPS, using the Fmoc/tBu strategy.
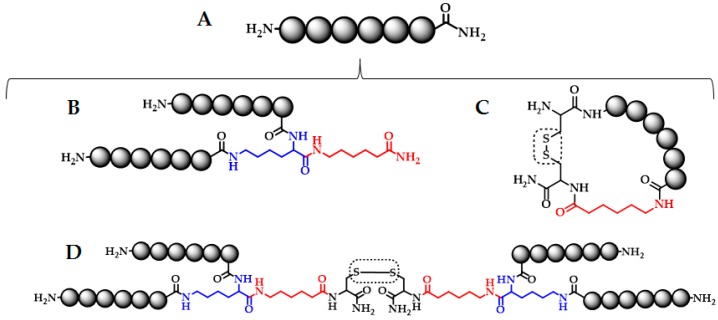
Specifically, sequences LfcinB (20–25): 20RRWQWR25, LfcinB (20–30): 20RRWQWRMKKLG30, and [Ala19]-LfcinB (17–31): 17FKARRWQWRMKKLGA31 were selected. In order to obtain families of each sequence, all groups contained four members, specifically (i) a linear; (ii) a dimeric; (iii) a cyclic; and (iv) a tetrameric peptide, as it is shown in Figure 1.
Figure 1.
Designed and synthesized peptide families derived from LfcinB. Three families of peptides were obtained, each containing four members: a linear (A); a dimeric (B); a cyclic (C); and a tetrameric (D) peptide. As linear peptides, LfcinB (20–25), LfcinB (20–30), and [Ala19]-LfcinB (17–31) were used. The sequence is represented by black spheres; aminohexanoic residues (in red) and lysine residues (in blue) are shown.
Dimeric peptides (Figure 1B) were synthesized using the MAPs (multiple antigen peptides) methodology. The cyclic peptides (Figure 1C) were obtained by oxidation of cysteine residues located at the sequence C and N-terminal ends. Finally, the tetrameric peptides (Figure 1D) were obtained by the formation of an inter-disulfide bridge by the oxidation of purified dimeric precursor peptides (Table 1). All crude products were characterized using RP-HPLC and then purified by means of SPE. In all cases, the chromatographic profile of the purified products exhibited the main specie and purity was determined by RP-HPLC. MALDI-TOF-MS analysis showed that the synthesized peptides had the expected molecular weight (Table 1). As an example, Figure 2 shows the analytical results for peptide LfcinB (20–25); the chromatographic profile of crude product (Panel A) presents a main peak (tR: 4.3 min; purity: 40%). This product was purified and characterized, the RP-HPLC analysis shows a peak with the same retention time and a purity of 92% and its MALDI-TOF MS spectrum (Figure 2C) has a main signal at m/z 986,55 corresponding to [M + H]+. Oxidation reactions were monitored by RP-HPLC; Figure 2D presents the oxidation of a dimeric precursor, (RRWQWR)2K-Ahx-C, at reaction times of 0, 1, and 6 h, producing the tetramer (Lfcin B (20–25)4: (RRWQWR)4K2-Ahx2-C2).
Table 1.
Analytical characterization summary of designed and synthesized LfcinB-derived peptides.
| Synthetic Peptide Sequence | Analytical Characterization | |||
|---|---|---|---|---|
| RP-HPLC | MALDI-TOF MS | |||
| tR (min) | Purity c (%) | Monoisotopic Mass (M) | Experimental m/z, [M + H]+ | |
| 20RRWQWR25 | 4.3 | 92 | 985.54 | 986.55 |
| (RRWQWR)2K-Ahx | 5.1 | 86 | 2195.24 | 2198.46 |
| (RRWQWR)2K-Ahx-C a | 5.2 | 86 | 2298.24 | 2300.93 |
| C-RRWQWR-Ahx-C b | 4.4 | 85 | 1304.64 | 1306.08 |
| 20RRWQWRMKKLG30 | 4.7 | 95 | 1542.87 | 1544.53 |
| (RRWQWRMKKLG)2K-Ahx | 5.3 | 86 | 3309.91 | 3311.62 |
| (RRWQWRMKKLG)2K-Ahx-C a | 5.4 | 90 | 3412.92 | 3415.07 |
| C-RRWQWRMKKLG-Ahx-C b | 5.0 | 95 | 1861.98 | 1862.14 |
| 17FKARRWQWRMKKLGA31 | 4.9 | 95 | 1960.11 | 1961.99 |
| (FKARRWQWRMKKLGA)2K-Ahx | 5.5 | 88 | 4144.38 | 4146.73 |
| (FKARRWQWRMKKLGA)2K-Ahx-C a | 5.5 | 85 | 4247.39 | 4255.51 |
| C-FKARRWQWRMKKLGA-Ahx-C b | 5.2 | 92 | 2279.21 | 2279.93 |
a Precursor of tetrameric peptide; b Precursor of cyclic peptide and c Peptide purity was calculated using the percentage of peak area at the chromatographic profile.
Figure 2.
Reverse phase high performance liquid chromatography (RP-HPLC) analysis of LfcinB (20–25): crude (A) and purified (B) product. Purified LfcinB (20–25) matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF) mass spectrum (C). Tetramer LfcinB (20–25)4 formation by oxidation; RP-HPLC analysis of reaction mixture at different times: 0 h (D: purified dimer precursor, tR 5.2 min); 1 h (E); and 6 h (F: final tetramer product, tR 5.6 min).
The tetrameric peptide LfcinB (20–25)4, had shown antibacterial activity against (i) E. coli (ATCC 11775, MIC: 5.5 µM and ATCC 25922, MIC: 4.4 µM); (ii) S. enteriditis ATCC 13076 (MIC: 44 µM); and (iii) S. aureus ATCC 25923 (MIC: 25 µM). The dimeric peptide LfcinB (20–25)2 also showed antibacterial activity against the same bacterial strains with MIC 2.8 µM, 22.4 µM, 5.6 µM, respectively. Peptide LfcinB (20–25) had shown less antibacterial activity against these bacterial strains (MIC: 12.5 µM, >203 µM, 102 µM, respectively) [15,25,26]. These results showed that dimeric and tetrameric peptides containing the RRWQWR sequence have greater antibacterial activity against E. coli strains (ATCC 11775 and ATCC 25922) than the other tested bacterial strains. In a similar way, the tetramer LfcinB (20–25)4 exhibited greater cytotoxicity against oral squamous carcinoma cell lines than its linear peptide analogue, LfcinB (20–25) [27].
For this research, peptide activity was evaluated in two different models: (i) bacteria and (ii) breast cancer cell lines. First, the designed peptide families were tested against two E. coli strains (ATCC 11775 and 25922). It was found that dimeric and tetrameric peptides exhibit greater antibacterial activity against the evaluated strains than their linear counterpart sequences (Table 1), confirming that the polyvalence enhanced the antibacterial activity. This behavior is in accordance with the mechanism suggested for LfcinB, which involves the initial electrostatic interaction with the negative charges of the bacterial cell wall. It has been proposed that the increase of the positive charge of the molecules enables the interaction with the charged negative molecules of the bacterial surface [10,11,12,13,14]. It has been suggested that LfcinB peptides self-assemble, forming a polymeric structures as a requisite for the interaction with the bacterial surface [9,28]. On the other hand, cyclic peptides exhibit antibacterial activity similar to monomeric peptides, suggesting that molecular restriction of these amino acid sequences does not increase antibacterial activity in the evaluated strains. This indicates that the relevant properties for antibacterial activity are both positively charged and have an amphipathic sequence.
Many new therapies are currently being used for cancer treatment; among these new methods, chemotherapy based on antimicrobial peptides (AMPs) has been of great interest due to the unique advantages of this kind of molecule, such as low molecular weight, ability to specifically target tumor cells, and low toxicity in normal tissues [29]. For example, the cytotoxic effect of AMPs normally occurs at micromolar levels, and it is not accompanied by significant levels of hemolysis or toxicity to other mammalian cells. In most cases, the mechanisms underlying such activity involve disruption of mitochondrial or plasmatic membranes of the target tumor cells [5]. AMPs are considered to be promising molecules for developing new drugs for treating different cancer types. LfcinB is an AMP with potential for designing molecules with antibacterial and anticancer properties. In this context, it is important to identify short sequences derived from LfcinB with anticancerigenic activity, specifically against breast cancer.
For the second part of the experiments, all members of each designed peptide family were tested against MDA-MB-468 and MDA-MB-231 breast cancer cell lines (Figure 3). It was found that the linear peptides LfcinB (20–25), LfcinB (20–30), and [Ala19]-LfcinB(17–31) exhibit a lower cytotoxic effect against both tested cell lines than the polyvalent peptides. When breast cancer cells lines were incubated with linear peptides or cyclic peptides (200 µg/mL), their viability was approximately 80%. Our results are consistent with other reports, which showed that neither T-leukemia nor MDA-MB-231 breast cancer cells were killed by free LfcinB (20–25); however, when this peptide was delivered (fusogenic liposome-mediated) into the cytosolic compartment, it caused extensive DNA fragmentation that was dependent on cathepsin B and caspase activation [17]. In a similar way, LfcinB (20–25) did not display any appreciable anticancer activity towards Jurkat cells, while the linear combination of hLF11 and LfcinB (20–25), which were joined as a single polypeptide chain by introducing either a Pro or a Gly-Gly spacer, considerably increased Jurkat-mediated cytotoxicity [24]. The linear peptides LFcinB (20–25) and LfcinB (20–30) exhibited a minimal cytotoxic effect (IC50 >500 µM) against human gastric cancer cell line AGS [17]. LfcinB and LfcinB (20–25) exhibited only weak cytotoxic activity against the adherent breast cancer cell line MDA-MB-231, even at the highest tested concentration (40 µM) [24].
Figure 3.
Cytotoxic effect of LfcinB-derived peptides against MDA-MB-468 (left) and MDA-MB-231 (right) breast cancer cell lines. Cytotoxic effect, after 2 h incubation time, of each designed peptide (Table 2) at different concentrations can be observed. LfcinB (20–25) family (A); LfcinB (20–30) family (B) and [Ala19]-LfcinB (17–31) family (C). The data are expressed as the mean ± S.D. (n = 3). Statistically significant differences were found in both cell lines at 100 µg/mL for: LfcinB(20–25)4 cf LfcinB (20–25), panel A; LfcinB(20–30)4 and LfcinB (20–30)2 cf LfcinB(20–30), panel B; and [Ala19]-LfcinB (17–31)4 and [Ala19]-LfcinB (17–31)2 cf [Ala19]-LfcinB (17–31), Panel C. (ANOVA, Post hoc Bonferroni, p < 0.05).
It was found that the tetrameric peptide LfcinB (20–25)4 exhibited high cytotoxicity against both tested breast cancer cell lines, and that it is dependent on peptide concentration (Figure 3A). LfcinB (20–25)4 cytotoxic effects were greater in the MDA-MB-468 cell line than in the MDA-MB-231 cell line. In Table 2, the IC50 values indicate that peptides LfcinB (20–30)2, LfcinB (20–30)4, [Ala19]-LfcinB (17–31)2, and [Ala19]-LfcinB (17–31)4, showed also high cytotoxicity in both breast cancer cell lines, suggesting that the polyvalence could be relevant to the observed activity.
Table 2.
LfcinB-derived peptides’ biological activity.
| Peptide Code | Antibacterial Effect MIC/MBC µg/mL (µM) | Cytotoxic Effect IC50 (µM) | ||
|---|---|---|---|---|
| E. coli ATCC 11775 | E. coli ATCC 25922 | MDA-MB-468 | MDA-MB-231 | |
| LfcinB (20–25) | 200(203)/200(203) | 200(203)/200(203) | >203 | >203 |
| LfcinB (20–25)2 | 50(22)/50(22) | 12.5(6)/25(11) | >100 | 130 |
| LfcinB (20–25)4 | 100(22)/200(44) | 100(22)/100(22) | 6 | 15 |
| LfcinB (20–25)cyc | 200(153)/200(153) | 100(77)/>200(>153) | >200 | >200 |
| LfcinB (20–30) | 200(130)/200(130) | 200(130)/200(130) | >130 | >130 |
| LfcinB (20–30)2 | 200(60)/200(60) | 100(30)/200(60) | 5 | 14 |
| LfcinB (20–30)4 | 200(15)/200(15) | 200(15)/200(15) | 2 | 6 |
| LfcinB (20–30)cyc | 200(107)/200(107) | 200(107)/200(107) | >107 | 27 |
| [Ala19]-LfcinB (17–31) | >200(>102)/>200(102) | 200(102)/200(102) | >102 | >102 |
| [Ala19]-LfcinB (17–31)2 | >200(>48)/>200(>48) | 100(24)/100(24) | 11 | 31 |
| [Ala19]-LfcinB (17–31)4 | ND | ND | 5 | 9 |
| [Ala19]-LfcinB (17–31)cyc | >200(>88)/>200(>88) | 200(88)/200(88) | >88 | >88 |
Specifically, the tetrameric peptide exhibits the maximum cytotoxic effect against MDA-MB-468 cell lines at a concentration of 11 µM (50 µg/mL), indicating that these cells are very sensitive to this molecule. The dimeric peptide LfcinB (20–25)2 (200 µg/mL) exhibited an intermediate cytotoxic effect in both tested cell lines. In a similar way, dimeric and tetrameric peptides containing the 20RRWQWRMKKLG30 sequence exhibited greater cytotoxic effect against both breast cancer cells lines than the linear sequence (Figure 3B). The dimer and tetramer exhibited the maximum cytotoxic effect at a concentration of 100 µg/mL, which corresponds to 30 µM and 15 µM, respectively, and their effect is constant at higher concentrations. In this family, the cyclic peptide showed a great cytotoxic effect against MDA-MB-468 cells, the cell viability being near to zero when the peptide concentration was 200 µg/mL (107 µM). The linear peptide exhibited a minimal cytotoxic effect in both breast cancer cell lines. For dimeric and tetrameric peptides containing the 17FKARRWQWRMKKLGA31 sequence, a greater cytotoxic effect against the breast cancer cell lines than their linear and cyclic analogues was also found (Figure 3C).
On the other hand, the dimeric peptides LfcinB (20–30)2 and [Ala19]-LfcinB (17–31)2 exhibited a greater cytotoxic effect than the dimeric peptide containing the minimal motif LfcinB (20–25)2, suggesting that the MKKLGA sequence enhanced the cytotoxic effect of those dimeric molecules. In all cases, tetrameric peptides exhibited a greater cytotoxic effect against MDA-MB-468 cell lines than that observed in MDA-MB-231 breast cancer cell lines.
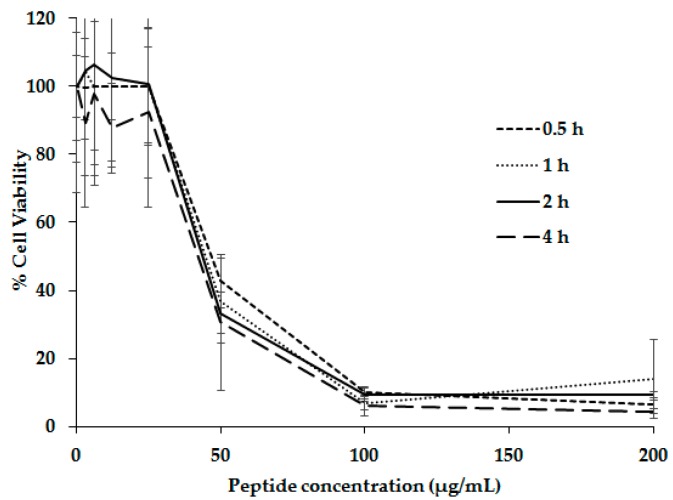
The cytotoxic effect of the tetrameric peptide LfcinB (20–25)4 against MDA-MB-468 breast cancer cell lines was tested at different incubation times, ranging from 30 to 240 min, with a peptide concentration of between 50 and 200 µg/mL (11–44 µM). It was found that at 30 min of treatment, the tetrameric peptide cytotoxic effect was significant, and after the first hour, cell viability was near 5% and was constant up to 4 h (Figure 4).
Figure 4.
Cytotoxic effect of peptide LfcinB (20–25)4 against MDA-MB-468 cell line. Cells were treated with different peptide concentrations and different incubation times.
The results indicate that the cytotoxic effect is fast and independent of incubation time. It depends on peptide concentration, 100 µg/mL (22 µM) being the minimum concentration with maximum cytotoxic effect. This behavior was also observed for oral squamous-cell carcinoma (OSCC) cell lines, SCC15 and CAL27. When they were treated with this tetramer, its cytotoxic effect was significant after the first hour of treatment, and it was constant up to 24 h [27,30].
The peptides LfcinB (20–25)2 and LfcinB (20–25)4 exhibited a greater and faster cytotoxic effect on MDA-MB-231 cells than has been reported for LfcinB (after 18 h of incubation, 45% cell death) [17]. Similarly, dimeric and tetrameric peptides also exhibited a greater and faster cytotoxic effect than BLF and LfcinB in other cancer models: BLF and LfcinB exhibited cytotoxic activity and significantly stimulated the apoptosis of HT-29 cells. The maximum effects were observed at 12 h of treatment, and the optimal concentrations for BLF and LfcinB were 800 µg/mL and 400 µg/mL, respectively [18]. It has been reported that incubation (24 h) of human MDA-MB-435 breast carcinoma cells in the presence of LfcinB caused cell death by apoptosis [29].
Dimeric and tetrameric peptides that exhibited a cytotoxic effect against MDA-468 breast cancer cells, while exhibiting minimal cytotoxic effect in fibroblasts cells (PCS 201-012). The peptide LfcinB (20–25)4 exhibited great cytotoxic effects in MDA-468 cells at 50 µg/mL, while the cytotoxic effect was minimal in PCS-201-012 cells at the same concentration (Figure 5). Dimeric and tetrameric peptides containing 17FKARRWQWRMKKLGA31 and 20RRWQWRMKKLG30 sequences exhibited a similar behavior at 100 µg/mL (Figure 5). These results indicate that the cytotoxic effect of these peptides could be selective for breast cancer cells lines.
Figure 5.
Comparative cytotoxic effect of LfcinB derived peptides against PCS 201-012 and MDA-MB-468 cell lines. (A) Peptide LfcinB (20–25)4; (B) LfcinB (20–30)2 and LfcinB (20–30)4 peptides and (C) [Ala19]-LfcinB (17–31)2 and [Ala19]-LfcinB (17–31)4 peptides.
In Summary, the results indicate that polyvalence of linear sequences increases the antibacterial activity and cytotoxic effects against both oral and breast cancer cell lines. Dimeric and tetrameric peptides containing sequences shorter than LfcinB could be considered as candidates for developing new therapeutic agents against both breast and oral cancer.
It has been reported that both LFB and LfcinB has activity against different cancer types [11,24], we specifically had found that the LfcinB-derived tetramer has activity in oral cancer [30] and, herein, we show that LfcinB-derived peptides have selective cytotoxicity against breast cancer cells. These results are promissory and it is important to evaluate the cytotoxic effect in other breast cancer cell lines as well as in other normal epithelial cell lines (e.g., mammary, bladder, bronchial, corneal, prostate, and renal epithelial cell lines) to determine the spectrum of activity and the selectivity of these peptides. It is also important to establish if these peptides have antitumoral activity in animal model assays.
3. Materials and Methods
3.1. Reagents and Materials
Mueller-Hinton, Agar SPC, Mueller Hinton Broth (MHB), E. coli ATCC 11775 and E. coli ATCC 25,922 were obtained from ATCC (Manassas, VA, USA), Fetal Bovine Serum (FBS) was obtained of Gibco. N,N-diisopropylethylamine (DIPEA), triisopropylsilane (TIPS), 1,2-ethanedithiol (EDT), 4-methylpiperidine, pyridine, and ninhydrin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Rink amide resin, Fmoc-amino acids, 6-chloro-1-hydroxy-benzotriazole (6-Cl-HOBt), and N,N-dicyclohexylcarbodiimide (DCC) were purchased from AAPPTec (Louisville, KY, USA). Methanol, diethyl ether, N,N-dimethylformamide (DMF), absolute ethanol, dichloromethane (DCM), acetonitrile (ACN), isopropylalcohol (IPA), and trifluoroacetic acid (TFA) were obtained from Honeywell-Burdick & Jackson (Muskegon, MI, USA). All reagents were used without further purification.
3.2. Solid Phase Peptide Synthesis
Peptides were synthesized using manual SPPS-Fmoc/tBu [25,31]. Briefly, Rink amide resin (0.46 meq/g) was used as solid support. (i) Fmoc group removal was carried out through treatment with 20% 4-methylpiperidine in DMF); (ii) For the coupling reaction, Fmoc-amino acids (0.21 mmol) were pre-activated with DCC/6-Cl-HOBt (0.20/0.21 mmol) in DMF at RT; (iii) Side-chain deprotection reactions and peptide separation from the resin were carried out with a cleavage cocktail containing TFA/water/TIPS/EDT (93/2/2.5/2.5 v/v/v); (iv) Crude peptides were precipitated by treatment with cool ethyl ether, dried at RT, and analyzed using RP-HPLC analytical chromatography.
3.3. LfcinB-Derived Peptide Characterization
3.3.1. Reverse Phase HPLC
RP-HPLC analysis was performed on a Merck Chromolith® C18 (50 mm × 4.6 mm) column using an Agilent 1200 liquid chromatograph (Omaha, NE, USA) with UV-Vis detector (210 nm). For peptide analysis (1.0 mg/mL crude or purified molecule), 10 μL samples were injected and a linear gradient was applied from 5% to 70% Solvent B (0.05% TFA in ACN) in Solvent A (0.05% TFA in water) for 11.5 min at a flow rate of 2.0 mL/min at room temperature.
3.3.2. Peptide Purification
Molecules were purified using solid-phase extraction columns (SUPELCO LC-18 with 2.0 g resin). SPE columns were activated prior to use with 30 mL acetonitrile (containing 0.1% TFA) and equilibrated with 30 mL water (containing 0.1%TFA). Crude peptides were passed through the column, and a gradient was used for their elution. Collected fractions were analyzed using RP-HPLC (as described above). Fractions that contained pure products were lyophilized.
3.3.3. MALDI-TOF MS
The purified peptides were analyzed by MALDI-TOF mass spectrometry. For sample preparation a solution of peptide (1 mg/mL) was mixed with the matrix (1.0 mg/mL of 2,5-dihydroxybenzoic acid, or sinapinic acid) in a relation of 2:18 (v/v) and 1 µL was seeded on the steel target. The experiment was performed on an Ultraflex III TOF-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) in reflectron mode, using an MTP384 polished steel target (Bruker Daltonics, Bremen, Germany), Laser: 500 shots and 25–30% power.
3.4. LfcinB-Derived Peptide Biological Activity
3.4.1. Antibacterial Activity Assays
The minimal inhibitory concentration (MIC) was determined using a microdilution assay [26]. In brief, bacterial strains were incubated for 18 to 24 h at 37 °C in an Muller Hinton broth (MHB) until an optical density of 0.15 to 0.30 (620 nm) was obtained. 90 µL of MHB was mixed with 90 µL of peptide (440 µg/mL), and using a 96-well microtiter plate peptide, serial dilution (200, 100, 50, 25, 12.5, and 6.2 µg/mL) was performed. 10 µL of inoculum (2 × 106 CFU/mL) was added to each well. Final volume in each well was 100 µL. Then they were incubated for 24 h at 37 °C, and the absorbance at 620 nm was measured using an Asys Expert Plus ELISA reader. For determining the minimum bactericidal concentration (MBC), a small sample was taken from each well using an inoculation loop, which was then spread on MHA plates and incubated overnight at 37 °C. MBC was considered to be the plate which exhibited no bacterial growth. Each of these tests was performed twice (n = 2).
3.4.2. MTT Assay
Cytotoxicity assays were performed as previously described [32]. Briefly, breast cancer cell lines (100 µL; 2.5 × 103 cells/well) were seeded in 96-well flat bottom tissue culture treated plates and were incubated at 37 °C in a 10% CO2 humidified atmosphere for 24 h, allowing for cell adhesion. Then the media was removed, then 100 µL of FBS (5%) and 100 µL of peptide were added. The peptide final concentration was ranging from 200 to 6.25 µg/mL and the final FBS concentration was 2.5%. Plates were incubated 2 h at 37 °C in a 10% CO2 humidified atmosphere (physiologic pH). Negative controls included medium and water. All controls were prepared in triplicate. Cell viability was determined using the MTT assay after 2 h [33]. For this, 10 µL of MTT solution (5 mg/mL) was added to each well, and the plates were incubated for 2 h at 37 °C. Formazan crystals were clarified by centrifugation, the supernatant was discarded, and the crystals were dissolved in DMSO (100 µL). Absorbance (570 nm) was registered on a Bio-Rad 680 microplate reader. (n = 3).
Acknowledgments
This research was conducted with the financial support of División de Investigación y Extensión sede Bogotá (DIEB), Universidad Nacional de Colombia (Project codes 34828 and 36087). Jorge Rodríguez thanks COLCIENCIAS for financing his Ph.D studies.
Author Contributions
Y.V.C. and A.L.L.C. conceived and designed the antibacterial activity experiments; J.A.R.G., Y.A.U.P. and G.A.R. performed the cytotoxic effect experiments; J.E.G.C. and Z.J.R.M. conceived, designed and performed synthesis, purification, and characterization peptides. All the authors contributed to the writing of the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds used in this paper are available from the authors.
References
- 1.World Health Organization Breast cancer. [(accessed on 23 July 2017)]; Available online: http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
- 2.Richie R.C., Swanson J.O. Breast Cancer: A Review of the Literature. J. Insur. Med. 2003;35:85–101. [PubMed] [Google Scholar]
- 3.Satija A., Ahmed S.M., Gupta R., Ahmed A., Rana S.P., Singh S.P., Bhatnagar S.M. Case report. Breast cancer pain management—A review of current & novel therapies. Indian J. Med. Res. 2014;103:216–225. [PMC free article] [PubMed] [Google Scholar]
- 4.Hormone Therapy for Breast Cancer. [(accessed on 23 July 2017)]; Available online: https://www.cancer.org/cancer/breast-cancer/treatment/hormone-therapy-for-breast-cancer.html.
- 5.Dennison S., Whittaker M., Harris F., Phoenix D. Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci. 2006;7:487–499. doi: 10.2174/138920306779025611. [DOI] [PubMed] [Google Scholar]
- 6.Felício M.R., Silva O.N., Gonçalves S., Santos N.C., Franco O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017;5:5. doi: 10.3389/fchem.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedl S., Zweytick D., Lohner K. Membrane-active host defense peptides—Challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids. 2011;164:766–781. doi: 10.1016/j.chemphyslip.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons J.A., Kanwar J.R., Kanwar R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer. 2015;15:425. doi: 10.1186/s12885-015-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnaud S., Evans R. Lactoferrin a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003;40:395–405. doi: 10.1016/S0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 10.Lonnerdal B., Lyer S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 11.García-Montoya I.A., Cendón T.S., Arévalo-Gallegos S., Rascón-Cruz Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta. 2012;1820:226–236. doi: 10.1016/j.bbagen.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita M., Wakabayashi H., Shin K., Yamauchi K., Yaeshima T., Iwatsuki K. Twenty-five years of research on bovine lactoferrin applications. Biochimie. 2009;91:52–57. doi: 10.1016/j.biochi.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- 14.Tomita M., Takase M., Wakabayashi H., Bellamy W. Antimicrobial peptides of lactoferrin. In: Hutchens T.W., Rumball S.V., Lönnerdal B., editors. Lactoferrin: Structure Function. Plenum Press; New York, NY, USA: 1994. pp. 209–218. [DOI] [PubMed] [Google Scholar]
- 15.León M.A., Leal A.L., Almanzar G., Rosas J.E., García J.E., Rivera Z.J. Antibacterial activity of synthetic peptides derived from Lactoferricin against Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212. Biomed. Res. Int. 2015;2015:1–8. doi: 10.1155/2015/453826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorland L.H., Ulvatne H., Rekdal O., Svendsen J.S. Initial binding sites of antimicrobial peptides in Staphylococcus aureus and Escherichia coli. Scand. J. Infect. Dis. 1999;31:467–473. doi: 10.1080/00365549950163987. [DOI] [PubMed] [Google Scholar]
- 17.Richardson A., de Antueno R., Duncan R., Hoskin D.W. Intracellular delivery of bovine lactoferricin’s antimicrobial core (RRWQWR) kills T-leukemia cells. Biochem. Biophys. Res. Commun. 2009;388:736–741. doi: 10.1016/j.bbrc.2009.08.083. [DOI] [PubMed] [Google Scholar]
- 18.Pan W.R., Chen P.W., Chen Y.L., Hsu H.C., Lin C.C., Chen W.J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013;96:7511–7520. doi: 10.3168/jds.2013-7285. [DOI] [PubMed] [Google Scholar]
- 19.Jiang R., Lönnerdal B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2017;95:99–109. doi: 10.1139/bcb-2016-0094. [DOI] [PubMed] [Google Scholar]
- 20.Iigo M., Kuhara T., Ushida Y., Sekine K., Moore M.A., Tsuda H. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin. Exp. Metastasis. 1999;17:35–40. doi: 10.1023/A:1026452110786. [DOI] [PubMed] [Google Scholar]
- 21.Mader J.S., Salsman J., Conrad D.M., Hoskin D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer Ther. 2005;4:612–624. doi: 10.1158/1535-7163.MCT-04-0077. [DOI] [PubMed] [Google Scholar]
- 22.Yoo Y.C., Watanabe S., Watanabe R., Hata K., Shimazaki K., Azuma I. Bovine lactoferrin and lactoferricin, a peptide derived from bovine lactoferrin, inhibit tumor metastasis in mice. Jpn. J. Cancer Res. 1997;88:184–190. doi: 10.1111/j.1349-7006.1997.tb00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilchie A.L., Vale R., Zemlak T.S., Hoskin D.W. Generation of a hematologic malignancy-selective membranolytic peptide from the antimicrobial core (RRWQWR) of bovine lactoferricin. Exp. Mol. Pathol. 2013;95:192–198. doi: 10.1016/j.yexmp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Arias M., Hilchie A.L., Haney E.F., Bolscher J.G., Hyndman M.E., Hancock R.E., Vogel H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2017;95:91–98. doi: 10.1139/bcb-2016-0175. [DOI] [PubMed] [Google Scholar]
- 25.Huertas N.J., Monroy Z.J.R., Medina R.F., Castañeda J.E.G. Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Molecules. 2017;22 doi: 10.3390/molecules22060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huertas Méndez N.J., Vargas Casanova Y., Gómez Chimbi A.K., Hernández E., Leal Castro A.L., Melo Diaz J.M., Rivera Monroy Z.J., García Castañeda J.E. Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076. Molecules. 2017;22 doi: 10.3390/molecules22030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solarte V., Rosas J.E., Rivera Z.J., Arango M.L., García J.E., Vernot J.A. A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines. Biomed. Res. Int. 2015;2015:630179. doi: 10.1155/2015/630179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapple D.S., Hussain R., Joannou C.L., Hancock R.E., Odell E., Evans R.W. Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide. Antimicrob. Agents Chemother. 2004;48:2190–2198. doi: 10.1128/AAC.48.6.2190-2198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Y.F., Jie M.M., Li B.S., Hu C.J., Xie R., Tang B., Yang S.M. Peptide-Based Treatment: A Promising Cancer Therapy. J. Immunol. Res. 2015;2015:761820. doi: 10.1155/2015/761820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solarte V.A., Conget P., Vernot J.P., Rosas J.E., Rivera Z.J., García J.E., Arango-Rodríguez M.L. A tetrameric peptide derived from bovine lactoferricin as a potential therapeutic tool for oral squamous cell carcinoma: A preclinical model. PLoS ONE. 2017;12:e0174707. doi: 10.1371/journal.pone.0174707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergel C., Rivera Z.J., Rosas J.E., García J.E. Efficient Synthesis of Peptides with 4-Methylpiperidine as Fmoc Removal Reagent by Solid Phase Synthesis. J. Mex. Chem. Soc. 2014;58:369–365. [Google Scholar]
- 32.Douglas S., Hoskin D.W., Hilchie A.L. Assessment of antimicrobial (host defense) peptides as anti-cancer agents. Methods Mol. Biol. 2014;1088:159–170. doi: 10.1007/978-1-62703-673-3_11. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]