Abstract
Xanthones, as some of the most active components and widely distributed in various herb medicines, have drawn more and more attention in recent years. So far, 168 species of herbal plants belong to 58 genera, 24 families have been reported to contain xanthones. Among them, Calophyllum, Cratoxylum, Cudrania, Garcinia, Gentiana, Hypericum and Swertia genera are plant resources with great development prospect. This paper summarizes the plant resources, bioactivity and the structure-activity relationships (SARs) of xanthones from references published over the last few decades, which may be useful for new drug research and development on xanthones.
Keywords: herbal medicines, xanthones, plant sources, pharmacology, gambogic acid, structure-activity relationships
1. Introdution
Xanthones (IUPAC name 9H-xanthen-9-one) are a kind of phenolic acid with a three-ring skeleton, widely distributed in herbal medicines. These constituents display a vast range of bioactitivies, including anticancer, anti-oxidative, antimicrobial, antidiabetic, antiviral, and anti-inflammatory effects. So far, at least 515 natural xanthones from 20 families of higher plants (122 species in 44 genera) have been summarized in a few reviews [1,2,3]. These reviews were limited to xanthones with anticancer and anti-inflammatory activities [4]. Their structure-activity relationships (SARs) were also not mentioned.
Over the past few decades, xanthones have become an important resource for drug development. For example, gambogic acid, a prenyl xanthone isolated from Garcinia hanburyi (Clusiaceae), exhibited remarkable apoptosis, cell proliferation and tumor angiogenesis bioactivities, along with anti-oxidant, and anti-inflammatory activities [5,6] and synergistic anticancer activity [7,8]. A phase II clinical trial using gambogic acid in combination with anticancer drugs was carried out in China [9]. Besides gambogic acid mentioned above, mangosteen, another of the most well-known xanthones, has been used as a dietary supplement to improve immune function, decrease serum C-reactive protein levels and increase the ratio of T helper cells [10].
Xanthones are mainly isolated from herbal medicines. Between 1988 and 2016, 168 species of herbal medicinal plant belonging to 58 genera, and 24 families were reported to contain xanthones. This review summarizes the phytochemistry, bioactivity and structure-activity relationships (SARs) of xanthones, which may be helpful to the further new drug research and development.
2. Plant Sources of Xanthones
Table 1 summarizes the phytochemical research on xanthones found in 168 plant species belonging to 58 genera and 24 families.
Table 1.
Plant distribution of xanthones.
| Family | Genus | Species | Reference |
|---|---|---|---|
| Acanthaceae | Andrographis | A. paniculata (Burm. f.) Nees | [11] |
| Anacardiaceae | Mangifera | M. indica L. | [12] |
| Rhus | R. coriaria L. | [13] | |
| Annonaceae | Anaxagorea | A. luzonensis A. Gray | [14] |
| Guatteria | G. blepharophylla Mart. | [15] | |
| Asparagus | Ledebouria | L. ovatifolia (Schrad.) Jessop | [16] |
| Asparagaceae | Anemarrhena | A. asphodeloides Bunge | [17] |
| Drimiopsis | D. maculate Lindl. & Paxton | [18] | |
| Asteraceae | Santolina | S. insularis (Gennari ex Fiori) Arrigoni | [19] |
| Bignoniaceae | Arrabidaea | A. samydoides (Cham.) Sandwith | [20] |
| Bombacaceae | Bombax | B. ceiba L. | [21] |
| Clusiaceae (or Guttiferae) | Allanblackia | A. floribunda Oliv. | [22] |
| A. gabonensis (Pellegr.) Bamps | [23] | ||
| A. monticola Staner L. C. | [24] | ||
| Bonnetia | B. stricta Mart. | [25] | |
| Calophyllum | C. brasiliense Cambess. | [26] | |
| C. caledonicum Vieill. ex Planch. & Triana | [27] | ||
| C. decipiens Wight | [28] | ||
| C. gracilipes Merr. | [29] | ||
| C. inophyllum L. | [30] | ||
| C. membranaceum Gardner & Champ. | [31] | ||
| C. panciflorum A. C. Smith | [32] | ||
| C. pinetorum Bisse | [33] | ||
| C. soulattri Burm. f. | [34] | ||
| C. symingtonianum M.R. Hend. & Wyatt-Sm. | [35] | ||
| C. thorelii Pierre | [36] | ||
| C. thwaitesii Planch. & Triana | [37] | ||
| Chrysochlamys | C. tenuis Hammel | [38] | |
| Clusia | C. pernambucensis G. Mariz | [39] | |
| Cratoxylum | C. cochinchinensis (Lour.) Blume | [40] | |
| C. formosum sp. Pruniflorum (Kurz) Gogelein | [41] | ||
| Garcinia | G. afzelii Engl. | [42] | |
| G. bracteata C.Y. Wu ex Y.H. Li | [43] | ||
| G. cambogia (Gaertn.) Desr. | [44] | ||
| G. cantleyana Whitmore | [45] | ||
| G. cowa Roxb. ex Choisy | [46] | ||
| G. dioica Blume | [47] | ||
| G. dulcis (Roxb.) Kurz | [48] | ||
| G. eugenifolia Wall. ex T. Anderson | [49] | ||
| G. fusca Pierre | [50] | ||
| G. goudotiana (Planch. & Triana) P. Sweeney & Z.S. Rogers | [51] | ||
| G. griffthii T. Anderson | [52] | ||
| G. hanburyi Hook. f. | [53] | ||
| G. hombroniana Pierre | [54] | ||
| G. lancilimba C.Y. Wu ex Y.H. Li | [55] | ||
| G. lateriflora Blume | [56] | ||
| G. linii C.E. Chang | [57] | ||
| G. mangostana L. | [58] | ||
| G. merguensis Wight | [59] | ||
| G. multiflora Champ. ex Benth. | [60] | ||
| G. nigrolineata Planch. ex T. Anderson | [61] | ||
| G. nitida Pierre | [62] | ||
| G. nobilis Engl. | [63] | ||
| G. nujiangensis C.Y. Wu & Y.H. Li | [64] | ||
| G. oblongifolia Champ. ex Benth. | [65] | ||
| G. oligantha Merr. | [66] | ||
| G. oliveri Pierre | [67] | ||
| G. parvifolia (Miq.) Miq. | [68] | ||
| G. paucinervis Chun & F.C. How | [69] | ||
| G. pedunculata Roxb. ex Buch.-Ham. | [70] | ||
| G. penangiana Pierre | [71] | ||
| G. polyantha Oliv. | [72] | ||
| G. porrecta Laness. | [68] | ||
| G. propinqua Craib | [73] | ||
| G. rigida Miq. | [74] | ||
| G. schomburgkiana Pierre | [75] | ||
| G. scortechinii King | [76] | ||
| G. smeathmannii (Planch. & Triana) Oliv. | [77] | ||
| G. staudtii Engl. | [78] | ||
| G. subelliptica Merr. | [79] | ||
| G. succifolia Kurz | [80] | ||
| G. tetralata C.Y. Wu ex Y.H. Li | [81] | ||
| G. vieillardii Pierre | [82] | ||
| G. virgate Vieill. | [83] | ||
| G. xanthochymus Hook. f. ex T. Anderson | [84] | ||
| G. xipshuanbannaensis Y.H. Li | [85] | ||
| Kielmeyera | K. coriacea Mart. | [86] | |
| K. variabilis Mart. & Zucc. | [87] | ||
| Mammea | M. siamensis T. Anderson | [88] | |
| Mesua | M. ferrea L. | [89] | |
| M. hexapetala (Hook. f.) P.S. Ashton | [90] | ||
| Psorospermum | P. adamauense Engl. | [91] | |
| P. febrifugum Spach | [92] | ||
| P. molluscum (Pers.) Hochr. | [93] | ||
| Rheedia | R. acuminata (Ruiz & Pav.) Planch. & Triana | [94] | |
| Symphonia | S. globulifera L.f. | [95] | |
| Vismia | V. laurentii De Wild. | [96] | |
| V. rubescens Oliv. | [96] | ||
| Fabaceae | Caesalpinia | C. sappan L. | [97] |
| Cassia | C. obtusifolia L. | [98] | |
| Cyclopia | C. genistoides (L.) Vent. | [99] | |
| Desmodium | D. caudatum (Thunb.) DC. | [100] | |
| Ganodermataceae | Gyrophora | G. proboscidea (L.) Ach. | [101] |
| Gentianaceae | Centaurium | C. spicatum (L.) Fritsch | [102] |
| Comastoma | C. pedunculatum (Royle ex G. Don) Holub | [103] | |
| C. pulmonarium (Turcz.) Toyok. | [104] | ||
| Gentiana | G. dinarica Beck | [105] | |
| G. kochiana Perr. & Songeon | [106] | ||
| G. lutea L. | [107] | ||
| G. tizuensis Franch. | [108] | ||
| G. utriculosa L. | [109] | ||
| Gentiana dinarica Beck. | [110] | ||
| Gentianella | G. acuta (Michx.) Hiitonen | [111] | |
| G. amarella (L.) Harry Sm. | [112] | ||
| G. turkestanorum (Gand.) Holub | [113] | ||
| Gentianopsis | G. barbata (Froel.) Ma | [114] | |
| G. paludosa (Hook. f.) Ma | [115] | ||
| Halenia | H. corniculata (L.) Cornaz | [116] | |
| H. elliptica D. Don | [117] | ||
| Lomatogonium | L. carinthiacum (Wulfen) A. Braun | [118] | |
| Schultesia | S. lisianthoides (Griseb.) Benth. & Hook. f. ex Hemsl. | [119] | |
| Swertia | S. chirayita (Roxb.) H. Karsten | [120] | |
| S. cordata (Wall. ex G. Don) C.B. Clarke | [121] | ||
| S. corymbosa Wight ex Griseb. | [122] | ||
| S. cuneata Wall. ex D. Don | [123] | ||
| S. elata Harry Sm. | [124] | ||
| S. franchetiana Harry Sm. | [125] | ||
| S. kouitchensis Franch. | [126] | ||
| S. longifolia Boiss. | [127] | ||
| S. minor (Griscb.) Knobl. | [128] | ||
| S. mussotii Franch. | [129] | ||
| S. paniculata | [130] | ||
| S. pseudochinensis H. Hara | [131] | ||
| S. punicea Hemsl. | [132] | ||
| S. speciosa Wall. | [133] | ||
| Tachia | T. grandiflora Maguire & Weaver | [134] | |
| Hippocrateaceae | Salacia | S. chinensis L. | [135] |
| S. elliptica (Mart.) G. Don | [136] | ||
| Hyacinthaceae | Scilla | S. scilloides (Lindl.) Druce | [137] |
| Hypericaceae | Hypericum | H. ascyron L. | [138] |
| H. attenuatum Fisch. ex Choisy | [139] | ||
| H. chinense L. | [140] | ||
| H. erectum Thunb. | [141] | ||
| H. lanceolatum Lam. | [142] | ||
| H. oblongifolium Choisy | [143] | ||
| H. patulum Thunb. | [144] | ||
| H. perforatum L. | [145] | ||
| H. sampsonii Hance | [146] | ||
| H. scabrum L. | [147] | ||
| H. styphelioides A. Rich. | [148] | ||
| Iridaceae | Iris | I. nigricans Dinsm. | [149] |
| Loganiaceae | Anthocleista | A. schweinfurthii Gilg | [150] |
| A. vogelii Planch. | [151] | ||
| Moraceae | Artocarpus | A. kemando Miq. | [152] |
| A. nobilis Thwaites | [153] | ||
| A. obtusus F.M. Jarrett | [154] | ||
| Cudrania | C. cochinchinensis (Lour.) Yakuro Kudo & Masam. | [155] | |
| C. fruticosa (Roxb.) Wight ex Kurz | [156] | ||
| C. tricuspidata (Carrière) Bureau ex Lavallée | [157] | ||
| Maclura | M. cochinchinensis (Lour.) Corner | [158] | |
| Onagraceae | Oenothera | O. biennis L. | [159] |
| Parmeliaceae | Usnea | U. hirta (L.) Weber ex F.H. Wigg | [160] |
| Polygalaceae | Bredemeyera | B. floribunda Willd. | [161] |
| Moutabea | M. guianensis Aubl. | [162] | |
| Polygala | P. caudata Rehder & E.H. Wilson | [163] | |
| P. crotalarioides Buch.-Ham. ex DC. | [164] | ||
| P. cyparissias A. St.-Hil. & Moq. | [165] | ||
| Securidaca | P. hongkongensis Hemsl. | [166] | |
| P. japonica Houtt. | [167] | ||
| P. karensium Kurz | [168] | ||
| P. tenuifolia Willd. | [169] | ||
| P. wattersii Hance | [170] | ||
| S. inappendiculata Hassk. | [171] | ||
| S. longepedunculata Fresen. | [172] | ||
| Rubiaceae | Coffea | C. pseudozanguebariae Bridson | [173] |
| Morinda | M. citrifolia L. | [174] | |
| Theaceae | Pentadesma | P. butyrace Sabine | [175] |
| Xanthorrhoeaceae | Bulbine | B. frutescens (L.) Willd. | [176] |
| Zingiberaceae | Hedychium | H. gardnerianum Sheppard ex Ker Gawl. | [177] |
Among them, the Calophyllaceae, Gentianaceae and Guttiferae are the most widely distributed families.
3. Bioactivities of Xanthones
Recently, some xanthones have been reported to be useful in the treatment of cancer, oxidation, microbial infection, diabetes, inflammation, virus infection et al. Target-based and structure-based activity evaluation has revealed that xanthones are good source of medicine for the treatment of various type of disease. In this part, we summarized pharmacological activities and SARs result of xanthones.
3.1. Effects on Cytotoxicity and Proliferation
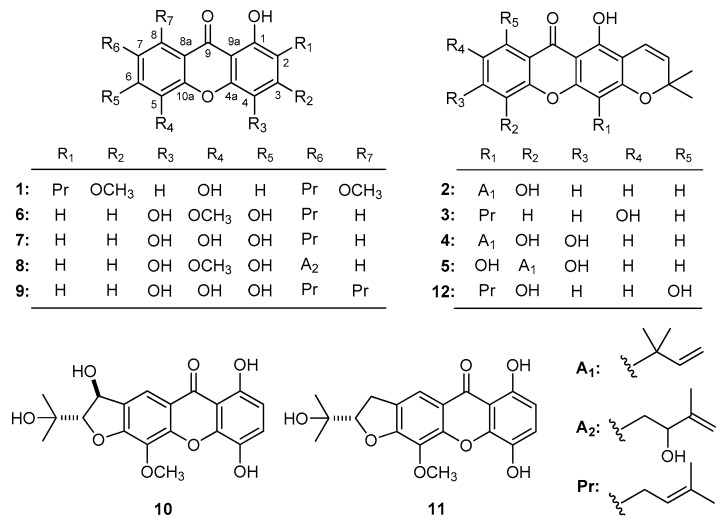
Cancer cytotoxicities of xanthones against leukemia cell lines were evaluated, which were isolated from Artocarpus [154], Calophyllum [34], Garcinia [178], Hypericum [140] genera herbal medicines. On the other hand, the xanthones phylattrin (1), caloxanthone C (2), brasixanthone B (3), macluraxanthone (4), and soulattrin (5) (Figure 1) obtained from C. soulattri [34] showed cytotoxic activities against the chronic myelogenous leukemia cell line (K562) (IC50: 22.10 ± 0.61, 18.20 ± 0.76, 31.00 ± 0.21, 5.28 ± 0.22, and 2.23 ± 0.13 μM, respectively). Their MTT test results indicated that with increasing number of hydroxyl groups, the anti-proliferative activity was enhanced (2 < 4).
Figure 1.
The structures of compounds 1–12.
In addition, the tests carried out by Niu et al. [43] supplemented the conclusions mentioned above. Thirty-one kinds of xanthones, including 1,4,6-trihydroxy-5-methoxy-7-prenylxanthone (6), 1,4,5,6-tetrahydroxy-7-prenylxanthone (7), bracteaxanthone III (8), 1,4,5,6-tetrahydroxy-7,8-di(3-methylbut-2-enyl)xanthone (9), bracteaxanthones V (10), IV (11), and garcinexanthone B (12) (Figure 1) were obtained from G. bracteata. Activity screening results revealed that the isoprenyl group played an important role in the HL-60 cytotoxicity. Among them, 7 and 9 showed stronger inhibitory abilities with IC50 at 10.1 ± 3.1, 2.8 ± 1.1 μM, respectively. The activities difference between these compounds suggested that along with the number of isoprenyl group increasing, the cytotoxicities became stronger. Meanwhile, the hydroxylation (6 > 8) or the cyclization into a furan or pyran ring of isoprenyl group (6 > 10–12) lowered the activity compared with corresponding compounds (IC50: 9.9 ± 0.8, 21.0 ± 0.5, 22.2 ± 0. 6, 18.0 ± 0.7 and 22.8 ± 0.4 μM for 6, 8, 10, 11 and 12, respectively).
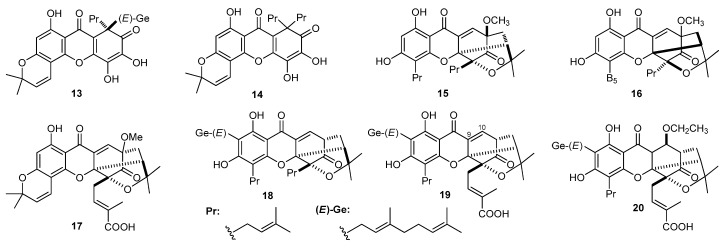
Xanthones from Garcinia [66], Polygala [166] genera plants were found to exhibit inhibitory activities in A549 lung cancer cell line. Oliganthins H (13), I (14), gaudichaudione H (15), cantleyanone (16), and oliganthone B (17) (Figure 2) isolated from G. oligantha [66] showed anti-proliferative potency for A549 with IC50 at 5.0 ± 0.32, 5.5 ± 0.47, 3.0 ± 0.49, 2.9 ± 0.42, 3.9 ± 0.86 μM, respectively. Compounds 15, 16 and 17 exhibited stronger inhibitory abilities compared with 13 and 14, which indicated that caged-xanthones may have better performances on inhibiting the growth of A549 cell line. A cytotoxicity screen on desoxygambogenin (18), isogambogenic acid (19), and 10α-ethoxy-9,10-dihydrogambogenic acid (20) (Figure 2) from G. hanburyi [53] against the A549 cell line indicated that the carboxylation of the side chain of the caged-xanthones decreased the activity (18 > 19), and the caged-xanthones with an olefinic bond between C-9 and C-10 depslayed higher inhibitory ability than hydroxyl substituted ones (19 > 20).
Figure 2.
The structures of compounds 13–20.
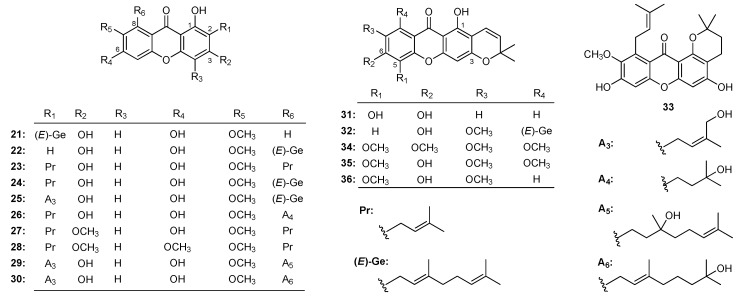
Xanthones isolated from Garcinia genus plants showed significant inhibitory effect in colon cancer cells. The cytotoxicity of cowaxanthone (21), rubraxanthone (22), α-mangostin (23), cowanin (24), cowanol (25) (Figure 3) from G. oliveri [67] against DLD-1 cell line was confirmed by the MTT method with IC50 value at 24.4 ± 0.7, 33.9 ± 2.2, 12.2 ± 0.4, 13.2 ± 0.2, 14.8 ± 2.1 μM, respectively. SARs results suggested that free 3,6-dihydroxyl group and isoprenyl side chain at C-2 and C-8 of were active units.
Figure 3.
The structures of compounds 21–36.
α-Mangostin (23), cowanin (24), cowanol (25), garcinone D (26), β-mangostin (27), fuscaxanthone C (28), fuscaxanthone I (29), kaennacowanol A (30), jacareubin (31), fuscaxanthone A (32), and 1-isomagostin (33) (Figure 3) were obtained from G. cowa [179]. To cervical cancer Hela cell line, IC50 value of cytotoxicities were 13.69, 11.68, 12.19, 22.58, 12.78, inactive, 17.20, 16.70, 11.43, inactive, 34.04 μM, respectively. SARs analysis results indicated that geranyl moiety at C-8 (24, 25 > 23; 29, 30 > 26) and the hydroxyl group at C-1, C-3, C-5 and C-6 enhanced their cytotoxicities (23 > 33, 24 > 32, 31 > 32, 27 > 28).
Cylindroxanthones A–C (34, 35 and 36) (Figure 3) were gained from G. cylindrocarpa [180], along with the increase of methoxy, the anti-proliferative potency against oral epidermoid KB cell line (IC50: 2.36, 59.05 and 57.24 μM for 34, 35 and 36 respectively) increased. The results indicated that the oxidation of the unsaturated isoprenyl group reduced anti-proliferative activity.
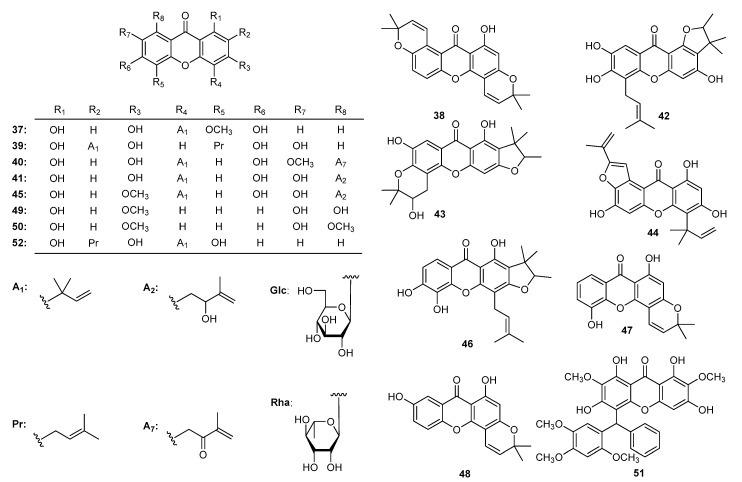
Neuroblastoma (SHSY5Y) cell line proliferation could be inhibited by neriifolone A (37), cudraxanthones A (38), L (39), cudratrixanthones C (40), G (41), H (42), I (43), O (44), 3-O-methyl-cudratrixanthone G (45), gerontoxanthone C (46), 6-deoxyisojacareubin (47), and nigrolineaxanthone F (48) (Figure 4) [181]. Moreover, gentiakochianin (49) and gentiacaulein (50) from G. kochiana [106] were also proved to promote cell cycle arrest in G2/M and G0/G1 phases in U251 human glioma cell line. Muchimangin B (51) [172] and allanxanthone A (52) (Figure 4) [23] inhibited the growth of pancreatic cancer (PANC-1), multiple myeloma (RPMI8226) and gastric cancer (BGC-823) cell lines. The SARs of above xanthones were not discussed for limitation on test sample number.
Figure 4.
The structures of compounds 37–52.
Although some xanthones showed significant inhibitory effects on cancer cell growth in vitro, the in vivo validatation report is rare, which limited their potential for development into new drug for anticancer.
3.2. Free Radical Scavenging Activity
Free radicals are defined as atoms with one unpaired electron, which can be formed through natural physiological processes. Overproduction of free radicals can accelerate the progression of cancer, cardiovascular disease, and age-related diseases.
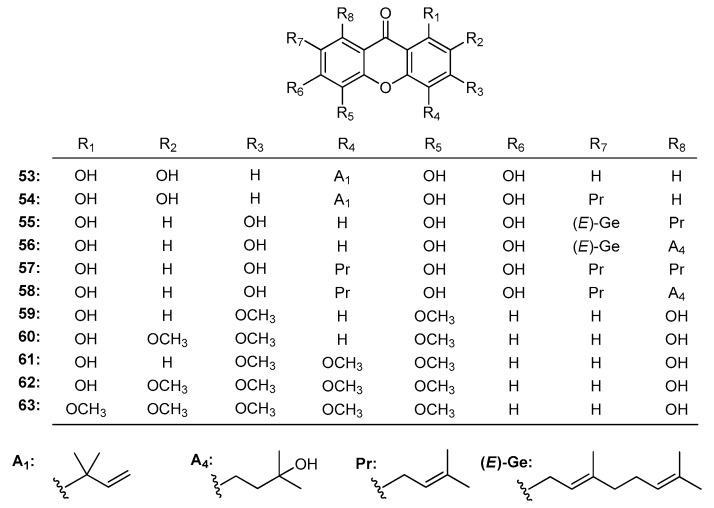
Symphoxanthone (53), subeliptenone B (54), garciniaxanthone E (55), garcinenone D (56), 1,3,5,6-tetrahydroxy-4,7,8-tri(3-methyl-2-butenyl)xanthone (57), garcinenone E (58) (Figure 5) were isolated from G. xanthochymus [84]. Their IC50 values against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical were 6.4, 6.0, 10.1, 6.8, 10.1, 8.5 μM, respectively. SARs analysis indicated that the radical-scavenging activity was partly related to phenolic hydroxy moiety numbers. Within them, compounds with ortho diphenolic hydroxy groups showed significant radical-scavenging activity.
Figure 5.
The structures of compounds 53–63.
As described above, 1,8-dihydroxy-4,6-dimethoxyxanthone (59), 1,8-dihydroxy-4,6,7-trimethoxyxanthone (60), 1,8-dihydroxy-4,5,6-trimetoxyxanthone (61), 1,8-dihydroxy-4,5,6,7-tetra-methoxyxanthone (62), 1-hydroxy-4,5,6,7,8-pentamethoxyxanthone (63) (Figure 5) [162]. The DPPH scavenging order was 59 (1.3 μg) < 63 (0.6 μg) < 60 and 61 (0.3 μg) < 62 (0.15 μg). Agreeing with literature reports, the results indicated that DPPH radical scavenging activities might be attributed to the phenol-like OH groups at the xanthone skeleton [182].
3.3. Anti-Microbial Activity
Xanthones show suppressive effects on microorganisms, such as Gram-positive or negative bacteria and fungi. The resources include Allanblackia [23], Cassia [102], Centaurium [106], Cratoxylum [40], Garcinia [183], Hypericum [97], Kielmeyera [87], Psorospermum [91], Swertia [133], Usnea [160], and Vismia [96] genera plants.
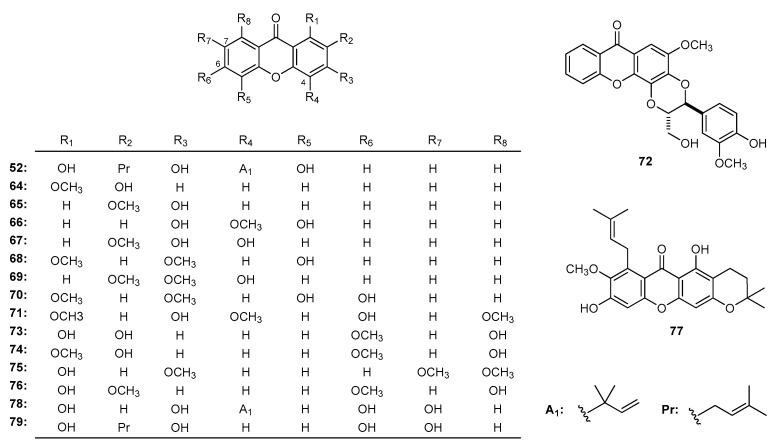
2-Hydroxy-1-methoxyxanthone (64), 3-hydroxy-2-methoxyxanthone (65), 3,5-dihydroxy-4-methoxyxanthone (66), 3,4-dihydroxy-2-methoxyxanthone (67), 5-hydroxy-1,3-dimethoxyxanthone (68), 4-hydroxy-2,3-dimethoxyxanthone (69), 3,4-dihydroxy-6,8-dimethoxyxanthone (70), 3,6-di-hydroxy-1,4,8-trimethoxyxanthone (71), and kielcorin (72) (Figure 6) obtained from K. variabilis [87] showed strong activities against EMRSA-16. According to the results, phenol-like OH groups at the xanthone skeleton may play an important role in the inhibitory ability on the proliferation of microorganisms (MIC: 32, 32, 32, 16, 64, 64, > 512 mg/L for 64, 65, 66 + 70 + 71, 67, 68, 69, 72, respectively). The isolates 1,7,8-trihydroxy-3-methoxyxanthone (73), gentiacaulein (74), and decussatin (75) (Figure 6) obtained from S. mussotii [133] were proved to inhibit the growth of M. tuberculosis with the same MICs at 125 μg/mL, while 1,8-dihydroxy-2,6-dimethoxyxanthone (76) exhibited negative results. The SAR analysis indicated that the C-2, 4, 5 hydroxyl or methoxyl on the xanthone skeleton may influence the activities in resisting bacterial infection.
Figure 6.
The structures of compounds 52, 64–79.
The antibacterial capacity of α-mangostin (23), cowanin (24), fuscaxanthone A (32), 9-hydroxycalabaxanthone (77) (Figure 6) [183] against Staphylococcus aureus suggested that the increase of the unsaturated isoprenyl groups lowered the anti-bacterial ability. Boonnak et al. [40] reported that 1,3,7-trihydroxyxanthones with isoprenyl or geranyl side chain and 1,3,7-trioxygenated xanthone with geranyl side chain showed strong inhibitory activity on P. aeruginosa (a kind of Gram-negative bacteria).
Allanxanthones A (52), D (78) and 1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)xanthone (79) (Figure 6) obtained from the stem bark of A. gabonensis [23] were studied for their antifungal ability against Candida krusei. Allanxanthone D (78) showed stronger antibiotic activity than the reference antibiotic (IC50 μg/mL: 1.22, 2.44, 2.44, 4.88, for 52, 78, 79, and the reference antibiotic nystatin, respectively), which indicated that the oxygen substitution at C-6, 7 and the prenyl substitution at C-4 may be the active units.
3.4. α-Glucosidase Inhibitory Activity
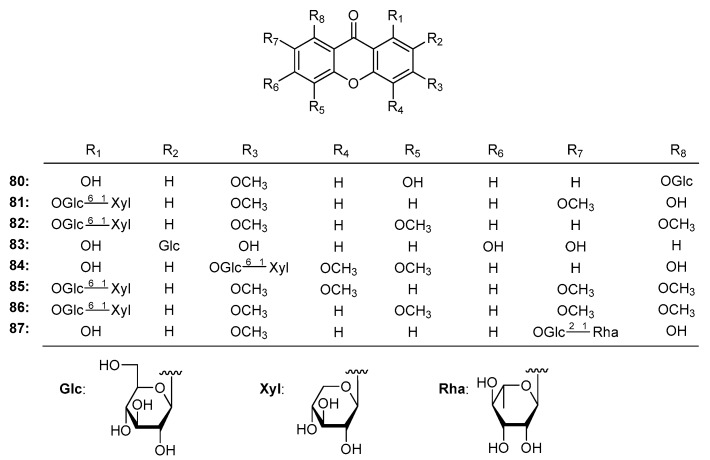
Plants resources with α-glucosidase inhibitory activity include the Cudrania [155], Garcinia [184], and Swertia [130] genera. Swertianolin (80), 1-O-[β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl]-8-hydroxy-3,7-dimethoxyxanthone (81), kouitchenside D (82), mangiferin (83), kouitchensides F (84), B (85), E (86), kouitchenside I (87) (Figure 7) isolated from S. kouitchensis [130] showed inhibitory effects on α-glucosidase with IC50 values of 126 ± 23, 451 ± 41, 360 ± 39, 296 ± 52, 184 ± 23, 383 ± 18 and 371 ± 22 μM, inactive, respectively. The results revealed that the substitution with a primeverosyl residue led to increased inhibitory effects than other diglycoside units (81, 82, 84, 85, and 86 > 87), and oxygen substitution at C-1 or C-8 (80, 83 and 84), while a diglycoside residue located at C-7 (87) reduced the inhibitory activity.
Figure 7.
The structures of compounds 80–87.
3.5. Anti-Virus Activity
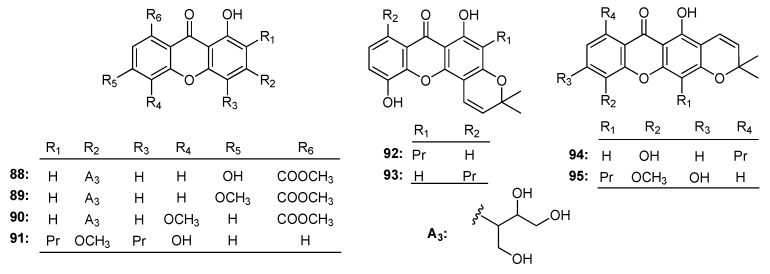
Xanthones obtained from Comastoma [108], Garcinia [178], and Swertia [128] genera exhibited inhibitory effects on tobacco mosaic virus (TMV). The anti-TMV half-leaf tests on paucinervins E (88), F (89), G (90), cudraxanthone G (91), ananixanthone (92), merguenone (93), nigrolineaxanthone K (94), 5-O-methylxanthone V1 (95) (Figure 8) (IC50: 21.4 ± 2.3, 42.8 ± 3.0, 53.6 ± 2.2, 52.8 ± 3.0, 68.9 ± 2.3, 82.4 ± 2.6 μM, for 88, 89, 90, 91, 92, 95, respectively) [107] suggested that the hydroxyl groups might be one of the active units (88 > 89 and 90). The introduction of the pyran ring (92, 93, 94, and 95) or the interaction through hydrogen bonding with an isoprenyl group (91) would lower the inhibitory activities.
Figure 8.
The structures of compounds 88–95.
3.6. Anti-Inflammatory Activity
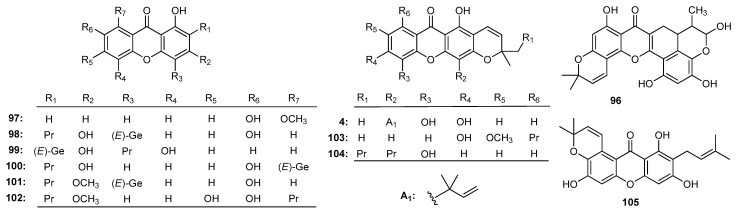
During the past ten years, plants belonging to the Artocarpus [154], Calophyllum [185], Cratoxylum [41], and Garcinia [46] genera have been reported to display anti-inflammatory activity. Pyranocycloartobiloxanthone A (96, Figure 9), a novel xanthone isolated from A. obtusus [154] presented not only anti-inflammatory and anti-oxidant activities, but also anti-apoptotic and anti-bacterial effects against Helicobacter pylori. The inhibitory effects of 1,7-dihydroxy-8-methoxyxanthone (97), cochinchinone A (98), formoxanthone A (99), macruraxanthone (4), cochinxanthone E (100), pruniflorone L (101), dulcisxanthone F (102), 5,9-dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methyl-but-2-enyl)-2H,6H-pyrano-[3,2b]-xanthone (103), pruniflorone K (104), and garcinone B (105) (Figure 9) obtained from C. formosum ssp. pruniflorum showed inhibitory effects on NO production by murine macrophage-like RAW264.7 cells [41]. Among them, compounds 99, 102, 103 displayed good suppressive ability on NO production with IC50 values of 8.0, 3.9, and 4.3 μM, respectively, while 98, 100 and 105 showed moderate activity with IC50 values of 12.6, 12.8 and 11.8 μM, respectively. The investigation indicated that tetraoxygenated xanthone skeleton exhibited inhibition of NO production greater than trioxygenated xanthone skeleton, while the methoxyl group at C-3 or C-7 on the tetraoxygenated isoprenylated-xanthone skeleton was an essential group.
Figure 9.
The structures of compounds 4, 96–105.
4. Conclusions
As a class of secondary metabolites obtained from a number of herbal medicines, xanthones are playing more and more important roles in new drug research and development. The pharmacokinetics and toxicity (PK/tox) properties of xanthones, as part of the most crucial preclinical studies, have proved that xanthones are promising drug candidates owing to their high efficacy and low toxicity [186,187].
In this paper, we have summarized the plant sources, bioactivity and the SARs of xanthones from literature published over the last few decades. As a result, 168 species of herbal medicine plants belonging to 58 genera, and 24 families were found to be enriched in xanthones. Among them, the Calophyllum, Cratoxylum, Cudrania, Garcinia, Gentiana, Hypericum and Swertia genera are the plant resource with the most development prospect. Xanthones display multiple bioactivities, which may be useful for new drug development for cancer, inflammation, bacterial, fungal and viral infection, diabetes, and so on.
Acknowledgments
Part of this research was supported by the Program of the National Natural Science Foundation of China (No. 81673688, 81673703), Natural Science Foundation of Tianjin City (No. 15JCYBJC54900), and Changjiang Scholars and Innovative Research Team in University (PCSIRT IRT_14R41).
Author Contributions
Yi Zhang and Tao Wang designed the manuscript; Jingya Ruan wrote the manuscript; Chang Zheng, Yanxia Liu, and Lu Qu retrieved the literature; Haiyang Yu and Lifeng Han perfected the language. All authors discussed, edited and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vieira L.M., Kijjoa A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005;12:2413–2446. doi: 10.2174/092986705774370682. [DOI] [PubMed] [Google Scholar]
- 2.Peres V., Nagem T.J., de Oliveira F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry. 2000;55:683–710. doi: 10.1016/S0031-9422(00)00303-4. [DOI] [PubMed] [Google Scholar]
- 3.Han Q.B., Xu H.X. Caged Garcinia xanthones: Development since 1937. Curr. Med. Chem. 2009;16:3775–3796. doi: 10.2174/092986709789104993. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez-Orozco F., Failla M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients. 2013;5:3163–3183. doi: 10.3390/nu5083163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Chen W. Gambogic Acid is a Novel Anti-cancer Agent that Inhibits Cell Proliferation, Angiogenesis and Metastasis. Anticancer Agents. Med. Chem. 2012;12:994–1000. doi: 10.2174/187152012802650066. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Liu W., Zhao Q., Qi Q., Lu N., Yang Y., Nei F.F., Rong J.J., You Q.D., Guo Q.L. Synergistic effect of 5-fluorouracil with gambogic acid on BGC-823 human gastric carcinoma. Toxicology. 2009;256:135–140. doi: 10.1016/j.tox.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y., Yang L., You Q.D., Nie F.F., Gu H.Y., Zhao L., Wang X.T., Guo Q.L. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256:259–266. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang T., Wei J., Qian X., Ding Y., Yu L., Liu B. Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett. 2008;262:214–222. doi: 10.1016/j.canlet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z.T., Wang J.W. Phase I human tolerability trial of gambogic acid. Chin. J. New Drugs. 2007;1:79–83. [Google Scholar]
- 10.Tang Y.P., Li P.G., Kondo M., Ji H.P., Kou Y., Ou B. Effect of a mangosteen dietary supplement on human immune function: A randomized, double-blind, placebo-controlled trial. J. Med. Food. 2009;12:755–763. doi: 10.1089/jmf.2008.0204. [DOI] [PubMed] [Google Scholar]
- 11.Dua V.K., Verma G., Dash A.P. In vitro antiprotozoal activity of some xanthones isolated from the roots of Andrographis paniculata. Phytother. Res. 2009;23:126–128. doi: 10.1002/ptr.2556. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez J.E., Zambrano R., Sepúlveda B., Simirgiotis M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of chilean pica mango fruits (Mangifera indica L. Cv. piqueño) Molecules. 2013;19:438–458. doi: 10.3390/molecules19010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh O., Ali M., Akhtar N. New antifungal xanthones from the seeds of Rhus coriaria L. Z. Naturforsch. C. 2011;66:17–23. doi: 10.5560/ZNC.2011.66c0017. [DOI] [PubMed] [Google Scholar]
- 14.Sabphon C., Sermboonpaisarn T., Sawasdee P. Cholinesterase inhibitory activities of xanthones from Anaxagorea luzonensis A. Gray. J. Med. Plant Res. 2012;6:3781–3785. [Google Scholar]
- 15.Emmanoel V.C., Assis M.F., Lúcia B.M., Braga R.M. Chemical constituents isolated from the bark of Guatteria blepharophylla (Annonaceae) and their antiproliferative and antimicrobial activities. J. Braz. Chem. Soc. 2011;22:1111–1117. [Google Scholar]
- 16.Waller C.P., Thumser A.E., Langat M.K., Crouch N.R., Mulholland D.A. COX-2 inhibitory activity of homoisoflavanones and xanthones from the bulbs of the Southern African Ledebouria socialis and Ledebouria ovatifolia (Hyacinthaceae: Hyacinthoideae) Phytochemistry. 2013;95:284–290. doi: 10.1016/j.phytochem.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Guo J., Xu C.H., Xue R., Jiang W.X., Wu B., Huang C.G. Cytotoxic activities of chemical constituents from rhizomes of Anemarrhena asphodeloides and their analogues. Arch. Pharm. Res. 2015;38:598–603. doi: 10.1007/s12272-014-0431-4. [DOI] [PubMed] [Google Scholar]
- 18.Mulholland D.A., Koorbanally C., Crouch N.R., Sandor P. Xanthones from Drimiopsis maculate. J. Nat. Prod. 2004;67:1726–1728. doi: 10.1021/np040076z. [DOI] [PubMed] [Google Scholar]
- 19.Cottiglia F., Casu L., Bonsignore L., Casu M., Floris C., Sosa S., Altinier G., Loggia R.D. Topical anti-inflammatory activity of flavonoids and a new xanthone from Santolina insularis. Z. Naturforsch. C. 2005;60:63–66. doi: 10.1515/znc-2005-1-212. [DOI] [PubMed] [Google Scholar]
- 20.Pauletti P.M., Castro-Gamboa I., Silva D., Helena S. New antioxidant C-glucosylxanthones from the stems of Arrabidaea samydoides. J. Nat. Prod. 2003;66:1384–1387. doi: 10.1021/np030100t. [DOI] [PubMed] [Google Scholar]
- 21.Sati S.C., Sati M.D., Sharma A. Isolation and characterization of flavone di-glucoside and acetoxyxanthone from the flowers of Bombex ceiba. J. Appl. Nat. Sci. 2011;3:128–130. [Google Scholar]
- 22.Nkengfack A.E., Azebaze G.A., Vardamides J.C., Fomum Z.T., Heerden F.R. A prenylated xanthone from Allanblackia floribunda. Phytochemistry. 2002;60:381–384. doi: 10.1016/S0031-9422(02)00036-5. [DOI] [PubMed] [Google Scholar]
- 23.Azebaze A.G.B., Ouahouo B.M.W., Vardamides J.C., Valentin A., Kuete V., Acebey L., Beng V.P., Nkengfack A.E., Meyer M. Antimicrobial and antileishmanial xanthones from the stem bark of Allanblackia gabonensis. Chem. Nat. Compd. 2008;44:582–587. doi: 10.1007/s10600-008-9141-9. [DOI] [PubMed] [Google Scholar]
- 24.Azebaze A.G., Menasria F., Noumi L.G., Nguemfo E.L. Xanthones from the seeds of Allanblackia monticola and their apoptotic and antiproliferative activities. Planta Med. 2009;75:243–248. doi: 10.1055/s-0028-1088375. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho M.P., Lima M.M.C., Santos M.G., Rocha L.M., Kuster R.M. Anthraquinones and xanthone from Bonnetia stricta and their chemosystematic significance. Biochem. Syst. Ecol. 2013;48:73–75. doi: 10.1016/j.bse.2012.11.019. [DOI] [Google Scholar]
- 26.Blanco-Ayala T., Lugo-Huitron R., Serrano-Lopez E.M., Reyes-Chilpa R., Rangel-Lopez E., Pineda B., Medina-Campos O.N., Sanchez-Chapul L., Pinzon E., Cristina T.S. Antioxidant properties of xanthones from Calophyllum brasiliense: Prevention of oxidative damage induced by FeSO4. BMC Complement. Altern. Med. 2013;13:262. doi: 10.1186/1472-6882-13-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oger J.M., Morel C., Helesbeux J.J., Litaudon M., Seraphin D., Dartiguelongue C., Larcher G., Richomme P., Duval O. First 2-hydroxy-3-methylbut-3-enyl substituted xanthones isolated from plants: Structure elucidation, synthesis and antifungal activity. Nat. Prod. Res. 2003;17:195–199. doi: 10.1080/1057563021000040808. [DOI] [PubMed] [Google Scholar]
- 28.Ajithabai M.D., Rameshkumar B., Jayakumar G., Varma L., Nair M.S., Ajaikumar Nair G.P. Decipic acid and 12-acetyl apetalic acid from Calophyllum decipiens Wight. Indian J. Chem. B. 2012;51B:393–397. [Google Scholar]
- 29.Nasir N.M., Rahmani M., Shaari K., Kassim N.K., Go R., Stanslas J., Jeyaraj E.J. Xanthones from Calophyllum gracilipes and their cytotoxic activity. Sains Malays. 2013;42:1261–1266. [Google Scholar]
- 30.Iinuma M., Tosa H., Tanaka T., Yonemori S. Two xanthones from roots of Calophyllum inophyllum. Phytochemistry. 1995;38:725–728. doi: 10.1016/0031-9422(94)00733-A. [DOI] [Google Scholar]
- 31.Ming M., Zhang X., Chen H.F., Zhu L.J., Zeng D.Q., Yang J., Wu G.X., Wu Y.Z., Yao X.S. RXRα transcriptional inhibitors from the stems of Calophyllum membranaceum. Fitoterapia. 2016;108:66–72. doi: 10.1016/j.fitote.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Ito C., Miyamoto Y., Rao K.S., Furukawa H. A novel dibenzofuran and two new xanthones from Calophyllum panciflorum. Chem. Pharm. Bull. 1996;44:441–443. doi: 10.1248/cpb.44.441. [DOI] [Google Scholar]
- 33.Alarcon A.B., Cuesta-Rubio O., Cardenas Perez J., Piccinelli A.L., Rastrelli L. Constituents of the Cuban endemic species Calophyllum pinetorum. J. Nat. Prod. 2008;71:1283–1286. doi: 10.1021/np800079c. [DOI] [PubMed] [Google Scholar]
- 34.Mah S.H., Ee G.C., The S.S., Rahmani M., Lim Y.M., Go R. Phylattrin, a new cytotoxic xanthone from Calophyllum soulattri. Molecules. 2012;17:8303–8311. doi: 10.3390/molecules17078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamura F., Muhamud A., Hashim R., Sulaiman O., Ohara S. Two antifungal xanthones from the heartwood of Calophyllum symingtonianum. Jpn. Agric. Res. 2012;46:181–185. doi: 10.6090/jarq.46.181. [DOI] [Google Scholar]
- 36.Nguyen L.T., Nguyen D.M., Nguyen L.H.D. A new xanthone from the bark of Calophyllum thorelii. Nat. Prod. Res. 2013;27:563–567. doi: 10.1080/14786419.2012.682992. [DOI] [PubMed] [Google Scholar]
- 37.Dharmaratne H.R.W., Wanigasekera W.M.A.P. Xanthones from root bark of Calophyllum thwaitesii. Phytochemistry. 1996;42:249–250. doi: 10.1016/0031-9422(95)00841-1. [DOI] [Google Scholar]
- 38.Molinar-Toribio E., Gonzalez J., Ortega-Barria E., Capson T.L., Coley P.D., Kursar T.A., McPhail K., Cubilla-Rios L. Antiprotozoal activity against Plasmodium falciparum and Trypanosoma cruzi of xanthones isolated from Chrysochlamys tenuis. Pharm. Biol. 2006;44:550–553. doi: 10.1080/13880200600885234. [DOI] [Google Scholar]
- 39.Silva E.M., Araujo Renata M., Freire-Filha L.G., Silveira E.R., Lopes N.P., Elias P.J., Braz-Filho R., Espindola L.S. Clusiaxanthone and tocotrienol series from Clusia pernambucensis and their antileishmanial activity. J. Braz. Chem. Soc. 2013;24:1314–1321. [Google Scholar]
- 40.Wilawan M., Rattanaburi S., Phongpaichit S., Kanjana-Opas A. Antibacterial and cytotoxic xanthones from Cratoxylum cochinchinense. Phytochem. Lett. 2008;1:211–214. [Google Scholar]
- 41.Boonnak N., Chantrapromma S., Tewtrakul S., Sudsai T. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW264.7 macrophages by isolated xanthones from the roots of Cratoxylum formosum ssp. pruniflorum. Arch. Pharm. Res. 2014;37:1329–1335. doi: 10.1007/s12272-014-0338-0. [DOI] [PubMed] [Google Scholar]
- 42.Kamdem W., Mulholland D., Wansi J.D., Mbaze L.M., Powo R. Afzeliixanthones A and B, 2 new prenylated xanthones from Garcinia afzelii ENGL. (Guttiferae) Chem. Pharm. Bull. 2006;54:448–451. doi: 10.1248/cpb.54.448. [DOI] [PubMed] [Google Scholar]
- 43.Niu S.L., Li Z.L., Ji F., Liu G.Y., Zhao N., Liu X.Q., Jing Y.K., Hua H.M. Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry. 2012;77:280–286. doi: 10.1016/j.phytochem.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Milena M., Bassarello C., Bifulco G., Piacente S. Polyisoprenylated benzophenone derivatives from the fruits of Garcinia cambogia and their absolute configuration by quantum chemical circular dichroism calculations. Tetrahedron. 2010;66:139–145. [Google Scholar]
- 45.Jantan I., Saputri F.C. Benzophenones and xanthones from Garcinia cantleyana var. cantleyana and their inhibitory activities on human low-density lipoprotein oxidation and platelet aggregation. Phytochemistry. 2012;80:58–63. doi: 10.1016/j.phytochem.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Ritthiwigrom T., Laphookhieo S., Pyne S.G. Chemical constituents and biological activities of Garcinia cowa Roxb. Maejo. Int. J. Sci. Technol. 2013;7:212–231. [Google Scholar]
- 47.Iinuma M., Tosa H., Tanaka T., Riswan S. Three new xanthones from the bark of Garcinia dioica. Chem. Pharm. Bull. 1996;44:232–234. doi: 10.1248/cpb.44.232. [DOI] [Google Scholar]
- 48.Deachathai S., Mahabusarakam W., Phongpaichit S., Taylor W.C. Phenolic compounds from the fruit of Garcinia dulcis. Phytochemistry. 2005;66:2368–2375. doi: 10.1016/j.phytochem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Mian J.V.Y., Lian E.G.C., Aspollah S.M., Hin T.Y.Y., Yen K.H., Yok C.M.K. Benzophenone constituents from the roots of Garcinia eugenifolia. Res. J. Chem. Environ. 2012;16:36–39. [Google Scholar]
- 50.Ito C., Itoigawa M., Takakura T., Ruangrungsi N., Enjo F., Tokuda H., Nishino H., Furukawa H. Chemical constituents of Garcinia fusca: Structure elucidation of eight new xanthones and their cancer chemopreventive activity. J. Nat. Prod. 2003;66:200–205. doi: 10.1021/np020290s. [DOI] [PubMed] [Google Scholar]
- 51.Mahamodo S., Riviere C., Neut C., Abedini A., Ranarivelo H., Duhal N., Roumy V. Antimicrobial prenylated benzoylphloroglucinol derivatives and xanthones from the leaves of Garcinia goudotiana. Phytochemistry. 2014;102:162–168. doi: 10.1016/j.phytochem.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Alkadi K.A.A., Adam A., Taha M., Hasan M.H., Shah S.A.A. Prenylated xanthone and rubraxanthone with antiplatelet aggregation activity in human whole blood isolated from Garcinia griffithii. Orient. J. Chem. 2013;29:1291–1295. doi: 10.13005/ojc/290404. [DOI] [Google Scholar]
- 53.Deng Y.X., Pan S.L., Zhao S.Y., Wu M.Q., Sun Z.Q., Chen X.H., Shao Z.Y. Cytotoxic alkoxylated xanthones from the resin of Garcinia hanburyi. Fitoterapia. 2012;83:1548–1552. doi: 10.1016/j.fitote.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 54.Klaiklay S., Sukpondma Y., Rukachaisirikul V., Phongpaichit S. Friedolanostanes and xanthones from the twigs of Garcinia hombroniana. Phytochemistry. 2013;85:161–166. doi: 10.1016/j.phytochem.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y., Li D., Jia C.C., Xue C.M., Bai J., Li Z.L., Hua H.M. Three new xanthones from the leaves of Garcinia lancilimba. J. Nat. Med. 2016;70:173–178. doi: 10.1007/s11418-015-0950-4. [DOI] [PubMed] [Google Scholar]
- 56.Ren Y., Lantvit D.D., Carcache E.J., Kardono L.B.S., Riswan S. Proteasome-inhibitory and cytotoxic constituents of Garcinia lateriflora: Absolute configuration of caged xanthones. Tetrahedron. 2010;66:5311–5320. doi: 10.1016/j.tet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J.J., Chen I.S., Duh C.Y. Cytotoxic xanthones and biphenyls from the root of Garcinia linii. Planta Med. 2004;70:1195–1200. doi: 10.1055/s-2004-835851. [DOI] [PubMed] [Google Scholar]
- 58.Chae H.S., Kim E.Y., Han L., Kim N.R., Lam B., Paik J.H., Yoon K.D. Xanthones with pancreatic lipase inhibitory activity from the pericarps of Garcinia mangostana L. (Guttiferae) Eur. J. Lipid Sci. Technol. 2016;118:1416–1421. doi: 10.1002/ejlt.201500516. [DOI] [Google Scholar]
- 59.Nguyen L.H.D., Vo H.T., Pham H.D., Connolly J.D., Harrison L.J. Xanthones from the bark of Garcinia merguensis. Phytochemistry. 2003;63:467–470. doi: 10.1016/S0031-9422(02)00433-8. [DOI] [PubMed] [Google Scholar]
- 60.Chiang Y.M., Kuo Y.H., Oota S., Fukuyama Y. Xanthones and benzophenones from the stems of Garcinia multiflora. J. Nat. Prod. 2003;66:1070–1073. doi: 10.1021/np030065q. [DOI] [PubMed] [Google Scholar]
- 61.Rukachaisirikul V., Ritthiwigrom T., Pinsa A., Sawangchote P., Taylor W.C. Xanthones from the stem bark of Garcinia nigrolineata. Phytochemistry. 2003;64:1149–1156. doi: 10.1016/S0031-9422(03)00502-8. [DOI] [PubMed] [Google Scholar]
- 62.Ee G.C.L., Foo C.H., Jong V.Y.M., Ismail N.H., Sukari M.A., Ya Y.H.T., Awang K. A new xanthone from Garcinia nitida. Nat. Prod. Res. 2012;26:830–835. doi: 10.1080/14786419.2011.559640. [DOI] [PubMed] [Google Scholar]
- 63.Fouotsa H., Tatsimo S.J.N., Neumann B., Michalek C., Mbazoa C.D., Nkengfack A.E., Sewald N., Lannang A.M. A new xanthone derivative from twigs of Garcinia nobilis. Nat. Prod. Res. 2014;28:1030–1036. doi: 10.1080/14786419.2014.903398. [DOI] [PubMed] [Google Scholar]
- 64.Tang Z., Xia Z.X., Qiao S.P., Jiang C., Shen G.R., Cai M.X., Tang X.Y. Four new cytotoxic xanthones from Garcinia nujiangensis. Fitoterapia. 2015;102:109–114. doi: 10.1016/j.fitote.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 65.Shan W.G., Lin T.D., Yu H.N., Chen Y., Zhan Z.J. Polyprenylated xanthones and benzophenones from the bark of Garcinia oblongifolia. Helv. Chim. Acta. 2012;95:1442–1448. doi: 10.1002/hlca.201200019. [DOI] [Google Scholar]
- 66.Tang Y.X., Fu W.W., Wu R., Tan H.S., Shen Z., Xu H.X. Bioassay-guided isolation of prenylated xanthone derivatives from the leaves of Garcinia oligantha. J. Nat. Prod. 2016;79:1752–1761. doi: 10.1021/acs.jnatprod.6b00137. [DOI] [PubMed] [Google Scholar]
- 67.Ha L.D., Hansen P.E., Vang O., Duus F., Pham H.D., Nguyen L.H.D. Cytotoxic geranylated xanthones and O-alkylated derivatives of α-mangostin. Chem. Pharm. Bull. 2009;57:830–834. doi: 10.1248/cpb.57.830. [DOI] [PubMed] [Google Scholar]
- 68.Kardono L.B.S., Hanafi M., Sherley G., Kosela S., Harrison L.J. Bioactive constituents of Garcinia porrecta and G. parvifolia grown in Indonesia. Pak. J. Biol. Sci. 2006;9:483–486. [Google Scholar]
- 69.Wu Y.P., Zhao W., Xia Z.Y., Kong G.H., Lu X.P., Hu Q.F., Gao X.M. Three novel xanthones from Garcinia paucinervis and their anti-TMV activity. Molecules. 2013;18:9663–9669. doi: 10.3390/molecules18089663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vo H.T., Nguyen N.T.T., Maas G., Werz U.R., Pham H.D., Nguyen L.H.D. Xanthones from the bark of Garcinia pedunculata. Phytochem. Lett. 2012;5:766–769. doi: 10.1016/j.phytol.2012.08.009. [DOI] [Google Scholar]
- 71.Jabit M.L., Khalid R., Abas F., Shaari K., Hui L.S., Stanslas J., Lajis N.H. Cytotoxic xanthones from Garcinia penangiana Pierre. Z. Naturforsch. C. 2007;62:786–792. doi: 10.1515/znc-2007-11-1202. [DOI] [PubMed] [Google Scholar]
- 72.Lannang A.M., Komguem J., Ngninzeko F.N., Tangmouo J.G., Lontsi D., Ajaz A., Choudhary M.I., Ranjit R., Devkota K.P., Sondengam B.L. Bangangxanthone A and B, two xanthones from the stem bark of Garcinia polyantha Oliv. Phytochemistry. 2005;66:2351–2355. doi: 10.1016/j.phytochem.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 73.Tantapakul C., Phakhodee W., Ritthiwigrom T., Cheenpracha S., Prawat U., Deachathai S., Laphookhieo S. Rearranged benzophenones and prenylated xanthones from Garcinia propinqua Twigs. J. Nat. Prod. 2012;75:1660–1664. doi: 10.1021/np300487w. [DOI] [PubMed] [Google Scholar]
- 74.Elya B., He H.P., Kosela S., Hanafi M., Hao X.J. A new cytotoxic xanthone from Garcinia rigida. Fitoterapia. 2008;79:182–184. doi: 10.1016/j.fitote.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 75.Sukandar E.R., Siripong P., Khumkratok S., Tip-pyang S. New depsidones and xanthone from the roots of Garcinia schomburgkiana. Fitoterapia. 2016;111:73–77. doi: 10.1016/j.fitote.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 76.Sukpondma Y., Rukachaisirikul V., Phongpaichit S. Xanthone and sesquiterpene derivatives from the fruits of Garcinia scortechinii. J. Nat. Prod. 2005;68:1010–1017. doi: 10.1021/np0580098. [DOI] [PubMed] [Google Scholar]
- 77.Fouotsa H., Lannang A.M., Dzoyem J.P., Tatsimo S.J.N., Neumann B., Mbazoa C.D. Antibacterial and antioxidant xanthones and benzophenone from Garcinia smeathmannii. Planta Med. 2015;81:594–599. doi: 10.1055/s-0035-1545841. [DOI] [PubMed] [Google Scholar]
- 78.Ngoupayo J., Tabopda T.K., Ali M.S. Antimicrobial and immunomodulatory properties of prenylated xanthones from twigs of Garcinia staudtii. Bioorg. Med. Chem. 2009;17:5688–5695. doi: 10.1016/j.bmc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Abe F., Nagafuji S., Okabe H., Higo H., Akahane H. Trypanocidal constituents in plants 2. Xanthones from the stem bark of Garcinia subelliptica. Biol. Pharm. Bull. 2003;26:1730–1733. doi: 10.1248/bpb.26.1730. [DOI] [PubMed] [Google Scholar]
- 80.Duangsrisai S., Choowongkomon K., Bessa L.J., Costa P.M., Amat N., Kijjoa A. Antibacterial and EGFR-tyrosine kinase inhibitory activities of polyhydroxylated xanthones from Garcinia succifolia. Molecules. 2014;19:19923–19934. doi: 10.3390/molecules191219923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo Y.E., Wang L.L., Li Z.L., Niu S.L., Liu X.Q., Hua H.M., Chen H., Chu J., Zhang T.C. Triterpenes and xanthones from the stem bark of Garcinia tetralata. J. Asian Nat. Prod. Res. 2011;13:440–443. doi: 10.1080/10286020.2011.568414. [DOI] [PubMed] [Google Scholar]
- 82.Hay A.E., Aumond M.C., Mallet S., Dumontet V., Litaudon M., Rondeau D., Richomme P. Antioxidant Xanthones from Garcinia vieillardii. J. Nat. Prod. 2004;67:707–709. doi: 10.1021/np0304971. [DOI] [PubMed] [Google Scholar]
- 83.Merza J., Aumond M.C., Rondeau D., Dumontet V., Le Ray A.M. Prenylated xanthones and tocotrienols from Garcinia virgate. Phytochemistry. 2004;65:2915–2920. doi: 10.1016/j.phytochem.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 84.Zhong F.F., Chen Y., Wang P., Feng H.J., Yang G.Z. Xanthones from the bark of Garcinia xanthochymus and their 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity. Chin. J. Chem. 2009;27:74–80. doi: 10.1002/cjoc.200990029. [DOI] [Google Scholar]
- 85.Han Q.B., Yang N.Y., Tian H.L., Qiao C.F., Song J.Z., Chang D.C., Chen S.L., Luo K.Q., Xu H.X. Xanthones with growth inhibition against HeLa cells from Garcinia xipshuanbannaensis. Phytochemistry. 2008;69:2187–2192. doi: 10.1016/j.phytochem.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 86.Biesdorf C., Cortez D.A.G., Audi E.A. Assessment of anxiolytic and panicolytic effects of dichloromethane fraction from stems of Kielmeyera coriacea. Phytomedicine. 2012;19:374–377. doi: 10.1016/j.phymed.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 87.Coqueiro A., Choi Y.H., Verpoorte R., Gupta K.B.S.S., De Mieri M., Hamburger M., Young M.C.M., Stapleton P., Gibbons S., Bolzani V.S. Antistaphylococcal prenylated acylphoroglucinol and xanthones from Kielmeyera variabilis. J. Nat. Prod. 2016;79:470–476. doi: 10.1021/acs.jnatprod.5b00858. [DOI] [PubMed] [Google Scholar]
- 88.Laphookhieo S., Promnart P., Syers J.K., Kanjana-Opas A., Ponglimanont C., Karalai C. Coumarins and xanthones from the seeds of Mammea siamensis. J. Braz. Chem. Soc. 2007;18:1077–1080. doi: 10.1590/S0103-50532007000500031. [DOI] [Google Scholar]
- 89.Ee G.C.L., The S.S., Rahmani M., Taufiq-Yap Y.H., Go R., Mah S.H. A new furanoxanthone from the root bark of Mesua ferrea. Lett. Org. Chem. 2012;9:457–459. [Google Scholar]
- 90.Karunakaran T., Ee G.C.L., The S.S., Daud S., Mah S.H., Lim C.K., Jong V.Y.M., Awang K. A new coumarin from stem bark of Mesua hexapetala. Nat. Prod. Res. 2016;30:1591–1597. doi: 10.1080/14786419.2015.1120727. [DOI] [PubMed] [Google Scholar]
- 91.Maurice T., Marlyse O.W.B., Robert N.J., Pierre M., Victor K., Sterner O., Michelle M., Ephrem N.A. Antimicrobial prenylated xanthones and anthraquinones from barks and fruits of Psorospermum adamauense (Engl.) Nat. Prod. J. 2013;3:60–65. doi: 10.2174/2210315511303010011. [DOI] [Google Scholar]
- 92.Abou-Shoer M., Suwanborirux K., Habib A.A.M., Chang C.J., Cassady J.M. Xanthones and vismiones from Psorospermum febrifugum. Phytochemistry. 1993;34:1413–1420. doi: 10.1016/0031-9422(91)80040-8. [DOI] [Google Scholar]
- 93.Leet J.E., Liu X.H., Drexler D.M., Cantone J.L., Huang S., Mamber S.W., Fairchild C.R., Hussain R., Newman D.J., Kingston D.G.I. Cytotoxic xanthones from Psorospermum molluscum from the madagascar rain forest. J. Nat. Prod. 2008;71:460–463. doi: 10.1021/np070523l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Almanza G.R., Quispe R., Mollinedo P., Rodrigo G., Fukushima O., Villagomez R., Akesson B., Sterner O. Antioxidant and antimutagenic polyisoprenylated benzophenones and xanthones from Rheedia acuminate. Nat. Prod. Commun. 2011;6:1269–1274. [PubMed] [Google Scholar]
- 95.Cottet K., Neudorffer A., Kritsanida M., Michel S., Lallemand M.C., Largeron M. Polycyclic polyprenylated xanthones from Symphonia globulifera: Isolation and biomimetic electrosynthesis. J. Nat. Prod. 2015;78:2136–2140. doi: 10.1021/acs.jnatprod.5b00239. [DOI] [PubMed] [Google Scholar]
- 96.Tala M.F., De Dien Tamokou J., Tchakam P.D., Tane P., Kuiate J.R., Wabo H.K. Antioxidant xanthones, anthraquinones and semi-synthetic derivatives from Vismia rubescens and Vismia laurentii. Pharmacologyonline. 2011;3:1410–1418. [Google Scholar]
- 97.Zhao H., Wang X., Li W., Koike K., Bai H. A new minor homoisoflavonoid from Caesalpinia sappan. Nat. Prod. Res. 2014;28:102–105. doi: 10.1080/14786419.2013.847439. [DOI] [PubMed] [Google Scholar]
- 98.Sob S.V.T., Wabo H.K., Tane P., Ngadjui B.T., Ma D. A xanthone and a polyketide derivative from the leaves of Cassia obtusifolia (Leguminosae) Tetrahedron. 2008;64:7999–8002. doi: 10.1016/j.tet.2008.05.125. [DOI] [Google Scholar]
- 99.Kokotkiewicz A., Luczkiewicz M., Pawlowska J., Luczkiewicz P., Sowinski P., Witkowski J., Bryl E., Bucinski A. Isolation of xanthone and benzophenone derivatives from Cyclopia genistoides (L.) Vent. (honeybush) and their pro-apoptotic activity on synoviocytes from patients with rheumatoid arthritis. Fitoterapia. 2013;90:199–208. doi: 10.1016/j.fitote.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Li W., Sun Y.N., Yan X.T., Yang S.Y., Choi C.W., Kim Y.H. Phenolic compounds from Desmodium caudatum. Nat. Prod. Sci. 2013;19:215–220. [Google Scholar]
- 101.Rezanka T., Jachymova J., Dembitsky V.M. Prenylated xanthone glucosides from Ural’s lichen Umbilicaria proboscidea. Phytochemistry. 2003;62:607–612. doi: 10.1016/S0031-9422(02)00539-3. [DOI] [PubMed] [Google Scholar]
- 102.El-Shanawany M.A., Mohamed G.A., Nafady A.M., Ibrahim S.R.M., Radwan M.M., Ross S.A. A new xanthone from the roots of Centaurium spicatum. Phytochem. Lett. 2011;4:126–128. doi: 10.1016/j.phytol.2010.12.008. [DOI] [Google Scholar]
- 103.Zhou M., Zhou K., Zhao Y.L., Xiang N.J., Zhang T.D., Wang Y.D., Dong W., Ji B.K., Li L.M., Lou J. Three new prenylated xanthones from Comastoma pedunculatum and their anti-tobacco mosaic virus activity. Phytochem. Lett. 2015;11:245–248. doi: 10.1016/j.phytol.2015.01.006. [DOI] [Google Scholar]
- 104.Zhou M., Zhou K., Zhao Y.L., Xiang N.J., Zhang T.D., Wang Y.D., Zhang C.M., Wang Y.D., Dong W., Ji B.K., et al. New xanthones from Comastoma pulmonarium and their anti-tobacco mosaic virus activity. Heterocycles. 2015;91:604–609. [Google Scholar]
- 105.Krstic-Milosevic D., Jankovic T., Vinterhalter B., Menkovic N., Aljancic I., Vinterhalter D. Influence of carbohydrate source on xanthone content in root cultures of Gentiana dinarica Beck. Plant Growth Regul. 2013;71:147–155. doi: 10.1007/s10725-013-9815-6. [DOI] [Google Scholar]
- 106.Isakovic A., Jankovic T., Harhaji L., Kostic-Rajacic S., Nikolic Z., Vajs V., Trajkovic V. Antiglioma action of xanthones from Gentiana kochiana: Mechanistic and structure-activity requirements. Bioorg. Med. Chem. 2008;16:5683–5694. doi: 10.1016/j.bmc.2008.03.069. [DOI] [PubMed] [Google Scholar]
- 107.Aberham A., Schwaiger S., Stuppner H., Ganzera M. Quantitative analysis of iridoids, secoiridoids, xanthones and xanthone glycosides in Gentiana lutea L. roots by RP-HPLC and LC-MS. J. Pharm. Biomed. Anal. 2007;45:437–442. doi: 10.1016/j.jpba.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 108.Tian X., Xu X.Z., Zhan H.Y., Wang A.L. Two new O- and C-glycosylxanthones from Gentiana tizuensis Franch. Indian J. Chem. B. 2003;42B:950–952. [Google Scholar]
- 109.Jankovic T., Krstic-Milosevic D., Aljancic I., Savikin K., Menkovic N., Radanovic D., Milosavljevic S. Phytochemical re-investigation of Gentiana utriculosa. Nat. Prod. Res. 2009;23:466–469. doi: 10.1080/14786410802079477. [DOI] [PubMed] [Google Scholar]
- 110.Petrovic S., Leskovac A., Joksic G. Radioprotective properties of Gentiana dinarica polyphennols on human lymphocytes in vitro. Res. Artic. 2008;95:1035–1041. [Google Scholar]
- 111.Lv L.J., Li M.H. Terpenoilds, flavonoids and xanthones from Gentianella acuta (Gentianaceae) Biochem. Syst. Ecol. 2009;37:497–500. [Google Scholar]
- 112.Urbain A., Marston A., Batsuren D., Purev O., Hostettmann K. Preparative isolation of closely-related xanthones from Gentianella amarella ssp. acuta by high-speed countercurrent chromatography. Phytochem. Anal. 2008;19:514–519. doi: 10.1002/pca.1077. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L., Zou D.Z., Bai S., Li Z.H., Zhang C.H., Li M.H. Chemical constituents from Gentianella turkestanorum (Gentianaceae) Biochem. Syst. Ecol. 2016;65:89–92. doi: 10.1016/j.bse.2016.02.014. [DOI] [Google Scholar]
- 114.Cui Z.H., Li Y., Wang Z.W., Zhang J., Zhang C.H., Zhang N., Li M.H. Chemical constituents from Gentianopsis barbata var. sinensis Ma (Gentianaceae) Biochem. Syst. Ecol. 2013;47:101–103. doi: 10.1016/j.bse.2012.11.003. [DOI] [Google Scholar]
- 115.Yeung M.F., Lau C.B.S., Chan R.C.Y., Zong Y.Y., Che C.T. Search for antimycobacterial constituents from a Tibetan medicinal plant, Gentianopsis paludosa. Phytother. Res. 2009;23:123–125. doi: 10.1002/ptr.2506. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez S., Wolfender J.L., Odontuya G., Purev O., Hostettmann K. Xanthones, secoiridoids and flavonoids from Halenia corniculata. Phytochemistry. 1995;40:1265–1272. doi: 10.1016/0031-9422(95)00402-S. [DOI] [Google Scholar]
- 117.Wang Y., Shi J.G., Wang M.Z., Che C.T., Yeung J.H.K. Vasodilatory actions of xanthones isolated from a Tibetan herb, Halenia elliptica. Phytomedicine. 2009;16:1144–1150. doi: 10.1016/j.phymed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 118.Li L., Li M.H., Zhang N., Huang L.Q. Chemical constituents from Lomatogonium carinthiacum (Gentianaceae) Biochem. Syst. Ecol. 2011;39:766–768. doi: 10.1016/j.bse.2011.07.002. [DOI] [Google Scholar]
- 119.Terreaux C., Maillard M., Gupta M.P., Hostettmann K. Xanthones from Schultesia lisianthoides. Phytochemistry. 1995;40:1791–1795. doi: 10.1016/0031-9422(95)00483-N. [DOI] [Google Scholar]
- 120.Singh P.P., Ambika Chauhan S.M.S. Activity-guided isolation of antioxidant xanthones from Swertia chirayita (Roxb.) H. Karsten (Gentianaceae) Nat. Prod. Res. 2012;26:1682–1686. doi: 10.1080/14786419.2011.592836. [DOI] [PubMed] [Google Scholar]
- 121.Rahman A.U., Pervin A., Feroz M., Choudhary M.I., Qureshi M.M., Perveen S., Mir I., Khan M.I. Phytochemical studies on Swertia cordata. J. Nat. Prod. 1994;57:134–137. doi: 10.1021/np50103a019. [DOI] [Google Scholar]
- 122.Uvarani C., Arumugasamy K., Chandraprakash K., Sankaran M., Ata A., Mohan P.S. A new DNA-intercalative cytotoxic allylic xanthone from Swertia corymbosa. Chem. Biodivers. 2015;12:358–370. doi: 10.1002/cbdv.201400055. [DOI] [PubMed] [Google Scholar]
- 123.Khetwal K.S., Rajput P.S., Sajwan K., Pathak S., Adhikari A. 1,5-Dihydroxy-3,8-dimethoxyxanthone from Swertia cuneata. Indian J. Chem. B. 2003;42B:953–955. doi: 10.1002/chin.200332173. [DOI] [Google Scholar]
- 124.Jiang W., Zhu D.L., Wang M.F., Yang Q.S., Zuo M.Y., Zeng L., Li G.P. Xanthones from the herb of Swertia elata and their anti-TMV activity. Nat. Prod. Res. 2015;30:1810–1815. doi: 10.1080/14786419.2015.1081198. [DOI] [PubMed] [Google Scholar]
- 125.Sun Y.G., Zhang X., Xue X.Y., Zhang Y., Xiao H.B., Liang X.M. Rapid identification of polyphenol C-glycosides from Swertia franchetiana by HPLC-ESI-MS-MS. J. Chromatogr. Sci. 2009;47:190–196. doi: 10.1093/chromsci/47.3.190. [DOI] [PubMed] [Google Scholar]
- 126.Wan L.S., Min Q.X., Wang Y.L., Yue Y.D., Chen J.C. Xanthone glycoside constituents of Swertia kouitchensis with α-glucosidase inhibitory activity. J. Nat. Prod. 2013;76:1248–1253. doi: 10.1021/np400082g. [DOI] [PubMed] [Google Scholar]
- 127.Hajimehdipoor H., Dijoux-Franca M.G., Mariotte A.M., Amanzadeh Y., Sadat-Ebrahimi S.E., Ghazi-Khansari M. Two new xanthone diglycosides from Swertia longifolia Boiss. Nat. Prod. Res. 2006;20:1251–1255. doi: 10.1080/14786410600906319. [DOI] [PubMed] [Google Scholar]
- 128.Uvarani C., Chandraprakash K., Sankaran M., Ata A., Mohan P.S. Antioxidant and structure-activity relationships of five tetraoxygenated xanthones from Swertia minor (Griscb.) Knobl. Nat. Prod. Res. 2012;26:1265–1270. doi: 10.1080/14786419.2011.561494. [DOI] [PubMed] [Google Scholar]
- 129.Luo C.T., Mao S.S., Liu F.L., Yang M.X., Chen H.R., Kurihara H., Li Y.L. Antioxidant xanthones from Swertia mussotii, a high altitude plant. Fitoterapia. 2013;91:140–147. doi: 10.1016/j.fitote.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 130.Pant N., Misra H., Jain D.C. A xanthone glycoside from aerial parts of Swertia paniculata. J. Saudi Chem. Soc. 2014;18:551–554. doi: 10.1016/j.jscs.2011.11.001. [DOI] [Google Scholar]
- 131.Li J.C., Feng L., Sun B.H., Ikeda T., Nohara T. Hepatoprotective activity of the constituents in Swertia pseudochinensis. Biol. Pharm. Bull. 2005;28:534–537. doi: 10.1248/bpb.28.534. [DOI] [PubMed] [Google Scholar]
- 132.Zheng X.Y., Yang Y.F., Li W., Zhao X., Sun Y., Sun H., Wang Y.H., Pu X.P. Two xanthones from Swertia punicea with hepatoprotective activities in vitro and in vivo. J. Ethnopharmacol. 2014;153:854–863. doi: 10.1016/j.jep.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 133.Rana V.S., Rawat M.S. A new xanthone glycoside and antioxidant constituents from the rhizomes of Swertia speciosa. Chem. Biodivers. 2005;2:1310–1315. doi: 10.1002/cbdv.200590102. [DOI] [PubMed] [Google Scholar]
- 134.Silva L.F., Luiz F., Lima E.S., Vasconcello M.C. In vitro and in vivo antimalarial activity and cytotoxicity of extracts, fractions and a substance isolated from the Amazonian plant Tachia grandiflora (Gentianaceae) Memórias Instituto Oswaldo Cruz. 2013;108:501–507. doi: 10.1590/S0074-02762013000400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Periyar S.S., Magesh V., Raja V., Mahendran T.S., Muthumary J., Kalaichelvan P.T., Murugesan K. Antimutagenicity of mangiferin purified from Salacia chinensis Linn. Int. Multidiscip. Res. J. 2011;1:1–5. [Google Scholar]
- 136.Duarte L.P., Figueiredo R.C., Faria De Sousa G., Soares D.B.S., Rodrigues S.B.V., Silva F.C., Silva G.D.F., Filho S.A.V. Chemical constituents of Salacia elliptica (Celastraceae) Quim. Nova. 2010;33:900–903. doi: 10.1590/S0100-40422010000400026. [DOI] [Google Scholar]
- 137.Nishida Y., Eto M., Miyashita H., Ikeda T., Yamaguchi K., Yoshimitsu H., Nohara T., Ono M. A new homostilbene and two new homoisoflavones from the bulbs of Scilla scilloides. Chem. Pharm. Bull. 2008;56:1022–1025. doi: 10.1248/cpb.56.1022. [DOI] [PubMed] [Google Scholar]
- 138.Hashida W., Tanaka N., Takaishi Y. Prenylated xanthones from Hypericum ascyron. J. Nat. Med. 2007;61:371–374. doi: 10.1007/s11418-007-0152-9. [DOI] [Google Scholar]
- 139.Zhou Z.B., Zhang Y.M., Luo J.G., Kong L.Y. Cytotoxic polycyclic polyprenylated acylphloroglucinol derivatives and xanthones from Hypericum attenuatum. Phytochem. Lett. 2016;15:215–219. doi: 10.1016/j.phytol.2016.02.004. [DOI] [Google Scholar]
- 140.Lou J., Wang H., Liu G.Y., Yang J.X., Li L.M., Hu Q.F., Gao X.M. Isolation of new xanthone from Hypericum chinense and its cytotoxicity. Asian J. Chem. 2015;27:2102–2104. doi: 10.14233/ajchem.2015.17780. [DOI] [Google Scholar]
- 141.Matsuoka E., Machida K., Kikuchi M. Chemical constituents of Hypericum erectum Thunb. J. Nat. Med. 2008;62:467–469. doi: 10.1007/s11418-008-0256-x. [DOI] [PubMed] [Google Scholar]
- 142.Wabo H., Kowa T.K., Lonfouo A.H.N., Tchinda A.T., Tane P., Kikuchi H., Frederich M., Oshima Y. Phenolic compounds and terpenoids from Hypericum lanceolatum. Rec. Nat. Prod. 2012;6:94–100. [Google Scholar]
- 143.Ali M., Latif A., Zaman K., Arfan M., Maitland D., Ahmad H., Ahmad M. Anti-ulcer xanthones from the roots of Hypericum oblongifolium Wall. Fitoterapia. 2014;95:258–265. doi: 10.1016/j.fitote.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 144.Ishiguro K., Nakajima M., Fukumoto H., Isoi K. A xanthone substituted with an irregular monoterpene in cell suspension cultures of Hypericum patulum. Phytochemistry. 1995;39:903–905. doi: 10.1016/0031-9422(95)00080-Q. [DOI] [Google Scholar]
- 145.Sun L.W., Sun Y.N., Yan X.T., Yang S.Y., Choi C.W., Hyun J.W., Kang H.K., Paek K.Y., Kim Y.H. Isolation of xanthones from adventitious roots of St. John’s Wort (Hypericum perforatum L.) and their antioxidant and cytotoxic activities. Food Sci. Biotechnol. 2013;22:945–949. [Google Scholar]
- 146.Xiao Z.Y., Shiu W.K.P., Zeng Y.H., Mu Q., Gibbons S. A naturally occurring inhibitory agent from Hypericum sampsonii with activity against multidrug-resistant staphylococcus aureus. Pharm. Biol. 2008;46:250–253. doi: 10.1080/13880200701739405. [DOI] [Google Scholar]
- 147.Tanaka N., Takaishi Y., Shikishima Y., Nakanishi Y., Bastow K., Lee K.H., Takeda Y., Kodzhimatov O.K. Prenylated benzophenones and xanthones from Hypericum scabrum. J. Nat. Prod. 2004;67:1870–1875. doi: 10.1021/np040024+. [DOI] [PubMed] [Google Scholar]
- 148.Gamiotea-Turro D., Cuesta-Rubio O., Prieto-Gonzalez S., De Simone F., Passi S., Rastrelli L. Antioxidative constituents from the leaves of Hypericum styphelioides. J. Nat. Prod. 2004;67:869–871. doi: 10.1021/np030364f. [DOI] [PubMed] [Google Scholar]
- 149.Al-Khalil S., Tosa H., Iinuma M. A xanthone C-glycoside from Iris nigricans. Phytochemistry. 1995;38:729–731. doi: 10.1016/0031-9422(94)00641-6. [DOI] [Google Scholar]
- 150.Mbouangouere R.N., Tane P., Ngamga D., Khan S.N., Choudhary M.I., Ngadjui B.T. A new steroid and α-glucosidase inhibitors from Anthocleista schweinfurthii. Res. J. Med. Plant. 2007;1:106–111. [Google Scholar]
- 151.Ateufack G., Nguelefack T.B., Mbiantcha M., Tane P., Kamanyi A. Spasmogenic activity of 1-hydroxy-3,7,8-trimethoxyxanthone isolated from the methanol extract of the stem bark of Anthocleista vogelii planch. (Loganiaceae) in rats. Pharmacologyonline. 2007;3:374–384. [Google Scholar]
- 152.Ee G.C., Teo S.H., Rahmani M., Lim C.K., Lim Y.M., Go R. Artomandin, a new xanthone from Artocarpus kemando (Moraceae) Nat. Prod. Res. 2011;25:995–1003. doi: 10.1080/14786419.2010.534471. [DOI] [PubMed] [Google Scholar]
- 153.Jayasinghe U.L., Samarakoon T.B., Kumarihamy B.M.M., Hara N., Fujimoto Y. Four new prenylated flavonoids and xanthones from the root bark of Artocarpus nobilis. Fitoterapia. 2008;79:37–41. doi: 10.1016/j.fitote.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 154.Hashim N.M., Rahmani M., Sukari M.A., Ali A.M., Alitheen N.B., Go R., Ismail H.B.M. Two new xanthones from Artocarpus obtusus. J. Asian Nat. Prod. Res. 2010;12:106–112. doi: 10.1080/10286020903450411. [DOI] [PubMed] [Google Scholar]
- 155.Chen L., Zhou Q., Li B., Liu S.J., Dong J.X. A new flavonoid from Cudrania cochinchinensis. Nat. Prod. Res. 2015;29:1217–1221. doi: 10.1080/14786419.2014.997234. [DOI] [PubMed] [Google Scholar]
- 156.Liang B., Li H.R., Xu L.Z., Yang S.L. Xanthones from the roots of Cudrania fruticosa Wight. J. Asian Nat. Prod. Res. 2007;9:393–397. doi: 10.1080/10286020600782355. [DOI] [PubMed] [Google Scholar]
- 157.Quang T.H., Ngan N.T.T., Yoon C.S., Cho K.H., Kang D.G., Lee H.S., Kim Y.G., Oh H. Protein tyrosine phosphatase 1B inhibitors from the roots of Cudrania tricuspidata. Molecules. 2015;20:11173–11183. doi: 10.3390/molecules200611173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakashima K., Tanaka T., Murata H., Kaburagi K., Inoue M. Xanthones from the roots of Maclura cochinchinensis var. gerontogea and their retinoic acid receptor-α agonistic activity. Bioorg. Med. Chem. Lett. 2015;25:1998–2001. doi: 10.1016/j.bmcl.2015.02.075. [DOI] [PubMed] [Google Scholar]
- 159.Ahmad A., Singh D.K., Fatima K., Tandon S., Luqman S. New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity. Ind. Crops Prod. 2014;58:125–132. doi: 10.1016/j.indcrop.2014.04.008. [DOI] [Google Scholar]
- 160.Rezanka T., Sigler K. Hirtusneanoside, an unsymmetrical dimeric tetrahydroxanthone from the Lichen Usnea hirta. J. Nat. Prod. 2007;70:1487–1491. doi: 10.1021/np070079m. [DOI] [PubMed] [Google Scholar]
- 161.Silveira E.R., Falcao M.J.C., Kingston D.G.I., Glass T.E. Pentaoxygenated xanthones from Bredemeyera floribunda. Phytochemistry. 1995;39:1433–1436. doi: 10.1016/0031-9422(95)00103-E. [DOI] [Google Scholar]
- 162.Filho H.D.S., Pacheco L.C., Andrade E.S., Correa M.J.C., Araujo R.N.M., Guilhon G.M.S.P., Da Silva J.K.R., Santos L.S. Xanthones from the roots of Moutabea guianensis Aubl. Molecules. 2015;20:127–134. doi: 10.3390/molecules20010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lin L.L., Huang F., Chen S.B., Yang D.J., Chen S.L., Yang J.S., Xiao P.G. Xanthones from the roots of Polygala caudata and their antioxidation and vasodilatation activities in vitro. Planta Med. 2005;71:372–375. doi: 10.1055/s-2005-864108. [DOI] [PubMed] [Google Scholar]
- 164.Deng S.M., Yang X.H., Zhao Y.X., Zhou J. New xanthones from Polygala crotalarioides. Chem. Res. Chin. Univ. 2006;22:400–402. doi: 10.1016/S1005-9040(06)60127-X. [DOI] [Google Scholar]
- 165.Klein L.C.J., Gandolfi R.B., Santin J.R., Lemos M., Cechinel Filho V., Andrade S.F. Antiulcerogenic activity of extract, fract ions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalaceae) Naunyn Schmiedebergs Arch. Pharmacol. 2010;381:121–126. doi: 10.1007/s00210-009-0485-x. [DOI] [PubMed] [Google Scholar]
- 166.Wu J.F., Tu P.F., Zhan H.T., Gao J.C. Dioxyxanthones from Polygala hongkongensis and their cytotoxicity. Chem. Res. Chin. Univ. 2011;27:777–779. [Google Scholar]
- 167.Qing C.X., Li C.J., Zuo L., Yang J.Z., Zhang D.M. Three new xanthones from the roots of Polygala japonica Houtt. J. Asian Nat. Prod. Res. 2009;11:465–469. doi: 10.1080/10286020902835547. [DOI] [PubMed] [Google Scholar]
- 168.Dao T.T., Dang T.T., Nguyen P.H., Kim E., Thuong P.T., Won K. Xanthones from Polygala karensium inhibit neuraminidases from influenza a viruses. Bioorg. Med. Chem. Lett. 2012;22:3688–3692. doi: 10.1016/j.bmcl.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 169.Jiang Y., Zhang W., Tu P.F., Xu X.J. Xanthone glycosides from Polygala tenuifolia and their conformational analyses. J. Nat. Prod. 2005;68:875–879. doi: 10.1021/np050026+. [DOI] [PubMed] [Google Scholar]
- 170.Zhou Y.H., Guo Q., Jiang Y., Tu P.F. Chemical constituents from the roots of Polygala wattersii Hance. J. Chin. Pharm. Sci. 2014;23:723–730. doi: 10.5246/jcps.2014.10.092. [DOI] [Google Scholar]
- 171.Yang X.D., An N., Xu L.Z., Yang S.L. New xanthone glycosides from Securidaca inappendiculata. J. Asian Nat. Prod. Res. 2002;4:141–145. doi: 10.1080/10286020290027434. [DOI] [PubMed] [Google Scholar]
- 172.Dibwe D.F., Awale S., Kadota S., Tezuka Y. Muchimangins A-D: Novel diphenylmethyl-substituted xanthones from Securidaca longepedunculata. Tetrahedron Lett. 2012;53:6186–6190. doi: 10.1016/j.tetlet.2012.08.115. [DOI] [Google Scholar]
- 173.Pascale T., Mondolot L., Gargadennec A., Kochko A., Hamon S., Fruchier A., Campa C. First report on mangiferin (C-glucosyl-xanthone) isolated from leaves of a wild coffee plant, Coffea pseudozanguebariae (Rubiaceae) Acta Bot. Gallica. 2008;155:513–519. [Google Scholar]
- 174.Siddiqui B.S., Sattar F.A., Begum S., Gulzar T., Ahmad F. Chemical constituents from the stems of Morinda citrifolia linn. Arch. Pharm. Res. 2007;30:793–798. doi: 10.1007/BF02978826. [DOI] [PubMed] [Google Scholar]
- 175.Tala M.F., Wabo H.K., Zeng G.Z., Ji C.J., Tane P., Tan N.H. A prenylated xanthone and antiproliferative compounds from leaves of Pentadesma butyracea. Phytochem. Lett. 2013;6:326–330. doi: 10.1016/j.phytol.2013.03.016. [DOI] [Google Scholar]
- 176.Abdissa N., Heydenreich M., Midiwo J.O., Ndakala A., Majer Z., Neumann B., Stammler H.G., Sewald N., Yenesew A. A xanthone and a phenylanthraquinone from the roots of Bulbine frutescens, and the revision of six seco-anthraquinones into xanthones. Phytochem. Lett. 2014;9:67–73. doi: 10.1016/j.phytol.2014.04.004. [DOI] [Google Scholar]
- 177.Carvalho M.J., Carvalho L.M., Ferreira A.M., Silva A.M.S. A new xanthone from Hedychium gardnerianum. Nat. Prod. Res. 2003;17:445–449. doi: 10.1080/1478641031000118906. [DOI] [PubMed] [Google Scholar]
- 178.Li D.H., Li C.X., Jia C.C., Sun Y.T., Xue C.M., Bai J., Hua H.M., Liu X.Q., Li Z.L. Xanthones from Garcinia paucinervis with in vitro anti-proliferative activity against HL-60 cells. Arch. Pharm. Res. 2016;39:172–177. doi: 10.1007/s12272-015-0692-6. [DOI] [PubMed] [Google Scholar]
- 179.Kaennakam S., Siripong P., Tip-pyang S. Kaennacowanols A–C, three new xanthones and their cytotoxicity from the roots of Garcinia cowa. Fitoterapia. 2015;102:171–176. doi: 10.1016/j.fitote.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 180.Sukandar E.R., Ersam T., Fatmawati S., Siripong P., Aree T., Tip-pyang S. Cylindroxanthones A–C, three new xanthones and their cytotoxicity from the stem bark of Garcinia cylindrocarpa. Fitoterapia. 2016;108:62–65. doi: 10.1016/j.fitote.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 181.Kwon J., Hiep N.T., Kim D.W., Hwang B.Y., Lee H.J., Mar W., Lee D. Neuroprotective xanthones from the root bark of Cudrania tricuspidata. J. Nat. Prod. 2014;77:1893–1901. doi: 10.1021/np500364x. [DOI] [PubMed] [Google Scholar]
- 182.Chen Y., Fan H., Yang G.Z., Jiang Y., Zhong F.F., He H.W. Prenylated xanthones from the bark of Garcinia xanthochymus and their 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities. Molecules. 2010;15:7438–7449. doi: 10.3390/molecules15107438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Trisuwan K., Ritthiwigrom T. Benzophenone and xanthone derivatives from the inflorescences of Garcinia cowa. Arch. Pharm. Res. 2012;35:1733–1738. doi: 10.1007/s12272-012-1004-z. [DOI] [PubMed] [Google Scholar]
- 184.Chen Y., He S.W., Tang C., Li J., Yang G.Z. Caged polyprenylated xanthones from the resin of Garcinia hanburyi. Fitoterapia. 2016;109:106–112. doi: 10.1016/j.fitote.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 185.Ee G.C., Mah S.H., Rahmani M., Taufiq-Yap Y.H., The S.S., Yang M. A new furanoxanthone from the stem bark of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011;13:956–960. doi: 10.1080/10286020.2011.600248. [DOI] [PubMed] [Google Scholar]
- 186.Gomes A.S., Brandão P., Fernandes C.S.G., da Silva M.R.P.C., de Sousa M.E.D.S.P., Pinto M.M.M. Drug-like properties and ADME of xanthone derivatives: The antechamber of clinical trials. Curr. Med. Chem. 2016;23:3654–3686. doi: 10.2174/0929867323666160425113058. [DOI] [PubMed] [Google Scholar]
- 187.Han S.Y., You B.H., Kim Y.C., Chin Y.W., Choi Y.H. Dose-independent ADME properties and tentative identification of metabolites of α-mangostin from Garcinia mangostana in mice by automated microsampling and UPLC-MS/MS methods. PLoS ONE. 2015;10:e0131587. doi: 10.1371/journal.pone.0131587. [DOI] [PMC free article] [PubMed] [Google Scholar]